Abstract
Projects to manage arthropod pests using entomopathogenic nematodes (EPNs) in Brazil, Korea and USA are reviewed to identify conditions and practices that affected the use of EPNs for pest management. A proliferation of covered agriculture in Korea, the growth in demand for high value, pesticide-free produce in Korea and Brazil, and the cost-effectiveness of EPNs created favorable conditions for the widespread adoption of EPN products in Brazilian guava orchards and Korean vegetable greenhouses. In Florida, EPNs imported from South America function successfully as classical biocontrol agents against invasive mole crickets attacking pasture and turf. However, the low value of pasture and the availability of cost-effective chemical insecticides in turf have depressed the demand for EPN products to control mole crickets. In Florida citrus orchards, a recent, dramatic increase in the use of chemical insecticides to control an arthropod vector of a devastating bacterial disease of citrus (huanglongbing) reduced the demand for EPN products to control Diaprepes root weevils. Nevertheless, a rich and diverse EPN fauna in the Florida peninsula provides significant control of subterranean stages of root weevils in some habitats, and is the focus of research to develop cultural practices that exploit the potential for increased pest management through EPN conservation.
Keywords: augmentation biological control; classical biological control; conservation biological control; covered agriculture; Heterorhabditis, organic agriculture; Steinernema
The longstanding recognition that entomopathogenic nematodes (EPNs) can provide excellent arthropod pest control in some situations remains frequently unexploited by growers for reasons that are well documented (Shapiro-Ilan et al., 2002; Georgis et al., 2006). Two major impediments are cost and reliability. For example, the potentially widespread use of EPNs in turf (Frank and Walker, 2006) and cotton (Shapiro-Ilan et al., 2002) were supplanted by less expensive chemical pesticides and insect resistant germplasm, respectively. However, inferior performance of EPNs compared to chemical insecticides in many situations and inconsistent performance of EPNs, due to product quality control or environmental variation, are the greatest impediments to the adoption of EPNs in pest management programs (Lewis et al., 1998). Nevertheless, pest management tactics continually evolve in response to issues involving pesticide resistance, safety, health and environmental concerns, and development of pest biotypes that break host plant resistance. These issues and technical advances in EPN production and utilization will provide further opportunities to integrate the use of EPNs in pest management programs. Here we describe selected agricultural industries in Brazil, Korea and the United States in which EPN use has been studied and recommended for pest management. In each case we outline the basis on which recommendations were made, the degree to which growers have adopted EPN use, and our perceived reasons for the current status of commercial EPN products.
Use of Heterorhabditis Baujardi LPP7 Against the Guava Weevil in Brazil
Psidium guajava L. is native to the American tropics but is currently grown in more than 50 subtropical and tropical countries (Gould a Raga, 2002). Brazil is the principal red guava producer followed by Mexico, whereas India is the major producer of white guava (Gould and Raga, 2002). Different pests attack fruits, leaves and trunks, causing more or less damage depending on the region or country. The main pests of fruits are guava weevil (Conotrachelus psidii Marshall) and fruit flies (Ceratitis capitata Wied. and Anastrepha spp.). On the leaves, the main pest is a psyllid (Triozoida sp.) that causes damage mainly after pruning when new leaves start growing (Souza et al. 2003).
Guava weevil is considered the main pest in Brazilian guava orchards. This pest directly affects the fruit quality causing great damage. The adults are present in orchards during summer, appearing in September-October and remaining until March. Females lay eggs in immature fruit (3-4 cm diameter) and larvae progress through four instars as the fruit develops. Infestation leads to acceleration in fruit maturation and fruit drop when ripe. Subsequently, larvae crawl into the soil where they develop into prepupae. Individuals may remain in this stage for up to six months before pupation and development into the adult (Boscán de Martinez and Cásares, 1982; Bailez et al., 2003). Control methods involve weekly applications of insecticides to suppress adults, but most of those currently in use for guava weevil control will be discontinued soon (Souza et al., 2003; Agência Nacional de Vigilância Sanitária, 2004). Without chemical control, the percentage of damaged fruit in heavily infested orchards can reach 100% (Bóscan de Martinez and Cásares, 1980). The amount of fruit attacked has been increasing over the past three years possibly due to the development of insecticide resistance (C. Dolinski, unpublished). Poorly timed chemical applications and the tendency for adult weevils to hide in the litter around trees and avoid contact with the chemicals could also be involved (Denholm and Rolland, 1992).
The virulence of nine species/strains of EPNs to fourth-instar weevils was assessed in the laboratory. Larval mortality in Petri dish assays with sterile sand at 100 infective juveniles (IJs)/larva, ranged from 33.5% to 84.5%, with the heterorhabditids being the most virulent. In sand column assays with H. baujardi Phan, Subbotin, Nguyen and Moens LPP7, H. indica Poinar, Karanukar and David Hom1, and S. riobrave Cabanillas, Poinar and Raulston 355 at 100, 200 and 500 IJs/larva, significant mortality was observed only for H. baujardi (62.7%) and H. indica (68.3%) at the highest dose. For H. baujardi LPP7, the LT50 and LT90 for 100 IJs were 6.3 and 9.9 days, whereas the LC50 and LC90 over seven days were 52 and 122.2 IJs (Dolinski et al., 2006). In a greenhouse study with guava trees in 20-L pots (ten weevil larvae/pot), and doses of 500, 1000 or 2000 IJs/pot (0.17, 0.35, or 0.7 IJ/cm2), H. baujardi LPP7 caused 30% and 58% mortality at the two highest doses (Dolinski et al., 2006). Heterorhabditis baujardi LPP7 is an indigenous nematode isolated from the tropical forest of Rondônia, in the north of Brazil (Dolinski et al., 2008).
Del Valle et al. (2008) assessed the susceptibility of the guava weevil to H. baujardi LPP7 IJs in the greenhouse and under field conditions, and applied nematodes in cadavers of 7th instar Galleria mellonella L. (greater wax moth). Field persistence of these nematodes in the soil was evaluated through G. mellonella-baiting. Insect cadaver concentrations of 2, 4, and 6 applied in pots in the greenhouse experiment caused significantly greater mortality than the control. Significant differences were observed in the field between control and treatments only when 6 cadavers per 0.25 m2 were applied. Infective juveniles from the cadavers persisted 6 weeks after application in the field, but decreased greatly thereafter (Del Valle et al., 2008).
