Abstract
Entomopathogenic nematodes (EPNs) in the families Heterorhabditidae and Steinernematidae have a mutualistic–symbiotic association with enteric γ-Proteobacteria (Steinernema–Xenorhabdus and Heterorhabditis–Photorhabdus), which confer high virulence against insects. EPNs have been studied intensively because of their role as a natural mortality factor for soil-dwelling arthropods and their potential as biological control agents for belowground insect pests. For many decades, research on EPNs focused on the taxonomy, phylogeny, biogeography, genetics, physiology, biochemistry and ecology, as well as commercial production and application technologies. More recently, EPNs and their bacterial symbionts are being viewed as a model system for advancing research in other disciplines such as soil ecology, symbiosis and evolutionary biology. Integration of existing information, particularly the accumulating information on their biology, into increasingly detailed population models is critical to improving our ability to exploit and manage EPNs as a biological control agent and to understand ecological processes in a changing world. Here, we summarize some recent advances in phylogeny, systematics, biogeography, community ecology and population dynamics models of EPNs, and describe how this research is advancing frontiers in ecology.
Keywords: biodiversity, entomopathogenic nematodes, Heterorhabditis, multivariate analysis, Photorhabdus, soil ecology, soil food web, Steinernema, Xenorhabdus
Although entomopathogenic nematodes (EPNs) have been exploited as biological control agents since the last half of the 20th century, much research remains to be done to understand how these organisms function in agricultural and other ecosystems. Specifically, it is critical to know when and how these nematodes will be effective and profitable biological controls. EPNs have been used with varying success to control soil insects. Parkman et al. (1996) documented the successful control of introduced mole crickets, Scapteriscus spp., in Florida pastures that was accomplished using an introduced species, Steinernema scapterisci, as a classical biological control agent. However, consistent efficacy of EPNs in augmentative applications against soil-dwelling insects in agricultural systems has not yet been achieved despite their predicted potential (Georgis et al., 2006). One of the reasons for this lack of success is insufficient understanding of the complexity of biotic and abiotic interactions that EPNs have in the soil environment in both managed and natural ecosystems.
Most research on EPNs has focused on their occurrence, efficacy and persistence (Gaugler, 2002; Grewal et al., 2005). Additionally, intensive research on naturally-occurring populations has revealed that EPNs can serve as an excellent model system for understanding biological, ecological and evolutionary processes involving other soil organisms (Burnell and Stock, 2000; Goodrich–Blair and Clarke, 2007; Denno et al., 2008; Stock and Goodrich–Blair, 2008). However, a focus on multidisciplinary approaches informed by biological, ecological, evolutionary and computational sciences is needed for advancing our knowledge of EPNs and their bacterial symbionts. In this respect, Thompson et al. (2001) defined four frontiers that should be taken into account when integrating studies in ecology: 1) The dynamics of coalescence in complex communities, 2) the evolutionary and historical determinants of ecological processes in the context of ecological memory, 3) the emergent properties of complex systems, and 4) the ecological topology (Table 1).
Table 1.
Definitions of the main four concepts proposed in Thompson et al. (2001).
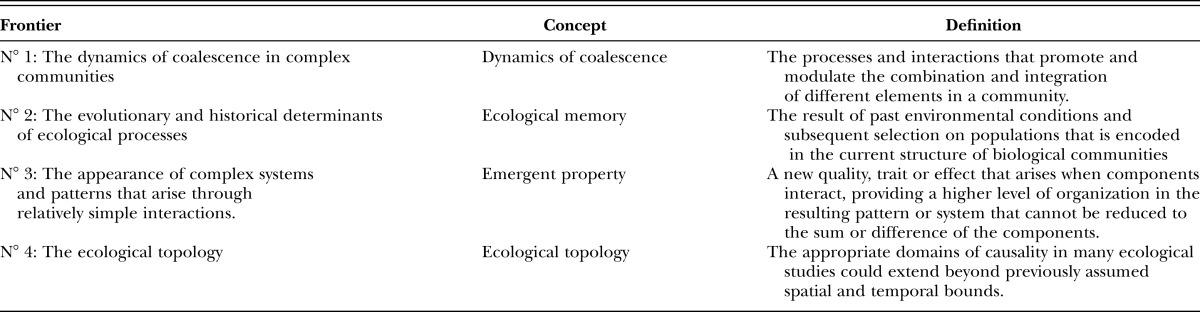
Thompson’s first frontier relates to the study of physical and chemical factors (e.g., soil texture and structure, soil water status, gases, temperature, pH), numerous biotic factors (e.g., prey populations and communities, food-plants of herbivorous prey, intraguild predation) that influence predator-prey, host-parasite, and other food web interactions. The second frontier focuses on the role of ‘ecological memory,’ the consideration of disciplines such as biogeography, phylogeny and evolution as the background to understanding the interactions among organisms and their environment. The third frontier addresses the need for the integration of field-based ecological and laboratory-based biological information through mathematical models to reveal new associations between organisms, their emergent properties and patterns, and to predict the response of soil ecosystems to a changing world. The fourth frontier requires sufficient progress on each of the first three to span from short-term microcosm studies to long-term and spatially extensive studies. Also required is the development of methodology that crosses or links spatial and temporal scales, synthesis of established knowledge to uncover consistent patterns, processes, and unifying principles, and the ability to share information. In this review, we illustrate the utility of EPNs as model systems considering Thompson’s four frontiers (Thompson et al., 2001). We then discuss how this information is critical for the application and conservation of EPNs, and also for future research in other disciplines considering EPNs and their bacterial symbionts.
Frontier 1: Epns and Community Coalescence
The soil sustains a high diversity of organisms that interact in complex food webs that are shaped by abiotic factors. The integration of habitat characteristics and limitations with spatial-temporal fluctuations in the numbers of their inhabitants, expand our understanding about complex ecological processes. Traditionally, the effects of abiotic factors on EPNs have been widely studied in laboratory experiments using soils or artificial substrates that have been treated to reduce interactions with other external factors (reviewed in Barbercheck, 1992; Glazer, 2002). Therefore, there is a need for a multidisciplinary approach to study such relationships in a wider context. Moreover, soil nematodes are currently recognized as key players in regulating soil food web composition, decomposition and mineralization, microbial transport, predation, and parasitism (Ferris et al., 2001). Even so, EPNs have rarely been incorporated into these studies. More recently, there has been a growing appreciation for complex interactions that are common in nature. This has been reflected by research on natural and managed systems that have focused on EPNs as a component of the broader food web and engaged in two–trophic and higher level trophic interactions (Denno et al., 2008; Ram et al. 2008; Stuart et al., 2008). This type of research demonstrates how EPNs can be used as a model for research that increases our knowledge about basic soil food-web structure and function, and belowground–aboveground interactions. In addition, this information potentially can be used to manipulate soil food webs to increase the efficacy of biological control in the soil.
In laboratory, greenhouse and field trials, many abiotic factors have been shown to be associated with the occurrence, movement, and persistence of EPNs. These include physical or chemical characteristics (e.g., soil moisture, temperature, pH, texture, structure and bulk density) and characteristics resulting from human activities (e.g., chemical or physical disturbance during ecosystem management such as fertilization, application of pesticides, etc.) (Stuart et al., 2006). In particular, soil moisture is a critical abiotic factor affecting EPN behavior, efficacy and survival because nematodes require water films of sufficient thickness and continuity to allow movement (reviewed in Kaya and Gaugler, 1993; Glazer, 2002; Shapiro–Ilan et al., 2002). Conversely, in very wet or saturated soils, oxygen may be limiting and nematode movement can be restricted due to lack of surface tension forces (Wallace, 1968). Soil texture and structure are also major factors affecting EPNs (Kaya and Gaugler, 1993; Shapiro–Ilan et al., 2002) and many studies on the effects of soil on EPNs have focused on soil texture. However, soil structure, geometry and porosity have rarely been studied, even though structural pore space affects the movement of water, air and organisms in soil. In managed systems, the observed effects of soil characteristics have been mixed. For example, suppression of the citrus root weevil, Diaprepes abbreviatus, by Steinernema riobrave is greater in coarse sandy soils than in fine textured soils (Shapiro et al., 2000; Duncan et al. 2001, 2003a). In contrast, the persistence of naturally occurring and inoculated populations of Heterorhabditis bacteriophora in turfgrass (Campbell et al., 1998), and Steinernema carpocapsae and H. bacteriophora in maize are not affected by soil texture (Millar and Barbercheck, 2002).
EPNs can serve as models for understanding the effects of disturbance on ecological interactions in soil. Similar to other soil fauna, the diversity of EPNs and their interactions with other organisms can be significantly altered by management practices such as irrigation, planting density, variety selection, tillage regime, fertility inputs, pesticide use and various other factors (reviewed in Shapiro–Ilan et al., 2002; Barbercheck and Hoy, 2005; Stuart et al., 2006). Mechanical disturbances such as tillage can have profound effects on the biological and functional properties of the soil. For example, soil faunal biomass often drops with increased agricultural usage, especially where conventional tillage is practiced (Stinner and House, 1990). In managed systems, lack of physical disturbance (stability) and favorable soil conditions (adequate moisture, aeration, structure) have favored successful use of inundative application of EPNs (Shapiro–Ilan et al., 2002). Under a conventional tillage regime, the soil surface also tends to have greater fluctuations in temperature and moisture than under conservation tillage management. Consequently, EPNs are more frequently detected in reduced tillage regimes than in conventional tillage (Brust, 1991; Shapiro et al., 1999; Hummel et al., 2002; Millar and Barbercheck, 2002; Campos-Herrera et al., 2008). Compaction and removal of surface residue resulting from tillage also contribute to reduction in soil moisture and pore space for soil organisms (Stinner and House, 1990; Millar and Barbercheck, 2002). The greater complexity of the soil environment associated with relatively high levels of crop residue in conservation tillage regimes might influence the abundance of EPNs through provision of a greater number and diversity of hosts (Brust, 1991). Surface residues could benefit nematode persistence through protection from desiccation or ultraviolet light, and increase insect pest suppression by EPNs (Shapiro et al., 1999) or enhance nematode movement (Jabbour and Barbercheck, 2008). However, the effects of tillage on EPNs are variable and depend on the EPN species present and their associated foraging strategies (Campbell and Gaugler, 1997; Hummel et al., 2002; Millar and Barbercheck, 2002).
