Abstract
Application of hormesis in aging research and interventions is becoming increasingly attractive and successful. The reason for this is the realization that mild stress-induced activation of one or more stress response (SR) pathways, and its consequent stimulation of repair mechanisms, is effective in reducing the age-related accumulation of molecular damage. For example, repeated heat stress-induced synthesis of heat shock proteins has been shown to have a variety of anti-aging effects on growth and other cellular and biochemical characteristics of normal human skin fibroblasts, keratinocytes and endothelial cells undergoing aging in vitro. Therefore, searching for potential hormetins – conditions and compounds eliciting SR-mediated hormesis – is drawing attention of not only the researchers but also the industry involved in developing healthcare products, including nutriceuticals, functional foods and cosmeceuticals. Here we present the example of a skin care cosmetic as one of the first successful product developments incorporating the ideas of hormesis. This was based on the studies to analyse the molecular effects of active ingredients extracted from the roots of the Chinese herb Sanchi (Panax notoginseng) on gene expression at the level of mRNAs and proteins in human skin cells. The results showed that the ginsenosides extracted from Sanchi induced the transcription of stress genes and increased the synthesis of stress proteins, especially the heat shock protein HSP1A1 or Hsp70, in normal human keratinocytes and dermal fibroblasts. Furthermore, this extract also has significant positive effects against facial wrinkles and other symptoms of facial skin aging as tested clinically, which may be due to its hormetic mode of action by stress-induced synthesis of chaperones involved in protein repair and removal of abnormal proteins. Acceptance of such a hormesis-based product by the wider public could be instrumental in the social recognition of the concept of hormesis as the beneficial effects of mild stress of choice, and will encourage the development of novel health care products with physical, nutritional and mental hormetins.
Keywords: anti-aging, hormesis, hormetin, proteasome, stress
INTRODUCTION
Hormesis in aging is defined as the life supporting beneficial effects resulting from the cellular responses to single or multiple rounds of mild stress (Rattan, 2004a; 2004b; 2008a; 2008b). Various mild stresses that have been reported to delay aging and prolong longevity in cells and organisms include thermal shock, irradiation, heavy metals, pro-oxidants, electromagnetic field, hypergravity, exercise and food restriction (Le Bourg and Rattan, 2008; Rattan and Demirovic, 2009; 2010a). Hormesis is also one of the most likely explanations for the health beneficial effects of various foods and their components, including spices, flavanoids and polyphenols (Hayes, 2007; Wiegant et al., 2009; Hayes, 2010; Demirovic and Rattan, 2011; Lima et al., 2011).
The operating principle behind hormesis is that low levels of stress from physical, chemical and biological stressors often result in the functional improvement of cells, tissues, organs and organisms – a phenomenon termed physiological hormesis (Calabrese et al., 2007; Mattson, 2008a; 2008b). A crucial aspect of the stress response (SR) is that it is not monotonic with respect to the dose of the stressor, rather it is almost always characterized by a nonlinear biphasic relationship. Several meta-analyses performed on a large number of papers published in the fields of toxicology, pharmacology, medicine, and radiation biology have led to the conclusion that the most fundamental shape of the dose response is neither threshold nor linear, but is U- or inverted U-shaped, depending on the endpoint being measured (Calabrese et al., 2007; Calabrese, 2008). In this article, we first present a brief review of the logic for the application of hormesis in aging interventions based on the current understanding of the biological basis of aging, and then discuss a case study in the development of a novel skin care cosmetic based on these ideas.
AGING AS THE SHRINKAGE OF THE HOMEODYNAMIC SPACE
Aging is an emergent, epigenetic and a meta-phenomenon, which is not controlled by a single mechanism. Although individually no tissue, organ or system becomes functionally exhausted even in very old organisms, it is their combined interaction and interdependence that determines the survival of the whole. Survival of an organism is a constant struggle between the occurrence of damage and the mechanisms of maintenance and repair. There are three major sources of damages within a cell: (1) reactive oxygen species (ROS) and free radicals (FR) formed due to external inducers of damage (for example ultra-violet rays), and as a consequence of cellular metabolism involving oxygen, metals and other metabolites; (2) nutritional components such as glucose and its metabolites, and their biochemical interactions with FR; and (3) spontaneous errors in biochemical processes, such as DNA duplication, transcription, post-transcriptional processing, translation, and post-translational modifications. Innumerable damaging events occur in cells constantly, but a wide range of molecular, cellular and physiological pathways of repair counteract them and assure survival. All these processes involve numerous genes whose products and their interactions give rise to a “homeodynamic space” or the “buffering capacity”, which is the ultimate determinant of an individual’s chance and ability to survive and maintain a healthy state (Rattan, 2006; 2011).