In 2008, we started to work with a small group of farmers with the objective of establishing an integrated pest management (IPM) program in their orchards. Approximately twenty farmers organized in an association named GOIACAM (Associação do Produtores de Goiabas de Cachoeiras de Macacu) in the state of Rio de Janeiro, Brazil, paid for the nematode registration and were trained to rear G. mellonella themselves. The G. mellonella larvae were taken to the lab, infected with H. baujardi LPP7 and then returned to the farms as infected cadavers for application. First results showed a decrease of 40-70% in emerged adults, in plots of 9 m2 where 20 cadavers were used, compared to control trees with no nematode application. If neem cake was also applied below the canopy for larvae control, an additive effect occurred, with the adult control reaching almost 80%. The farmers also initiated cultural control by removing all damaged fruit from the orchards, which reduced the pest innoculum for the following year. Between rows, Crotalaria sp. and other Leguminosae were planted to increase the soil fertility and serve as refuge for natural enemies. Because insecticides are not being used in those experimental areas, beneficial arthropods such as coccinelids and crisopids are being seen more often within the orchards where nematodes were applied. Using these strategies, the production costs were reduced by 40%. Some growers use sprinkler irrigation, so Lara et al., (2008) evaluated the influence of irrigation application of EPNs on the viability, infectivity and host search capability of H. baujardi LPP7 IJs. The results demonstrated that the irrigation system did not adversely affect any of the factors described previously. Today, growers can choose from these different application methods, based on their situation.
The guava weevil population in the areas where the IPM was implemented in 2008 is very low compared to what it was when we first applied nematodes. We routinely recovered 20 to 30 adults from each tree prior to initiating GOIACAM, compared to just 2 or 3 adults now. This population reduction reflects directly on the number of damaged fruits, which is now typically 1 % of the fruits produced. The weevil is still present, but there is an understanding that it is at a new lower equilibrium in the orchards. Currently there are at least 10 ha of guava under IPM. This area will likely expand because, after so many years, positive results are apparent to the growers.
A new project is being initiated to convert orchards under both IPM and conventional management to organic management systems. The consumption and demand for organically grown fruit is increasing. Because guava planted in these areas is exclusively for direct consumption, there is a desire for less pesticides use. The growers are beginning to understand that if they want better quality and better prices, they will have to invest in new methods to produce and protect guava.
Management of Leafy Vegetable Pests Using EPNs in Korean Greenhouses
Indigenous EPNs have been surveyed and isolated from various Korean habitats since the mid-1980s. Many of these isolates have been evaluated for their efficacy against important local and introduced insect pests of rice, vegetables, forest, turfgrass, and greenhouse crops (Kaya et al., 2006). Among the Korean isolates, Steinernema carpocapsae (Weiser) Wouts, Mracek, Gerdin and Bedding and Heterorhabditis bacteriophora Poinar from soil, and an unidentified species of Heterorhabditis from the white grub Exomala orientalis (Waterhouse) Piattella and Sabatinelli are produced commercially in vivo and in vitro. Sesil Corporation (www.sesilipm.co.kr) was one of the first companies to produce EPNs and develop the industry in Korea. Nematodes were reared in G. mellonella larvae and packaged on sponges containing 2 x 107 IJs. The company was able to produce 200 and 380 packages per day of S. carpocapsae Pocheon strain and an unidentified Heterorhabditis sp., respectively, and these products were used against caterpillars on vegetables, fungus gnats on mushrooms, and other insect pests of greenhouse plants (Kaya et al., 2006). Dongbu Ceres Co. Ltd. (www.dongbuceres.co.kr) merged with Sesil Corporation in June 2011 and discontinued EPN production. A second company, Bicosys Co. Ltd., began producing S. carpocapsae and H. bacteriophora in vitro at about the same time as Sesil, but also discontinued EPN production in 2011. However, in 2008 former scientists and staff of Bicosys founded Ecowin Co. Ltd. (http://www.eco-win.kr) which produces ECOWIN-S (S. carpocapsae) and ECOWIN-H (H. bacteriophora) in vitro. The products contain 2 x 107 IJs on sponges designed to treat 330 m2 and are marketed for control of cutworm, onion maggot, webworm, diamondback moth, armyworm, and fungus gnat.
Korean isolates of EPNs have been tested against a number of endemic and introduced insect pests in Korea. Notable successes have occurred in the greenhouse, especially against an invasive fungus gnat Bradysia agrestis Sasakawa (=B. difformis Frey) (see Kaya et al., 2006). The introduction of B. difformis, gave rise to a serious problem for seedlings of vegetable crops in greenhouses. One such crop was watermelon, whose seedlings are generally raised for 35 days before being sold, at which time it is critical to encounter few or no fungus gnats. Among five EPN species, S. carpocapsae proved most effective against all stages of B. difformis except for the egg and first instar (Kim et al., 2004a, 2004b). When watermelon seeds were treated with S. carpocapsae at sowing, the larval and adult densities of B. difformis were greatly reduced and mortality of seedlings ranged between 0.2 - 1.9% 34 days post-treatment, compared to 25.5-71.6% in the untreated plots. Moreover, S. carpocapsae persisted during the 35 day greenhouse period and for one week after transplanting to the field (see Kaya et al., 2006). Based on these results, S. carpocapsae have been applied to all subsequent watermelon crops by this grower.
Growers’ acceptance of EPNs for pest management is crop dependent. The greatest penetration of EPN usage is in leafy, greenhouse-grown vegetables due to the favorable environment (temperature and moisture) for these nematodes and the high prices organically grown vegetables command compared to those treated with chemical insecticides. Leafy vegetables grown in greenhouses are especially susceptible to pest outbreaks. Typical pests include Autographa nigrisigna (Walker), Spodoptera litura (Fabricius), S. exigua (Hűbner), and Cucullia fraterna Butler on lettuce; Artogeia rapae (L.), Plutella xylostella (L.), Mamestra brassicae L., S. litura, S. exigua, A. nigrisgna on kale; Pl. xylostella, Phaedon brassicae Baly, and Phyllotreta striolata (Fabricius) on mustard; Hymenia recurvalis Fabricius on Swiss chard; Athalia rosae ruficornis Jakovlev and Phy. striolata on Pak choi; Pl. xylostella, M. brassicae, A. nigrisigna, Pha. brassicae, and Phy. striolata on Ssamchoo (Korean cabbage); and Agrotis segetum (Denis et Schifferműller), S. litura and S. exigua on crown daisy.