The effects of biotic factors on EPNs have received less attention, probably due to methodological limitations. Knowledge of the interactions of soil organisms with their environments is critical for the development of models for soil management that are focused on system stability and resilience. Numerous biotic factors influence predator–prey, host–parasite, and other food web interactions (Rosenheim et al., 1995). In relation to this, a broad range of host and non–host arthropods, competitors, predators, parasites and pathogens (reviewed by Kaya, 2002; Stuart et al., 2006) can influence EPN survival and reproduction. Additionally, omnivory is common in detrital food webs, adding to the list of organisms that might interact with EPNs and impact their population levels (Walter et al., 1987; Walter and Ikonen, 1989). Recent ecological studies have illustrated the utility of EPNs as regulators of soil food webs, and the effects of soil food webs on above-belowground interactions. (De Deyn and Van der Putten, 2005; Hooper et al. 2005; Stuart et al., 2006; Denno et al., 2008). Some of these studies will be reviewed here to illustrate the utility of EPNs for understanding biotic interactions across a range of trophic interaction complexity.
Research on EPNs can provide insights into parasite–prey interactions, as these nematodes act both parasites and prey in soil. The primary biotic factor influencing the occurrence and persistence of EPNs at a particular location is probably the presence of suitable insect hosts (Peters, 1996; Mráček et al., 1999). Regulation of soil biota through predation is widespread in soil food webs and there are many examples of regulation of densities of soil animals and microbes by their consumers (reviewed by Wardle et al., 2004). Predation on EPNs has not been studied extensively, but a wide range of soil organisms could play an important role in regulating EPN populations. For example, the ubiquity and abundance of nematophagous fungi (NF), bacteria, nematodes, mites, collembolans and other microarthropods in soil suggest that these organisms might have considerable impact on EPNs in the natural environment (reviewed in Kaya, 2002). Under laboratory conditions, omnivorous and nematophagous predators can be voracious feeders (Gilmore and Raffensperger, 1970). The capacity of a predator to exert a regulatory effect on a population of nematodes is determined partly by its ability to increase their population level and/or predation rate as prey density increases. Many nematophagous organisms have rapid development times and high reproductive capacities, many species (e.g., predatory mesostigmatid mites) exhibit at least some degree of specificity towards nematodes, and many are capable of reproducing rapidly by parthenogenesis (Walter et al., 1987; Walter and Ikonen, 1989). The potential impact of natural enemies of EPNs has generally been assessed in observation chambers or in pots of sterilized soil (Gilmore and Raffensperger, 1970; Epsky et al., 1988; Gilmore and Potter, 1993) and, although these artificial systems do not reproduce more complex field conditions, there is some evidence that supports the role that predation by soil fauna might play in the lack of persistence of applied EPNs (Rosenheim et al., 1995; Kaya, 2002; Wilson and Gaugler, 2004; Duncan et al., 2007; El–Borai et al., 2007; Karagoz et al., 2007).
Additionally, EPN–infected insect cadavers represent a resource with which soil organisms can interact. The cadavers of at least some EPN species have been shown to be repellent to certain ant species, and thereby provide some measure of protection for the developing nematodes (Baur et al., 1998; Zhou et al., 2002). This effect has been demonstrated for a limited number of arthropod and EPN species (Baur et al., 1998; Kaya, 2002; Greenwood et al., 2011; Jabbour and Barbercheck, 2011; Gulçu et al., 2012). Baur et al. (1998) hypothesized the existence of an “ant–repellent factor” associated with Heterorhabditis–Photorhabdus infections because ants scavenged significantly more steinernematid–killed (60–85%) than heterorhabditid–killed (10–20%) insects. When these authors tested the effect of the bacteria–infected cadavers in the field, none of the insects killed by P. luminescens were scavanged compared with 70% of those killed by X. nematophila. These results and later studies by Zhou et al. (2002) suggested that P. luminescens is responsible for preventing ants from foraging on heterorhabditid–killed hosts. More recently, Gulçu et al. (2012) showed that the predator repellant–activity of the EPN–bacteria complex extends to crickets, wasps and calliphorid flies. The authors proposed the new terminology “scavenger deterrent factor” to describe this phenomenon. Fewer arthropods were associated with non-augmented soil controls and soil treated with Heterorhabditis–infected cadavers, than soil treated with Steinernema-infected cadavers (Greenwood et al., 2011). Karagoz et al. (2007) suggested that the observed pattern is due to arthropod repellency associated with heterorhabditid nematodes. Soil microarthropod abundance and community composition differed between treatments that provided resources (S. carpocapsae–killed) compared with those that did not (sham burial control) (Jabbour and Barbercheck, 2011). Similar to the results of Greenwood et al. (2011), soil surrounding S. carpocapsae–killed insect larvae contained more dipterans, acarid mites, staphylinid beetles, onychiurid and entomobryid collembolans, and immature and male mesostigmatid mites than soil at sham burial sites. Even though most of these arthropods are capable of nematophagy, the relative abundance of EPNs was not associated with arthropod community composition. Perhaps the great reproductive potential of EPNs dampens the potential negative effects of their natural enemies.
Frontier 2: Evolutionary Understanding of Epns and Their Symbiotic Bacteria: Phylogeny, Systematics and Ecological Memory
EPNs currently comprise two families: Steinernematidae and Heterorhabditidae (Dillman et al., 2012). The most current taxonomic account for the Steinernematidae recognizes the genus Neosteinernema, which contains only one species, N. longicurvicauda, and the genus Steinernema (type genus) with more than 60 recognized species (Table 2). The second family, Heterorhabditidae, contains a single genus, Heterorhabditis, with more than 20 currently recognized species (Table 2). These nematodes have a mutualistic association with Gram–negative γ–Proteobacteria in the genera Xenorhabdus (for steinernematids) and Photorhabdus (for heterorhabditids) (Table 2). Each individual nematode harbors one bacterial species; moreover, a nematode species is associated with only one bacterial species, with the exception of H. bacteriophora. Some bacterial species are able to share different nematode hosts (Lee and Stock, 2010a). Recent studies have described 21 species of Xenorhabdus and three species of Photorhabdus, containing 12 subspecies (Tailliez et al., 2006, 2010).
Table 2.
List of described species of entomopathogenic nematodes and their bacterial symbionts

The increasing number of described species and the dearth of expertise on traditional morphological diagnostic methods have necessitated supplementary approaches such as molecular methods to properly characterize and diagnose EPN taxa. In this respect, nucleotide sequence analysis has proven to be a useful tool not only for diagnostics at different taxonomic levels, but also for providing valuable data for phylogenetic inference about EPNs (Nguyen et al., 2001; Stock et al., 2001; Spiridonov et al., 2004; Adams et al., 2007). A limitation of these molecular hypotheses is that they were inferred using data from a single genetic locus (nuclear ribosomal DNA). To address the potential limitations of single–locus molecular hypotheses, multilocus approaches have been proposed to assess phylogenetic relationships among Steinernema taxa (Nadler et al. 2006; Lee and Stock, 2010a).
The diagnosis and identification of bacterial symbiont species and strains has undergone changes in methodology, similar to that used for EPN’s, ranging from phenotypic traits, biochemical and biophysical techniques to molecular methods. For example, molecular methods such as restriction analysis of PCR amplified gene products and riboprinting have been employed to determine diversity among entomopathogenic bacterial species. These methods have also been used for rapid identification of bacteria and to avoid tedious phenotypic characterization techniques (Szállás et al., 2001). More recently, sequence data of single and multigene datasets has been used to identify Xenorhabdus and Photorhabdus species and/or strains and to develop hypotheses about their evolutionary relationships (Liu et al., 2001; Tailliez et al 2006, 2010; Lee and Stock 2010b). In addition, sequence data has been used to develop coevolutionary hypotheses between these bacteria and their nematode hosts. With respect to the Steinernema–Xenorhabdus complex, Lee and Stock (2010b) developed the first comprehensive hypothesis for host–symbiont evolutionary trajectories. In this study, 30 Steinernema–Xenorhabdus pairs were considered. Reconstruction of the associations showed two scenarios that maximized cospeciation to 12 events, each of the scenarios contained 17 host switches (i.e. a bacterium switched to a different nematode host) and 7 occurrences of sorting (i.e. absence of a symbiont lineage from that of the host). In a similar study, Maneesakorn et al. (2011) examined the coevolutionary history between Heterorhabditis and their Photorhabdus symbionts and although limited to a single gene approach, the authors concluded that this mutualistic partnership evolved in concert.
We now have access to nearly complete genomes of several EPN and bacterial symbiont species, which allows for large–scale comparative analysis (Schwartz et al., 2011). New genome sequences of these mutualistic partners together with novel analytical methods will help improve our understanding of their phylogenies, will generate a better understanding of genome evolution in these organisms and will contribute to the advancement of the evolutionary history of EPNs, their obligate symbionts, their insect hosts, and their ecological roles.
Although molecular techniques have provided a tremendous amount of unbiased data for systematic studies of EPNs, it would be a mistake to replace classical morphological approaches with molecular methods. Together, morphological and molecular data will continue to provide a more comprehensive view of EPN evolution, and more robust taxonomic statements (Stock and Reid, 2004; Adams et al., 2006; Stock, 2009).
Correct identification of species is critical to understanding observations made in ecological studies. Description and identification of new isolates and species has been the focus of surveys worldwide for decades. The main goal has been the discovery of strains adapted to local conditions and insect pests. At a global scale, EPNs are ubiquitous (with the exception of Antartica where they have not been detected) and EPNs have a patchy or local aggregative distribution. Although current knowledge of EPN geographic distribution is in part an artifact of sampling efforts, species such as Steinernema feltiae and S. carpocapsae have been found to have a cosmopolitan distribution (Hominick, 2002). Four EPN species, S. feltiae, S. carpocapsae, Heterorhabditis indica and H. bacteriophora are pervasive: these steinernematids have been found in all continents except Africa, whereas H. bacteriophora has not been detected in Asia, and H. indica has not been detected in South America, Europe or Africa (Hominick, 2002; Adams et al., 2006). This distribution pattern suggests that dispersal mechanisms can be highly effective and probably occur by a combination of active and passive dissemination mechanisms (Adams et al., 2006).
