Abstract
Entomopathogenic nematodes (EPNs) have been utilized in classical, conservation, and augmentative biological control programs. The vast majority of applied research has focused on their potential as inundatively applied augmentative biological control agents. Extensive research over the past three decades has demonstrated both their successes and failures for control of insect pests of crops, ornamental plants, trees and lawn and turf. In this paper we present highlights of their development for control of insect pests above and below ground. The target insects include those from foliar, soil surface, cryptic and subterranean habitats. Advances in mass-production and formulation technology of EPNs, the discovery of numerous efficacious isolates/strains, and the desirability of reducing pesticide usage have resulted in a surge of commercial use and development of EPNs. Commercially produced EPNs are currently in use for control of scarab larvae in lawns and turf, fungus gnats in mushroom production, invasive mole crickets in lawn and turf, black vine weevil in nursery plants, and Diaprepes root weevil in citrus in addition to other pest insects. However, demonstrated successful control of several other insects, often has not lead to capture of a significant share of the pesticide market for these pests.
Keywords: biological control, commercialization, cryptic habitats, Epigeal habitats, entomopathogenic nematode, Heterorhabditis bacteriophora, Heterorhabditis megidis, Heterorhabditis zealandica, Steinernema carpocapsae, Steinernema feltiae, Steinernema glaseri, Steinernema riobrave, Steinernema scapterisci, Steinernema scarabaei, subterranean habitats
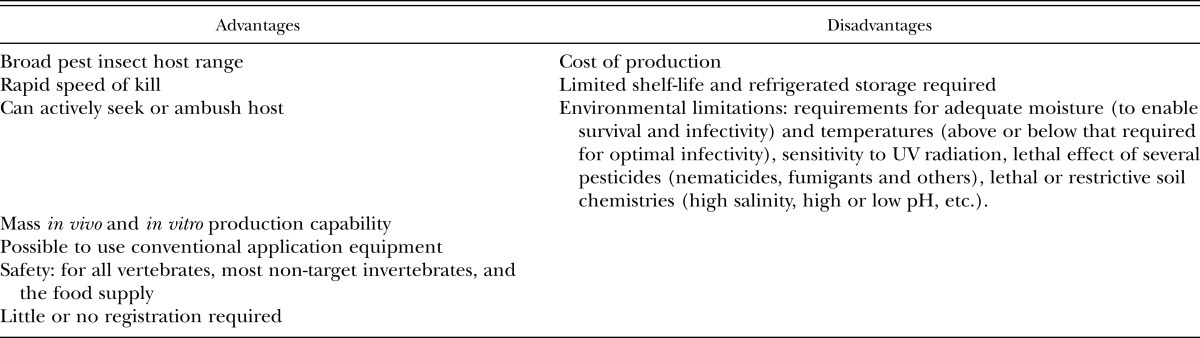
Nematodes that parasitize insects, known as entomopathogenic nematodes (EPNs), have been described from 23 nematode families (Koppenhöfer, 2007). Of all of the nematodes studied for biological control of insects, the Steinernematidae and Heterorhabditidae have received the most attention because they possess many of the attributes of effective biological control agents (Kaya and Gaugler, 1993; Grewal et al., 2005a; Koppenhöfer, 2007) and have been utilized as classical, conservational, and augmentative biological control agents. The vast majority of applied research has focused on their potential as inundatively applied augmentative biological control agents (Grewal et al., 2005a). Extensive research over the past three decades has demonstrated both their successes and failures for control of insect pests of crops, ornamental, and lawn and turf (Shapiro-Ilan et al., 2002; Georgis et al., 2006). The main advantages and disadvantages of EPNs are presented in Table 1. They can be considered good candidates for integrated pest management and sustainable agriculture due to a variety of attributes in addition to those presented in Table 1: some species can recycle and persist in the environment; they may have direct and/or indirect effects on populations of plant parasitic nematodes and plant pathogens; can play an indirect role in improving soil quality; and are compatible with a wide range of chemical and biological pesticides used in IPM programs. This paper will review selected literature on the successful use of EPNs for control of insect pests above and below ground and discuss aspects of their commercialization.
Table 1.
Advantages and disadvantages of entomopathogenic nematodes (modified from Shapiro-Ilan et al., 2012).
Entomopathogenic Nematodes for Control of Insect Pests
Selection of an EPN for control of a particular pest insect is based on several factors that include the nematode’s host range, host finding or foraging strategy, tolerance of environmental factors and their effects on survival and efficacy (temperature, moisture, soil type, exposure to ultraviolet light, salinity and organic content of soil, means of application, agrochemicals, and others). The 4 most critical factors are moisture, temperature, pathogenicity for the targeted insect, and foraging strategy (Kung et al., 1991; Kaya and Gaugler, 1993; Campbell et al., 2003; Grewal et al., 2005a). Within a favorable range of temperatures, adequate moisture and a susceptible host, those EPNs with a mobile foraging strategy (cruisers and intermediate foraging strategies) could be considered for use in subterranean and certain above-ground habitats (foliar, epigeal and cryptic habitats). Those with a sit and wait foraging strategy (ambushers) will be most effective in cryptic and soil surface habitats.
The effects of environmental factors (such as temperature, moisture, aeration, and soil type [esp. texture and chemistry]) and biotic factors (species of EPN, targeted insect, age of insect, soil fauna) have been documented by numerous researchers (Gaugler and Kaya, 1990; Kaya and Gaugler, 1993; Shapiro-Ilan et al., 2012; Grewal et al., 2005a; Georgis et al., 2006). Temperature range for survival and infectivity will depend on the species of EPN and its native habitat and center of origin (Kaya, 1990). For example, Steinernema feltiae can be infective from 2-30°C, whereas some heterorhabditids can infect host insects from 7 to 35°C and Steinernema carpocapsae is nearly inactive at 10°C (Kaya, 1990; Georgis et al., 2006; Lacey et al., 2006a).
Subterranean habitats. EPNs are predominantly isolated from soil habitats and not surprisingly, many subterranean insects have received attention for control efforts with EPNs, notably white grubs of the family Scarabaeidae and weevils (Coleoptera:Curculionidae). Scarab larvae are the principle insect pests of lawn and turf while many of the adults are polyphagous pests of flowers and foliage. Damage caused by larvae (grubs) in golf courses has resulted in implementation of control using chemical pesticides and increasingly, EPNs.