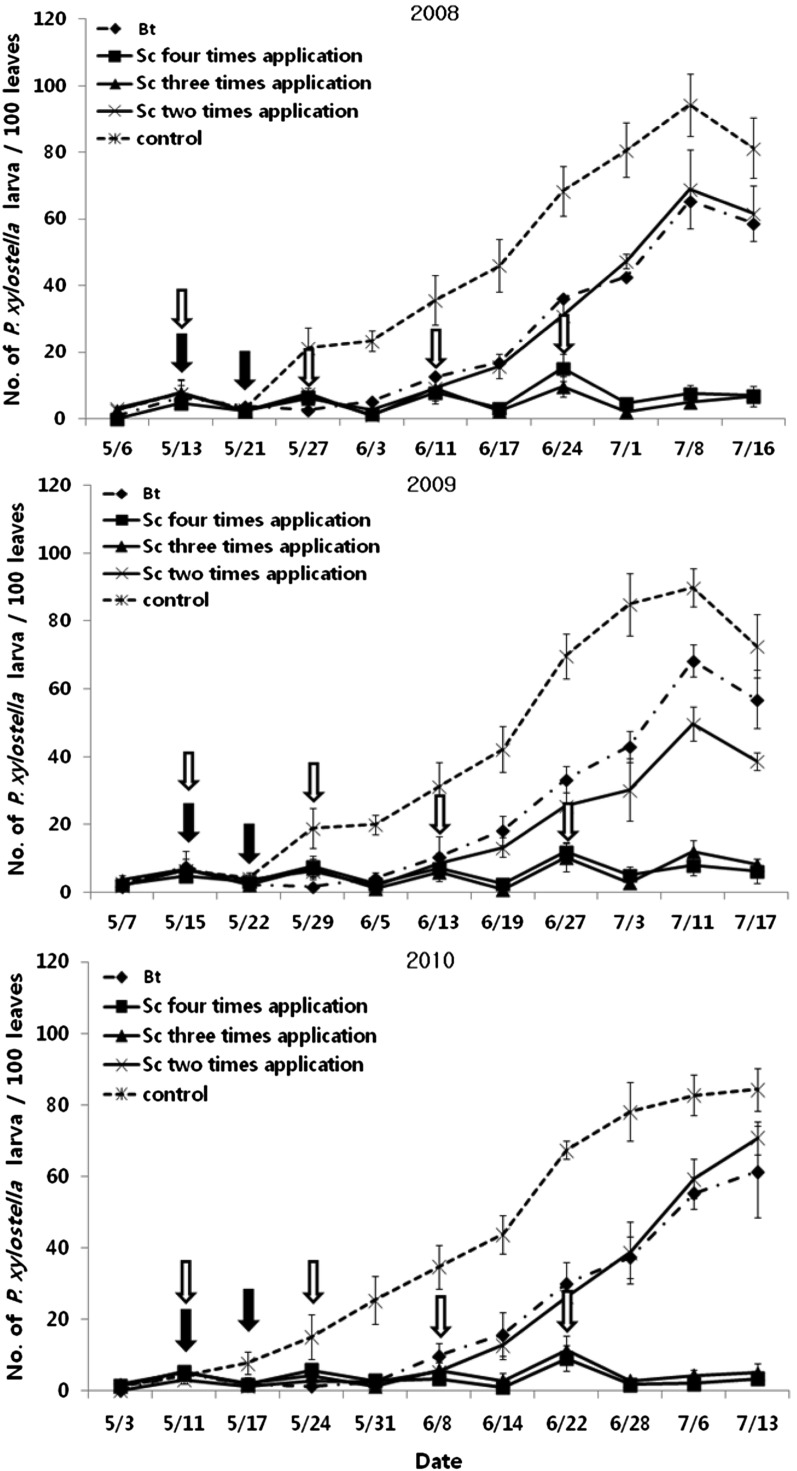
The use of S. carpocapsae outperformed that of Bacillus thuringiensis Berliner (Bt) during recent trials in greenhouses lasting three years for control of Au. nigrisigna on lettuce, Ar. rapae on kale and Pl. xylostella on mustard. Treatments performed similarly in all years against each pest and results of managing Pl. xylostella on mustard are shown here (Fig. 1). The research was conducted in Gongju, Chungnam province from 2008 to 2010. Three greenhouses per treatment, containing newly transplanted, 30-day-old mustard seedlings enclosed with 40 mesh insect netting were assigned as: (1) untreated control, (2) S. carpocapsae (ECOWIN-S) at 9.2 x 108 IJs/ha, applied either 2, 3, or 4 times and (3) Bt var. kurstaki serotype IIIa, IIIb (Bayer Korea, Seoul, Korea) at 6 kg ai/ha + Bt subsp. aizawai NT423 (Dongbu HiTek, Seoul, Korea) at 3kg ai/ha. The S. carpocapsae were applied with a motorized sprayer in late afternoons. The numbers of caterpillars were determined every 6 to 8 days. During more than 60 days the density of Pl. xylostella remained uniformly low in greenhouses treated 3 or 4 times with S. carpocapsae, whereas insect numbers increased throughout the cropping cycle each year in the untreated controls. Less frequent use of EPNs or use of Bt provided significant control of Pl. xylostella, but far less than that of more frequent EPN use.
Fig. 1.
Mean number of Plutella xylostella on greenhouse-cultivated mustard during three years as affected by 1) no pest management, 2) treatment with Bacillus thuringiensis, or 3) treatment with entomopathogenic nematodes either twice, three or four times.
Results such as these emphasize the tremendous potential for continued expansion of EPN use in Korean covered agriculture. The greenhouse cultivation of 34 vegetables occupies 15,377 ha for leafy vegetables, 1,796 ha for seasoning vegetables and 46,906 ha for fruit vegetables in 2010 (KMFAFF, 2011). The EPN use in greenhouses was 50% in fruit vegetables, 30% in seasoning vegetables, 10% in leaf vegetables, 5% in root vegetables, and 5% in others (www.eco-win.kr, National Institute of Horticultural and Herbal Science, 2011). Often, it required just one cropping season of EPN use to convince growers to use them routinely. There are no biocontrol products comparable to EPNs in terms of cost, efficacy or broad spectrum activity. Consequently, EPNs are the most widely used biocontrol agents for control of caterpillars, sawflies, and leaf beetles in leafy vegetables. The main target insects of commercial entomopathogenic nematodes currently are B. difformis, Palpita indica Saunders, and S. litura in root vegetables, Pl. xylostella, M. brassicae, A. nigrisigna, S. litura, S. exigua, A. rapae, Hymenia recurvalis, Pha. brassicae, and Phy. striolata in leaf vegetables in greenhouse.
Beyond covered agriculture, Korean farming practices are increasingly dependent on non-chemical forms of pest control. Between 2005 and 2011, the Korea Ministry for Food, Agriculture, Forestry and Fisheries provided financial incentives to growers who managed any of nine high value crops using approved “eco-friendly” pest management practices. This project encouraged the development of natural enemy products, including those with EPNs, and growers responded favorably. The arable hectareage in the country is 984,000 ha for paddy rice, 731,000 ha for upland rice and 206,148 ha for vegetables. More than 173,000 farms utilize either chemical-free (94,533 ha) or low level (83,955 ha) chemical management for pest control (KMFAFF, 2011). Thirty-five natural enemy products are currently sold in Korea. The price of products is variable from about US$ 15 to US$ 160 per sales unit. By contrast, the price of ECOWIN-S is US$ 15 per pack containing 2 x 107 IJs and is thus considered a low price product. Recent sales of EPN products in Korea were valued at US$ 3,500,000 per year.
Classical, Augmentation, and Conservation Biological Control with EPNs in Florida
In Florida, EPNs have been studied for more than two decades in the context of formal, multidisciplinary programs to manage invasive species of mole crickets and root weevils (Shapiro et al., 2002 and 2005; Campos-Herrera et al., 2010). These programs also supported continuous outreach efforts to growers in the form of meetings and field days in order to encourage the use of EPNs in management programs. In the course of developing both classical and augmentation biological control tactics to manage these pests, it became apparent that the Florida peninsula supports rich and abundant communities of native EPN species that could potentially be exploited by using tactics that promote EPN conservation (Duncan et al., 2007; Stuart et al., 2008).