An effective homeodynamic space or buffering capacity has three major characteristics: stress response (SR), the ability for damage prevention, repair and removal, and the ability for continuous remodeling and adaptation (Rattan, 2011). One useful way of conceptualizing aging is the progressive shrinkage of the homeodynamic space during the period of survival beyond the natural lifespan termed essential lifespan (Carnes, 2011; Rattan, 2000a, 2000b; Rattan, 2011; Rattan and Clark, 2005). A critical component of the homeodynamic space is the SR. In this context, the term “stress” is defined as a signal generated by any physical, chemical or biological factor (stressor), which in a living system initiates a series of events in order to counteract, adapt and survive. There are seven main pathways of SR intrinsic to cells: heat shock response (HSR), unfolded protein response (UPR), autophagy, antioxidant response, inflammatory response, DNA repair response and sirtuin response (Rattan and Demirovic, 2010a; 2010b). Not all pathways of SR respond to every stressor, and although there may be some overlap, generally SR pathways are quite specific. The specificity of the response is mostly determined by the nature of the damage induced by the stressor and the variety of downstream effectors involved (Rattan and Demirovic, 2010a; 2010b).
The seven major pathways of SR listed above can be used as the screening platform for discovering, testing and monitoring the effects of novel hormetins. Such hormetins may be categorized as: (1) physical hormetins, such as exercise, hypergravity, heat and radiation; (2) biological and nutritional hormetins, such as infections, micronutrients, spices and other natural and synthetic compounds; and (3) psychological hormetins, such as mental challenge and focused attention or meditation. Several review and research articles have been published with respect to testing potential anti-aging hormetins, such as heat shock, irradiation, hypergravity, curcumin, kinetin, rosmarinic acid, ferulic acid and food restriction (Barone et al., 2009; Berge et al., 2006; 2008; Birringer, 2011; Demirovic and Rattan, 2011; Le Bourg and Rattan, 2008; Mattson, 2008a; 2008b; Rattan, 2008a; 2008b; Lima, et al., 2011).
PANAX NOTOGINSENG (SANCHI) AS A HORMETIN
A novel source of biological hormetin is the Sanchi ginseng, Panax notoginseng, cultivated in China for more than 400 years, and whose root components are claimed to have several medicinal properties including stanching the blood, dispersion of gore and reduction of the pain caused by blood diseases (Guo et al., 2010). The main active components in this plant were identified to be saponins, flavonoids, dencichine and polysac-charides (Guo, et al., 2010). Previously, an extract from the roots of a Korean wild ginseng Sansam (Panax ginseng) has been tested for its use as a cosmetic and a patent has been granted for its formulation (Choi et al., 2008).
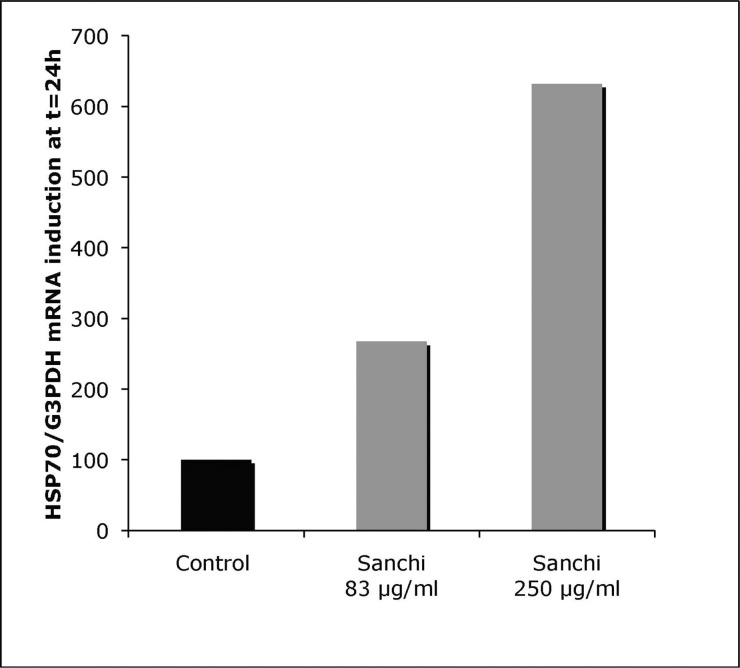
In LVMH Research Laboratories, one of the research areas being pursued is to test natural and synthetic compounds for their effects on human skin cells in vitro, and on human volunteers, with a view to identify, select and develop potential skin care cosmetics and cosmeceuticals. During the course of such studies in 2007, the roots of Panax notoginseng (Burkill, F.H. Chen), grown in the Wenshan, Yunnan province of China, were ground and extracted with ethanol, followed by the separation, concentration, discoloration and crystallisation yielding a mixture of saponins. The extracted and purified material had more than 95% saponins with ginsenosides Rb1 and Rg1 as the main constituents. In the preliminary studies performed to test the effects of the above extract on the expression of genes at the level of mRNA, it was found to induce the expression of one of the main stress response genes HSP1A1 or Hsp70 in human epidermal keratinocytes up to 6-fold, depending on the dose of Sanchi extract (Fig. 1).