White grubs are among the more difficult insects to control with EPNs because they have developed various morphological and behavioral barriers to infection (Klein et al. 2007). Among white grub species that are important pests of turf in the USA, the Japanese beetle, Popillia japonica appears to be the most EPN-susceptible species (Grewal et al., 2005b; Klein et al., 2007). The first EPN species that was used for control of scarabs on a widespread basis was Steinernema glaseri. (Gaugler et al., 1992). Its efficacy against scarab larvae, most notably the P. japonica, has been documented by several researchers (Gaugler et al., 1992). EPNs that have provided good field control of P. japonica include S. scarabaei (100%), H. bacteriophora (strain GPS11) (34–97%), H. bacteriophora (strain TF) (65–92%), and H. zealandica (strain X1) (73–98%) (Grewal et al., 2005b; Koppenhöfer et al., 2006). Among these, two species, H. bacteriophora and H. zealandica, are commercially available for grub control (Grewal et al., 2005b).
Numerous examples of research and practical use of EPNs for control of P. japonica larvae and those of several scarab species are reviewed by Klein (1990), Kaya and Gaugler (1993), Grewal et al., (2005b), Lewis and Clarke (2012), Shapiro, et al. (2002, 2012). The infectivity of Steinernema scarabaei, S. glaseri, Heterorhabditis zealandica, and Heterorhabditis bacteriophora for the Oriental Beetle, Anomala orientalis, and P. japonica was tested in loamy sand, sandy loam, loam, silt loam, clay loam, acidic sand, and an organic potting mix by Koppenhöfer and Fuzy (2006). The two Heterorhabditis spp. were most infective in potting mix, and S. scarabaei was most infective in loamy sand in the greenhouse. In a greenhouse study, S. glaseri was equally infective in all soil types. Acidic sand had a negative effect on infectivity of all species in laboratory experiments. Persistence of S. scarabaei was highest in all soil types but its recovery declined significantly over time only in clay loam. Cappaert and Koppenhöfer (2003) also demonstrated overwinter persistence in treated field plots.
The combination of EPNs and other control agents has proved to be synergistic and produces higher mortality than either agent alone. For example, Koppenhöfer and Kaya (1997) showed additive and synergistic interaction between EPNs and Bacillus thuringiensis for scarab grub control. Koppenhöfer and Kaya (1998), Koppenhöfer et al. (2000), Polavarapu et al. (2007), and Koppenhöfer and Fuzy (2008), demonstrated synergism between the neonicotinoid insecticide, imidacloprid and EPNs. However, Cappaert and Koppenhöfer (2003) observed antagonism between imidacloprid and S. scarabaei for control of the European chafer, Rhizotrogus majalis (Scarabaeidae). Despite the demonstrated synergistic effect of the combined use of EPNs and other control methods, this strategy has yet to be used on a practical basis for control of scarab larvae.
Successful control of weevils, pests of small fruit crops, ornamentals and turf has been reported by several researchers. One of the most studied pest species is the black vine weevil, Otiorhynchus sulcatus (Curculionidae). Kakouli-Duarte et al. (1997) conducted field trials for control of O. sulcatus larvae in strawberry plants using S. carpocapsae and Heterorhabditis megidis. In field experiments, drip irrigation was used to distribute infective juveniles (IJs) along and across raised strawberry beds. Steinernema carpocapsae treatment in late summer produced 49.5% reduction of early instar O. sulcatus larvae, and late spring application resulted in 65% control of late instar larvae. Whereas spring application of H. megidis caused only 26% mortality of late instar larvae. Results of studies by Haukeland and Lola-Luz (2010) in Ireland and Norway demonstrated that H. megidis provided good control of O. sulcatus as long as temperatures were above 10°C.
Ansari et al. (2008) observed that application of the fungus, Metarhizium anisopliae (Hypocreales), following 1 or 2 weeks later by either Heterorhabditis bacteriophora, S. feltiae or Steinernema kraussei provided 100% control of third-instar O. sulcatus. The authors suggest that EPN and M. anisopliae act synergistically for control of the weevil in potted winter creeper, Euonymus fortune, under greenhouse conditions. Other examples of weevil control are provided by: Klein (1990), Simser and Roberts (1994), Booth et al. (2002), van Tol and Raupp (2005), Georgis et al. (2006), and McGraw and Koppenhöfer (2008). See below EPN application for control of insects in soil surface habitats.
Continued prospection for new EPN species or races that are infective for scarabs and weevils is highly warranted. For example, Cappaert and Koppenhöfer (2003) and Koppenhöfer and Fuzy (2003) demonstrated elevated pathogenicity of S. scarabaei against P. japonica, A. orientalis, R. majalis, northern masked chafer, Cyclocephala borealis, and Asian garden beetle, Maladera castanea compared to other EPNs. Additionally, this species can persist in the environment for considerable periods (Cappaert and Koppenhöfer, 2003; Koppenhöfer and Fuzy, 2009). Experimental evaluations of several species for scarab control in turf and lawn include H. bacteriophora, H. megidis, and new isolates of Heterorhabditis spp. and Steinernema kushidai and Steinernema spp. (Shapiro-Ilan et al., 2002; Grewal et al. 2004).
Fungus gnats, Lycoriella spp. and Bradysia spp. (Diptera: Sciaridae) are significant subterranean pests in mushroom production and cultivation of greenhouse plants, respectively. Steinernema feltiae and Heterorhabditis spp. have demonstrated good efficacy for control of L. auripilla, L. mali, L. solani, B. coprophila and B. difformis (Scheepmaker et al., 1998a, 1998b; Jagdale et al., 2004, 2007; Jess et al. 2005; Tomalak et al., 2005; Grewal, 2007). Application rates of 1.0 to 1.5 x 106 of S. feltiae IJs/m2 provide affordable and effective control of Lycoriella spp. that is comparable to or better than that of insecticides commonly used in mushroom production (Jagdale et al., 2004; Grewal, 2007). Also, because S. feltiae recycles in gnat larvae, it maintains its effectiveness longer than diflubenzuron and methoprene (Grewal and Richardson, 1993). Commercially produced S. feltiae are now routinely used for control of Lycoriella spp. in the United States and Europe (Grewal and Georgis, 1998; Georgis et al., 2006; Grewal, 2007).
Foliar application: Arthurs et al. (2004), analyzed data from dozens of field trials in which EPNs were applied for control of insect pests in above ground habitats. The lowest efficacy was reported for foliar habitats. The major limiting factor of foliar application of EPNs to leaf surfaces is the rapid desiccation of the IJs, although anti-desiccants have been shown to increase the effectiveness of EPNs against certain pest species. Steinernema carpocapsae, is the most commonly applied species for control of foliar and other above-ground pests. Due to its ambusher host-finding strategy, they are ideal candidates for pest insects that are encountered on the surface soil when they descend from foliage.