Classical biocontrol of mole crickets
Invasive mole crickets are major pests of turf, pastures and vegetables throughout the Caribbean Basin. Two South American mole crickets, Scapteriscus didactylis Latreille and S. abbreviatus Scudder arrived in Puerto Rico in the late 18th and 19th centuries, respectively (Frank et al., 2007). Scapteriscus abbreviatus and two other South American natives, S. vicinus Scudder and S. borellii Giglio-Tos, were introduced into Florida and Georgia near the beginning of the last century. Mole crickets cost vegetable, pasture and turf growers in Puerto Rico and the southeastern US an estimated $200 million annually due to crop loss and management costs (Frank and Parkman, 1999). Accordingly, the Florida legislature initiated the University of Florida, IFAS Mole Cricket Research Program in 1978 to discover and introduce natural enemies of mole crickets from South America to Florida. Several parasitic wasp and fly species were introduced and established as a result of the program (Frank et al., 1995, 1996). Most notable, however, was the discovery and use of a nematode that represents a truly unique case in which EPNs have been utilized successfully for classical biological control (Nguyen and Smart, 1990; Leppla et al., 2007).
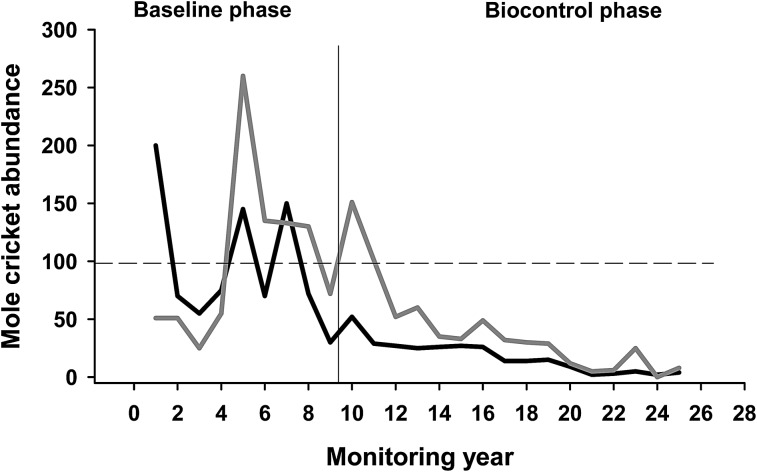
The EPN Steinernema scapterisci Nguyen and Smart was discovered in Uruguay, one of the countries at the putative center of origin of the mole cricket (Nguyen and Smart, 1990). All mole cricket species tested were susceptible to infection by the nematode, whereas non-orthopteran species were poor hosts (Nguyen and Smart, 1991). Steinernema scapterisci was introduced in several Florida pastures in the mid-1980s, where it persisted and by 1990 was reported to provide effective biological control of several invasive mole cricket species (Parkman et al., 1993; Frank and Walker, 2006). Infected mole crickets can transport S. scapterisci over short and long distances before they die, which permitted growers to economize by strip treating as little as 12% of pasture surface to achieve good control within a year (Parkman et al., 1993; Adjei et al., 2006; Leppla et al., 2007). Dispersal of the nematode by infected mole crickets resulted in its detection in pastures and even citrus orchards miles from where it was introduced (Parkman et al., 1993; Campos-Herrera et al., 2010 and 2011). Two mole cricket monitoring sites in Gainesville FL collected baseline population data for 9 years beginning in 1978, after which nematodes and imported parasitic wasps and flies were detected in samples. During the next 16 years mole cricket numbers (S. vicinus and S. borellii) at both sites remained at ca. 5% of baseline levels and mortality due to nematodes was estimated to be as high as 88% (Frank and Walker, 2006) (Fig. 2). Pest control companies reported dramatic reductions in the number of lawn-care accounts for mole cricket management (Frank and Walker, 2006).
Fig. 2.
Abundance of mole crickets (Scapteriscus borellii, black line; S. vicinus, gray line) trapped at two pasture monitoring stations near Gainesville FL during 25 years (1979-2004). The first nine years represent baseline data, recorded before parasitic nematodes, wasps and flies released at other localities were detected at the Gainesville site. The succeeding years reveal the effects of classical biological control on mole cricket populations. Dashed line is the mean of annual mole cricket counts during the baseline period, standardized to the value 100 for each species. Redrawn from Frank and Walker (2006) with permission from the Entomological Society of America.
Because of the proven ability of S. scapterisci to regulate mole cricket populations and improve the quality of pasture, the nematode became commercially available in the early 1990s and scientists involved with the mole cricket program switched their research focus to parasitoids (Frank et al., 2007). However, the local company licensed to produce nematodes was unable to resolve production problems and discontinued manufacturing EPNs in 1996. After several years during which EPNs were unavailable and mole cricket damage increased in some parts of Florida, additional funds were appropriated by the legislature in 2001 to reinitiate efforts by the Mole Cricket Program to promote the use of nematodes. The nematode was licensed to Becker Underwood Inc. (Ames, IA) and trials were established in pastures and turf in several Florida counties, again demonstrating consistent persistence and spread of S. scapterisci and reduction of mole crickets and plant damage in the treated areas (Adjei et al., 2006). Since 2002 S. scapterisci has been available commercially through Becker Underwood under the name Nematac S.
Despite the success of the Mole Cricket Research Program in demonstrating the utility of S. scapterisci, grower use of nematodes remains disappointing. Product penetration of the potential hectarage (turf, pasture and sod) is much less than one percent (Al Clarke, Becker Underwood, personal communication). Golf course managers prefer to use currently available pesticides, and ranchers are constrained in the cost they can expend for pest control. Still, all available evidence indicates that S. scapterisci continues to infect mole crickets at high levels that suppress plant damage in pastures previously treated with the nematode. Consequently, UF/IFAS scientists continue to explore methods to increase grower adoption of commercial nematodes. Recently, the USDA, NIFA, Southern Region IPM Program supported the development of two EPN application rigs to deliver S. scapterisci in narrow strips by means of chisels to just a portion of fields. The rigs were made available at no cost to growers who purchase the nematodes. This fact, and the ability of S. scapterisci to quickly colonize the entire treated field (and eventually neighboring fields) brought application costs to levels that growers could afford (currently ca. $62.00/Ha). Field days demonstrating the application rigs at work in pastures were employed in 2011 throughout Florida to extend the information as widely as possible. Nevertheless, in spite of the unique performance of the formulated S. scapterisci product and the increased effort to extend this information and promote its use, Becker Underwood discontinued production of Nematac-S in February 2012 due to insufficient profitability.