FIG. 1.
Normal human keratinocytes in culture have a several fold increase in the level of HSP1A1 / Hsp70 mRNA after 24 hr exposure to different doses of Sanchi extract. The data are presented after normalising with the expression of mRNA of a house-keeping gene G3PDH.
Following the above observations on the effect of Sanchi extract in the induction of stress gene expression, further detailed studies were performed with respect to the synthesis of the stress protein Hsp70 in human skin fibroblasts. These tests were done using a blinded-protocol with coded samples at an independent test laboratory “StratiCell” (http://www.straticell.com/) in Belgium under the supervision of the lead author of this article (SISR) during the period August to November 2009. For this purpose, normal human diploid fibroblasts (BJ-HDF), at about 50% lifespan completed in vitro were used under standard cell culture protocols for testing the effects of a compound on cell growth, survival and other biochemical characteristics (Demirovic and Rattan, 2011; Magalhaes et al., 2002; Rattan and Sodagam, 2005). Cells were treated with various doses of Sanchi extracts (LVMH test compound labelled as LVMH2009/1), and were analysed for cell survival by using mitochondrial activity assay MTS and for stress proteins by Western blotting, respectively. The compounds were dissolved in the solvent dimethyl sulfoxide (DMSO) whose final concentration in cell culture medium was never more than 0.05%, and had no apparent biological effects on cells. Celastrol, a well known inducer of stress proteins, was used as an internal control (Hansen and Bross, 2010; Westerheide et al., 2004) Levels of three stress markers, Hsp70, Hsp32 and Nrf2 were determined by Western blot analysis of proteins, using respective antibodies and standard methods (Magalhaes et al., 2002).
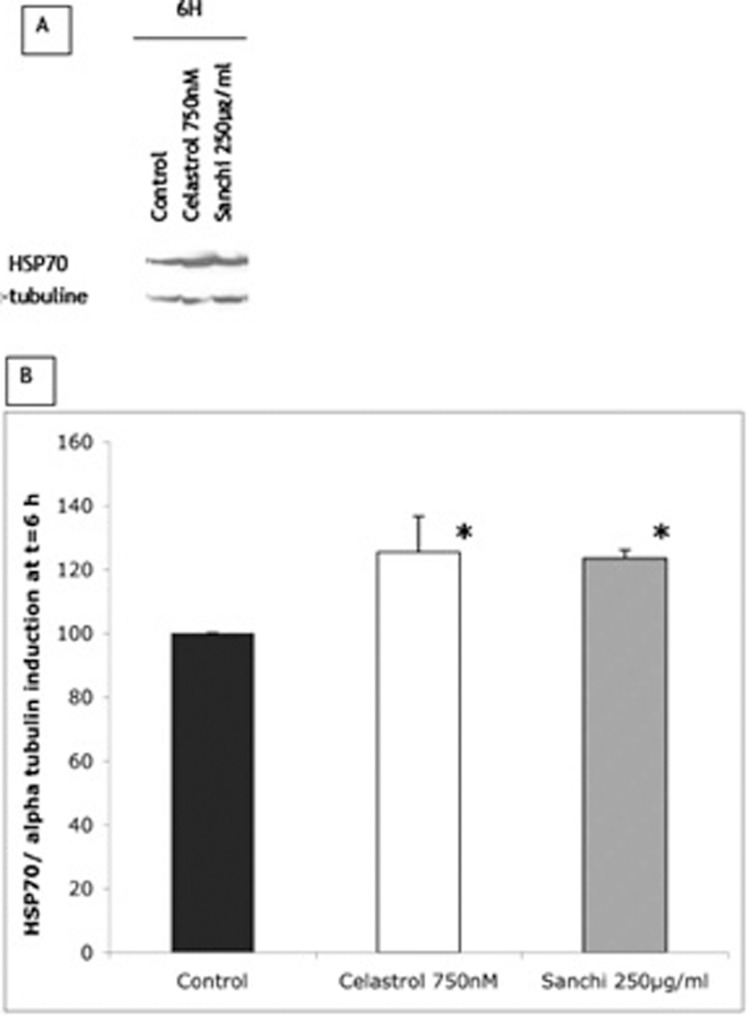
There was no negative effect on the viability of human cells exposed to DMSO, celastrol (750 nM) or Sanchi extract (250 μg/ml) for 24 hr, as determined by the MTT mitochondrial activity assay (data not shown). As regards the induction of stress proteins, Fig. 2 shows the results of the effects of Sanchi extract (250 μg/ml) and celasterol (750 nM) on the induction of Hsp70 synthesis within 6 hr of treatment. While celastrol also induced the synthesis of the other two stress proteins Hsp32 and Nrf2, Sanchi extract did not induce the synthesis of these proteins to any significant level (data not shown).
FIG. 2.