Treatment of diamondback moth larvae, Plutella xylostella (Lepidoptera:Plutellidae), on watercress with B. thuringiensis and S. carpocapsae, both at half rate, resulted in 58% control, significantly higher than that of each applied at full rate (Baur et al., 1998). These authors concluded that repeated applications of EPNs alone would probably be ineffective in attaining control. However, they suggested that EPNs could serve as components of IPM of P. xylostella if efficacy can be increased and they could help manage resistance to B. thuringiensis. Baur et al. (1997) demonstrated that additives generally improved EPN persistence and efficacy on watercress, but the improvement was probably not sufficient to increase the feasibility of foliar applications of EPNs against P. xylostella. Similar results were reported by Schroer and Ehlers (2005) and Schroer et al. (2005) for S. carpocapsae and combination of the nematode and B. thuringiensis with a polymer. Other researchers have reported on the efficacy of EPNs for control of P. xylostella with similar results, i.e less than 50% of targeted populations were controlled with high concentrations of EPNs (Somvanshi et al., 2006; Nyasani et al., 2008). However, Lello et al. (1996) reported that high output hydraulic nozzles deposited the greatest number of IJs onto foliage and produced up to 98% mortality.
Control of other pest species with EPNs on foliar surfaces has been variable (Georgis et al., 2006). For example, Bélair et al. (2003) demonstrated that foliar applications of S. carpocapsae did not provide an acceptable level of control of imported cabbageworm Artogeia rapae (Lepidoptera: Pieridae) under environmental conditions in Québec. On the other hand, research on S. carpocapsae and S. feltiae demonstrated their potential for control of the leafminers (Diptera: Agromyzidae): Liriomyza trifolii (Hara et al., 1993; LeBeck et al., 1993; Sher et al., 2000; Tomalak et al., 2005) Liriomyza huidobrensis (Williams and Walters, 2000), and Tuta absoluta (Batalla-Carrera et al., 2010) and other leafminer species.
EPN application for control of insects in epigeal (soil surface) habitats: The most successful use of EPNs above ground involves soil surface treatment of insects while they are transiting over or through the soil surface. One of the best examples of successful control of an insect pest at the soil surface is that of EPNs for control of Diaprepes root weevil, Diaprepes abbreviatus (Coleoptera:Cucurlionidae) in citrus. The weevil is a major pest in Florida citrus since its introduction from Caribbean islands in the 1960s. Its subterranean grubs can severely damage roots and heavy persistent infestation can result in tree mortality. The eggs of the weevil are laid on leaves and after hatching, larvae drop to the ground, and enter the soil. Because neonate D. abbreviatus larvae are highly susceptible to EPNs, surface application of IJs while they are entering the soil provides an effective means for their control. In Florida, EPNs have been marketed for weevil control for nearly 20 years. Two commercially produced species, Steinernema riobrave and Heterorhabditis indica have been used for effective control. These nematodes appear to be most effective at high temperatures (27 ± 2°C) in coarse sandy soils. Larval mortality of over 90% has been reported for field trials with S. riobrave when applied at 1.2 x 1010 IJs/ha (McCoy et al. 2002, 2007; Shapiro-Ilan et al, 2002; Stuart et al., 2008). The use of irrigation systems for application of EPNs has been effective in delivering IJs into the zone below trees where larvae enter the soil.
Cutworms (Lepidoptera: Noctuidae) (Agrotis, Amathes, Noctua, Peridroma, Prodenia spp.) are leaf, bud, and stem feeders and some species feed on roots. They spend some or all of their feeding stages in contact with the soil. Many species overwinter as penultimate or last instar larvae or pupae in the soil or under fallen leaves and other debris at the soil surface. During their feeding or resting activity on the surface of the soil they are good targets for ambusher EPNs when soil moisture is sufficient for IJ survival and infectivity. Although several studies have demonstrated good control of cutworms in crops and turf (Capinera et al., 1988; Buhler and Gibb 1994; West and Vrain, 1997; Shapiro-Ilan et al., 2002; Ebssa and Koppenhöfer, 2011) they are not yet implemented on a large scale.
Steinernema spp. and Heterorhabditis spp. have also been used with varying degrees of success in field trials for pest insects that exit fruit and enter the soil to pupate. For example, plum curculio, Conotrachelus nenuphar, (Coleoptera:Curculionidae) (Shapiro-Ilan, 2004, 2008; Alston et al., 2005; Kim and Alston 2008; Pereault et al., 2009), fruit flies (Rhagoletis indifferens, Anastrepha ludens; Diptera:Tephritidae) (Yee and Lacey, 2003; Toledo et al., 2005) and several other species reviewed by Georgis et al. (2006) and Dolinski and Lacey (2007).
The invasive mole cricket, Scapteriscus vicinus (Orthoptera:Gryllotalpidae), from South America, is a serious pest of lawn and turf in the Southern United States. Successful classical biological control of the cricket with Steinernema scapterisci, an EPN collected in the putative center of origin of the cricket in Uruguay, is documented by Hudson et al. (1988), Parkman and Smart (1996), and Parkman et al. (1996).The nematode was successfully established after introduction of S. scapterisci-infested cadavers and applications in small plots at a rate equivalent to 2 x 109 IJs/ha (Hudson et al., 1988; Parkman et al., 1993; Parkman and Smart, 1996). In addition, S. scapterisci was auto-dispersed by infected mole crickets to create new foci of infection (Parkman et al., 1993). Due to the territoriality of S. vicinus, Parkman and Frank (1992) developed a unique method of treatment using sound traps to attract and infect the crickets. Three years after the initial introduction of S. scapterisci, mole cricket populations at release sites were reduced by up to 98%. Application of S. scapterisci to untreated sites and augmentative applications have been facilitated by commercial production of the nematode (Grewal et al., 2005b).
Cryptic habitats: Cryptic habitats include but are not limited to: under bark and leaf litter, in prop piles, fruit bins, nut shells, and pruning wounds. The use of EPNs for successful control of a number of orchard insect pests in cryptic habitats has been reported in several reviews (Cross et al., 1999; Shapiro et al. 2005; Lacey et al. 2007; Lacey and Shapiro, 2008).
Codling moth, Cydia pomonella (Lepidoptera:Tortricidae), a worldwide pest of apple and other pome fruit, provides an excellent example of the successful use of EPNs for control in cryptic habitats. Diapausing full grown larvae overwinter in hibernacula under bark and in other cryptic habitats. After harvest, they account for 100% of the codling moth population. Control of these larvae would result in reduced emergence of adult moths the following spring. The most evaluated species for codling moth control are S. carpocapsae, S. feltiae, H. bacteriophora, and H. zealandica. The abiotic factors that have the greatest influence on their larvicidal activity against C. pomonella are temperature, moisture, and type of habitat (Kaya et al., 1984; Lacey and Unruh, 1998; Unruh and Lacey, 2001; Lacey et al., 2006a; Navaneethan et al., 2010; de Waal et al., 2011). Application of IJs of S. carpocapsae or S. feltiae at 2.5 x 106 IJs/tree or 1-2.5 x 109/ha under optimal conditions of temperature and moisture (20-25°C, saturated humidity) can provide up to 90% reduction of overwintering larvae (Unruh and Lacey, 2001; Lacey et al. 2006a). Although both species are efficacious, S. feltiae is more temperature tolerant and can be applied later in the fall when temperatures are too low (10°C) for S. carpocapsae. Larvicidal activity has been improved by the addition of humectants to IJ suspensions, supplemental application of water and through the use of mulches in orchards with few above ground cryptic habitats (Unruh and Lacey, 2001; Lacey et al. 2005, 2006a, 2006b, 2010; de Waal et al., 2011).