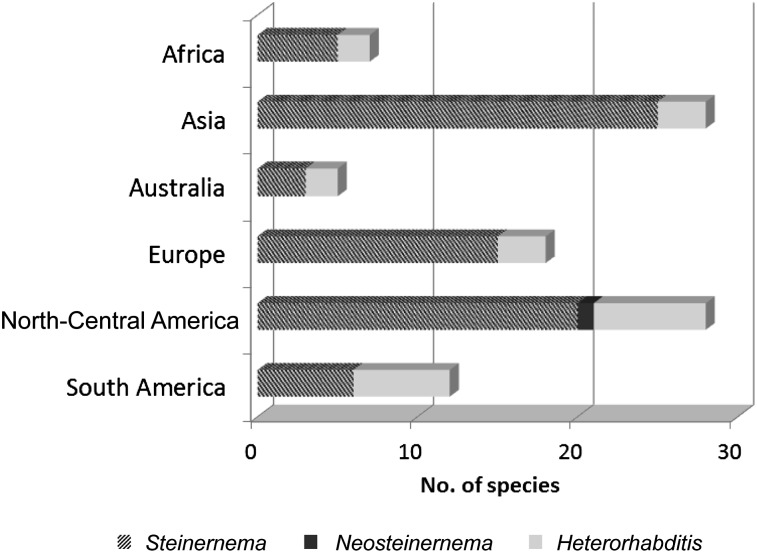
At present, North America and Asia account for more than 40 nominal Steinernema species (∼ 70 % of all described species) (Figure 1). Steinernema feltiae and S. carpocapase remain the most omnipresent species. Within the Heterorhabditidae, more than 15 Heterorhabditis spp. have been recovered, with H. bacteriophora as the most widely distributed species, followed by H. indica and Heterorhabditis baujardi. South and North America take the lead in diversity of Heterorhabdtitis species encountered (Figure 1). Europe has been the most extensively and intensively sampled continent. In Europe, nine named Steinernema spp. have been recorded, with S. feltiae and Steinernema affine the most widespread species, whereas three named Heterorhabditis species have been reported, with Heterorhabditis megidis as the species most widely distributed. Based on the latest account (Adams et al., 2006), the number of EPN species in the Australian continent includes three Steinernema and three Heterorhabditis species. In Africa, sampling efforts have recently increased dramatically, and many new and already–known species have been discovered and are being identified (Hattings et al., 2008; Abu–Shadi et al., 2011; Malan et al., 2011; Kanga et al., 2012). For example, in South Africa, novel species such as Steinernema khoisanae and Heterorhabditis safricana were discovered, and at least three novel species of Steinernema are currently being described (Hattings et al., 2008).
Fig. 1.
World distribution of Steinernema spp. and Heterorhabditis spp.
As more surveys with diverse sampling strategies are undertaken, the complexity of habitat preferences of EPNs have become more apparent. Factors such as soil type, distribution of suitable hosts, physiological and behavioral adaptations are key factors affecting the distribution of EPN species (Adams et al., 2006; Stuart et al., 2006). In general, heterorhabditids prefer sandy coastal soils. Some taxa prefer more calcareous soils or more acidic soils (H. bacteriophora and Heterorhabditis marelata, respectively), while others range beyond coastal regions (H. bacteriophora) and are broadly distributed in turf and weedy habitats (H. megidis) (Stuart and Gaugler, 1994; Stock et al., 1996; Constant et al., 1998). Prevalence of steinernematids is highest in woodlands (Hominick et al., 1996). Recent extensive and intensive surveys conducted in Europe and the USA revealed habitat preference for several steinernematids (Hominick et al, 1995; Stock et al., 1999; Sturhan and Linskova, 1999; Sturhan, 1999). S. feltiae prevails in grasslands and woodlands (Hominick, 2002). This species and S. affine are virtually the only steinernematids found in arable soils in Germany (Sturhan, 1999). Steinernema kraussei and S. intermedium are mainly forest/woodland species. S. kraussei has been found in coniferous forests in Europe and North America (USA and Canada) on both the east and west coasts (Sturhan, 1999; Sturhan and Linskova, 1999) and was recently isolated in high altitude woodland in Spain (Campos–Herrera et al., 2007). Along with the increasing complexity of habitat relationships described above, new techniques are being introduced to study these relationships.
Frontier 3: Emergent Properties of Population Dynamics and Trophic Interations
There is a growing interest in studying the linkage between biodiversity and ecosystem function and the integration of aboveground – belowground feedback (Wardle et al., 2004; De Deyn and van der Putten, 2005; Hooper et al., 2005). EPNs are ideally suited for serving as model systems in this type of study, specifically to examine higher level trophic interactions in soil and above–belowground feedbacks (Denno et al., 2008). For example, in an agronomic context, the reduction in root damage mediated by augmented EPNs is reflected aboveground by a decrease in the number of adults emerging from the soil and an increase of plant biomass and yield (Duncan et al., in press). Plants play a key role in EPN interactions because they directly affect the soil abiotic-biotic environment. For example, plant root density can affect the ability of EPNs to find a host insect (Choo and Kaya, 1991). Furthermore, Ennis et al. (2010) observed that when artificially damaging the roots of a plant to emulate insect feeding, S. carpocapsae’s ability to find an insect host increased.
The efficacy of natural enemies of herbivorous insects can be directly related to the plant’s secondary chemistry. This phenomenon has been demonstrated for several insect pathogens, including EPNs (Barbercheck, 1993; Barbercheck et al., 1995; Grewal et al., 1995; Rasmann et al., 2005; Gassmann et al., 2010). The attack of a plant by herbivorous arthropods can result in considerable changes in the plant's chemical phenotype. The emission of herbivore–induced plant volatiles (HIPV) results in the attraction of predators and parasites of the herbivores that induced these changes (Dicke et al., 2009). The suppressive effect of EPNs on root–feeding insects mediated through the production of HIPVs has been studied. For example, the sesquiterpene (E)–β–caryophyllene is a volatile compound emitted by maize in response to aboveground herbivory by lepidopteran larvae (Turlings et al., 1998) and in roots wounded by western corn rootworm (Kolner et al. 2008). Rasmann et al. (2005) demonstrated the activity of this volatile as an attractant for EPNs that parasitize rootworms. In relation to this finding, Ali et al. (2010) found that citrus roots recruited significantly more nematodes (Steinernema diaprepesi) when infested with Diaprepes abbreviatus larvae than non–infested roots, and identified the HIPVs released mainly pregeijerene. When applied in field experiments, this chemical attracted several native EPNs and significantly increased weevil mortality, which demonstrated the potential for using this chemical to improve biocontrol (Ali et al., 2012).
Studies of trophic cascades, in which EPNs benefit plants by reducing herbivore pressure, have produced significant insights for biological control of soil–dwelling insect pests. In coastal California, native H. marelatus populations are dynamically linked with populations of the ghost caterpillar, Hepialus californicus, and the bush lupine, Lupinus arboreus (reviewed in Strong, 2002; Ram et al. 2008). Ghost moth larvae inflict severe root damage and can kill bush lupines. H. marelatus causes high mortality of these caterpillars, and the spatial distribution of this EPN is positively correlated with fluctuations in the caterpillar population and local distribution of lupine bushes. Indeed, in the absence of H. marelatus, lupine mortality increased as a function of H. californicus density. Mature bush lupines possess a dense canopy and thick detrital layer that reduces desiccation and helps maintain a zone of relatively moist soil around their taproots. Abiotic factors, such as soil moisture, affected the nematodes’ survival and played a critical role in the trophic cascade (Preisser and Strong, 2004). The survival of infective juveniles (IJs) increased in the lupine rhizospheres during dry summer conditions (Dugaw et al., 2005).
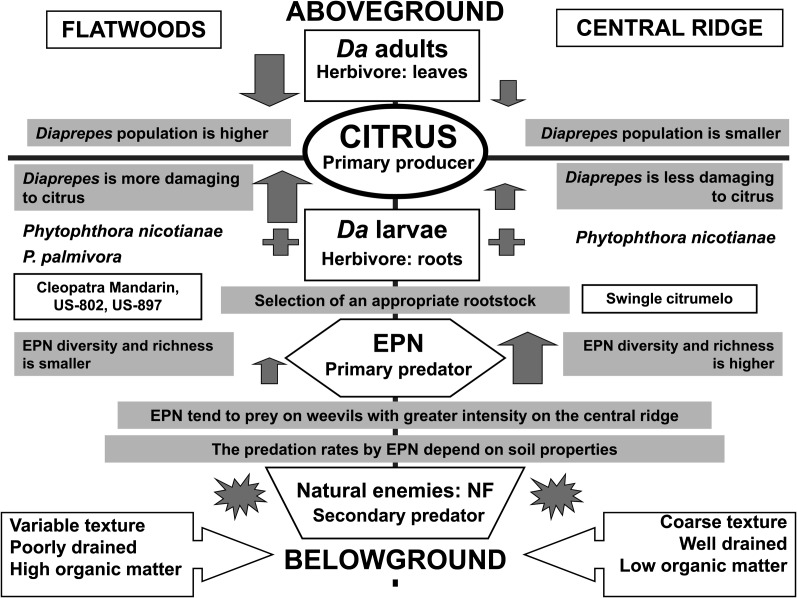
The role of EPNs in a trophic cascade has also been documented in citrus groves in Florida (reviewed in Stuart et al., 2008) (Figure 2). These groves are widely affected by a pest–disease complex involving the weevil D. abbreviatus and plant–pathogenic fungi in the genus Phytophthora. This pathogen complex represents one of the foremost biotic threats to citrus. Diaprepes weevils are typically less abundant in Central Ridge groves than in Flatwoods groves (McCoy et al., 2003; Futch et al., 2005). Similarly, native communities of EPNs are generally more diverse and more prevalent in citrus groves on the well–drained, coarse sandy soils of the Central Ridge than in those on the poorly–drained, finer textured soils of the Flatwoods (Duncan et al., 2003a). The difference in weevil abundance appears to be directly related to EPN prevalence in groves with different soil types, and soil type appears to influence prevalence of EPNs either directly (restricting nematode movement and host searching ability) or indirectly (soil food web interactions). These foodweb interactions also vary when considering different habitats and include: i) competition with the free–living bactivorous nematodes (FLBN) Pellioditis sp. in the weevil cadavers (Duncan et al., 2003b), ii) antagonism by nematophagous fungi (NF) and iii) parasitism by Paenibacillus species that reproduce in D. abbreviatus and impair EPN motility in soil (El–Borai et al., 2005; Duncan et al., 2007). The presence of the nematode S. riobrave increased the number of Pellioditis sp., which developed in Diaprepes cadavers (Duncan et al., 2003b). Also, EPN augmentation consistently increased the prevalence of trapping NF (Duncan et al., 2007). Integration of these results on post-application biology of EPNs is fundamental to providing growers with environmentally-friendly IPM alternatives. Research approaches such as these provide an excellent opportunity for expanding our understanding of the dynamics of soil food web interactions and contribute to advancing the frontier of emergent properties of complex systems.
Fig. 2.
EPNs in trophic cascades in an agricultural system, citrus groves in Florida (USA). Da = Diaprepes abbreviatus (citrus root weevil); NF= nematophagous fungi. For detailed description, see the corresponding text.
Frontier 4: Spatial and Temporal Modeling of Epn Population Dynamics and Ecosystem Processes
Research on the quantitative ecology of EPNs has contributed substantially to this frontier in ecology. Understanding EPN population change requires an understanding of their reproduction, dispersal and survival. EPN reproduction takes place entirely within an insect host, whereas IJ survival and dispersal take place mostly in the soil. Once inside a host, reproduction can be limited by host size, suitability and host defensive mechanisms. A number of intrinsic mechanisms contribute to EPN IJ survival in the soil, including dispersal (active and passive) to avoid harsh environments or seek more suitable environments and insect hosts, and physiological mechanisms to withstand desiccation, high or low temperature extremes, and other harsh environmental conditions. Many studies have contributed to our understanding of EPN population dynamics and their interactions with other organisms. In this section we review some of the key studies on modeling EPN population dynamics, habitat characteristics and spatial-temporal ecological community relationships.