Induction of HSP 70 normalized with alpha-tubulin in normal human skin fibroblasts treated for 6 hr with 750 nM celastrol (positive control) and 250 μg /ml Sanchi extract. Monoclonal mouse anti-human Hsp70 antibody (1/1500 dilution; Gentaur) and sheep ECL anti-mouse IgG HRP (1/200,000 dilution; GE Healthcare-Amersham) were used for western blotting of Hsp70 (A). Western blot imaging and quantification was done by ImageJ software (B). Statistical analysis was performed with STATGRAPHICS PLUS CENTURION package UNIWIN 6.0, with results of 3 independent experiments. Levels of Hsp70 were increased significantly (p<0.05), by 25% and 24% in celastrol- and Sanchi-treated cells, respectively.
Induction of synthesis of Hsp70 protein by Sanchi qualifies it to be called a hormetin in accordance with the definition of hormetin as discussed above. Furthermore, the observed extent (about 24%) of induced synthesis of Hsp70 is within the limits of a hormetic response (Calabrese et al., 2007). A similar induction of Hsp70 in human skin fibroblasts by another hormetic condition (mild heat shock) has been shown to associate with several biologically beneficial effects in human skin fibroblasts and keratincoytes undergoing aging in vitro, including lifespan extension, maintenance of youthful morphology, improved cellular functioning and enhanced abilities of maintenance and repair (Beedholm et al., 2004; Fonager et al., 2002; Nielsen et al., 2006; Rattan, 1998; 2005; 2008a; 2008b; Rattan and Ali, 2007; Rattan et al., 2009 Verbeke et al., 2000; 2001a; 2002). Furthermore, pleiotropic role of Hsp70 including its activity as a protein chaperone, as an anti-inflammatory agent, and a stimulator of proteasomal degradation pathways suggest its potential benefits as an anti-aging protein (Daugaard et al., 2007; Liberek et al., 2008; Singh et al., 2006; Söti et al., 2005; Verbeke et al., 2001b).
HORMETIN-CONTAINING COSMETIC PRODUCT
In parallel with the experiments performed on human skin cells in vitro, which demonstrated the hormetic nature of the Sanchi extract by its ability to induce the synthesis of a stress protein, LVMH labs had also undertaken studies on testing the effects of Sanchi extract on the facial characteristics of human volunteers. These tests were performed in accordance with the widely accepted and standardised protocols for such studies, including obtaining the appropriate ethical permissions (Glaser, 2004; Hunt et al., 2010). A cosmetic formulation containing Sanchi extract was tested on up to 43 healthy women volunteers for a period of up to 1 month. Some of the main results obtained in these studies included the maintenance of skin firmness as measured by a cutometer, and a significant improvement in the orientation and depth of facial wrinkles (data not shown here). As a result of these studies, a skin care cosmetic product, brand-named “Vax’in For Youth”, was launched in 2010 by Givenchy (http://www.givenchy.com/), as a novel product developed from the ideas of hormesis.