Fruit bins that are in the orchard during the time when diapause-destined larvae are seeking cryptic habitats, can also provide overwintering sites. These larvae withstand storage conditions (0-4 °C, high N2, low O2) and may emerge in orchards the following year when bins are placed in the orchard. EPNs have been evaluated against cocooned larvae in fruit bins by drenching and immersing the bins in water containing IJs (Lacey and Chauvin, 1999; Cossentine et al., 2002; Lacey et al., 2005; de Waal et al., 2010). Although interest in the use of EPNs for codling moth control has been expressed by orchardists, particularly those in organic fruit production, EPNs are not currently implemented for control of overwintering larvae.
Several studies and field trials of EPNs for control of other insect pests of orchards in cryptic habitats have been reported. These include the filbertmoth, Melissopus latiferreanus (Tortricidae), (Chambers et al., 2010); navel orangeworm, Amyelois transitella (Lepidoptera:Pyralidae), (Siegel et al., 2004, 2006); Oriental fruitmoth, Grapholita molesta (Tortricidae), (Riga et al., 2006); peachtree borer, Synanthedon pictipes (Lepidoptera:Sesiidae), (Shapiro-Ilan et al., 2010), and additional species reviewed by Shapiro-Ilan et al. (2005, 2012). Lacey et al. (2007) and Lacey and Shapiro-Ilan (2008).
Commercialization
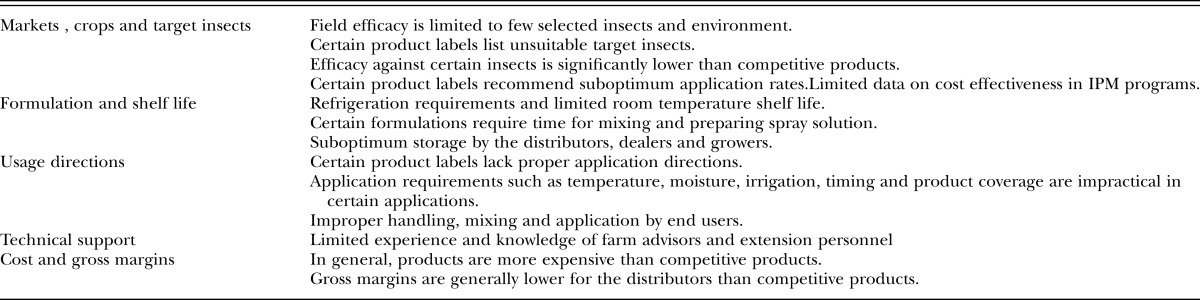
Advances in mass-production and formulation technology of EPNs, the discovery of numerous efficacious isolates/strains and the desirability of reducing pesticide usage have resulted in a surge of scientific and commercial interest in these nematodes. The lessons learned from earlier problems have encouraged scientists and companies to increase their effort toward improving cost efficiency and better products positioning in the market within the confines of product capabilities. At present, EPNs are produced and marketed by few companies and as a result these companies are making reasonable profits which is critical to continued commercial production. From the producer’s point of view, marketing nematodes is a success. However, from the global point of view, the revenues and the market share has been limited to certain markets with little opportunities to expand to new markets. Although the demand for biopesticide products has increased significantly since 2003 (Table 2), the global revenues of the nematodes have been flat, whereas significant increase in the revenues and market share of many of other biopesticide products have been realized (Table 2). Factors such as cost, shelf life, handling, mixing, coverage, new caution signal- based pesticides, compatibility and profit margins to manufacturers and distributors have contributed to the failure of EPNs to penetrate many markets or gain significant market share in the current markets (Table 3).
Table 2.
Biopesticide and entomopathogenic nematode market size compared to chemical pesticides at the distributor level ($US million) (CPL Business Consultant report, 2008, BBC Research report, 2009).
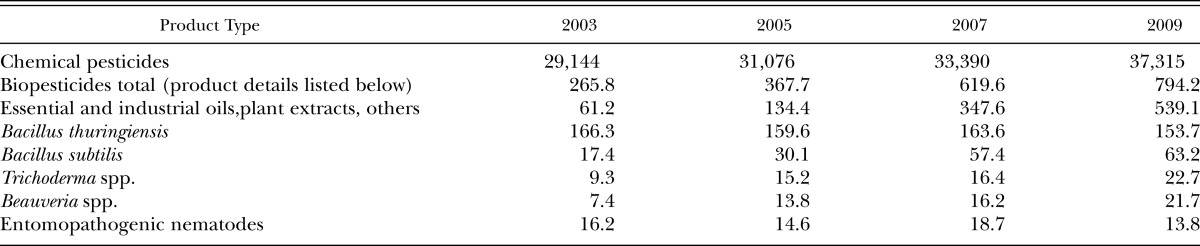
Table 3.
Factors affecting market expansion of entomopathogenic nematodes (modified from Georgis 2004, Georgis et al., 2006).
