Abstract
This study aims to explore the expression of GRP78, a marker of endoplasmic reticulum (ER) stress, in the cortex of rat brains acutely exposed to methylmercury (MeHg). Thirty Sprague-Dawley (SD) rats were randomly divided into six groups, and decapitated 6 hours (h) after intraperitoneal (i.p.) injection of MeHg (2, 4, 6, 8 or 10 mg/kg body weight) or normal saline. Protein and mRNA expression of Grp78 were detected by western blotting and real-time PCR, respectively. The results showed that a gradual increase in GRP78 protein expression was observed in the cortex of rats acutely exposed to MeHg (2, 4 or 6 mg/kg). Protein levels peaked in the 6 mg/kg group (p < 0.05 vs. controls), decreased in the 8 mg/kg group, and bottomed below the control level in the 10 mg/kg group. Parallel changes were noted for Grp78 mRNA expression. It may be implied that acute exposure to MeHg induced hormetic dose-dependent changes in Grp78 mRNA and protein expression, suggesting that activation of ER stress is involved in MeHg-induced neurotoxicity. Low level MeHg exposure may induce GRP78 protein expression to stimulate endogenous cytoprotective mechanisms.
Keywords: Methylmercury, endoplasmic reticulum stress, GRP78, rat, cortex
INTRODUCTION
Methylmercury (MeHg) is recognized as a global environmental pollutant. Its primary target organ is the central nervous system (CNS). MeHg disrupts multiple biochemical and physiological processes (Aschner et al. 2010). In humans, MeHg damages the visual cortex and interferes with somatosensory processing. Signs of intoxication include the constriction of the visual field, hearing loss, sensory impairment of the extremities, muscle weakness, tremors and mental deterioration (Castoldi et al. 2001). Several mechanisms have been proposed for MeHg-induced neurotoxicity, such as the induction of oxidative stress (Farina et al. 2011), disruption of neurotrophic signaling (Andersson et al. 1997), disruption of intracellular calcium homeostasis (Hare et al. 1993), and alterations in neurotransmitter systems (Minnema et al. 1989). Nevertheless, the precise nature of MeHg-induced neurotoxicity remains to be fully elucidated, likely reflecting upon its multifaceted disruptive cellular mechanisms (Atchison and Hare 1994; Ceccatelli et al. 2010; Grandjean et al. 2010).
The endoplasmic reticulum (ER) is a major organelle serving several specialized functions, such as calcium storage, biosynthesis, folding and assembly of transmembrane and secretory proteins, and the production of phospholipids and sterols. Disturbance in any of these functions may lead to ER stress (Xu et al. 2005). One of the most characterized and highly conserved ER stress responses is the unfolded-protein response (UPR) (Kaufman 2002). The UPR diminishes cellular stress by activating protein folding and degradation pathways in the ER and by inhibiting protein synthesis (Mori 2000; Rutkowski and Kaufman 2004). The UPR induces the expression of the 78-kDa glucose-regulated protein (GRP78). GRP78 is an ER-resident molecular chaperone that prevents the aggregation of unfolded or misfolded proteins so that they can be properly refolded, ubiquitinated and presented to the proteasome for degradation (Lee 2001; Brostrom and Brostrom 2003; Schröder and Kaufman 2005).
GRP78 is also known as an immunoglobin-binding protein, BiP. It is a major functional molecule that is regulated by the ER stress response, and its up-regulation affords protection from cytotoxic injury (Lee 2001). GRP78 also functions as a molecular chaperone under physiological conditions (Pfaffenbach and Lee 2011). Upon stress, a greater abundance of GRP78 proteins is required to promote refolding and repair of denatured proteins, which serves impedie a cellular damage cascade (Pfaffenbach and Lee 2011). A protective role of GRP78 is supported by studies in neurons and glias (Lee et al. 1999; Yu et al. 1999; Suyama et al. 2011). However, when ER stress is prolonged and/or too pronounced, ER resident chaperones are unable to counteract the accumulation of misfolded proteins and an ER-mediated apoptotic program is triggered through the activation of caspase-12 (Nakagawa et al. 2000; Lamkanfi et al. 2004; Cribb et al. 2005).