CONCLUSION AND PERSPECTIVE
In this article we have presented a case study of the development of a novel skin care product, which is based in the concept of hormesis and hormetins. This is an example of incorporating the latest scientific concept in the development of health care prodcuts and challenging the prevalent view that stress is always harmful. Furthermore, this also demonstrates that being aware of the phenomenon of hormesis can result in discovering the usefulness of new compounds which otherwise may have been rejected due to their molecular effects of stress induction.
This study also shows that rigorous scientific experiments using appropriate model systems are necessary in order to elucidate the molecular mechanisms of action and to establish the chemical nature of the compound. For example, treatment of human skin cells in vitro with Sanchi extract resulted in HSR but not in Nrf2 response, thus indicating that Sanchi does not induce oxidative damage in human cells and may not affect anti-oxidative pathways immediately. Instead, Sanchi brings about its cell strengthening effects by hormetic stimulation of HSR via mild denaturation and enhanced removal of abnormal proteins. Furthermore, these results demonstrate that the induction of HSR by Sanchi at the translational level (about 24% increase in Hsp70 level) is within the expected range of physiological hormesis (Calabrese et al., 2007) and should not be harmful over a long term and repeated exposure. Induction of Hsp70 within this hormetic range by other stresses such as exercise is generally considered to be safe and beneficial in almost all circumstances, including cancer. However, further studies are required to confirm that the molecular effects of Sanchi observed in human keratinocytes and dermal fibroblasts in vitro also occur in the skin in vivo.
Another important lesson to be learnt from this case study is that observing a particular biological end-result in response to a treatment with a test compound may not imply a straight forward chemical nature and mode of action of that compound. For example, the biological end-result of being antioxidative is often interpreted as an evidence for the compound itself being a chemical antioxidant in terms of being a direct scavenger of ROS, which can be incorrect and misleading (Halliwell, 2000; Pun et al., 2010). Various polyphenols, flavanoids and spices are examples of such compounds which have anti-oxidative effects but without being direct chemical antioxidants (Birringer 2011, Lima et al., 2011). The concept of physiological hormesis, however, implies that a biologically beneficial antioxidative end-result can be achieved by hormetins, such as curcumin and ferulic acid, which actually cause some oxidative damage (Barone et al., 2009; Lima et al., 2011). Since identical biological end-results could be achieved by activating mechanistically very different pathways, it is very important to know the earliest step(s) in the mode of action of a compound for establishing its uniqueness and specificity. Such knowledge can also be useful in claiming the intellectual property rights and to facilitate further modifications and applications of the compound.
Applying the concept of hormesis in testing the effects of natural and synthetic compounds by analysing various SR pathways can help to screen and select potentially useful compounds with specific targets. Some suggestions for discovering novel hormetins by activating different SR pathways are: autophagic response for food-restriction mimetics, DNA repair response for UV-protectors, sirtuin response for mitochondrial energy production enhancers, Nrf2 response for antioxidants, and NF-κB response for anti-inflammatory compounds (Rattan and Demirovic, 2010a; 2010b; Yang et al., 2011).
Finally, the success of a hormetin-based skin care product can be instrumental in the social recognition and acceptance of the concept of hormesis as the beneficial effects of mild stress of choice. A change in the social perception of stress through scientific understanding of the hormetic nature of stress will open up possibilities for developing many other lines of health care products and technologies using physical, mental and nutritional hormetins.
Acknowledgments
Laboratory of Cellular Ageing, Aarhus University, is partially supported by a research grant from LVMH Reserche, France.