Genetic improvements through traditional selection regimes and changes in bacterial payload may be the solution to develop stable formulations, improve nematode searching abilities, producing more virulent nematodes, and increasing tolerance to environmental extremes. However, it is important to carefully assess the return of investment when newer technologies are used for genetic modifications that could result in the loss of organic approval and registration exemption status from the registration requirements in many countries. Nevertheless, genetic improvements will play major roles in strengthening and expanding the market share of EPNs in the current crops-pest complex. However, the fact will remain that other advancements are needed to strengthen the efficacy and the field host range beyond the current target insects, crops and environment (examples: root maggots, flea beetles, corn rootworms, cucumber beetles and wireworms)
Literature Cited
- Alston DG, Rangel DEN, Lacey LA, Golez HG, Kim JJ, Roberts DW. Evaluation of novel fungus and nematode isolates for control of Conotrachelus nenuphar (Coleoptera: Curculionidae) larvae. Biological Control. 2005;35:163–171. [Google Scholar]
- Ansari MA, Shah FA, Butt TM. Combined use of entomopathogenic nematodes and Metarhizium anisopliae as a new approach for black vine weevil, Otiorhynchus sulcatus, control. Entomologia Experimentalis et Applicata. 2008;129:340–347. [Google Scholar]
- Arthurs S, Heinz KM, Prasifka JR. An analysis of using entomopathogenic nematodes against above-ground pests. Bulletin of Entomological Research. 2004;94:297–306. doi: 10.1079/ber2003309. [DOI] [PubMed] [Google Scholar]
- Batalla-Carrera L, Morton A, García-del-Pino F. Efficacy of nematodes against the tomato leafminer Tuta absoluta in laboratory and greenhouse conditions. BioControl. 2010;55:523–530. [Google Scholar]
- Baur ME, Kaya HK, Gaugler R, Tabashnik B. Effects of adjuvants on entomopathogenic nematode persistence and efficacy against Plutella xylostella. Biocontrol. Science and Technology. 1997;7:513–525. [Google Scholar]
- Baur ME, Kaya HK, Tabashnik BE, Chilcutt CF. Suppression of diamondback moth (Lepidoptera: Plutellidae) with an entomopathogenic nematode (Rhabditida: Steinernematidae) and Bacillus thuringiensis Berliner. Journal of Economic Entomology. 1998;91:1089–1095. doi: 10.1093/jee/91.5.1089. [DOI] [PubMed] [Google Scholar]
- Bélair G, Fournier Y, Dauphinais N. Efficacy of steinernematid nematodes against three insect pests of crucifers in Quebec. Journal of Nematology. 2003;35:259–265. [PMC free article] [PubMed] [Google Scholar]
- Booth SR, Tanigoshi LK, Shanks CH., Jr Evaluation of entomopathogenic nematodes to manage root weevil larvae in Washington State cranberry, strawberry, and red raspberry. Environmental Entomology. 2002;31:895–902. [Google Scholar]
- Buhler WG, Gibb TJ. Persistence of Steinernema carpocapsae and S.glaseri (Rhabditida: Steinernematidae) as measured by their control of black cutworm (Lepidoptera: Noctuidae) larvae in bentgrass. Journal of Economic Entomology. 1994;87:638–642. [Google Scholar]
- Campbell JF, Lewis EE, Stock SP, Nadler S, Kaya HK. Evolution of host search strategies in entomopathogenic nematodes. Journal of Nematology. 2003;35:142–145. [PMC free article] [PubMed] [Google Scholar]
- Capinera JL, Pelissier D, Menout GS, Epsky ND. Control of black cutworm, Agrotis ipsilon (Lepidoptera: Noctuidae), with entomogenous nematodes (Nematoda: Steinernematidae, Heterorhabditidae) Journal of Invertebrate Pathology. 1988;52:427–435. [Google Scholar]
- Cappaert DL, Koppenhöfer AM. Steinernema scarabaei, an entomopathogenic nematode for control of the European chafer. Biological Control. 2003;28:379–386. [Google Scholar]
- Chambers U, Bruck DJ, Olsen J, Walton VM. Control of overwintering filbertworm (Lepidoptera: Tortricidae) larvae with Steinernema carpocapsae. Journal of Economic Entomology. 2010;103:416–422. doi: 10.1603/ec09255. [DOI] [PubMed] [Google Scholar]
- Cossentine JE, Jensen LB, Moyls L. Fruit bins washed with Steinernema carpocapsae (Rhabditida: Steinernematidae) to control Cydia pomonella (Lepidoptera: Tortricidae). Biocontrol. Science and Technology. 2002;12:251–258. [Google Scholar]
- Cross JV, Solomon MG, Chandler D, Jarrett P, Richardson PN, Winstanley D, Bathon H, Huber J, Keller B, Langenbruch GA, Zimmerman G. Biocontrol of pests of apples and pears in northern and central Europe: 1. Microbial agents and nematodes. Biocontrol. Science and Technology. 1999;9:125–149. [Google Scholar]
- de Waal JY, Malan AP, Levings J, Addison MF. Key elements in the successful control of diapausing codling moth, Cydia pomonella (Lepidoptera: Tortricidae) in wooden fruit bins with a South African isolate of Heterorhabditis zealandica (Rhabditida: Heterorhabditidae). Biocontrol. Science and Technology. 2010;20:489–502. [Google Scholar]
- de Waal JY, Malan AP, Addison MF. Evaluating mulches together with Heterorhabditis zealandica (Rhabditida: Heterorhabditidae) for the control of diapausing codling moth larvae, Cydia pomonella (L.) (Lepidoptera: Tortricidae). Biocontrol. Science and Technology. 2011;21:255–270. [Google Scholar]
- Dolinski C, Lacey LA. Microbial control of arthropod pests of tropical tree fruit. Neotropical Entomology. 2007;36:161–179. doi: 10.1590/s1519-566x2007000200001. [DOI] [PubMed] [Google Scholar]
- Ebssa L, Koppenhöfer AM. Efficacy and persistence of entomopathogenic nematodes for black cutworm control in turfgrass. Biocontrol. Science and Technology. 2011;21:779–796. [Google Scholar]
- Gaugler R, Kaya HK. 1990 Entomopathogenic nematodes in biological control. Boca Raton: CRC Press. [Google Scholar]
- Gaugler R, Campbell JF, Selvan S, Lewis EE. Large-scale inoculative releases of the entomopathogenic nematode Steinernema glaseri: Assessment 50 years later. Biological Control. 1992;2:181–187. [Google Scholar]
- Georgis R. Current and prospective markets for entomopathogenic nematodes. International Journal of Nematology. 2004;14:1–8. [Google Scholar]
- Georgis R, Koppenhöfer AM, Lacey LA, Bélair G, Duncan LW, Grewal PS, Samish M, Tan L, Torr P, van Tol RWHM. Successes and failures in the use of parasitic nematodes for pest control. Biological Control. 2006;38:103–123. [Google Scholar]