Previous reports suggested that several in vitro heavy metals, including lead, manganese, cadmium and mercury, can induce ER stress and contribute to damage or adaptation in exposed cells (Castiglioni et al. 2001; Chun et al. 2001; Qian et al. 2000, 2001; Liu et al. 2006; Hiramatsu et al. 2007; Yokouchi et al. 2007; Shinkai et al. 2010; Aremu et al. 2011; Ji et al. 2011). Liu et al.(2006) reported that in LLC-PK1 cells inorganic mercury (Hg) failed to significantly induce GRP78 expression, yet it significantly increased the protein levels of two other markers of ER stress, namely phospho-eukaryotic initiation factor 2 (eIF2α) and ATF4. Exposure of Atlantic cod to Hg-enriched sediments for five weeks was associated with enhanced mRNA expression of calreticulin (Olsvik et al. 2011b), a Ca2+-buffering chaperone in the ER lumen (Qiu and Michalak, 2009). Calreticulin protein expression was also increased in MeHg-treated Atlantic salmon (Olsvik et al. 2011a). Increased expression of calreticulin increases Ca2+ transients and has been linked to ER stress (Zhang et al. 2007). In MeHg-susceptible C2C12-DMPK160 cells, the organometal significantly up-regulated Grp78 mRNA and ER stress-related proteins, XBP1 and GRP78 (Usuki et al. 2008). In agreement with the previous studies, Cambier and colleagues noted that Hsp5a(Grp78) and Hsp90b1(grp94) were overexpressed in the skeletal muscles of zebrafish fed a MeHg-contaminated diet (Cambier et al. 2010). Our previous study showed that acute MeHg (4 mg/kg body weight) exposure increased GRP78 protein expression in the cerebral cortex, hippocampus, brain stem, striatum and cerebellum (Zhang et al. 2010a). Thus, a link exists between MeHg exposure and ER stress. However, little is known about the dose-response relationship between ER stress and in vivo acute systemic exposure to MeHg.
The objective of the present study was to determine the dose-effect relationship between MeHg exposure and GRP78 expression at the protein and mRNA levels over an expanded dosimetry range. Our results establish that acute exposure to MeHg induced Grp78 protein expression in a non-linear dose-dependent manner. These results suggest that ER stress activation plays a role in MeHg-induced neurotoxicity. Furthermore, low level MeHg exposure may induce a hormetic effect to stimulate endogenous cytoprotective mechanisms.
MATERIALS AND METHODS
Drugs and Reagents
Methylmercury (MeHg, 99.9% purity) was purchased from Sigma (St. Louis, MO, USA). GRP78 polyclonal antibody and β–actin antibody were obtained from the Santa Cruz Biotechnology Company (Santa Cruz, CA, USA). Trizol was bought from Invitrogen (Carlsbad, CA, USA) and the protein extraction kit was obtained from the Kang Cheng Bioengineering Company (Shanghai, China). We bought the BCA protein assay kit from Thermo Scientific (Waltham, MA, USA). Amersham ECL Plus Western Blotting Detection Reagents were obtained from the GE Healthcare Company (Waukesha, WI, USA). The reverse transcription and PCR kits were obtained from Fermentas (Glen Burnie, MD, USA). SYBR Green I Mix was purchased from Stratagene (La Jolla, CA, USA) and the PCR primers were synthesized at the Health Bioengineering Company (Shanghai, China).
Animals
Adult (4–5 weeks) male Sprague-Dawley (SD) rats (n=30) weighing 200–300 g were provided by the Laboratory Animal Center of Jiangsu University.
Animal treatment protocol
After a 1-week adaption period, thirty SD rats were randomly divided into six experimental groups. In a previous study, we found that, compared with the control group, MeHg-induced alterations of Grp78 protein only in the cerebral cortex and brain stem were statistically significant at the dosage of 4 mg/kg, and magnitude of induction in cerebral cortex was the largest, though the trend was similar in the cerebellum, cerebral cortex, brain stem, hippocampus and striatum (Zhang, et al. 2010a). Therefore, we chose cerebral cortex as the optimal structure for the present study and the range of dosages was extended to 2, 4, 6, 8 or 10 mg/kg body weight from 4mg/kg body weight. Regarding to sampling time points, we chose 6 h after administration of MeHg as critical time point because the peak of Grp78 induction was at 6 h after MeHg injection (Zhang et al. 2010a). The animals were decapitated 6 h after a single i.p. MeHg injection (2, 4, 6, 8 or 10 mg/kg body weight), and the cerebral cortex was immediately dissected out and placed on ice, following our previous treatment protocol (Zhang et al. 2010a). The rats in the control group were i.p. injected with normal saline. The brain cortices were rapidly frozen in liquid nitrogen and transferred to the −80°C freezer. All animal exposure protocols were approved by the Jiangsu University Institutional Animal Care and Use Committee (IACUC) and were adhered to strictly to minimize pain. All exposures and procedures followed the NIH Laboratory Animal Care and Use guidelines.
Primers
Primers were designed and synthesized based on Grp78 and rat housekeeping gene (β-actin) sequence information. The sequences of the primers are shown in Table 1.
TABLE 1.