Academic interest in the performance of S. scapterisci in Florida is currently high. It remains to be seen whether the license to produce the nematode will be acquired by another company. Future research will utilize a newly developed molecular primer/probe set that can quantify S. scapterisci present in soil samples (Campos-Herrera et al., 2011). Use of this tool will facilitate efforts to understand the relative contributions of S. scapterisci and the introduced parasitoids Larra bicolor F. and Ormia depleta Wiedemann in controlling mole crickets, a critical question given the currently unique status of S. scapterisci in classical biological control. The molecular probe can also help growers identify fields in which nematode applications are likely to be profitable. Previous methods of detecting the nematode in the field, requiring trapping and monitoring crickets, are too costly for routine monitoring.
Conservation and augmentation/inoculative biocontrol of citrus root weevils
Excluding Asian citrus psyllid, which vectors a tree-debilitating bacterial disease known as “huanglongbing” or “citrus greening”, Diaprepes abbreviatus L. is the most important insect pest of citrus in Florida (Graham et al., 1996, 2003; Duncan et al., 1999; McCoy, 1999). A polyphagous weevil pest, native to the Caribbean, D. abbreviatus was first detected in Florida in 1964 (Wolcott, 1936; Woodruff, 1964). For nearly half a century, the weevil has caused devastating losses of citrus trees and sometimes entire orchards. In Florida, annual losses and cost of control for the Diaprepes root weevil in citrus were estimated at $72 million a decade ago (Pena and Amalin, 2000). The weevil is now established in Texas and California where it is the subject of quarantine and eradication programs. In Florida, a statewide Diaprepes Task Force comprised of citrus growers and scientists from University of Florida, USDA, and Florida Department of Agriculture operated between 1993 and 2008. The organization supported annual meetings and field days to develop research programs and disseminate the resulting information. Federal funding in excess of US $400,000 per year directly supported Diaprepes research from 1998-2008.
Adult D. abbreviatus feed on new foliage and eggs are laid on leaves. Individual females live about one year and lay over 11,000 eggs. The neonate larvae fall to the soil where they feed on increasingly larger roots as they develop through up to 11 instars during several months (Quintela et al., 1998; Nigg et al., 2003). Adults emerge from soil in citrus groves throughout the year, but largely in spring and sometimes autumn (Duncan et al., 2001). Florida citrus growers use chemical insecticides and growth regulators, respectively, to kill adult D. abbreviatus and prevent egg hatch. Chemical soil barriers are also available to kill neonates entering soil and adults emerging from soil. However, cost and disruption of biological control limit the frequency with which chemicals can be used. Thus, chemicals currently available are only marginally effective because they are non-persistent, leaving large windows of opportunity between applications during which weevils enter the safety of the soil or emerge from soil to lay eggs (Duncan et al., 1999; McCoy, 1999).
Following the loss of persistent organochlorine pesticides, the only recommended control for larvae feeding on roots has been the seasonal application of EPNs (Duncan and McCoy, 1996; Duncan et al., 1996, 2007; Bullock et al., 1999; McCoy et al., 2002). Steinernema carpocapsae, H. bacteriophora and H. indica were the first commercially formulated nematodes to be used to manage D. abbreviatus (Schroeder, 1987; Figueroa and Roman, 1990; Adair, 1994). Eventually, S. riobrave (Cabanillas et al., 1994), was shown to provide greater control of D. abbreviatus than these other EPNs when applied to orchard soils (Schroeder, 1994; Duncan et al., 1996; Bullock et al., 1999). Direct measurements of larval suppression in the field ranged from 46% at recommended rates (25 IJs/cm2 soil surface) (Duncan et al., 2003) to ≥ 90% at higher rates (Duncan and McCoy, 1996; Duncan et al., 1996; Bullock et al., 1999). Use of S. riobrave at recommended rates reduced adult emergence from soil during 12 months by half (Duncan et al., 2007). However, estimates of the efficacy of S. riobrave for weevil control vary widely due to factors such as product quality (producers and products have changed over time), application rates, experimental methods, and edaphic conditions (McCoy et al., 2000, 2002; Jenkins et al., 2007, 2008).
With the commercialization of S. riobrave, citrus growers in Florida readily adopted use of EPNs as part of Diaprepes management programs due to the severity of the pest and an inability to adequately control weevils with chemical pesticides. In 1999, at the peak of EPN use in Florida citrus, ca. 19,000 ha of citrus were treated with S. riobrave (Shapiro-Ilan et al., 2002). Subsequently, grower confidence in EPN products was gradually undermined by reports of poor product quality control (frequent low viability of IJs and fungal contamination) and several recalls of the commercial product. In 2004 Becker Underwood Inc. obtained a license to produce formulated S. riobrave. The product, marketed as BioVector 355 resolved the former quality issues and, although sales increased over time, they did not attain the level achieved in the mid-late 1990s. Recent changes in citrus pest management practices in response to the introduction of huonglongbing, resulted in low demand and the discontinuation of BioVector 355 in 2011. Specifically, the number of insecticide applications has quadrupled (from 2 sprays annually to 8-9 annually) as growers attempt to prevent Asian citrus psyllids from infecting trees. As a consequence, adult D. abbreviates appear to be less abundant. Regardless, the cost of pest management has more than doubled (currently $466/A) since the recent establishment of citrus greening and a second invasive bacterial disease, citrus canker, and growers have no choice but to forgo other pest management costs (R.P. Muraro, UF/IFAS economist, unpublished). The increased use of pesticides has resulted in the loss of traditionally beneficial arthropods and the appearance of secondary pests not encountered in Florida citrus for decades. Growers and pest control advisors are also aware of the increased risk of pesticide resistance development. All of these well-recognized consequences of overreliance on pesticides are also reasons that biological control tactics predominated in Florida citriculture until recently. It remains to be seen how long heavy pesticide use can be maintained and whether EPN augmentation will again become an important tactic in weevil management programs.