- Grewal PS. 2007 Mushroom pests. pp. 457–461 in L. A. Lacey and H. K. Kaya, eds. Field manual of techniques in invertebrate pathology: Application and evaluation of pathogens for control of insects and other invertebrate pests, second ed. Dordrecht: Springer. [Google Scholar]
- Grewal PS, Georgis R. 1998 Entomopathogenic nematodes, pp. 271–297 in F. R. Hall and J. J. Menn, eds, Methods in biotechnology, biopesticides: Use and delivery. Totowa: Humana Press. [Google Scholar]
- Grewal PS, Richardson PN. Effects of application rates of Steinernema feltiae (Nematoda: Steinernematidae) on control of the mushroom sciarid fly Lycoriella auripilla. Biocontrol. Science and Technology. 1993;3:29–40. [Google Scholar]
- Grewal PS, Power KT, Grewal SK, Suggars A, Haupricht S. Enhanced consistency in biological control of white grubs (Coleoptera: Scarabaeidae) with new strains of entomopathogenic nematodes. Biological Control. 2004;30:73–82. [Google Scholar]
- Grewal PS, Ehlers R-U, Shapiro-Ilan DI. 2005a Nematodes as biological control agents. Wallingford: CABI Publishing. [Google Scholar]
- Grewal PS, Koppenhöfer AM, Choo HY. 2005b Lawn, turfgrass, and pasture applications. Pp. 115–146 in P. S. Grewal, R.-U. Ehlers, and D. I. Shapiro-Ilan, eds., Nematodes as biocontrol agents. Wallngford:CABI Publishing. [Google Scholar]
- Hara AH, Kaya HK, Gaugler R, LeBeck LM, Mello CL. Entomopathogenic nematodes for biological control of the leafminer, Liriomyza trifolii (Dipt., Agromyzidae) (Rhabditida: Steinernematidae) Entomaphaga. 1993;38:359–369. [Google Scholar]
- Haukeland S, Lola-Luz T. Efficacy of the entomopathogenic nematodes Steinernema kraussei and Heterorhabditis megidis against the black vine weevil Otiorhynchus sulcatus in open field-grown strawberry plants. Agricultural and Forest Entomology. 2010;12:363–369. [Google Scholar]
- Hudson WG, Frank JH, Castner JL. Biological control of Scapteriscus spp. mole crickets (Orthoptera: Gryllotapidae) in Florida. Bulletin of the Entomological Society of America. 1988;34:192–198. [Google Scholar]
- Jagdale GB, Casey ML, Grewal PS, Lindquist RK. Application rate and timing, potting medium, and host plant effects on the efficacy of Steinernema feltiae against the fungus gnat, Bradysia coprophila, in floriculture. Biological Control. 2004;29:296–305. [Google Scholar]
- Jagdale GB, Casey ML, Cañas L, Grewal PS. Effect of entomopathogenic nematode species, split application and potting medium on the control of the fungus gnat, Bradysia difformis (Diptera: Sciaridae), in the greenhouse at alternating cold and warm temperatures. Biological Control. 2007;43:23–30. [Google Scholar]
- Jess S, Schweizer H, Kilpatrick M. 2005 Mushroom applications. Pp. 191–213 in P. S. Grewal, R.-U. Ehlers, and D. I. Shapiro-Ilan, eds., Nematodes as biocontrol agents. Wallingford:CABI Publishing. [Google Scholar]
- Kaya HK. 1990 Soil ecology. Pp. 93–115 in R. Gaugler and H. K. Kaya, eds. Entomopathogenic nematodes in biological control. Boca Raton: CRC Press. [Google Scholar]
- Kaya HK, Gaugler R. Entomopathogenic nematodes. Annual Review of Entomology. 1993;38:181–206. [Google Scholar]
- Kaya HK, Joos JL, Falcon LA, Berlowitz A. Suppression of the codling moth (Lepidoptera: Olethreutidae) with the entomogenous nematode, Steinernema feltiae (Rhabditida: Steinernematidae) Journal of Economic Entomology. 1984;77:1240–1244. [Google Scholar]
- Kakouli-Duarte T, Labuschagne L, Hague NGM. Biological control of the black vine weevil, Otiorhynchus sulcatus (Coleoptera: Curculionidae) with entomopathogenic nematodes (Nematoda: Rhabditida) Annals of Applied Biology. 1997;131:11–27. [Google Scholar]
- Kim HG, Alston DG. Potential of two entomopathogenic nematodes for suppression of plum curculio (Conotrachelus nenuphar, Coleoptera Curculionidae) life stages in Northern Climates. Environmental Entomology. 2008;37:1272–1279. doi: 10.1603/0046-225x(2008)37[1272:potenf]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Klein MG. 1990 Efficacy against soil-inhabiting insect pests. Pp 195–214 in R. Gaugler and H. K. Kaya, eds. Entomopathogenic nematodes in biological control. Boca Raton: CRC Press. [Google Scholar]
- Klein MG, Grewal PS, Jackson TA, Koppenhöfer AM. 2007 Lawn, turf and grassland pests. Pp. 655–675 in L. A. Lacey and H. K. Kaya, eds. Field manual of techniques in invertebrate pathology: Application and evaluation of pathogens for control of insects and other invertebrate pests, second ed. Dordrecht: Springer. [Google Scholar]
- Koppenhöfer AM. 2007 Nematodes. Pp. 249–264 in L. A. Lacey and H. K. Kaya, eds. Field manual of techniques in invertebrate pathology: Application and evaluation of pathogens for control of insects and other invertebrate pests, second ed. Dordrecht: Springer. [Google Scholar]
- Koppenhöfer AM, Fuzy EM. Steinernema scarabaei for the control of white grubs. Biological Control. 2003;28:47–59. [Google Scholar]
- Koppenhöfer AM, Fuzy EM. Effect of soil type on infectivity and persistence of the entomopathogenic nematodes Steinernema scarabaei, Steinernema glaseri, Heterorhabditis zealandica, and Heterorhabditis bacteriophora. Journal of Invertebrate Pathology. 2006;92:11–22. doi: 10.1016/j.jip.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Koppenhöfer AM, Fuzy EM. Early timing and new combinations to increase the efficacy of neonicotinoid-entomopathogenic nematode (Rhabditida: Heterorhabditidae) combinations against white grubs (Coleoptera: Scarabaeidae) Pest Management Science. 2008;64:725–735. doi: 10.1002/ps.1550. [DOI] [PubMed] [Google Scholar]
- Koppenhöfer AM, Fuzy EM. Long-term effects and persistence of Steinernema scarabaei applied for suppression of Anomala orientalis (Coleoptera: Scarabaeidae) Biological Control. 2009;48:63–72. [Google Scholar]
- Koppenhöfer AM, Kaya HK. Additive and synergistic interaction between entomopathogenic nematodes and Bacillus thuringiensis for scarab grub control. Biological Control. 1997;8:131–137. [Google Scholar]
- Koppenhöfer AM, Kaya HK. Synergism of imidacloprid and an entomopathogenic nematode: A novel approach to white grub (Coleoptera: Scarabaeidae) control in turfgrass. Journal of Economic Entomology. 1998;91:618–623. [Google Scholar]