Grp78 and β-actin primers sequences
| Gene | Primer’s sequence (5′-3′) | DNA fragments (bp) | Melting point (°C) |
|---|---|---|---|
| Grp78 | For: AACCCAGATGAGGCTGTAGCATA | 162 | 64 |
| Rev: CACAGTGTTCCTCGGAATCAGTT | |||
| β-actin | For: GAGGGAAATCGTGCGTGAC | 444 | 58 |
| Rev CTGGAAGGTGGACAGTGAG |
Western blot analysis
Western blot analysis was conducted as previously described (Zhang et al. 2010a). Briefly, total protein was extracted from the cerebral cortex with a protein extraction kit. The protein concentration was assessed with the BCA protein assay kit (ThermoFisher Scientific, Rockford, IL, USA). Equal amounts (20 μg protein/lane) of protein and prestained molecular-weight markers were loaded onto 12% sodium dodecyl sulfate-poly-acrylamide gels and subsequently transferred to PVDF immunoblotting membranes. The membranes were incubated for 60 min at room temperature in blocking buffer containing 5% nonfat dry milk. Next, the membranes were washed in Tris-buffered saline and incubated overnight at 4°C with a rabbit polyclonal antibody to GRP78 (1:1000) followed by incubation with a goat anti-rabbit IgG HRP-linked antibody (1:10000). The membranes were exposed to Amersham ECL Plus Western Blotting Detection Reagents, and a Typhoon 9400 molecular imaging system (excitation wavelength 430 nm, emission wavelength 503 nm) was used for chemical fluorescent scanning. The lane densities were assessed with ImageQuant TL software (GE Healthcare, Waukesha, WI, USA). The ratio of GRP78 gray value and the corresponding β-actin expression level in the various groups was compared to the control group, which was set to 1.
Quantitative real-time PCR
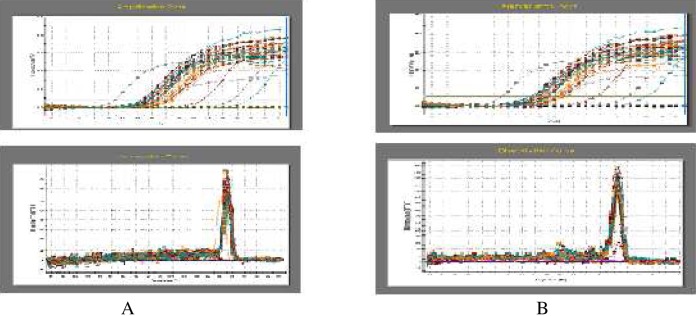
Total mRNA from the cerebral cortex was extracted with Trizol reagent, following the manufacturer’s instructions. Then the first-strand cDNA was synthesized using 1 μg mRNA. Fluorescent real time quantitative PCR (qPCR) was performed to detect the mRNA levels of Grp78 and β-actin in a Stratagene Mx3000P PCR machine (La Jolla, CA, USA). The qPCR reaction included: 7.5 μL SYBR Green 1 mix, 0.3 μL forward primer (10 mM), 0.3 μL reverse primer (10 mM), 0.3 μL Reference Dye (ROX) (10 mM), 1 μL cDNA template and 5.6 μL ddH2O. The PCR was performed for 35 cycles (denaturation: 95°C, 30 seconds (s); annealing: Grp78, 64°C for 30 s or β-actin 58°C for 30 s; extension: 72°C for 25 s). The relative expression of the target gene was calculated as the ratio between the copy numbers of target gene over that of β-actin. The levels of mRNA in the various groups were compared with the control group, which was set to 1. The specific working conditions of the real-time quantitative PCR are shown in Fig. 1.
FIG. 1.
Fluorescent quantitative amplification and melting curves. A: The amplification and melting curves of β–actin; B: The amplification and melting curves of GRP78.
Data analysis
The data are expressed as means ± S.D. Statistical analysis was conducted with SPSS13.0. Significant differences between groups were evaluated by one-way analysis of variance (ANOVA) with Student-Newman-Keuls test as a post hoc test. p < 0.05 was considered to be statistically significant.
RESULTS
Effect of MeHg exposure on GRP78 protein expression in rat cerebral cortex
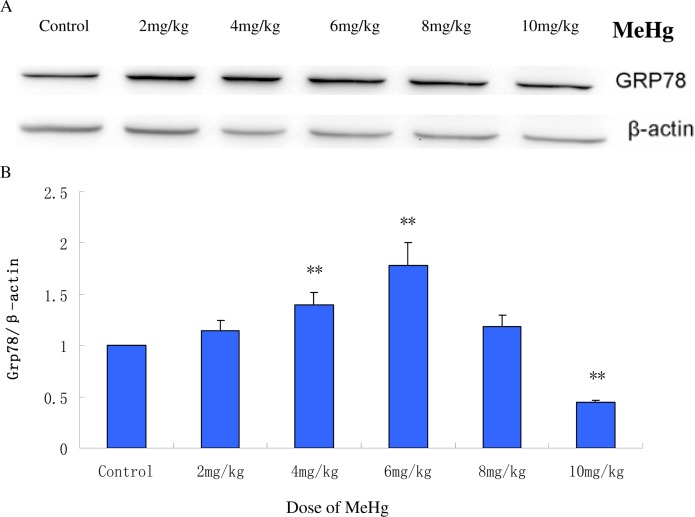
Compared with the control group, GRP78 protein expression showed an initial increasing trend followed by a gradual decrease (Fig. 2). GRP78 protein expression significantly increased (p< 0.05) in the 4 mg MeHg/kg group and peaked in the 6 mg MeHg/ kg group (180±3% of control). Interestingly, higher doses of MeHg (8 and 10 mg/kg) failed to further increase GRP78 protein expression. GRP78 expression in the 8 mg MeHg/ kg group was lower than the 6 mg MeHg/ kg group, but the expression levels remained higher than in the control group (p< 0.05). Notably, GRP78 expression in the highest dosing group (10 mg MeHg/kg) was significantly reduced, dropping to levels below the control group (p< 0.05).