Population models: Temporal population dynamics can be modeled as a flow through a series of states, starting at an arbitrary point in the life cycle: active IJ outside of a host ⇒ juvenile inside host ⇒ adults ⇒ juveniles (⇒ repeat over multiple generations) ⇒ active IJs outside of hosts (which may also periodically enter an inactive stage, facultative quiescence or anhydrobiosis under adverse conditions). The rates of flow between each state are where mechanistic and quantitative understanding of EPN biology is needed to develop mathematical equations that represent those rates. If spatial as well as temporal dynamics are to be considered, then states can be referenced by location, and additional rates representing movement of IJs or infected hosts to and from each location are needed. Mortality results in loss of EPNs from any of the states described above, and requires additional rate equations. The many rate equations described above are the focus of the following review of some selected literature useful in developing mathematical models of EPN population dynamics across temporal and spatial scales, and that contribute to an improved ecological topology for EPNs.
Beginning with host finding by IJs, a number of early studies focused on understanding the soil environment and characteristics that influence host searching behavior (reviewed by Kaya and Gaugler, 1993; Lewis et al., 2006; Stuart et al., 2006). For example, soil physical characteristics can influence the movement of IJs toward insect hosts in the soil. Host finding behavior was recognized to vary among nematode species and potentially requires somewhat different functions for each in representing host encounter rates. Studies on host invasion provide additional needed biological information to model invasion and mortality rates of insect hosts by EPNs. The broad host community associated with EPNs (Peters, 1996; Georgis et al., 2006) and variation in the physical or behavioral barriers to entry among hosts (Eidt and Thurston, 1995; Gouge et al., 1999) require careful treatment of the potential for infection and reproduction as a function of a particular host community coalescing in space and time. Tritrophic interactions (described above) between nematodes, insects and host plants influence host finding as well as host invasion rates. Furthermore, complexity in invasion rates has been observed as a result of the presence of endophytic fungi and their influence on the plant-insect-nematode interaction (Grewal et al., 1995). Data on the impact of physical and chemical factors on the susceptibility of insect hosts can also contribute to quantifying invasion rates (e.g. Eidt and Thurston, 1995; Grewal et al., 2001). Additionally, it has been demonstrated that EPN IJs vary over time in the proportion of the population that infects, which potentially requires accounting for the age structure of the population (Bohan and Hominick, 1997) or tracking the conditions under which IJs develop (Jagdale and Gordon, 1997). This variation in the nematodes themselves might represent a means for increasing the odds of survival for populations of IJs (Campbell et al., 1999).
Data on reproductive rates of EPN has been reported as a function of host species (Wang and Bedding, 1996; Boff et al., 2000a) and environmental conditions in the insect host (Grewal et al., 1994; Boff et al., 2000b; Bornstein–Forst et al., 2005), as well as in relation to density dependent effects within the host (Selvan et al., 1993; Ryder and Griffin, 2002). Despite the availability of data for some of these key parameters, this is a relatively weak area that needs to be further investigated in modeling studies. Most of the research on EPN development has been conducted using Galleria mellonella as the standard rearing host rather than considering representative species from other insect orders (some exceptions are cited above). Mortality within the host and emergence of nematode progeny has been described as a function of environmental conditions, in particular temperature and humidity (Brown and Gaugler, 1997; Koppenhöfer et al., 1997), and also limited to G. mellonella as the insect host model.
Dispersal of IJs in the absence of hosts has been described both as passive or phoretic (Lacey et al., 1995; Shapiro et al., 1995) and active processes (Del Valle et al., 2008). Active dispersal has been studied in laboratory settings, and has considered different EPN species (Grewal et al., 1994) and various soil conditions and depths (Hsiao and All, 1996; Anderson et al., 1997; Portillo–Aguilar et al., 1999; Del Valle et al., 2008). These studies have been conducted in field and laboratory experiments (Ferguson et al., 1995; Hsiao and All, 1998). Studies of dispersal have been complemented by studies on spatial distribution (Stuart and Gaugler, 1994; Campbell et al., 1998; Bohan, 2000), and provide important background information for comparisons with the spatial distributions of insect hosts and habitat conditions. Below we provide a few examples of these interactions.
Predators and pathogens of IJs are common mortality factors that can be included in a model, as described in detail above (Frontier 3). Delayed emergence of IJs from cadavers can be interpreted as a survival mechanism that helps maintain EPN populations in the absence of insect hosts and also when there are unfavourable environmental factors (Koppenhöfer et al., 1997). A mathematical interpretation of these interactions could be used in refining rates of transition in a dynamic population model.
Since 2000, researchers have delved more deeply into these individual components of basic EPN population biology and ecology, and several studies have combined these data to develop population models. For example, analytical population models were developed to explore strategies for application of EPNs in crop protection (Fenton et al., 2000, 2001, 2002; Fenton and Rands, 2004). Such models are based on mathematically tractable sets of a few differential equations; in this case, three equations that describe the change in IJs, uninfected hosts, and infected cadavers over time. The models incorporated previous data on reproduction and mortality rates for many species. Although both precise in their solutions and general in their description of EPN dynamics, such models tend to require simplification of a great deal of the biological complexity to maintain tractability in the equations. Similarly, Wilson et al. (2004) developed a model using the slug parasitic nematode Phasmarhabditis hermaphrodita to optimise slug control based on descriptive rather than mechanistic functions that relate nematode and slug numbers to damage over time. Such descriptive models have been extended to include spatial effects (Stuart et al., 2006) and better describe observed populations in field surveys. However, more mechanistic models that incorporate additional details of described EPN biology have not yet been attempted to our knowledge, and could provide a needed complement to simple descriptive models (Van Nes and Scheffer 2005).
Spatial and temporal models: Research described above provides the context for developing a mechanistic understanding of the relationships between EPNs, habitats, and host communities. Although the relationship between EPNs and soil or habitat characteristics has been examined in the studies cited above, the analytical methods used have not always been designed to relate multiple species of entomopathogenic and free–living nematode species to multiple environmental variables in combination such as in the study by Hoy et al. (2008). Statistical techniques for relating species to environmental variables, described only briefly here, include both direct and indirect methods. Indirect methods are those that focus on a single set of variables (typically related to the environment), and seek a set of linear combinations of those variables that captures the variation within and among them. Principle Components Analysis (PCA) is a common example. A typical approach might be to use PCA to examine variation within a set of edaphic factors in sites where EPN were surveyed, factors hypothesized to be important in EPN distribution (e.g., Alumai et al., 2006; Kanga et al., 2012). The PCA, however, would provide insight into the variation among the edaphic variables, and those variables only. The methodology offers no direct evidence for how nematode species relate to that variation, other than strictly post hoc attempts by inspection of where sites with nematodes fall on axes representing a linear combination of the edaphic variables that maximizes the variation within and among them. By contrast, in direct methods the relationship between species and environment is included in the statistical model. In this type of model, the environmental/ soil variables (explanatory variables, predictors) are used to explain the variability observed in species (dependent variables, response variables that might be quantitative, semiquantitative or qualitative). The direct methods restrict the analysis of variation among environmental variables describing sites to only the variation that is associated with the variation among species. A second consideration in the selection of the model relating species to environment is whether the relationship is linear (i.e. abundance strictly increasing or decreasing as a function of the environmental variable) or unimodal (i.e. an optimum in the environmental variable exists above and below which species abundance declines). Examples of the latter are pH and temperature.
Several studies have suggested direct gradient analysis, including Redundancy Analysis (RDA, linear model) and Canonical Correspondence Analysis (CCA, unimodal model) (Ter Braak, 1986) as more useful methods to relate the presence and abundance of free–living nematode taxa to multiple soil conditions (Fiscus and Neher, 2002; Neher et al., 2005). Several studies have used RDA to examine EPN relationships with habitat variables, and included soil characteristics (Kanga et al. 2012), tillage (Greenwood et al., 2011), plant cover and the abundance of suitable insect hosts (Mráček et al., 2005). Hoy et al. (2008) used CCA to relate edaphic conditions with a wide range of free–living nematode species and two EPN species. In this study, the presence and abundance of nematodes, and EPNs in particular, were associated with a unique combination of the many edaphic biotic and abiotic factors that have been identified and described individually in the literature reviewed above, with respect to community coalescence, ecological memory, and emergent properties. Furthermore, this study demonstrated that the edaphic conditions associated with EPNs were different from those associated with the other nematode taxa, possibly a function of unique life history traits. Such studies of EPNs and their relationship to the environment could be enhanced in two ways: with more mechanistic understanding of the relationships between species and environmental variables and with more inclusive consideration of the biotic community assemblages that form the environment for EPNs. Steps are being taken to elaborate on the relationships between species and environmental variables using more sophisticated statistical techniques as described above and in terms of more sensitive detection of EPNs and other nematodes and more detailed measurement of environmental variables that are related.
Studies of EPN communities are becoming more detailed (Spiridonov et al., 2007) and better focused on naturally occurring host communities rather than strictly focusing on potential biological control targets. Studies relating EPNs to both soils and soil management are taking a more holistic approach (e.g., Campos–Herrera et al., 2008). Use of quantitative real time PCR as a means for detecting and enumerating EPN presence without baiting soil (Torr et al., 2007; Campos–Herrera et al., 2010) or in comparison with soil baits (Campos–Herrera et al., 2011a) offers new insights. Interactions between EPNs and soil food webs are also becoming more detailed and are leading to a more complete understanding of these communities (El–Borai et al., 2005; Duncan et al., 2007; Karagoz et al., 2007; Strong, 2007). In addition, use of molecular techniques is contributing to more detailed information on the role of EPN in food webs (Read et al., 2006; Campos–Herrera et al., 2011b, Pathak et al., 2012).
Research on EPN dispersal mechanisms and their role in spatial distribution continues to increase. Factors such as different species or strains (Lacey et al., 2001; Rolston et al., 2006), and sex variation within species (Fujimoto et al., 2007; Alsaiyah et al., 2009) have been studied in relation to dispersal mechanisms. Moreover, factors such as soil conditions (Jabbour and Barbercheck, 2008) and biotic community effects have been investigated for their effect on both active and phoretic movement of EPNs (Eng et al., 2005).
Research related to mass production has also taken a population dynamics approach (Hirao and Ehlers, 2010). Trait selection for factors important in population dynamics, such as host location, could have direct application in insect control (Mukuka et al., 2010; Salame et al., 2010), although the focus of these studies has been technological rather than ecological. More recently, ecological genetics has been used to investigate EPN distribution in natural settings (Crossan et al., 2007; Bashey and Lively, 2009). Research with this focus should lead to a better understanding of EPN populations dynamics.