- Koppenhöfer AM, Brown IM, Gaugler R, Grewal PS, Kaya HK, Klein MG. Synergism of entomopathogenic nematodes and imidacloprid against white grubs: Greenhouse and field evaluation. Biological Control. 2000;19:245–251. [Google Scholar]
- Koppenhöfer AM, Grewal PS, Fuzy EM. Virulence of the entomopathogenic nematodes Heterorhabditis bacteriophora, Heterorhabditis zealandica, and Steinernema scarabaei against five white grub species (Coleoptera: Scarabaeidae) of economic importance in turfgrass in North America. Biological Control. 2006;38:397–404. [Google Scholar]
- Kung SP, Gaugler R, Kaya HK. Effects of soil temperature, moisture and relative humidity on entomopathogenic nematode persistence. Journal of Invertebrate Pathology. 1991;57:242–249. [Google Scholar]
- Lacey LA, Chauvin RL. Entomopathogenic nematodes for control of codling moth in fruit bins. Journal of Economic Entomology. 1999;92:104–109. doi: 10.1093/jee/92.1.104. [DOI] [PubMed] [Google Scholar]
- Lacey LA, Shapiro-Ilan DI. Microbial control of insect pests in temperate orchard systems: Potential for incorporation into IPM. Annual Review of Entomology. 2008;53:121–144. doi: 10.1146/annurev.ento.53.103106.093419. [DOI] [PubMed] [Google Scholar]
- Lacey LA, Unruh TR. Entomopathogenic nematodes for control of codling moth, Cydia pomonella (Lepidoptera: Tortricidae): Effect of nematode species, concentration, temperature, and humidity. Biological Control. 1998;13:190–197. [Google Scholar]
- Lacey LA, Neven LG, Headrick HL, Fritts R., Jr Factors affecting entomopathogenic nematodes (Steinernematidae) for the control of overwintering codling moth (Lepidoptera: Tortricidae) in fruit bins. Journal of Economic Entomology. 2005;98:1863–1869. doi: 10.1603/0022-0493-98.6.1863. [DOI] [PubMed] [Google Scholar]
- Lacey LA, Arthurs SP, Unruh TR, Headrick H, Fritts R., Jr Entomopathogenic nematodes for control of codling moth (Lepidoptera: Tortricidae) in apple and pear orchards: effect of nematode species and seasonal temperatures, adjuvants, application equipment and post-application irrigation. Biological Control. 2006a;37:214–223. [Google Scholar]
- Lacey LA, Granatstein D, Arthurs SP, Headrick H, Fritts R., Jr Use of entomopathogenic nematodes (Steinernematidae) in conjunction with mulches for control of overwintering codling moth (Lepidoptera: Tortricidae) Journal of Entomological Science. 2006b;41:107–119. [Google Scholar]
- Lacey LA, Arthurs SP, Knight A, Huber J. 2007 Pp. 527–546 in L. A. Lacey and H. K. Kaya, eds. Field manual of techniques in invertebrate pathology: Application and evaluation of pathogens for control of insects and other invertebrate pests, second ed. Dordrecht: Springer. [Google Scholar]
- Lacey LA, Shapiro-Ilan DI, Glenn GM. The effect of post-application anti-desiccant agents and formulated host-cadavers on entomopathogenic nematode efficacy for control of diapausing codling moth larvae (Lepidoptera: Tortricidae). Biocontrol. Science and Technology. 2010;20:909–921. [Google Scholar]
- LeBeck LM, Gaugler R, Kaya HK, Hara AH, Johnson MW. Host stage suitability of the leafminer Liriomyza trifolii (Diptera: Agromyzidae) to the entomopathogenic nematode Steinernema carpocapsae (Rhabditida: Steinernematidae) Journal of Invertebrate Pathology. 1993;62:58–63. [Google Scholar]
- Lello ER, Patel MN, Matthews GA, Wright DJ. Application technology for entomopathogenic nematodes against foliar pests. Crop Protection. 1996;15:567–574. [Google Scholar]
- Lewis EE, Clarke DJ. 2012 Nematode parasites and entomopathogens. Pp. 395–443 in F. E. Vega and H. K. Kaya, eds. Insect pathology, second ed. San Diego: Academic Press. [Google Scholar]
- McCoy CW, Stuart RJ, Duncan LW, Nguyen K. Field efficacy of two commercial preparations of entomopathogenic nematodes against larvae of Diaprepes abbreviatus (Coleoptera: Curculionidae) in alfisol type soil. Florida Entomologist. 2002;85:537–544. [Google Scholar]
- McCoy CW, Stuart RJ, Duncan LW, Shapiro-Ilan DI. 2007 Application and evaluation of entomopathogens for citrus pest control. Pp. 567–581 in L.A. Lacey and H. K. Kaya, eds. Field manual of techniques in invertebrate pathology: Application and evaluation of pathogens for control of insects and other invertebrate pests, 2nd ed. Dordrecht:Springer. [Google Scholar]
- McGraw BA, Koppenhöfer AM. Evaluation of two endemic and five commercial entomopathogenic nematode species (Rhabditida: Heterorhabditidae and Steinernematidae) against annual bluegrass weevil (Coleoptera: Curculionidae) larvae and adults. Biological Control. 2008;46:467–475. [Google Scholar]
- Navaneethan T, Strauch O, Besse S, Bonhomme A, Ehlers R. Influence of humidity and a surfactant-polymer-formulation on the control potential of the entomopathogenic nematode Steinernema feltiae against diapausing codling moth larvae (Cydia pomonella L.) (Lepidoptera: Tortricidae) BioControl. 2010;55:777–788. [PubMed] [Google Scholar]
- Nyasani J O, Kimenju JW, Olubayo FM, Wilson MJ. Laboratory and field investigations using indigenous entomopathogenic nematodes for biological control of Plutella xylostella in Kenya. International Journal of Pest Management. 2008;54:355–361. [Google Scholar]
- Parkman JP, Frank JH. Infection of sound-trapped mole crickets, Scapteriscus spp., by Steinernema scapterisci. Florida Entomologist. 1992;75:163–165. [Google Scholar]
- Parkman JP, Smart GC. Entomopathogenic nematodes, a case study: introduction of Steinernema scapterisci in Florida, USA. Biocontrol. Science and Technology. 1996;6:413–419. [Google Scholar]
- Parkman JP, Frank JH, Nguyen KVB, Smart GC., Jr Dispersal of Steinernema scapterisci (Rhabditida: Steinernematidae) after inoculative applications for mole cricket (Orthoptera: Gryllotalpidae) control in pastures. Biological Control. 1993;3:226–232. [Google Scholar]
- Parkman JP, Frank JH, Walker TJ, Schuster DJ. Classical biological of Scapteriscus spp. (Orthoptera: Gryllotalpidae) in Florida. Biological Control. 1996;25:1415–1420. [Google Scholar]
- Pereault RJ, Whalon ME, Alston DG. Field efficacy of entomopathogenic fungi and nematodes targeting caged last-instar plum curculio (Coleoptera: Curculionidae) in Michigan cherry and apple orchards. Environmental Entomology. 2009;38:1126–1134. doi: 10.1603/022.038.0420. [DOI] [PubMed] [Google Scholar]