FIG. 2.
GRP78 protein expression in the cerebral cortex of rats acutely (6 h) exposed to MeHg. A: Western blot analysis of GRP78 expression. β-actin was used as a loading control. B: Protein bands from the western blots were quantified by scanning densitometry. The relative gray value for the GRP78 protein was expressed relative to β-actin and the control group was set to 1 (n=5). Means ± SD. **P < 0.05: MeHg treated groups vs. normal saline treated control group (control).
Effect of MeHg treatment on Grp78 mRNA expression in the rat cerebral cortex
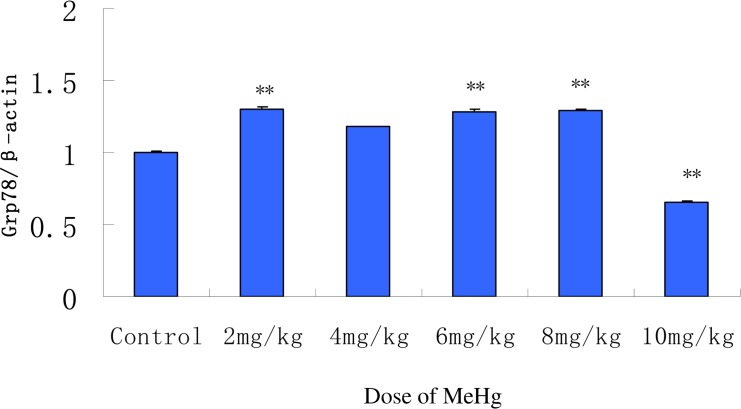
To further explore the relationship between GRP78 expression and MeHg exposure in rat cerebral cortex, changes in Grp78 mRNA were examined with real time PCR (Fig. 3). Grp78 mRNA levels significantly increased even in the lowest MeHg exposure group (2 mg/kg) vs. the controls (p< 0.05). Nevertheless, unlike GRP78 protein expression levels, Grp78 mRNA levels were maintained at approximately the same level in the 2, 4, 6 and 8 mg/kg groups (p> 0.05 vs. controls) (Fig. 3). Consistent with the protein changes, a significant decrease in Grp78 mRNA was noted in the highest MeHg exposure group (10 mg/kg). Both the Grp78 mRNA and protein expression were significantly reduced to levels below the controls in this group (p< 0.05), suggesting that cellular damage may be inherent to the highest MeHg dosing group.
FIG. 3.
Grp78 mRNA expression analyzed by qPCR in the cerebral cortex of rats acutely exposed to MeHg. β-Actin was used as a loading control. The relative value for Grp78 mRNA was expressed relative to β-actin, and the control group was set to 1 (n=5). Means ± SD. **P < 0.05: MeHg treated groups vs. normal saline treated control group (control).
DISCUSSION
This is the first time a dosimetry study on the effects of MeHg exposure in rat brain has been performed to assess the potential for a hormetic effect. Our results showed a typical inverted U-type curve of rat brain (cortical) GRP78 expression at the protein and mRNA levels, which has previously been defined as hormesis (Calabrese and Baldwin 2001; Calabrese and Blain 2004). Hormesis refers to a process whereby a sub-lethal stressor renders an organism resistant to subsequent stress. This effect has been demonstrated in multiple stress models, both in cell culture to in vivo human models, including studies on dietary restriction, exercise, radiation, chemical and heat exposures (Damelin et al. 2000; Damelin and Alexander 2001; Cypser et al. 2006; Mattson 2008; Le Bourg 2009). The mechanisms that mediate hormesis are ill defined; nevertheless, previous research has implicated members of the heat shock protein 70 (HSP70) family and metallothionein (MT) proteins as contributors to hormesis. Levels of these proteins are known to increase in response to heavy metal exposures (Damelin et al. 2000). Additionally, hormesis has been invoked as a potential explanation for the latent period observed in MeHg exposure and the delayed onset of symptoms (Weiss et al. 2002). Heinz and colleagues found that when mallard (Anas platyrhynchos) eggs were injected with MeHg at doses ranging from 0 to 6.4 ...g/g egg, the eggs’ hatching success was significantly increased at the lowest doses and progressively decreased at incremental doses (Heinz et al. 2012). The same group also reported that a parental diet containing 0.5 mg MeHg/g body weight enhanced mallard reproduction (Heinz et al. 2010). These results are consistent with low-dose MeHg induction of cytoprotective agents, such as glutathione, heat shock proteins (HSP) and metalloth-ioneins (MT1) as recently documented in Caenorhabditis elegans (Helmcke and Aschner 2010).