REFERENCES
- Barone E, Calabrese V, Mancuso C. Ferulic acid and its therapeutic potential as a hormetin for age-related diseases. Biogerontology. 2009;10:97–108. doi: 10.1007/s10522-008-9160-8. [DOI] [PubMed] [Google Scholar]
- Beedholm R, Clark BFC, Rattan SIS. Mild heat stress stimulates proteasome and its 11S activator in human fibroblasts undergoing aging in vitro. Cell Stress & Chaperones. 2004;9:49–57. doi: 10.1379/475.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berge U, Kristensen P, Rattan SIS. Kinetin-induced differentiation of normal human keratinocytes undergoing aging in vitro. Ann NY Acad Sci. 2006;1067:332–336. doi: 10.1196/annals.1354.045. [DOI] [PubMed] [Google Scholar]
- Berge U, Kristensen P, Rattan SIS. Hormetic modulation of differentiation of normal human epidermal keratinocytes undergoing replicative senescence in vitro. Exp Gerontol. 2008;43:658–662. doi: 10.1016/j.exger.2007.12.009. [DOI] [PubMed] [Google Scholar]
- Birringer M. Hormetics: dietary triggers of an adaptive stress response. Pharm Res. 2011;28:2680–2694. doi: 10.1007/s11095-011-0551-1. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ. Hormesis and medicine. Br J Clin Pharmacol. 2008;66:594–617. doi: 10.1111/j.1365-2125.2008.03243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese EJ, Bachmann KA, Bailer AJ, Bolger PM, Borak J, Cai L, Cedergreen N, Cherian MG, Chiueh CC, Clarkson TW, Cook RR, Diamond DM, Doolittle DJ, Dorato MA, Duke SO, Feinendegen L, Gardner DE, Hart RW, Hastings KL, Hayes AW, Hoffmann GR, Ives JA, Jaworowski Z, Johnson TE, Jonas WB, Kaminski NE, Keller JG, Klaunig JE, Knudsen TB, Kozumbo WJ, Lettieri T, Liu SZ, Maisseu A, Maynard KI, Masoro EJ, McClellan RO, Mehendale HM, Mothersill C, Newlin DB, Nigg HN, Oehme FW, Phalen RF, Philbert MA, Rattan SI, Riviere JE, Rodricks J, Sapolsky RM, Scott BR, Seymour C, Sinclair DA, Smith-Sonneborn J, Snow ET, Spear L, Stevenson DE, Thomas Y, Tubiana M, Williams GM, Mattson MP. Biological stress response terminology: Integrating the concepts of adaptive response and preconditioning stress within a hormetic dose-response framework. Toxicol Appl Pharmacol. 2007;222:122–128. doi: 10.1016/j.taap.2007.02.015. [DOI] [PubMed] [Google Scholar]
- Carnes BA. What is lifespan regulation and why does it exist? Biogerontology. 2011;12:367–374. doi: 10.1007/s10522-011-9338-3. [DOI] [PubMed] [Google Scholar]
- Choi A, Seo BS, Joung MS, Lee YH, Park CM. A cosmetic composition comprising tissue cultured Panax ginseng C.A. 2008. Meyer adventitious root itself and a preparing method thereof. Office, K. P. Korea.
- Daugaard M, Rohde M, Jaattela M. The heat shock protein 70 family: Highly homologous proteins with overlapping and distinct functions. FEBS Lett. 2007;581:3702–3710. doi: 10.1016/j.febslet.2007.05.039. [DOI] [PubMed] [Google Scholar]
- Demirovic D, Rattan SIS. Curcumin induces stress response and hormetically modulates wound healing ability of human skin fibroblasts undergoing ageing in vitro. Biogerontology. 2011;12:437–444. doi: 10.1007/s10522-011-9326-7. [DOI] [PubMed] [Google Scholar]
- Fonager J, Beedholm R, Clark BFC, Rattan SIS. Mild stress-induced stimulation of heat shock protein synthesis and improved functional ability of human fibroblasts undergoing aging in vitro. Exp Gerontol. 2002;37:1223–1238. doi: 10.1016/s0531-5565(02)00128-6. [DOI] [PubMed] [Google Scholar]
- Glaser DA. Anti-aging products and cosmeceuticals. Facial Plast Surg Clin N Am. 2004;12:363–372. doi: 10.1016/j.fsc.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Guo HB, Cui XM, An N, Cai GP. Sanchi ginseng (Panax notoginseng (Burkill, F.H. Chen) in China: distribution, cultivation and variations. Genet Resour Crop Evol. 2010;57:453–460. [Google Scholar]
- Halliwell B. The antioxidant paradox. Lancet. 2000;355:1179–1180. doi: 10.1016/S0140-6736(00)02075-4. [DOI] [PubMed] [Google Scholar]