The research described above has enhanced our understanding of EPN population dynamics in both natural and managed ecosystems. Future research should consider other parameters such as habitat conditions associated with EPN presence and abundance to provide snapshots of EPN dynamics in space and time. Inclusion of environmental conditions in models will further improve our understanding of EPN population dynamics.
Conclusions
Use of modeling to understand EPN interactions in the environment is contributing significantly to the key frontiers of ecology (Thompson et al., 2001). The dynamics of coalescence in ecological communities are being explored extensively to understand the relationships between EPNs and the abiotic and biotic soil habitat. To better understand EPN associations with the environment and other members of the soil community, methods for recovering and validating new isolates and for rapid identification should be standardized. This effort is particularly important for biogeographical studies because EPNs are introduced as biological control agents and might displace native, undescribed species. The ecological memory underlying EPN life histories will continue to provide a context for ongoing research on their systematics and biogeography. In particular, the increasing discovery rate of these nematodes has prompted the need for rapid and more accurate methods for species diagnostics through molecular biology methods. Quantitative and mechanistic analyses of EPN populations and their relationship to habitat characteristics are identifying emergent properties in their unique life history traits. Finally, as studies and techniques are combined into a more comprehensive view of naturally occurring EPN populations and their dynamics in space and time, an ecological topology for EPNs and their ecosystems is beginning to emerge.
Literature Cited
- Abu–Shadi N, Shamseldean MM, Abd-Elbary NA, Stock SP. 2011 Diversity and distribution of entomopathogenic nematodes (Heterorhabditidae and Steinernematidae) in Egypt. Society of Nematologists 50th Annual Meeting, Corvallis OR. pp. 45. [Google Scholar]
- Adams BJ, Fodor A, Koppenhöfer HS, Stackenbrandt E, Stock SP, Klein MG. Biodiversity and systematic of nematode–bacterium entomopathogens. Biological Control. 2006;38:4–21. [Google Scholar]
- Adams BJ, Peat SM, Dillman AR. 2007 Phylogeny and evolution. Pp. 693–733 in Nguyen, K. B., and Hunt, D. J., eds. Entomopathogenic nematodes: systematics, phylogeny and bacterial symbionts. Nematology Monographs and Perspectives, vol. 5: Koninklijke Brill NV, Leiden. [Google Scholar]
- Ali JG, Alborn HT, Stelinski LL. Subterranean herbivore–induced volatiles released by citrus roots upon feeding by Diaprepes abbreviatus recruit entomopathogenic nematodes. Journal of Chemical Ecology. 2010;36:361–368. doi: 10.1007/s10886-010-9773-7. [DOI] [PubMed] [Google Scholar]
- Ali JG, Alborn HT, Campos–Herrera R, Kaplan F, Duncan LW, Rodríguez-Saona C, Koppenhöfer A, Stelinski LL. 2012. Extending explorations of belowground herbivore-induced plant volatiles: attracting natural enemies of root pests in multiple contexts. PLoS One. http://dx.plos.org/10.1371/journal.pone.0038146. [Google Scholar]
- Alsaiyah MAM, Ebssa L, Zenner A, O'Callaghan KM, Griffin CT. Sex ratios and sex–biased infection behaviour in the entomopathogenic nematode genus Steinernema. International Journal for Parasitology. 2009;39:725–734. doi: 10.1016/j.ijpara.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Alumai A, Grewal PS, Hoy CW, Willoughby DA. Factors affecting the natural occurrence of entomopathogenic nematodes in turfgrass. Biological Control. 2006;36:368–374. [Google Scholar]
- Anderson ARA, Young IM, Sleeman BD, Griffiths BS, Robertson WM. Nematode movement along a chemical gradient in a structurally heterogeneous environment. Experimental and Fundamental Applied Nematology. 1997;20:157–163. [Google Scholar]
- Barbercheck ME. Effect of soil physical factors on biological control agents of soil insect pests. Florida Entomologist. 1992;75:539–548. [Google Scholar]
- Barbercheck ME. Tritrophic level effects on entomopathogenic nematodes. Environmental Entomology. 1993;22:1166–1171. [Google Scholar]
- Barbercheck ME, Hoy CW. 2005 A systems approach to conservation of nematodes. Pp. 331–347 in Grewal P. S., Ehlers, R.–U. and David, Shapiro–Ilan, D. I. eds. Nematodes as Biocontrol Agents: CABI Publishing, CAB International, Wallingford, Oxon, UK. [Google Scholar]
- Barbercheck ME, Wang J, Hirsh IS. Host plant effects on entomopathogenic nematodes. Journal of Invertebrate Pathology. 1995;66:169–177. [Google Scholar]
- Bashey F, Lively CM. Group selection on population size affects life–history patterns in the entomopathogenic nematode Steinernema carpocapsae. Evolution. 2009;63:1301–1311. doi: 10.1111/j.1558-5646.2009.00637.x. [DOI] [PubMed] [Google Scholar]
- Baur ME, Kaya HK, Strong DR. Foraging ants as scavengers on entomopathogenic nematode–killed insects. Biological Control. 1998;12:231–236. [Google Scholar]
- Boff MIC, Wiegers GL, Smits PH. Influences of host size and host species on the infectivity and development of Heterorhabditis megidis (strain NLH–E87.3) Biocontrol. 2000a;45:469–482. [Google Scholar]
- Boff MIC, Wiegers GL, Smits PH. Effect of storage time and temperature on infectivity, reproduction and development of Heterorhabditis megidis in Galleria mellonella. Nematology. 2000b;2:635–644. [Google Scholar]
- Bohan DA. Spatial structuring and frequency distribution of the nematode Steinernema feltiae Filipjev. Parasitology. 2000;121:417–425. doi: 10.1017/s0031182099006551. [DOI] [PubMed] [Google Scholar]
- Bohan DA, Hominick WM. Long–term dynamics of infectiousness within the infective–stage pool of the entomopathogenic nematode Steinernema feltiae (site 76 strain) Filipjev. Parasitology. 1997;114:301–308. [Google Scholar]
- Bornstein–Forst S, Kiger H, Rector A. Impacts of fluctuating temperature on the development and infectivity of entomopathogenic nematode Steinernema carocapsae A10. Journal of Invertebrate Pathology. 2005;88:147–153. doi: 10.1016/j.jip.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Brown IM, Gaugler R. Temperature and humidity influence emergence and survival of entomopathogenic nematodes. Nematologica. 1997;43:363–375. [Google Scholar]
- Brust GE. Soil moisture, no–tillage and predator effects on southern corn rootworm survival in peanut agroecosystems. Entomologia Experimentalis et Applicata. 1991;58:109–121. [Google Scholar]
- Burnell AM, Stock P. Heterorhabditis, Steinernema and their bacterial symbionts: lethal pathogens of insects. Nematology. 2000;2:31–42. [Google Scholar]
- Campbell JF, Gaugler R. Inter–specific variation in entomopathogenic nematode foraging strategy: dichotomy or variation along a continuum? Fundamental and Applied Nematology. 1997;20:393–398. [Google Scholar]
- Campbell JF, Orza G, Yoder F, Lewis E, Gaugler R. Spatial and temporal distribution of endemic and released entomopathogenic nematode populations in turfgrass. Entomologia Experimentalis et Applicata. 1998;86:1–11. [Google Scholar]
- Campbell JF, Koppenhöfer AM, Kaya HK, Chinnasri B. Are there temporarily non–infectious dauer stages in entomopathogenic nematode populations: a test of the phased infectivity hypothesis. Parasitology. 1999;118:499–508. doi: 10.1017/s0031182099003984. [DOI] [PubMed] [Google Scholar]
- Campos–Herrera R, Escuer M, Labrador S, Robertson L, Barrios L, Gutiérrez C. Distribution of the entomopathogenic nematodes from La Rioja (Northern Spain) Journal of Invertebrate Pathology. 2007;95:125–139. doi: 10.1016/j.jip.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Campos–Herrera R, Gómez–Ros JM, Escuer M, Cuadra L, Barrios L, Gutiérrez C. Diversity, occurrence, and life characteristics of natural entomopathogenic nematode populations from La Rioja (Northern Spain) under different agricultural management and their relationships with soil factors. Soil Biology and Biochemistry. 2008;40:1474–1484. [Google Scholar]
- Campos–Herrera R, Stuart RJ, El–Borai FE, Gutiérrez C, Duncan LW. 2010 Entomopathogenic nematode ecology and biological control in Florida citrus. Pp. 101–130 in Ciancio, A., and Mukerji, K. G. Eds. Integrated Management of Arthropod Pests and Insect Borne Diseases, Vol. 5: Springer Science+Business Media B.V. [Google Scholar]
- Campos–Herrera R, Johnson EG, El–Borai FE, Stuart RJ, Graham JH, Duncan LW. Long–term stability of entomopathogenic nematode spatial patterns in soil as measured by sentinel insects and real–time PCR assays. Annals of Applied Biology. 2011a;158:55–68. [Google Scholar]
- Campos–Herrera R, El–Borai FE, Stuart RJ, Graham JH, Duncan LW. Entomopathogenic nematodes, phoretic Paenibacillus spp., and the use of real time quantitative PCR to explore soil food webs in Florida citrus groves. Journal of Invertebrate Pathology. 2011b;108:30–39. doi: 10.1016/j.jip.2011.06.005. [DOI] [PubMed] [Google Scholar]
- Choo HY, Kaya HK. Influence of soil texture and presence of roots on host finding by Heterorhabditis bacteriophora. Journal of Invertebrate Pathology. 1991;58:279–280. [Google Scholar]
- Constant P, Marchay L, Fischer–Le Saux M, Briand–Panoma S, Mauleon H. Natural occurrence of entomopathogenic nematodes (Rhabditida: Steinernematidae and Heterorhabditidae) in Guadalupe islands. Fundamental and Applied Nematology. 1998;21:667–672. [Google Scholar]
- Crossan J, Paterson S, Fenton A. Host availability and the evolution of parasite life–history strategies. Evolution. 2007;61:675–684. doi: 10.1111/j.1558-5646.2007.00057.x. [DOI] [PubMed] [Google Scholar]
- De Deyn GB, Van der Putten WH. Linking above and belowground biodiversity. Trends in Ecology and Evolution. 2005;20:625–633. doi: 10.1016/j.tree.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Del Valle EE, Dolisnki C, Souze RM. Dispersal of Heterorhabditis baujardi LPP7 (Nematodo: Rhabditida) applied to the soil as infected host cadavers. International Journal of Pest Management. 2008;54:115–122. [Google Scholar]
- Denno RF, Gruner DS, Kaplan I. Potential for entomopathogenic nematodes in biological control: A meta–analytical synthesis and insights from trophic cascade theory. Journal of Nematology. 2008;40:61–72. [PMC free article] [PubMed] [Google Scholar]
- Dicke M, van Loon JJA, Soler R. Chemical complexity of volatiles from plants induced by multiple attack. Nature Chemical Biology. 2009;5:317–324. doi: 10.1038/nchembio.169. [DOI] [PubMed] [Google Scholar]
- Dillman AR, Chaston JM, Adams BJ, Ciche TA, Goodrich-Blair H, Stock SP, Sternberg PW. 2012 doi: 10.1371/journal.ppat.1002527. An entomopathogenic nematode by any other name. PLoS Pathogens, 8, e1002527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugaw CJ, Preisser EL, Hastings A, Strong DR. Widening the window of persistence in seasonal host–pathogen systems. Theoretical Population Biology. 2005;68:267–276. doi: 10.1016/j.tpb.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Duncan LW, Genta JG, Zellers J, Fares A, Stansly PA. Efficacy of Steinernema riobrave against larvae of Diaprepes abbreviatus in Florida soils of different texture. Nematropica. 2001;31:130. [Google Scholar]
- Duncan LW, Graham JH, Dunn DC, Zellers J, McCoy CW, Nguyen K. Incidence of endemic entomopathogenic nematodes following application of Steinernema riobrave for control of Diaprepes abbreviatus. Journal of Nematology. 2003a;35:178–186. [PMC free article] [PubMed] [Google Scholar]
- Duncan LW, Dunn DC, Bague G, Nguyen K. Competition between entomopathogenic and free–living bactivorous nematodes in larvae of the weevil Diaprepes abbreviatus. Journal of Nematology. 2003b;35:187–193. [PMC free article] [PubMed] [Google Scholar]
- Duncan LW, Graham JH, Zellers J, Bright D, Dunn DC, El–Borai FE, Porazinska DL. Food web responses to augmenting the entomopathogenic nematodes in bare and animal manure–mulched soil. Journal of Nematology. 2007;39:176–189. [PMC free article] [PubMed] [Google Scholar]
- Duncan, L. W., Stuart, R. J., El-Borai, F. E., Campos-Herrera, R., Pathak, E., Graham, J. H. Modifying orchard planting sites conserves entomopathogenic nematodes, reduces weevil herbivory, and increases citrus tree growth, survival, and fruit yield. Biological Control in press.