The disruption of protein synthesis is a primary mechanism of MeHg-induced neurotoxicity. Decreased incorporation of [14C] leucine has been shown in cerebral and cerebellar slices of rats exposed in vivo to this organometal (Yoshino et al. 1966; Verity et al. 1977). However, the mechanisms underlying this inhibition of protein synthesis have not been established. Notably, increased accumulation of unfolded or misfolded proteins in the ER lumen results in compensatory activation of the UPR (Lee 2001). A hallmark of UPR activation is global reduction in the rate of protein synthesis, which is controlled by reversible phosphorylation of the α-subunit of eIF2. The phosphorylation of eIF2α prevents formation of the eIF2-tRNAmet-40s complex, thereby impeding the initiation of protein synthesis (Harding et al. 1999; Kaufman 1999). Thus, high-dose MeHg-induced UPR activation and eIF2α phosphorylation may arrest protein synthesis (Verity et al. 1977; Syversen 1981; Fair et al. 1987). These mechanisms reduce the flux of nascent proteins entering the ER, and then alleviate the increased burdens of protein processing in the ER (Kaufman 1999).
The inverted U-typed curve noted in our study is a common phenomenon (Calabrese and Blain 2004). Upregulation of the expression of stress proteins, such as HSP70 may play a role in the underlying mechanisms of hormesis (Damelin et al. 2000). Qian et al. (2001) demonstrated the hormetic effects of lead and mercury on Grp78 mRNA in C6 rat glioma cells. Low-level inorganic mercury also transcriptionally activated Grp78 in HepG2 cells (Sutton et al. 2002). Though obtained from in vitro cultured cells, these data corroborate MeHg’s ability to induce a hormetic dose-response effect in ER stress responses. Taken together, our studies along with earlier work on GRP78 expression in response to cadmium, lead, inorganic and organic mercury, nickel and iron (Zhang et al. 2010b), suggest that GRP78 is a universal target for heavy metal exposure, and that the ER serves as an intracellular sensor for injuries incurred by toxic metals. Furthermore, though the present acute exposure protocol has merits as it provides the necessary means for obtaining dose range for chronic exposure and data on the difference between acute and chronic effects, the effects of chronic (long-term) exposure to MeHg in rats should be conducted to most closely mimic the human exposure via fish and rice consumption (Grotto et al. 2011; Li et al. 2010).
In summary, acute exposure to MeHg produced a hormetic effect on cortical ER stress-activated UPR in the rat brain. This effect may be attributed to low-level MeHg activated UPR which serves to combat MeHg-induced oxidative stress and calcium imbalance to keep cellular home-ostasis. High levels of GRP78 may bind to MeHg thus chelating free MeHg and reducing its ability to bind to cellular targets (Zhang et al. 2009). However, the mechanisms and relevance of the MeHg-induced biphasic change in GRP78 expression warrant further study.
Acknowledgments
This work was supported in part by the Natural Science Foundation of China (No. 30872139 to Lu Rongzhu), the Nutrition-Disease Team Fund of Jiangsu University (to Lu Rongzhu) and the National Institute of Environmental Health Sciences (ES10563 and ES07331 to Michael Aschner).
REFERENCES
- Aschner M, Onishchenko N, Ceccatelli S. Toxicology of alkylmercury compounds. Met Ions Life Sci. 2010;7:403–434. doi: 10.1039/BK9781847551771-00403. [DOI] [PubMed] [Google Scholar]
- Andersson H, Lindqvist E, Olson L. Downregulation of brain-derived neurotrophic factor mRNA in adult rat brain after acute administration of methylmercury. Mol Chem Neuropathol. 1997;31:225–233. doi: 10.1007/BF02815126. [DOI] [PubMed] [Google Scholar]
- Aremu DA, Ezomo OF, Meshitsuka S. Gene expression in primary cultured astrocytes affected by aluminum: alteration of chaperons involved in protein folding. Environ Health Prev Med. 2011;16:16–24. doi: 10.1007/s12199-010-0161-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atchison WD, Hare MF. Mechanisms of methylmercury-induced neurotoxicity. FASEB J. 1994;8:622–629. doi: 10.1096/fasebj.8.9.7516300. [DOI] [PubMed] [Google Scholar]
- Brostrom MA, Brostrom CO. Calcium dynamics and endoplasmic reticular function in the regulation of protein synthesis: implications for cell growth and adaptability. Cell Calcium. 2003;34:345–363. doi: 10.1016/s0143-4160(03)00127-1. [DOI] [PubMed] [Google Scholar]