- Polavarapu S, Koppenhöfer AM, Barry JD, Holdcraft RJ, Fuzy EM. Entomopathogenic nematodes and neonicotinoids for remedial control of oriental beetle, Anomala orientalis (Coleoptera: Scarabaeidae), in highbush blueberry. Crop Protection. 2007;26:1266–1271. [Google Scholar]
- Riga K, Lacey LA, Guerra N, Headrick HL. Control of the oriental fruit moth, Grapholita molesta, using entomopathogenic nematodes in laboratory and bin assays. Journal of Nematology. 2006;38:168–171. [PMC free article] [PubMed] [Google Scholar]
- Scheepmaker JWA, Geels FP, Rutjens AJ, Smits PH, Van Griensven LJLD. Comparison of the efficacy of entomopathogenic nematodes for the biological control of the mushroom pests Lycoriella auripila (Sciaridae) and Megaselia halterata (Phoridae). Biocontrol. Science and Technology. 1998a;8:277–287. [Google Scholar]
- Scheepmaker JWA, Geels FP, Smits PH, van Griensven LJLD. Influence of Steinernema feltiae and diflubenzuron on yield and economics of the cultivated mushroom Agaricus bisporus in Dutch mushroom culture. Biocontrol. Science and Technology. 1998b;8:269–275. [Google Scholar]
- Schroer S, Ehlers R-U. Foliar application of the entomopathogenic nematode Steinernema carpocapsae for biological control of diamondback moth larvae (Plutella xylostella) Biological Control. 2005;33:81–86. [Google Scholar]
- Schroer S, Sulistydanto D, Ehlers R-U. Control of Plutella xylostella using polymer-formulated Steinernema carpocapsae and Bacillus thuringiensis in cabbage fields. Journal of Applied Entomology. 2005;129:128–204. [Google Scholar]
- Shapiro-Ilan DI, Mizell RF, Cottrell TE, Horton DL. Measuring field efficacy of Steinernema feltiae and Steinernema riobrave for suppression of plum curculio, Conotrachelus nenuphar, larvae. Biological Control. 2004;30:496–503. [Google Scholar]
- Shapiro-Ilan DI, Duncan LW, Lacey LA, Han R. 2005 Orchard crops. pp. 215–229 in P. S. Grewal, Ehlers, R.-U., and Shapiro-Ilan, D. I., eds. Nematodes as biological control agents. Wallingford: CABI Publishing. [Google Scholar]
- Shapiro-Ilan DI, Gouge DH, Koppenhöfer AM. 2002 Factors affecting commercial success: case studies in cotton, turf and citrus. pp. 333–355 in Gaugler, R., ed., Entomopathogenic nematology. Wallingford:CABI Publishing. [Google Scholar]
- Shapiro-Ilan DI, Mizell RF, Cottrell TE, Horton DH. Control of plum curculio, Conotrachelus nenuphar, with entomopathogenic nematodes: Effects of application timing, alternate host plant, and nematode strain. Biological Control. 2008;44:207–215. [Google Scholar]
- Shapiro-Ilan DI, Cottrell TE, Mizell RF, III, Horton DL, Behleand RW, Dunlap CA. Efficacy of Steinernema carpocapsae for control of the lesser peachtree borer, Synanthedon pictipes: Improved aboveground suppression with a novel gel application. Biological Control. 2010;54:23–28. [Google Scholar]
- Shapiro-Ilan DI, Bruck DJ, Lacey LA. 2012 Principles of epizootiology and microbial control. Pp. 29–72 in F. E. Vega and H. K. Kaya, eds. Insect pathology, second ed. San Diego: Academic Press. [Google Scholar]
- Sher RB, Parrella MP, Kaya HK. Biological Control of the Leafminer Liriomyza trifolii (Burgess): Implications for intraguild predation between Diglyphus begini Ashmead and Steinernema carpocapsae (Weiser) Biological Control. 2000;17:155–163. [Google Scholar]
- Siegel J, Lacey LA, Fritts R, Jr, Higbee BS, Noble P. Use of steinernematid nematodes for post harvest control of navel orangeworm (Lepidoptera: Pyralidae, Amyelois transitella) in fallen pistachios. Biological Control. 2004;30:410–417. [Google Scholar]
- Siegel J, Lacey LA, Higbee BS, Noble P, Fritts R., Jr Effect of application rates and abiotic factors on Steinernema carpocapsae for control of overwintering navel orangeworm (Lepidoptera: Pyralidae, Amyelois transitella) in fallen pistachios. Biological Control. 2006;36:324–330. [Google Scholar]
- Simser D, Roberts S. Suppression of strawberry root weevil, Otiorhynchus ovatus, in cranberries by entomopathogenic nematodes (Nematoda, Steinernematidae and Heterorhabditidae) Nematologica. 1994;40:456–462. [Google Scholar]
- Somvanshi VS, Ganguly S, Paul AVN. Field efficacy of the entomopathogenic nematode Steinernema thermophilum Ganguly and Singh (Rhabditida: Steinernematidae) against diamondback moth (Plutella xylostella L.) infesting cabbage. Biological Control. 2006;37:9–15. [Google Scholar]
- Stuart RJ, El Borai FE, Duncan LW. From augmentation to conservation of entomopathogenic nematodes: Trophic cascades, habitat manipulation and enhanced biological control of Diaprepes abbreviatus root weevils in Florida citrus groves. Journal of Nematology. 2008;40:73–84. [PMC free article] [PubMed] [Google Scholar]
- Toledo J, Ibarra JE, Liedo P, Gomez A, Rasgado MA, Williams T. Infection of Anastrepha ludens (Diptera: Tephritidae) larvae by Heterorhabditis bacteriophora conditions. Biocontrol. Science and Technology. 2005;15:627–634. [Google Scholar]
- Tomalak M, Piggott S, Jagdale GB. 2005 Glasshouse applications. Pp. 147–166 in P. S. Grewal, Ehlers, R.-U., and Shapiro-Ilan, D. I., eds. Nematodes as biological control agents. Wallingford: CABI Publishing. [Google Scholar]
- Unruh TR, Lacey LA. Control of codling moth, Cydia pomonella (Lepidoptera: Tortricidae) with Steinernema carpocapsae: effects of supplemental wetting and pupation site on infection rate. Biological Control. 2001;20:48–56. [Google Scholar]
- van Tol RWHM, Raupp MJ. 2005 Nursery and tree application. Pp. 167–190 in P. S. Grewal, Ehlers, R.-U., and Shapiro-Ilan, D. I., eds. Nematodes as biological control agents. Wallingford: CABI Publishing. [Google Scholar]
- West RJ, Vrain TC. Nematode control of black army cutworm (Lepidoptera: Noctuidae) under laboratory and field conditions. Canadian Entomologist. 1997;129:229–239. [Google Scholar]
- Williams EC, Walters KFA. Foliar application of the entomopathogenic nematode Steinernema feltiae against leafminers on vegetables. Biocontrol. Science and Technology. 2000;10:61–70. [Google Scholar]
- Yee WL, Lacey LA. Stage-specific mortality of Rhagoletis indifferens (Diptera: Tephritidae) exposed to three species of Steinernema Nematodes. Biological Control. 2003;27:349–356. [Google Scholar]