In addition to EPN augmentation, there is some interest in the possibility of managing D. abbreviatus by EPN conservation. The survival rate of D. abbreviatus in soil, based on a census of neonates falling from leaves and adults emerging from soil, was estimated to be between 0.7% and 1% (McCoy et al., 2003). When EPN efficacy against D. abbreviatus was being characterized in orchards, it became apparent that native EPNs contributed substantially to weevil larvae mortality (McCoy et al., 2000; Duncan et al. 2003, 2007). Duncan et al. (2003) reported that buried larvae were killed by EPNs at substantially higher rates in an orchard with low weevil abundance than in a second orchard with abundant weevils. These differences in weevil parasitism rates suggest that EPNs may be a factor in regional spatial patterns of weevils which are more abundant and damaging to citrus in some eco-regions than others (Futch et al., 2005). Indeed, Florida citrus orchards have been shown to support abundant EPN populations that comprise any of eight known species (Campos-Herrera et al., 2009 and unpublished data). Moreover, EPNs were detected in all of 53 orchards sampled across the state, with highest species richness and diversity in the central ridge, an eco-region with coarse sandy soil where D. abbreviatus is least damaging and least abundant (Campos-Herrera et al., unpublished). The prevalence and diversity of EPNs in the Florida peninsula and the possibility that nematodes modulate D. abbreviatus abundance more in some habitats than others suggests the need to study how soil physical properties affect EPNs in order to implement cultural practices that conserve these nematodes. Duncan et al. (2012) replaced native soil with coarse sand from the central ridge in oversized tree planting holes in a citrus orchard on sandy loam soil that supported few EPNs and abundant D. abbreviatus (Duncan et al., 2003). Four EPN species were then added to soil at the base of trees planted in native soil or sand. During 4 years, EPN species richness and diversity were greater in sand than in the native soil. Numbers of EPNs were not consistently different between soils, but EPNs consistently killed an average of 4-fold more sentinel larvae of D. abbreviatus buried in sand than in native soil. Forty percent fewer D. abbreviatus emerged from sand compared to native soil each year. Surviving trees in sand were 60% larger after 4 years and produced 85% more fruit than those in native soil. Although it is not possible to ascribe differences in weevil numbers and tree growth in the two soils exclusively to biological control by EPNs, the experiment demonstrated the possibility of dramatically increasing the rate of biological control over a span of years by a simple, one-time, cultural practice. The ecology of EPNs and soil food webs has received increased attention as advances in molecular techniques and multivariate analyses facilitate the study of relationships between cryptic organisms and the physical environment (Duncan et al., 2007; Greenwood et al., 2011; Jabbour and Barbercheck, 2011; Pathak et al., 2012). Increased understanding of how soil conditions affect food webs will present opportunities to exploit the potential of native biocontrol agents.
Conclusions
Growers will use EPNs for pest control only when EPN use is more profitable than alternative tactics. The examples given here illustrate how changing agricultural practices are likely to increase the acceptance of EPNs by growers in many agro-industries. The most important transitions leading to the adoption of EPN products here were the increased demand for organic products in Brazil and Korea, and the increased use of greenhouses to produce high value crops. Utilization of greenhouses increased production that doubled income for Korean growers. In turn, greenhouses provide optimal environmental conditions for using EPNs. Growing economies and increased living standards will similarly increase the demand for these high value crops in many parts of the world. Greater familiarity with agriculture by many people in developing countries has promoted a desire for reduced pesticide use as incomes have risen. The spectacular pest control achieved by EPN products in greenhouses allows Korean growers to take advantage of the organic market, something that is not yet possible for vegetable pest management in the open field. The low cost of Korean EPN products compared to those of other biocontrol agents further favors their adoption in covered agricultural operations. The fact that younger growers in Korea have more readily adopted EPN use than those over sixty years (KMFAFF, 2011) also suggests greater use of these products in the future.
Despite greater environmental challenges to the use of EPNs in the field, Brazilian guava growers have benefitted greatly from EPNs that control an important subterranean pest. Indeed, the high level of guava weevil control by EPNs suggests that growers and consumers in Brazil may benefit from an expansion of the organic market there. Similarly, the lack of effective soil-applied chemical insecticides provided an opportunity for development and use of EPN products that effectively managed weevils attacking citrus roots in Florida. The current intensive insecticide use in the citrus canopy has effectively eliminated commercial EPN use but will likely be a temporary aberration as growers attempt to maintain their orchards until a sustainable method to control huanglongbing is developed.
Finally, as with many pest control tactics, low value field crops often cannot support the cost of pest management with EPNs. Even though S. scapterisci has outstanding potential for long-term control of the serious damage caused by mole crickets in pastures, grower adoption of the tactic has been inadequate to support their continued production. Nevertheless, the commercialization of S. scapterisci and all of the products discussed here were the result of long term, broad based, publically supported coalitions of scientists and growers to encourage the development and use of biological controls. Continuing interest by these groups in the dynamics of classical biological control of the mole cricket supports the likelihood of future commercialization of this nematode.
Literature cited
- Adair RC. A four-year field trial of entomopathogenic nematodes for control of Diaprepes abbreviatus in a Flatwoods citrus grove. Proceedings of the Florida State Horticultural Society. 1994;107:63–68. [Google Scholar]
- Adjei MB, Smart GC, Frank JH, Leppla NC. Control of pest mole crickets (Orthoptera : Gryllotalpidae) in bahiagrass pastures with the nematode Steinernema scapterisci (Rhabditida : Steinernematidae)Survey of pest mole crickets (Orthoptera: Gryllotalpidae) activity on pasture in South-Central Florida. Florida Entomologist. 2006;89:532–535. [Google Scholar]