- Cambier S, Gonzalez P, Durrieu G, Maury-Brachet R, Boudou A, Bourdineaud JP. Serial analysis of gene expression in the skeletal muscles of zebrafish fed with a methylmercury-contaminated diet. Environ Sci Technol. 2010;44:469–475. doi: 10.1021/es901980t. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Baldwin LA. Hormesis: U-shaped dose responses and their centrality in toxicology. Trends Pharmacol Sci. 2001;22:285–291. doi: 10.1016/s0165-6147(00)01719-3. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Blain R. Metals and hormesis. J Env Monit. 2004;6:14N–19N. [Google Scholar]
- Castiglioni ET, Falahatpisheh MH, Zheng Y. Induction of 78 kD glucose-regulated protein (GRP78) expression and redox-regulated transcription factor activity by lead and mercury in C6 rat glioma cells. Neurotox Res. 2001;3:581–589. doi: 10.1007/BF03033212. [DOI] [PubMed] [Google Scholar]
- Castoldi AF, Coccini T, Ceccatelli S, Manzo L. Neurotoxicity and molecular effects of methylmercury. Brain Res Bull. 2001;55:197–203. doi: 10.1016/s0361-9230(01)00458-0. [DOI] [PubMed] [Google Scholar]
- Ceccatelli S, Daré E, Moors M. Methylmercury-induced neurotoxicity and apoptosis. Chem Biol Interact. 2010;188:301–308. doi: 10.1016/j.cbi.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Chun HS, Lee H, Son JH. Manganese induces endoplasmic reticulum (ER) stress and activates multiple caspases in nigral dopaminergic neuronal cells, SN4741. Neurosci Lett. 2001;316:5–8. doi: 10.1016/s0304-3940(01)02341-2. [DOI] [PubMed] [Google Scholar]
- Cribb AE, Peyrou M, Muruganandan S, Schneider L. The endoplasmic reticulum in xeno-biotic toxicity. Drug Metab Rev. 2005;37:405–342. doi: 10.1080/03602530500205135. [DOI] [PubMed] [Google Scholar]
- Cypser JR, Tedesco P, Johnson TE. Hormesis and aging in Caenorhabditis elegans. Exp Gerontol. 2006;41:935–939. doi: 10.1016/j.exger.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damelin LH, Alexander JJ. Metal-induced hormesis requires cPKC-dependent glucose transport and lowered respiration. Hum Exp Toxicol. 2001;20:347–358. doi: 10.1191/096032701680350596. [DOI] [PubMed] [Google Scholar]
- Damelin LH, Vokes S, Whitcutt JM, Damelin SB, Alexander JJ. Hormesis: a stress response in cells exposed to low levels of heavy metals. Hum Exp Toxicol. 2000;19:420–430. doi: 10.1191/096032700678816133. [DOI] [PubMed] [Google Scholar]
- Fair PH, Balthrop JE, Wade JL, Braddon-Galloway S. In vivo incorporation of [14C]leucine into brain protein of mice treated with methylmercury and thiol complexes of methylmercury. Toxicol Lett. 1987;36:213–220. doi: 10.1016/0378-4274(87)90188-3. [DOI] [PubMed] [Google Scholar]
- Farina M, Aschner M, Rocha JB. Oxidative stress in MeHg-induced neurotoxicity. Toxicol Appl Pharmacol. 2011;256:405–417. doi: 10.1016/j.taap.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P, Satoh H, Murata K, Eto K. Adverse effects of methylmercury: environmental health research implications. Environ Health Perspect. 2010;118:1137–1145. doi: 10.1289/ehp.0901757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotto D, Valentini J, Serpeloni JM, Monteiro PA, Latorraca EF, de Oliveira RS, Antunes LM, Garcia SC, Barbosa F., Jr Evaluation of toxic effects of a diet containing fish contaminated with methylmercury in rats mimicking the exposure in the Amazon riverside population. Environ Res. 2011;111:1074–1082. doi: 10.1016/j.envres.2011.09.013. [DOI] [PubMed] [Google Scholar]
- Harding HP, Zhang Y, Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 1999;397:271–274. doi: 10.1038/16729. [DOI] [PubMed] [Google Scholar]
- Hare MF, McGinnis KM, Atchison WD. Methylmercury increases intracellular concentrations of Ca++ and heavy metals in NG108-15 cells. J Pharmacol Exp Ther. 1993;266:1626–1635. [PubMed] [Google Scholar]
- Heinz GH, Hoffman DJ, Klimstra JD, Stebbins KR, Kondrad SL, Erwin CA. Hormesis Associated with a Low Dose of Methylmercury Injected into Mallard Eggs. Arch Environ Contam Toxicol. 2012;62:141–144. doi: 10.1007/s00244-011-9680-0. [DOI] [PubMed] [Google Scholar]
- Heinz GH, Hoffman DJ, Klimstra JD, Stebbins KR. Enhanced reproduction in mallards fed a low level of methylmercury: an apparent case of hormesis. Environ Toxicol Chem. 2010;29:650–653. doi: 10.1002/etc.64. [DOI] [PubMed] [Google Scholar]