- Hansen J, Bross P. A cellular viability assay to monitor drug toxicity. Methods Mol Biol. 2010;648:303–311. doi: 10.1007/978-1-60761-756-3_21. [DOI] [PubMed] [Google Scholar]
- Hayes DP. Nutritional hormesis. Eur J Clin Nutr. 2007;61:147–159. doi: 10.1038/sj.ejcn.1602507. [DOI] [PubMed] [Google Scholar]
- Hayes DP. Vitamin D and ageing. Biogerontology. 2010;11:1–16. doi: 10.1007/s10522-009-9252-0. [DOI] [PubMed] [Google Scholar]
- Hunt KJ, Hung SK, Ernst E. Botanical extracts as anti-aging preparations for the skin: a systematic review. Drugs Aging. 2010;27:973–985. doi: 10.2165/11584420-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Le Bourg E, Rattan SIS, editors. Mild stress and healthy aging: applying hormesis in aging research and interventions. Springer; The Netherlands: 2008. [Google Scholar]
- Liberek K, Lewandowska A, Zietkiewicz S. Chaperones in control of protein disaggregation. EMBO J. 2008;27:328–335. doi: 10.1038/sj.emboj.7601970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima CF, Pereira-Wilson C, Rattan SIS. Curcumin induces heme oxygenase-1 in normal human skin fibroblasts through redox signaling: Relevance for anti-aging intervention. Mol Nutr Food Res. 2011;55:430–442. doi: 10.1002/mnfr.201000221. [DOI] [PubMed] [Google Scholar]
- Magalhaes JP, Chainiaux F, Remacle J, Toussaint O. Stress-induced premature senescence in BJ and hTERT-BJ1 human foreskin fibroblasts. FEBS Lett. 2002;523:157–162. doi: 10.1016/s0014-5793(02)02973-3. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Dietary factors, hormesis and health. Ageing Res Rev. 2008a;7:43–48. doi: 10.1016/j.arr.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP. Hormesis defined. Ageing Res Rev. 2008b;7:1–7. doi: 10.1016/j.arr.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen ER, Eskildsen-Helmond Y, Rattan SIS. MAP-kinases and heat shock-induced hormesis in human fibroblasts during serial passaging in vitro. Ann NY Acad Sci. 2006;1067:343–348. doi: 10.1196/annals.1354.048. [DOI] [PubMed] [Google Scholar]
- Pun PB, Gruber J, Tang SY, Schaffer S, Ong RL, Fong S, Ng LF, Cheah I, Halliwell B. Ageing in nematodes: do antioxidants extend lifespan in Caenorhabditis elegans? Biogerontology. 2010;11:17–30. doi: 10.1007/s10522-009-9223-5. [DOI] [PubMed] [Google Scholar]
- Rattan SIS. Repeated mild heat shock delays ageing in cultured human skin fibroblasts. Biochem Mol Biol Int. 1998;45:753–759. doi: 10.1080/15216549800203162. [DOI] [PubMed] [Google Scholar]
- Rattan SIS. Ageing, gerontogenes, and hormesis. Ind J Exp Biol. 2000a;38:1–5. [PubMed] [Google Scholar]
- Rattan SIS. Biogerontology: the next step. Ann NY Acad Sci. 2000b;908:282–290. doi: 10.1111/j.1749-6632.2000.tb06655.x. [DOI] [PubMed] [Google Scholar]
- Rattan SIS. Aging intervention, prevention, and therapy through hormesis. J. Gerontol. Biol. Sci. 2004a;59A:705–709. doi: 10.1093/gerona/59.7.b705. [DOI] [PubMed] [Google Scholar]
- Rattan SIS. Aging, anti-aging, and hormesis. Mech Age Dev. 2004b;125:285–289. doi: 10.1016/j.mad.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Rattan SIS. Hormetic modulation of aging and longevity by mild heat stress. Dose-response. 2005;3:533–546. doi: 10.2203/dose-response.003.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattan SIS. Theories of biological aging: genes, proteins and free radicals. Free Rad Res. 2006;40:1230–1238. doi: 10.1080/10715760600911303. [DOI] [PubMed] [Google Scholar]
- Rattan SIS. Hormesis in aging. Ageing Res Rev. 2008a;7:63–78. doi: 10.1016/j.arr.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Rattan SIS. Principles and practice of hormetic treatment of aging and age-related diseases. Hum Exp Toxicol. 2008b;27:151–157. doi: 10.1177/0960327107083409. [DOI] [PubMed] [Google Scholar]
- Rattan SIS. Biogerontology: from here to where? The Lord Cohen Medal Lecture-2011. Biogerontology. 2011. [DOI] [PubMed]