- Eidt DC, Thurston GS. Physical deterrents to infection by entomopathogenic nematodes in wireworms (Coleoptera, Elateridae) and other soil insects. Canadian Entomologist. 1995;127:423–429. [Google Scholar]
- El–Borai FE, Duncan LW, Preston JF. Bionomics of a phoretic association between Paenibacillus sp. and the entomopathogenic nematode Steinernema diaprepesi. Journal of Nematology. 2005;37:18–25. [PMC free article] [PubMed] [Google Scholar]
- El–Borai FE, Brentu CF, Duncan LW. Augmenting entomopathogenic nematodes in soil from a Florida citrus orchard: Non–target effects of a trophic cascade. Journal of Nematology. 2007;39:203–210. [PMC free article] [PubMed] [Google Scholar]
- Eng MS, Preisser EL, Strong DR. Phoresy of the entomopathogenic nematode Heterorhabditis marelatus by a non–host organism, the isopod Porcellio scaber. Journal of Invertebrate Pathology. 2005;88:173–176. doi: 10.1016/j.jip.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Ennis DE, Dillon AB, Griffin CT. Simulated roots and host feeding enhance infection of subterranean insects by the entomopathogenic nematode Steinernema carpocapsae. Journal of Invertebrate Pathology. 2010;103:140–143. doi: 10.1016/j.jip.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Epsky ND, Walter DE, Capinera JL. Potential role of nematophagous microarthropods as biotic mortality factors of entomopathogenic nematodes (Rhabditida, Steinernematidae and Heterorhabditidae) Journal of Economic Entomology. 1988;81:821–825. [Google Scholar]
- Fenton A, Norman R, Fairbairn JP, Hudson PJ. Modelling the efficacy of entomopathogenic nematodes in the regulation of invertebrate pests in glasshouse crops. Journal of Applied Ecology. 2000;37:309–320. [Google Scholar]
- Fenton A, Norman R, Fairbairn JP, Hudson PJ. Evaluating the efficacy of entomopathogenic nematodes for the biological control of crop pests: A nonequilibrium approach. American Naturalist. 2001;158:408–425. doi: 10.1086/321993. [DOI] [PubMed] [Google Scholar]
- Fenton A, Gwynn RL, Gupta A, Norman R, Fairbairn JP, Hudson PJ. Optimal application strategies for entomopathogenic nematodes: integrating theoretical and empirical approaches. Journal of Applied Ecology. 2002;39:481–492. [Google Scholar]
- Fenton A, Rands SA. Optimal parasite infection strategies: a state–dependent approach. International Journal for Parasitology. 2004;34:813–821. doi: 10.1016/j.ijpara.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Ferguson CS, Schroeder PC, Shields EJ. Vertical distribution, persistence, and activity of entomopathogenic nematodes (Nematoda, Heterorhabditidae and Steinernematidae) in alfalfa snout beetle (Coleoptera, Curculionidae) infested fields. Environmental Entomology. 1995;24:149–158. [Google Scholar]
- Ferris H, Bongers T, de Goede RGM. A framework for soil food web diagnostics: extension of the faunal analysis concept. Applied Soil Ecology. 2001;18:13–29. [Google Scholar]
- Fiscus DA, Neher DA. Distinguishing sensitivity of free–living soil nematode genera to physical and chemical disturbances. Ecology Applied. 2002;12:565–575. [Google Scholar]
- Fujimoto A, Lewis EE, Cobanoglu G, Kaya HK. Dispersal, infectivity and sex ratio of early– or late–emerging infective juveniles of the entomopathogenic nematode Steinernema carpocapsae. Journal of Nematology. 2007;39:333–337. [PMC free article] [PubMed] [Google Scholar]
- Futch SH, Duncan LW, Zekri M. Validation of an area–wide extension program to estimate the seasonal abundance of adult citrus root weevils with un–baited pyramidal traps. Proceedings of the Florida State Horticultural Society. 2005;117:143–147. [Google Scholar]
- Gassmann AJ, Stock SP, Tabashnik BE, Singer MS. Tritrophic effects of host plants on an herbivore–pathogen interaction. Annals of the Entomological Society of America. 2010;103:371–378. [Google Scholar]
- Gaugler R, editor. 2002 Entomopathogenic Nematology. CABI Publishing, Wallingford, UK. [Google Scholar]
- Georgis R, Koppenhöfer AM, Lacey LA, Bélair G, Duncan LW, Grewal PS, Samish M, Tan L, Torr P, van Tol RWHM. Successes and failures in the use of parasitic nematodes for pest control. Biological Control. 2006;38:103–123. [Google Scholar]
- Gilmore SK, Raffensperger EM. Foods ingested by Tomocerus spp. (Collembola, Entomobryidae), in relation to habitat. Pedobiologia. 1970;10:135–140. [Google Scholar]
- Gilmore SK, Potter DA. Potential role of collembola as biotic mortality agents for entomopathogenic nematodes. Pedobiologia. 1993;37:30–38. [Google Scholar]
- Glazer I. 2002 Survival biology. Pp. 169–187 in Gaugler, R. (ed.) Entomopathogenic Nematology. CABI Publishing, Wallingford, UK. [Google Scholar]
- Goodrich–Blair H, Clarke DJ. Mutualism and pathogenesis in Xenorhabdus and Photorhabdus: two roads to the same destination. Molecular Microbiology. 2007;64:260–268. doi: 10.1111/j.1365-2958.2007.05671.x. [DOI] [PubMed] [Google Scholar]
- Gouge DH, Lee LL, Henneberry TJ. Effect of temperature and lepidopteran host species on entomopathogenic nematode (Nematoda: Steinernematidae, Heterorhabditidae) infection. Environmetal Entomology. 1999;28:876–883. [Google Scholar]
- Greenwood CM, Barbercheck ME, Brownie C. Short term response to soil microinvertebrates to application of entomopathogenic nematode–infected insects in two tillage systems. Pedobiologia. 2011;54:177–186. [Google Scholar]
- Grewal PS, Lewis EE, Gaugler R, Campbell JF. Host finding behavior as a predictor of foraging strategy in entomopathogenic nematodes. Parasitology. 1994;108:207–215. [Google Scholar]
- Grewal SK, Grewal PS, Gaugler R. Endophytes of fescue grasses enhance susceptibility of Popillia japonica larvae to an entomopathogenic nematode. Entomologia Experimentalis et Applicata. 1995;74:219–224. [Google Scholar]
- Grewal PS, Power KT, Shetlar DJ. Neonicotinoid insecticides alter diapause behavior and survival of overwintering white grubs (Coleoptera: Scarabaeidae) Pest Management Science. 2001;57:852–857. doi: 10.1002/ps.373. [DOI] [PubMed] [Google Scholar]
- Grewal P, Ehlers, R–U, Shapiro–Ilan DI. 2005 Nematodes as Bio–Control Agents. CABI Publishing, Wallingford, UK. [Google Scholar]
- Gulçu B, Hazir S, Kaya HK. 2012 doi: 10.1016/j.jip.2012.03.014. Scavenger deterrent factor (SDF) from symbiotic bacteria of entomopathogenic nematodes. Journal of Invertebrate Pathology. 110, 326–333. [DOI] [PubMed] [Google Scholar]
- Hatting J, Hazir S, Stock SP. Diversity and distribution of entomopathogenic nematodes (Steinernematidae, Heterorhabditidae) in South Africa. Journal of Invertebrate Pathology. 2008;102:120–128. doi: 10.1016/j.jip.2009.07.003. [DOI] [PubMed] [Google Scholar]
- Hirao A, Ehlers R. Influence of inoculum density on population dynamics and dauer juvenile yields in liquid culture of biocontrol nematodes Steinernema carpocapsae and S. feltiae (Nematoda: Rhabditida) Applied Microbiology Biotechnology. 2010;85:507–515. doi: 10.1007/s00253-009-2095-4. [DOI] [PubMed] [Google Scholar]
- Hominick WM. 2002 Biogeography. Pp. 115–144 in R. Gaugler (ed.), Entomopathogenic Nematology. CABI Publishing, Wallingford, UK. [Google Scholar]
- Hominick WM, Reid AP, Briscoe BR. Prevalence and habitat specificity of steinernematid and heterorhabditid nematodes isolated during soil surveys of the UK and the Netherlands. Journal of Helminthology. 1995;69:27–32. doi: 10.1017/s0022149x00013791. [DOI] [PubMed] [Google Scholar]
- Hominick WM, Reid AP, Bohan DA, Briscoe BR. Entomopathogenic nematodes: biodiversity, geographical distribution and the Convention on Biological Diversity. Biocontrol Science and Technology. 1996;6:317–331. [Google Scholar]
- Hooper DU, Chapin FS, III, Ewel JJ, Hector A, Inchausti P, Lavorel S, Lawton JH, Lodge D, Loreau M, Naeem S, Schmid B, Setälä H, Symstad AJ, Vandermeer J, Wardle DA. Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecological Monographs. 2005;75:3–35. [Google Scholar]
- Hoy CW, Grewal PS, Lawrence JL, Jagdale G, Acosta N. Canonical correspondence analysis demonstrates unique soil conditions for entomopathogenic nematode species compared with other free–living nematode species. Biological Control. 2008;46:371–379. [Google Scholar]
- Hsiao W, All JN. Effects of temperature and placement site on the dispersal of the entomopathogenic nematode, Steinernema carpocapsae in four soils. Zhonghua Kunchong. 1996;16:95–106. [Google Scholar]
- Hsiao W, All JN. Survey of the entomopathogenic nematode, Steinernema carpocapsae (Rhabditida: Steinernematidae) natural populations and its dispersal in the field. Zhonghua Kunchong. 1998;18:39–49. [Google Scholar]
- Hummel RL, Walgenbach JF, Barbercheck ME, Kennedy GG, Hoyt GD, Arellano C. Effects of production practices on soil–borne entomopathogens in western North Carolina vegetable systems. Environmental Entomology. 2002;31:84–91. [Google Scholar]