- Helmcke KJ, Aschner M. Hormetic effect of methylmercury on Caenorhabditis elegans. Toxicol Appl Pharmacol. 2010;248:156–164. doi: 10.1016/j.taap.2010.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiramatsu N, Kasai A, Du S, Takeda M, Hayakawa K, Okamura M, Yao J, Kitamura M. Rapid, transient induction of ER stress in the liver and kidney after acute exposure to heavy metal: evidence from transgenic sensor mice. FEBS Lett. 2007;581:2055–2059. doi: 10.1016/j.febslet.2007.04.040. [DOI] [PubMed] [Google Scholar]
- Ji YL, Wang H, Zhao XF, Wang Q, Zhang C, Zhang Y, Zhao M, Chen YH, Meng XH, Xu DX. Crosstalk between endoplasmic reticulum stress and mitochondrial pathway mediates cadmium-induced germ cell apoptosis in testes. Toxicol Sci. 2011;124:446–459. doi: 10.1093/toxsci/kfr232. [DOI] [PubMed] [Google Scholar]
- Kaufman RJ. Orchestrating the unfolded protein response in health and disease. J Clin Invest. 2002;110:1389–1398. doi: 10.1172/JCI16886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman RJ. Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev. 1999;13:1211–1233. doi: 10.1101/gad.13.10.1211. [DOI] [PubMed] [Google Scholar]
- Lamkanfi M, Kalai M, Vandenabeele P. Caspase-12: an overview. Cell Death Differ. 2004;11:365–368. doi: 10.1038/sj.cdd.4401364. [DOI] [PubMed] [Google Scholar]
- Le Bourg E. Hormesis, aging and longevity. Biochim Biophys Acta. 2009;1790:1030–1039. doi: 10.1016/j.bbagen.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Lee J, Bruce-Keller AJ, Kruman Y, Chan SL, Mattson MP. 2-Deoxy-D-glucose protects hippocampal neurons against excitotoxic and oxidative injury: evidence for the involvement of stress proteins. J Neurosci Res. 1999;57:48–61. doi: 10.1002/(SICI)1097-4547(19990701)57:1<48::AID-JNR6>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Lee AS. The glucose-regulated proteins: stress induction and clinical applications. Trends Biochem Sci. 2001;26:504–510. doi: 10.1016/s0968-0004(01)01908-9. [DOI] [PubMed] [Google Scholar]
- Li P, Feng X, Qiu G. Methylmercury exposure and health effects from rice and fish consumption: a review. Int J Environ Res Public Health. 2010;7:2666–2691. doi: 10.3390/ijerph7062666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Inageda K, Nishitai G, Matsuoka M. Cadmium induces the expression of Grp78, an endoplasmic reticulum molecular chaperone, in LLC-PK1 renal epithelial cells. Environ Health Perspect. 2006;114:859–864. doi: 10.1289/ehp.8920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP. Hormesis defined. Ageing Res Rev. 2008;7:1–7. doi: 10.1016/j.arr.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minnema DJ, Cooper GP, Greenland RD. Effects of methylmercury on neurotransmitter release from rat brain synaptosomes. Toxicol Appl Pharmacol. 1989;99:510–521. doi: 10.1016/0041-008x(89)90158-0. [DOI] [PubMed] [Google Scholar]
- Mori K. Tripartite management of unfolded proteins in the endoplasmic reticulum. Cell. 2000;101:451–454. doi: 10.1016/s0092-8674(00)80855-7. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA, Yuan J. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature. 2000;403:98–103. doi: 10.1038/47513. [DOI] [PubMed] [Google Scholar]
- Olsvik PA, Amlund H, Torstensen BE. Dietary lipids modulates methylmercury toxicity in Atlantic salmon. Food Chem Toxicol. 2011a;49:3258–3271. doi: 10.1016/j.fct.2011.09.025. [DOI] [PubMed] [Google Scholar]
- Olsvik PA, Brattås M, Lie KK, Goksøyr A. Transcriptional responses in juvenile Atlantic cod (Gadus morhua) after exposure to mercury-contaminated sediments obtained near the wreck of the German WW2 submarine U-864, and from Bergen Harbor, Western Norway. Chemosphere. 2011b;83:552–563. doi: 10.1016/j.chemosphere.2010.12.019. [DOI] [PubMed] [Google Scholar]
- Pfaffenbach KT, Lee AS. The critical role of GRP78 in physiologic and pathologic stress. Curr Opin Cell Biol. 2011;23:150–156. doi: 10.1016/j.ceb.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Y, Falahatpisheh MH, Zheng Y, Ramos KS, Tiffany-Castiglioni E. Induction of 78 kD glucose-regulated protein (GRP78) expression and redox-regulated transcription factor activity by lead and mercury in C6 rat glioma cells. Neurotox Res. 2001;3:581–589. doi: 10.1007/BF03033212. [DOI] [PubMed] [Google Scholar]
- Qian Y, Harris ED, Zheng Y, Tiffany-Castiglioni E. Lead targets GRP78, a molecular chaperone, in C6 rat glioma cells. Toxicol Appl Pharmacol. 2000;163:260–266. doi: 10.1006/taap.1999.8878. [DOI] [PubMed] [Google Scholar]