- Rattan SIS, Ali RE. Hormetic prevention of molecular damage during cellular aging of human skin fibroblasts and keratinocytes. Ann NY Acad Sci. 2007;1100:424–430. doi: 10.1196/annals.1395.047. [DOI] [PubMed] [Google Scholar]
- Rattan SIS, Clark BFC. Understanding and modulating ageing. IUBMB Life. 2005;57:297–304. doi: 10.1080/15216540500092195. [DOI] [PubMed] [Google Scholar]
- Rattan SIS, Demirovic D. Hormesis and aging. In: Mattson MP, Calabrese E, editors. Hormesis: a revolution in biology, toxicology and medicine. New York: Springer; 2009. pp. 153–175. [Google Scholar]
- Rattan SIS, Demirovic D. Hormesis as a mechanism for the anti-aging effects of calorie restriction. In: Everitt AV, Rattan SIS, Le Couteur DG, de Cabo R, editors. Calorie Restriction, Aging and Longevity. Springer; The Netherlands: 2010a. pp. 233–245. [Google Scholar]
- Rattan SIS, Demirovic D. Hormesis can and does work in humans. Dose Response. 2010b;8:58–63. doi: 10.2203/dose-response.09-041.Rattan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattan SIS, Fernandes RA, Demirovic D, Dymek B, Lima CF. Heat stress and hormetin-induced hormesis in human cells: effects on aging, wound healing, angiogenesis and differentiation. Dose-response. 2009;7:93–103. doi: 10.2203/dose-response.08-014.Rattan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattan SIS, Sodagam L. Gerontomodulatory and youth-preserving effects of zeatin on human skin fibroblasts undergoing aging in vitro. Rejuven Res. 2005;8:46–57. doi: 10.1089/rej.2005.8.46. [DOI] [PubMed] [Google Scholar]
- Singh R, Kølvraa S, Bross P, Christensen K, Gregersen N, Tan Q, Jensen UB, Eiberg H, Rattan SIS. Heat-shock protein 70 genes and human longevity. A view from Denmark. Ann NY Acad Sci. 2006;1067:301–308. doi: 10.1196/annals.1354.040. [DOI] [PubMed] [Google Scholar]
- Söti C, Pál C, Papp B, Csermely P. Molecular chaperones as regulatory elements of cellular networks. Curr Opin Cell Biol. 2005;17:210–215. doi: 10.1016/j.ceb.2005.02.012. [DOI] [PubMed] [Google Scholar]
- Verbeke P, Clark BFC, Rattan SIS. Modulating cellular aging in vitro: hormetic effects of repeated mild heat stress on protein oxidation and glycation. Exp Gerontol. 2000;35:787–794. doi: 10.1016/s0531-5565(00)00143-1. [DOI] [PubMed] [Google Scholar]
- Verbeke P, Clark BFC, Rattan SIS. Reduced levels of oxidized and glycoxidized proteins in human fibroblasts exposed to repeated mild heat shock during serial passaging in vitro. Free Rad Biol Med. 2001a;31:1593–1602. doi: 10.1016/s0891-5849(01)00752-3. [DOI] [PubMed] [Google Scholar]
- Verbeke P, Deries M, Clark BFC, Rattan SIS. Hormetic action of mild heat stress decreases the inducibility of protein oxidation and glycoxidation in human fibroblasts. Biogerontology. 2002;3:105–108. doi: 10.1023/a:1015284119308. [DOI] [PubMed] [Google Scholar]
- Verbeke P, Fonager J, Clark BFC, Rattan SIS. Heat shock response and ageing: mechanisms and applications. Cell Biol Int. 2001b;25:845–857. doi: 10.1006/cbir.2001.0789. [DOI] [PubMed] [Google Scholar]
- Westerheide SD, Bosman JD, Mbadugha BNA, Kawahara TLA, Matsumoto G, Kim S, Gu W, Devlin JP, Silverman RB, Morimoto RI. Celastrols as inducers of the heat shock response and cytoprotection. J Biol Chem. 2004;279:56053–56060. doi: 10.1074/jbc.M409267200. [DOI] [PubMed] [Google Scholar]
- Wiegant FA, Surinova S, Ytsma E, Langelaar-Makkinje M, Wikman G, Post JA. Plant adaptogens increase lifespan and stress resistance in C. elegans. Biogerontology. 2009;10:27–42. doi: 10.1007/s10522-008-9151-9. [DOI] [PubMed] [Google Scholar]
- Yang NC, Song TY, Chen MY, Hu ML. Effects of 2-deoxyglucose and dehydroepiandrosterone on intracellular NAD(+) level, SIRT1 activity and replicative lifespan of human Hs68 cells. Biogerontology. 2011;12:527–536. doi: 10.1007/s10522-011-9342-7. [DOI] [PubMed] [Google Scholar]