- Jabbour R, Barbercheck ME. Soil and habitat complexity effects on movement of the entomopathogenic nematode Steinernema carpocapsae in maize. Biological Control. 2008;47:235–243. [Google Scholar]
- Jabbour R, Barbercheck ME. Soil microarthropod response to the application of entomopathogenic nematode–killed insects in maize and flower strip habitats. Pedobiologia. 2011;54:243–251. [Google Scholar]
- Jagdale GB, Gordon R. Effect of recycling temperature on the infectivity of entomopathogenic nematodes. Canadian Journal of Zoology–Revue Canadienne De Zoologie. 1997;75:2137–2141. [Google Scholar]
- Kanga FN, Waeyenberge L, Hauser S, Moens M. Distribution of entomopathogenic nematodes in Southern Cameroon. Journal of Invertebrate Pathology. 2012;109:41–51. doi: 10.1016/j.jip.2011.09.008. [DOI] [PubMed] [Google Scholar]
- Karagoz M, Gulçu B, Cakmak I, Kaya H, Hazir S. Predation of entomopathogenic nematodes by Sancassania sp. (Acari, Acaridae) Experimental and Applied Acarology. 2007;43:85–95. doi: 10.1007/s10493-007-9105-y. [DOI] [PubMed] [Google Scholar]
- Kaya HK. 2002 Natural enemies and other antagonists. Pp. 169–203 in Gaugler, R. (ed.), Entomopathogenic Nematology. CABI Publishing, Wallingford, UK. [Google Scholar]
- Kaya HK, Gaugler R. Entomopathogenic nematodes. Annual Review of Entomology. 1993;38:181–206. [Google Scholar]
- Köllner TG, Held M, Lenk C, Hiltpold I, Turlings TCJ, Gershenzon J, Degenhardt J. A maize (E)–β–caryophyllene synthase implicated in indirect defense responses against herbivores is not expressed in most American maize varieties. Plant Cell. 2008;20:482–494. doi: 10.1105/tpc.107.051672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppenhöfer AM, Baur ME, Stock SP, Choo HY, Chinnasri B, Kaya HK. Survival of entomopathogenic nematodes within host cadavers in dry soil. Applied Soil Ecology. 1997;6:231–240. [Google Scholar]
- Lacey LA, Kaya HK, Bettencourt R. Dispersal of Steinernema glaseri (Nematoda, Steinernematidae) in adult japanese beetles, Popillia japonica (Coleoptera, Scarabaeidae) Biocontrol Science and Technology. 1995;5:121–130. [Google Scholar]
- Lacey LA, Rosa JS, Simoes NO, Amaral JJ, Kaya HK. Comparative dispersal and larvicidal activity of exotic and Azorean isolates of entomopathogenic nematodes against Popillia japonica (Coleoptera: Scarabaeidae) European Journal of Entomology. 2001;98:439–444. [Google Scholar]
- Lee MM, Stock SP. A multilocus approach to assessing co–evolutionary relationships between Steinernema spp. (Nematoda: Steinernematidae) and their bacterial symbionts Xenorhabdus spp. (γ–Proteobacteria: Enterobacteriaceae) Systematic Parasitology. 2010a;77:1–12. doi: 10.1007/s11230-010-9256-9. [DOI] [PubMed] [Google Scholar]
- Lee MM, Stock SP. A multigene approach for assessing evolutionary relationships of Xenorhabdus spp. (γ–Proteobacteria), the bacterial symbionts of entomopathogenic Steinernema nematodes. Journal of Invertebrate Pathology. 2010b;104:67–74. doi: 10.1016/j.jip.2010.01.005. [DOI] [PubMed] [Google Scholar]
- Lewis EE, Campbell J, Griffin C, Kaya HK, Peters A. Behavioral ecology of entomopathogenic nematodes. Biological Control. 2006;38:66–79. [Google Scholar]
- Liu J, Berry RE, Blouin MS. Identification of symbiontic bateria (Phothorhabdus and Xenorhabdus) from the entomopathogenic nematodes Heterorhabditis marelatus and Steinernema oregonense based on 16S rDNA sequence. Journal of Invertebrate Pathology. 2001;77:87–91. doi: 10.1006/jipa.2001.5007. [DOI] [PubMed] [Google Scholar]
- Malan A, Knoetze R, Moore SD. Isolation and identification of entomopathogenic nematodes from citrus orchards in South Africa and their biocontrol potential against false codling moth. Journal of Invertebrate Pathology. 2011;108:115–125. doi: 10.1016/j.jip.2011.07.006. [DOI] [PubMed] [Google Scholar]
- Maneesakorn P, An R, Daneshvar H, Taylor K, Bai X, Adams BJ, Grewal PS, Chandrapatya A. Phylogenetic and cophylogenetic relationships of entomopathogenic nematodes (Heterorhabditis: Rhabditida) and their symbiotic bacteria (Photorhabdus: Enterobacteriaceae) Molecular Phylogenetics and Evolution. 2011;59:271–280. doi: 10.1016/j.ympev.2011.02.012. [DOI] [PubMed] [Google Scholar]
- McCoy CW, Stuart RJ, Nigg HN. Seasonal life stage abundance of Diaprepes abbreviatus in irrigated and non–irrigated citrus plantings in central Florida. Florida Entomologist. 2003;86:34–42. [Google Scholar]
- Millar LC, Barbercheck ME. Effects of tillage practices on entomopathogenic nematodes in a Zea mays (L.) agroecosystem. Biological Control. 2002;25:11–20. [Google Scholar]
- Mráček Z, Bečvár S, Kindlmann P. Survey of entomopathogenic nematodes from the families Steinernematidae and Heterorhabditidae (Nematoda: Rhabditida) in the Czech Republic. Folia Parasitilogica. 1999;46:145–148. [Google Scholar]
- Mráček Z, Bečvár S, Kindlmann P, Jersakova J. Habitat preference for entomopathogenic nematodes, their insect hosts and new faunistic records for the Czech Republic. Biological Control. 2005;34:27–37. [Google Scholar]
- Mukuka J, Strauch O, Hoppe C, Ehlers R-U. Improvement of heat and desiccation tolerance in Heterorhabditis bacteriophora through cross–breeding of tolerant strains and successive genetic selection. Biocontrol. 2010;55:511–521. [Google Scholar]
- Nadler SA, Bolotin E, Stock SP. Phylogenetic relationships of Steinernema (Cephalobina, Steinernematidae) based on nuclear, mitochondrial, and morphological data. Systematic Parasitology. 2006;63:159–179. doi: 10.1007/s11230-005-9009-3. [DOI] [PubMed] [Google Scholar]
- Neher DA, Wu J, Barbercheck ME, Anas O. Ecosystem type affects interpretation of soil nematode community measures. Applied Soil Ecology. 2005;30:47–64. [Google Scholar]
- Nguyen KB, Maruniak J, Adams BJ. Diagnostic and phylogenetic utility of the rDNA internal transcribed spacer sequences of Steinernema. Journal of Nematology. 2001;33:73–82. [PMC free article] [PubMed] [Google Scholar]
- Parkman JP, Frank JH, Walker TJ, Schuster DJ. Classical biological control of Scapteriscus spp. (Orthoptera: Gryllotalpidae) in Florida. Environmental Entomology. 1996;25:1415–1420. [Google Scholar]
- Pathak E, El–Borai FE, Campos–Herrera R, Jonhson EG, Stuart RJ, Graham JH, Duncan LW. Use of real–time PCR to discriminate predatory and saprophagous behavior by nematophagous fungi. Fungal Biology. 2012;116:563–573. doi: 10.1016/j.funbio.2012.02.005. [DOI] [PubMed] [Google Scholar]
- Peters A. The natural host range of Steinernema and Heterorhabditis spp. and their impact on insect populations. Biocontrol Science and Technology. 1996;6:389–402. [Google Scholar]
- Portillo–Aguilar C, Villani MG, Tauber MJ, Tauber CA, Nyrop JP. Entomopathogenic nematode (Rhabditida: Heterorhabditidae and Steinernematidae) response to soil texture and bulk density. Environmental Entomology. 1999;28:1021–1035. [Google Scholar]
- Preisser EL, Strong DR. Climate affects predator control of an herbivore outbreak. American Naturalist. 2004;163:754–762. doi: 10.1086/383620. [DOI] [PubMed] [Google Scholar]
- Ram K, Gruner DS, McLaughlin JP, Preisser EL, Strong DR. Dynamics of a subterranean trophic cascade in space and time. Journal of Nematology. 2008;40:85–92. [PMC free article] [PubMed] [Google Scholar]
- Rasmann S, Köllner TJ, Degenhardt J, Hiltpold I, Toepfer S, Kuhlmann U, Gershenzon J, Turlings TCJ. Recruitment of entomopathogenic nematodes by insect–damaged maize roots. Nature. 2005;434:732–737. doi: 10.1038/nature03451. [DOI] [PubMed] [Google Scholar]
- Read DS, Sheppard SK, Bruford MW, Glen DM, Symondson WOC. Molecular detection of predation by soil micro–arthropods on nematodes. Molecular Ecology. 2006;15:1963–1972. doi: 10.1111/j.1365-294X.2006.02901.x. [DOI] [PubMed] [Google Scholar]
- Rolston AN, Griffin CT, Downes MJ. Emergence and dispersal patterns of two isolates of the entomopathogenic nematode Steinernema feltiae. Journal of Nematology. 2006;38:221–228. [PMC free article] [PubMed] [Google Scholar]
- Rosenheim JA, Kaya HK, Ehler LE, Marois JJ, Jaffee BA. Intraguild predation among biological control agents: theory and evidence. Biological Control. 1995;5:303–335. [Google Scholar]
- Ryder JJ, Griffin CT. Density–dependent fecundity and infective juvenile production in the entomopathogenic nematode, Heterorhabditis megidis. Parasitology. 2002;125:83–92. doi: 10.1017/s0031182002001877. [DOI] [PubMed] [Google Scholar]
- Salame L, Glazer I, Chubinishvilli MT, Chkhubianishvili T. Genetic improvement of the desiccation tolerance and host–seeking ability of the entomopathogenic nematode Steinernema feltiae. Phytoparasitica. 2010;38:359–368. [Google Scholar]
- Schwartz HT, Antoshechkin I, Sternberg PW. Applications of high-throughput sequencing to symbiotic nematode of the genus Heterorhabditis. Simbiosys. 2011;55:111–118. [Google Scholar]