- Qiu Y, Michalak M. Transcriptional control of the calreticulin gene in health and disease. Int J Biochem Cell Biol. 2009;41:531–538. doi: 10.1016/j.biocel.2008.06.020. [DOI] [PubMed] [Google Scholar]
- Rutkowski DT, Kaufman RJ. A trip to the ER: coping with stress. Trends Cell Biol. 2004;14:20–28. doi: 10.1016/j.tcb.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Schröder M, Kaufman RJ. ER stress and the unfolded protein response. Mutat Res. 2005;569:29–63. doi: 10.1016/j.mrfmmm.2004.06.056. [DOI] [PubMed] [Google Scholar]
- Shinkai Y, Yamamoto C, Kaji T. Lead induces the expression of endoplasmic reticulum chaperones GRP78 and GRP94 in vascular endothelial cells via the JNK-AP-1 pathway. Toxicol Sci. 2010;114:378–386. doi: 10.1093/toxsci/kfq008. [DOI] [PubMed] [Google Scholar]
- Sutton DJ, Tchounwou PB, Ninashvili N, Shen E. Mercury Induces Cytotoxicity and Transcriptionally Activates Stress Genes in Human Liver Carcinoma (HepG2) Cells. Int J Mol Sci. 2002;3:965–984. [Google Scholar]
- Suyama K, Watanabe M, Sakabe K, Okada Y, Matsuyama D, Kuroiwa M, Mochida J. Overexpression of GRP78 protects glial cells from endoplasmic reticulum stress. Neurosci Lett. 2011;504:271–276. doi: 10.1016/j.neulet.2011.09.045. [DOI] [PubMed] [Google Scholar]
- Syversen TL. Effects of methyl mercury on protein synthesis in vitro. Acta Pharmacol Toxicol (Copenh) 1981;49:422–426. doi: 10.1111/j.1600-0773.1981.tb00926.x. [DOI] [PubMed] [Google Scholar]
- Usuki F, Fujita E, Sasagawa N. Methylmercury activates ASK1/JNK signaling pathways, leading to apoptosis due to both mitochondria- and endoplasmic reticulum (ER)-generated processes in myogenic cell lines. Neurotoxicology. 2008;29:22–30. doi: 10.1016/j.neuro.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Verity MA, Brown WJ, Cheung M, Czer G. Methyl mercury inhibition of synaptosome and brain slice protein synthesis: in vivo and in vitro studies. J Neurochem. 1977;29:673–679. doi: 10.1111/j.1471-4159.1977.tb07785.x. [DOI] [PubMed] [Google Scholar]
- Weiss B, Clarkson TW, Simon W. Silent latency periods in methylmercury poisoning and in neurodegenerative disease. Environ Health Perspect. 2002;110(Suppl 5):851–854. doi: 10.1289/ehp.02110s5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Bailly-Maitre B, Reed JC. Endoplasmic reticulum stress: cell life and death decisions. J Clin Invest. 2005;115:2656–2664. doi: 10.1172/JCI26373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z, Luo H, Fu W, Mattson MP. The endoplasmic reticulum stress-responsive protein GRP78 protects neurons against excitotoxicity and apoptosis: suppression of oxidative stress and stabilization of calcium homeostasis. Exp Neurol. 1999;155:302–314. doi: 10.1006/exnr.1998.7002. [DOI] [PubMed] [Google Scholar]
- Yokouchi M, Hiramatsu N, Hayakawa K, Kasai A, Takano Y, Yao J, Kitamura M. Atypical, bidirectional regulation of cadmium-induced apoptosis via distinct signaling of unfolded protein response. Cell Death Differ. 2007;14:1467–1474. doi: 10.1038/sj.cdd.4402154. [DOI] [PubMed] [Google Scholar]
- Yoshino Y, Mozai T, Nakao K. Biochemical changes in the brain in rats poisoned with an alkymercury compound with special reference to the inhibition of protein synthesis in brain cortex slices. J Neurochem. 13:1223–1230. doi: 10.1111/j.1471-4159.1966.tb04281.x. [DOI] [PubMed] [Google Scholar]
- Zhang X, Szabo E, Michalak M, Opas M. Endoplasmic reticulum stress during the embryonic development of the central nervous system in the mouse. Int J Dev Neurosci. 2007;25:455–463. doi: 10.1016/j.ijdevneu.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Jiang X, Zhao X, Qian H, Wang S, Xing G, Wang S, Lu R. Time-course effect and region-specificity of endoplasmic reticulum stress in rat brains acutely exposed by methylmercury. Wei Sheng Yan Jiu. 2010a;39:271–274. (article in Chinese). [PubMed] [Google Scholar]
- Zhang Y, Jiang X, Qian H, Ma J, Lu R. Research advance on relationship between endoplasmic reticulum stress and metal toxicity. J Environ Health. 2010b;27:922–925. (article in Chinese). [Google Scholar]
- Zhang Y, Ye L, Wang B, Li Y, Sun L. Chelation of GRP78 with lead and its localization changes in the astroglia of rats exposed to lead. J Huazhong Univ Sci Technolog Med Sci. 2009;29:492–497. doi: 10.1007/s11596-009-0420-x. [DOI] [PubMed] [Google Scholar]