- Agência Nacional de Vigilância Sanitária – ANVISA. 2004. www.anvisa.gov.br. Acessado em Janeiro 2007.
- Bailez OE, Viana-Bailez AM, Lima JOG, Moreira DDO. Life-history of the guava weevil, Conotrachelus psidii Marshall (Coleoptera: Curculionidae), under laboratory conditions. Neotropical Entomology. 2003;32:203–207. [Google Scholar]
- Boscán de Martinez N, Cásares R. Distribuicion en el tiempo de las fases del gorgojo de la guayaba Conotrachelus psidii Marshall (Coleoptera: Curculionidae) en el campo. Agro Tropic. 1982;31:123–130. [Google Scholar]
- Bullock RC, Pelosi RR, Killer EE. Management of citrus root weevils (Coleoptera: Curculionidae) on Florida citrus with soil-applied entomopathogenic nematodes (Nematoda: Rhabditida) Florida Entomologist. 1999;82:1–7. [Google Scholar]
- Cabanillas HE, Poinar GO, Jr, Raulston JR. Steinernema riobravis sp. nov. (Rhabditida: Steinernematidae) from Texas. Fundamental and Applied Nematology. 1994;17:123–131. [Google Scholar]
- Campos-Herrera R, Duncan LW, Stuart RJ, El-Borai F, Gutierrez C. 2010 Entomopathogenic Nematode Ecology and Biological Control in Florida Citrus Orchards. Pg. 97–126 in A. Ciancio and K. G. Mukerji, eds. Integrated Management of Arthropod Pests and Insect Borne Diseases. London, Springer. [Google Scholar]
- Campos-Herrera R, El-Borai F, Stuart RJ, Graham JH, Duncan LW. Entomopathogenic nematodes, phoretic Paenibacillus spp, and the use of real time quantitative PCR to explore soil food webs in Florida citrus groves. Journal of Invertebrate Pathology. 2011;108:30–39. doi: 10.1016/j.jip.2011.06.005. [DOI] [PubMed] [Google Scholar]
- Del Valle EE, Dolinski C, Barreto ELS, Souza RM, Samuels RI. Efficacy of Heterorhabditis baujardi LPP7 (Nematoda: Rhabditida) applied in Galleria mellonella (Lepidoptera: Pyralidae) insect cadavers to Conotrachelus psidii, (Coleoptera: Curculionidae) larvae. Biocontrol Science and Technology. 2008;18:33–41. [Google Scholar]
- Denholm I, Rolland MW. Tactics for managing pesticide resistance in arthropods: Theory and practice. Annual Review Entomology. 1992;37:92–112. doi: 10.1146/annurev.en.37.010192.000515. [DOI] [PubMed] [Google Scholar]
- Dolinski CM, Del Valle EE, Stuart R. Virulence of entomopathogenic nematodes to larvae of the guava weevil Conotrachelus psidii (Coleoptera: Curculionidae) in laboratory and greenhouse experiments. Biological Control. 2006;38:422–427. [Google Scholar]
- Dolinski C, Kamitami F, Machado IR, Winter CE. Molecular and morphological characterization of heterorhabditid entomopathogenic nematodes from the tropical rainforest in Brazil. Memorias do Instituto Oswaldo Cruz. 2008;103:150–159. doi: 10.1590/s0074-02762008000200005. [DOI] [PubMed] [Google Scholar]
- Duncan LW, McCoy CW. Vertical distribution in soil, persistence, and efficacy against citrus root weevil (Coleoptera: Curculionidae) of two species of entomogenous nematodes (Rhabditida: Steinernematidae; Heterorhabditidae) Environmental Entomology. 1996;25:174–178. [Google Scholar]
- Duncan LW, McCoy CW, Terranova AC. Estimating sample size and persistence of entomogenous nematodes in sandy soils and their efficacy against the larvae of Diaprepes abbreviatus in Florida. Journal of Nematology. 1996;28:56–67. [PMC free article] [PubMed] [Google Scholar]
- Duncan LW, Shapiro DI, McCoy CW, Graham JH. 1999 Entomopathogenic nematodes as a component of citrus root weevil IPM. Pp. 69–78 in S. Polavarapu, S. ed. Proceedings of workshop on optimal use of insecticidal nematodes in pest management. August 28-30, New Brunswick, NJ, Rutgers University. [Google Scholar]
- Duncan LW, McCoy CW, Stansly PA, Graham JH, Mizell RF. Estimating the relative abundance of citrus root weevils with modified Tedder’s traps. Environmental Entomology. 2001;30:939–946. [Google Scholar]
- Duncan LW, Graham JH, Dunn DC, Zellers J, McCoy CW, Nguyen K. Incidence of endemic entomopathogenic nematodes following application of Steinernema riobrave for control of Diaprepes abbreviatus. Journal of Nematology. 2003;35:178–186. [PMC free article] [PubMed] [Google Scholar]
- Duncan LW, Graham JH, Zellers J, Bright D, Dunn DC, El-Borai FE. Food web responses to augmentation biological control using entomopathogenic nematodes in bare and composted-manure amended soil. Journal of Nematology. 2007;39:176–189. [PMC free article] [PubMed] [Google Scholar]
- Duncan LW, Stuart RJ, El-Borai FE, Campos-Herrera R, Pathak E, Graham JH. 2012 Modifying orchard planting sites conserves entomopathogenic nematodes, reduces weevil herbivory and increases citrus tree growth, survival and fruit yield. Biocontrol (in review). [Google Scholar]
- Figueroa W, Roman J. Biocontrol of the sugarcane rootstalk borer, Diaprepes abbreviatus (L.), with entomophilic nematodes. Journal of Agriculture of the University of Puerto Rico. 1990;74:395–404. [Google Scholar]
- Frank JH, Parkman JP. Integrated pest management of pest mole crickets with emphasis on the southeastern USA. Integrated Pest Management Reviews. 1999;4:39–52. [Google Scholar]
- Frank JH, Walker TJ. Permanent control of pest mole crickets (Orthoptera: Gryllotalpidae: Scapteriscus) in Florida. American Entomologist. 2006;52:138–144. [Google Scholar]
- Frank JH, Parkman JP, Bennett FD. Larra bicolor (Hymenoptera: Sphecidae), a biological control agent of Scapteriscus mole crickets (Orthoptera: Gryllotalpidae), established in northern Florida. Florida Entomologist 78. 1995:619–623. [Google Scholar]
- Frank JH, Walker TJ, Parkman JP. The introduction, establishment, and spread of Ormia depleta in Florida. Biological Control. 1996;6:368–377. [Google Scholar]
- Frank JH, Vicente NE, Leppla NC. A history of mole crickets (Orthoptera: Gryllotalpidae) in Puerto Rico. Insecta Mundi. 2007;4:1–10. [Google Scholar]
- Futch SH, Duncan LW, Zekri M. Validation of an area-wide extension program to estimate the seasonal abundance of adult citrus root weevils with un-baited pyramidal traps. Proceedings of the Florida State Horticultural Society. 2005;117:143–147. [Google Scholar]
- Georgis R, Koppenhofer AM, Lacey LA, Belair G, Duncan LW, Grewal PS, Samish M, Tan L, Torr P, van Tol RWHM. Successes and failures in the use of parasitic nematodes for pest control. Biological Control. 2006;38:103–123. [Google Scholar]
- Gould WP, Raga A. 2002 Pests of guava. Pp. 295–313 in J. E. Peña, J. L. Sharp, and M. Wysoki, eds. Tropical Fruit Pests and Pollinators. Wallingford, UK, CABI Publishing. [Google Scholar]
- Graham JH, McCoy CW, Rogers JS. Insect-plant pathogen interactions: preliminary studies of Diaprepes root weevils injuries and Phytophthora infections. Proceedings of the Florida State Horticulture Society. 1996;109:57–62. [Google Scholar]
- Graham JH, Bright DB, McCoy CW. Phytophthora-Diaprepes weevil complex: Phytophthora spp. relationship with citrus rootstocks. Plant Disease. 2003;87:85–90. doi: 10.1094/PDIS.2003.87.1.85. [DOI] [PubMed] [Google Scholar]