- Selvan S, Campbell JF, Gaugler R. Density–dependent effects on entomopathogenic nematodes (Heterorhabditidae and Steinernematidae) within an insect host. Journal of Invertebrate Pathology. 1993;62:278–284. [Google Scholar]
- Shapiro DI, Tylka GL, Berry EC, Lewis LC. Effects of earthworms on the dispersal of Steinernema spp. Journal of Nematology. 1995;27:21–28. [PMC free article] [PubMed] [Google Scholar]
- Shapiro DI, Obrycki JJ, Lewis LC, Jackson JJ. Effects of crop residue on the persistence of Steinernema carpocapsae. Journal of Nematology. 1999;31:517–519. [PMC free article] [PubMed] [Google Scholar]
- Shapiro DI, McCoy CW, Fares A, Obreza T, Dou H. Effects of soil type on virulence and persistence of entomopathogenic nematodes in relation to control of Diaprepes abbreviatus. Environmental Entomology. 2000;29:1083–1087. [Google Scholar]
- Shapiro–Ilan DI, Gouge DH, Koppenhöfer AM. 2002 Factors affecting commercial success: case studies in cotton, turf and citrus. Pp. 333–355 in Gaugler, R. (ed.) Entomopathogenic Nematology. CABI Publishing, Wallingford, UK. [Google Scholar]
- Spiridonov SE, Reid AP, Podrunka K, Subbotin SA, Moens M. Phylogenetic relationships within the genus Steinernema (Nematoda: Rhabditida) as inferred from analyses of sequences of the ITS1–5.8S–ITS2 region of rDNA and morphological features. Nematology. 2004;6:547–566. [Google Scholar]
- Spiridonov SE, Moens M, Wilson MJ. Fine scale spatial distributions of two entomopathogenic nematodes in a grassland soil. Applied Soil Ecology. 2007;37:192–201. [Google Scholar]
- Stinner BR, House GJ. Arthropods and other invertebrates in conservation–tillage agriculture. Annual Revue Entomology. 1990;35:299–318. [Google Scholar]
- Stock SP. 2009 Molecular approaches and the taxonomy of insect–parasitic and pathogenic nematodes. Pp. 71–100 in Stock, S. P., Vanderberg, J., Boemare, N. E., and Glazer, I (eds) Insect pathogens: molecular approaches and techniques. Cabi Publishing–CABI, Wallingford, Oxon, England, UK. [Google Scholar]
- Stock SP, Goodrich–Blair H. Entomopathogenic nematodes and their bacterial symbionts: the inside out of a mutualistic association. Symbiosis. 2008;46:65–75. [Google Scholar]
- Stock SP, Reid AP. Biosystematics (Steinernematidae, Heterorhabditidae): current status and future directions. Nematology Monographs and Perspectives. 2004;2:435–446. [Google Scholar]
- Stock SP, Strong D, Gardner SL. Identification of Heterorhabditis (Nematoda: Heterorhabditidae) from California with a new species isolated from the larvae of the ghost moth Hepialis californicus (Lepidoptera: Hepialidae) from the Bodega Bay Natural Reserve. Fundamental and Applied Nematology. 1996;19:585–595. [Google Scholar]
- Stock SP, Pryor BM, Kaya HK. Distribution of entomopathogenic nematodes (Steinernematidae and Heterorhabditidae) in natural habitats in California. USA. Biodiversity and Conservation. 1999;8:535–549. [Google Scholar]
- Stock SP, Campbell JF, Nadler SA. Phylogeny of Steinernema Travassos, 1927 (Cephalobina: Steinernematidae) inferred from ribosomal DNA sequences and morphological characters. Journal of Parasitology. 2001;87:877–889. doi: 10.1645/0022-3395(2001)087[0877:POSTCS]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Strong DR. 2002 Populations of entomopathogenic nematodes in foodwebs. Pp. 225–240 in Gaugler R. (ed.) Entomopathogenic Nematology, CABI Publishing, Wallingford, UK. [Google Scholar]
- Strong DR. Spacial ecology of food webs with entomopathogenic nematodes. Phytopathology. 2007;97:S143–S143. [Google Scholar]
- Stuart RJ, Gaugler R. Patchiness in populations of entomopathogenic nematodes. Journal of Invertebrate Pathology. 1994;64:39–45. [Google Scholar]
- Stuart RJ, Barbercheck ME, Grewal PS, Taylor RAJ, Hoy CW. Population biology of entomopathogenic nematodes: Concepts, issues, and models. Biological Control. 2006;38:80–102. [Google Scholar]
- Stuart RJ, El–Borai FE, Duncan LW. From augmerntatition to conservation of entomopathogenic nematodes: trophic cascades, habitat manipulation and enhanced biological control of Diaprepes abbreviatus root weevils in Florida citrus groves. Journal of Nematology. 2008;40:73–84. [PMC free article] [PubMed] [Google Scholar]
- Sturhan D. 1999 Prevalence and habitat specificity of entomopathogenic nematodes in Germany. Pp. 123–132 in Gwynn, R. L., Smits, P. H., Griffin, C., Ehlers, R.–U., Boemare, N. E., and Masson, J.–P. (eds). COST 819: Entomopathogenic nematodes: application and persistence of entomopathogenic nematodes. Brussels, Belgium, European Communities. [Google Scholar]
- Sturhan D, Lisková M. Occurrence and distribution of entomopathogenic nematodes in the Slovak Republic. Nematology. 1999;1:273–277. [Google Scholar]
- Szállás E, Pukall R, Pamjav H, Kovács G, Buzás Z, Fodor A, Stackebrandt E. 2001 Passengers who missed the train: comparative sequence analysis, PhastSystem PAGE- PCR-RFLP and automated RiboPrint phenotypes of Photorhabdus strains. Pp. 36–53 in Griffin, C. T., Burnell, A. M., Downes, M. J., and Mulder, R. Developments in Entomopathogenic Nematode/Bacterial Research Luxemburg: European Commission Publications. [Google Scholar]
- Tailliez P, Pagès S, Ginibre N, Boemare N. New insight into diversity in the genus Xenorhabdus, including the description of ten novel species. International Journal of Systematic and Evolutionary Microbiology. 2006;56:2805–2818. doi: 10.1099/ijs.0.64287-0. [DOI] [PubMed] [Google Scholar]
- Tailliez P, Laroui C, Ginibre N, Paule A, Pagès S, Boemare N. Phylogeny of Photorhabdus and Xenorhabdus based on universally conserved protein–coding sequences and implications for the taxonomy of these two genera. Proposal of new taxa: X. vietnamensis sp nov., P. luminescens subsp caribbeanensis subsp nov., P. luminescens subsp hainanensis subsp nov., P. temperata subsp khanii subsp nov., P. temperata subsp tasmaniensis subsp nov., and the reclassification of P. luminescens subsp thracensis as P. temperata subsp thracensis comb. nov. International Journal of Systematic and Evolutionary Microbiology. 2010;60:1921–1937. doi: 10.1099/ijs.0.014308-0. [DOI] [PubMed] [Google Scholar]
- Ter Braak CJF. Canonical Correspondence Analysis a new eigenvector technique for multivariate direct gradient analysis. Ecology (Washington D. C.) 1986;67:1167–1179. [Google Scholar]
- Thompson JN, Reichman OJ, Morin PJ, Polis GA, Power ME, Sterner RW, Couch CA, Gough L, Holt R, Hooper DU, Keesing F, Lovell CR, Milne BT, Molles MC, Roberts DW, Strauss SY. Frontiers of ecology. BioScience. 2001;51:15–24. [Google Scholar]
- Torr P, Spiridonov SE, Heritage S, Wilson MJ. Habitat associations of two entomopathogenic nematodes: a quantitative study using real–time quantitative polymerase chain reactions. Journal of Animal Ecology. 2007;76:238–245. doi: 10.1111/j.1365-2656.2006.01196.x. [DOI] [PubMed] [Google Scholar]
- Turlings TCJ, Urs B, Lengwiler UB, Bernasconi ML, Wechsler D. Timing of induced volatile emissions in maize seedlings. Planta. 1998;207:146–152. [Google Scholar]
- Van Nes EH, Scheffer M. A strategy to improve the contribution of complex simulation models to ecological theory. Ecological Modelling. 2005;185:153–164. [Google Scholar]
- Walter DE, Ikonen EK. Species, guilds, and functional groups: taxonomy and behavior in nematophagous arthropods. Journal of Nematology. 1989;21:315–327. [PMC free article] [PubMed] [Google Scholar]
- Walter DE, Hunt HW, Elliot ET. The influence of prey type on the development and reproduction of some predatory soil mites. Pedobiologia. 1987;30:419–424. [Google Scholar]
- Wallace HR. The dynamics of nematode movement. Annual Review of Phytopathology. 1968;6:91–114. [Google Scholar]
- Wang JX, Bedding RA. Population development of Heterorhabditis bacteriophora and Steinernema carpocapsae in the larvae of Galleria mellonella. Fundamental and Applied Nematology. 1996;19:363–367. [Google Scholar]
- Wardle DA, Bardgett RD, Klironomos JN, Setälä H, van der Putten WH, Wall DH. Ecological linkages between aboveground and belowground biota. Science. 2004;304:1629–1633. doi: 10.1126/science.1094875. [DOI] [PubMed] [Google Scholar]
- Wilson M, Gaugler R. Factors limiting short–term persistence of entomopathogenic nematodes. Journal of Applied Entomology. 2004;128:250–253. [Google Scholar]
- Wilson MJ, Glen DM, Hamacher GM, Smith JU. A model to optimise biological control of slugs using nematode parasites. Applied Soil Ecology. 2004;26:179–191. [Google Scholar]
- Zhou X, Kaya HK, Heungens K, Goodrich–Blair H. Response of ants to deterrent factor(s) produced by the symbiotic bacteria of entomopathogenic nematodes. Applied and Environmental Microbiology. 2002;68:6202–6209. doi: 10.1128/AEM.68.12.6202-6209.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]