- Greenwood CM, Barbercheck ME, Brownie C. Short term response to soil microinvertebrates to application of entomopathogenic nematode–infected insects in two tillage systems. Pedobiologia. 2011;54:177–186. [Google Scholar]
- Jabbour R, Barbercheck ME. Soil microarthropod response to the application of entomopathogenic nematode–killed insects in maize and flower strip habitats. Pedobiologia. 2011;54:243–251. [Google Scholar]
- Jenkins DA, Shapiro-Ilan D, Goenaga R. Virulence of entomopathogenic nematodes against Diaprepes abbreviatus in an Oxisol. Florida Entomologist. 2007;90:401–403. [Google Scholar]
- Jenkins DA, Shapiro-Ilan D, Goenaga R. Efficacy of entomopathogenic nematodes versus Diaprepes abbreviatus (Coleoptera: Curculionidae) larvae in a high clay-content Oxisol soil: Greenhouse trials with potted Litchi chinensis. Florida Entomologist. 2008;91:75–78. [Google Scholar]
- Kaya HK, Aguillera MM, Alumai A, Choo HH, de la Torrre M, Fodor A, Ganguly S, Hazir S, Lakatos T, Pye A, Wilson M, Yamanaki S, Yang H, Ehlers RU. Status of entomopathogenic nematodes and their symbiotic bacteria from selected countries or regions of the world. Biological Control. 2006;38:134–155. [Google Scholar]
- Kim HH, Choo HY, Kaya HK, Lee DW, Lee SM, Jeon HY. Steinernema carpocapsae (Rhabditida: Steinernematidae) as a biological control agent against the fungus gnat Bradysia agrestis (Diptera: Sciaridae) in propagation houses. Biocontrol Science and Technology. 2004a;14:171–183. [Google Scholar]
- Kim HH, Choo HY, Lee DW, Lee SM, Jeon HY, Cho MR, Yiem MS. Control efficacy of Korean entomopathogenic nematodes against fungus gnat, Bradysia agrestis (Diptera: Sciaridae) and persistence in bed soil. Korean Journal of Horticultural Science and Technology. 2004b;44:393–401. [Google Scholar]
- Lara JC, Dolinski C, Sousa EF, Daher RF. Effect of mini-sprinkler irrigation system on Heterorhabditis baujardi LPP7 (Nematoda: Heterorhabditidae) infective juvenile. Scientia Agricola. 2008;65:433–437. [Google Scholar]
- Leppla NC, Frank JH, Adjei MB, Vicente NE. Management of pest mole crickets in Florida and Puerto Rico with a nematode and parasitic wasp. Florida Entomologist. 2007;90:229–233. [Google Scholar]
- Lewis EE, Campbell JF, Gaugler R. 1998 A conservation approach to using entomopathogenic nematodes in turf and landscapes. Pp. 235–254 in P. Barbosa, ed. Conservation Biological Control. New York, NY, USA, Academic Press. [Google Scholar]
- McCoy CW. 1999 Arthropod pests of citrus roots. Pp. 149–156 in L. W. Timmer, and L. W. Duncan, eds. Citrus Health Management. St. Paul, MN, USA, APS Press. [Google Scholar]
- McCoy CW, Shapiro DI, Duncan LW, Nguyen K. Entomopathogenic nematodes and other natural enemies as mortality factors for larvae of Diaprepes abbreviatus (Coleoptera: Curculionidae) Biological Control. 2000;19:182–190. [Google Scholar]
- McCoy CW, Stuart RJ, Duncan LW, Nguyen K. Field efficacy of two commercial preparations of entomopathogenic nematodes against larvae of Diaprepes abbreviatus (Coleoptera: Curculionidae) in alfisol type soil. Florida Entomologist. 2002;85:537–544. [Google Scholar]
- McCoy CW, Stuart RJ, Nigg HN. Seasonal life stage abundance of Diaprepes abbreviatus in irrigated and non-irrigated citrus plantings in central Florida. Florida Entomologist. 2003;86:34–42. [Google Scholar]
- Ministry for Food, Agriculture, Forestry and Fisheries. 2011. The major statistics of food, agriculture, forestry, and fisheries in Korea.Seoul Korea, Hanra Printing. [Google Scholar]
- Nguyen KB, Smart GC., Jr Steinernema scapterisci n. sp. (Steinernematidae: Nematoda) Journal of Nematology. 1990;22:187–199. [PMC free article] [PubMed] [Google Scholar]
- Nguyen KB, Smart GC., Jr Pathogenicity of Steinernema scapterisci to selected invertebrates. Journal of Nematology. 1991;23:7–11. [PMC free article] [PubMed] [Google Scholar]
- Nigg HN, Simpson SE, Stuart RJ, Duncan LW, McCoy CW, Gmitter FG., Jr Abundance of Diaprepes abbreviatus (L.) (Coleoptera: Curculionidae) neonates falling to the soil under tree canopies in Florida citrus groves. Journal of Economic Entomology. 2003;96:835–843. doi: 10.1093/jee/96.3.835. [DOI] [PubMed] [Google Scholar]
- Parkman JP, Hudson WG, Frank JH, Nguyen KB, Smart GC., Jr Establishment and persistence of Steinernema scapterisci (Rhabditida: Steinernematidae) in field populations of Scapteriscus spp. mole crickets (Orthoptera: Gryllotalpidae) Journal of Entomological Science. 1993;28:182–190. [Google Scholar]
- Pathak E, El–Borai FE, Campos–Herrera R, Johnson EG, Stuart RJ, Graham JH, Duncan LW. Use of real-time pcr to discriminate parasitic and saprophagous behavior by nematophagous fungi. Fungal Biology. 2012 doi: 10.1016/j.funbio.2012.02.005. (in press). [DOI] [PubMed] [Google Scholar]
- Pena JE, Amalin DM. 2000 Biological control of Diaprepes abbreviatus by parasitoids. Diaprepes Short Course, University of Florida, IFAS, Lake Alfred, FL. March 22th, 2000. [Google Scholar]
- Quintela ED, Fan J, McCoy CW. Development of Diaprepes abbreviatus (Coleoptera: Curculionidae) on artificial and citrus root substrates. Journal of Economic Entomology. 1998;91:1173–1179. [Google Scholar]
- Schroeder WJ. Laboratory bioassays and field trials of entomogenous nematodes for control of Diaprepes abbreviatus. Environmental Entomology. 1987;16:987–989. [Google Scholar]
- Schroeder WJ. Comparison of two steinernematid species for control of the root weevil Diaprepes abbreviatus. Journal of Nematology. 1994;26:360–362. [PMC free article] [PubMed] [Google Scholar]
- Shapiro-Ilan DI, Gouge DH, Koppenhofer AM. 2002 Factors affecting commercial success: case studies in cotton, turf and citrus. Pp. 333–356 in R. Gaugler, ed. Entomopathogenic Nematology New York, NY, USA, CAB International. [Google Scholar]
- Shapiro-Ilan DI, Duncan LW, Lacey LA, Han R. 2005 Orchard crops. Pp. 215–230 in P. Grewal, R-U. Ehlers, and D. Shapiro-Ilan, eds. Nematodes as Biological Control Agents. St. Albans, UK, CAB International. [Google Scholar]
- Souza JC, Haga A, Souza MA. 2003 Pragas da goiabeira. Boletim Técnico 71. EPAMIG, Minas Gerais, Brazil, 60p. [Google Scholar]
- Stuart RJ, El-Borai FE, Duncan LW. From augmerntatition to conservation of entomopathogenic nematodes: trophic cascades, habitat manipulation and enhanced biological control of Diaprepes abbreviatus root weevils in Florida citrus groves. Journal of Nematology. 2008;40:73–84. [PMC free article] [PubMed] [Google Scholar]
- Wolcott GN. The life history of Diaprepes abbreviatus at Rio Piedras, Puerto Rico. Journal of Agricultural University of Puerto Rico. 1936;20:883–914. [Google Scholar]
- Woodruff RE. A Puerto Rican weevil new to the United States (Coleoptera: Curculionidae). Florida Department of Agriculture. Plant Industry Entomology Circular. 1964;77:1–4. [Google Scholar]