Abstract
Experiments were conducted in the greenhouse to assess root galling and egg production of three root-knot nematode species, Meloidogyne arenaria, M. incognita, and M. javanica, on several weeds common to Florida agricultural land. Weeds evaluated were Amaranthus retroflexus (redroot pigweed), Cyperus esculentus (yellow nutsedge), Eleusine indica (goosegrass), Portulaca oleracea (common purslane), and Solanum americanum (American black nightshade). Additionally, although it is recommended as a cover crop in southern regions of the U.S., Aeschynomene americana (American jointvetch) was evaluated as a weed following the detection of root galling in a heavy volunteer infestation of an experimental field in southeastern Florida. Weeds were propagated from seed and inoculated with 1000 nematode eggs when plants reached the two true-leaf stage. Tomato (Solanum lycopersicum ‘Rutgers’) was included as a positive control. Aeschynomene americana and P. oleracea roots supported the highest number of juveniles (J2) and had the highest number of eggs/g of root for all three species of Meloidogyne tested. However, though P. oleracea supported very high root levels of the three nematode species tested, its fleshy roots did not exhibit severe gall symptoms. Low levels of apparent galling, combined with high egg production, increase the potential for P. oleracea to support populations of these three species of root-knot nematodes to a degree that may not be appropriately recognized. This research quantifies the impact of P. oleracea as a host for M. arenaria, M. incognita, and M. javanica compared to several other important weeds commonly found in Florida agricultural production, and the potential for A. americana to serve as an important weed host of the three species of root-knot nematode tested in southern regions of Florida.
Keywords: Aeschynomene americana, Amaranthus retroflexus, Cyperus esculentus, Eleusine indica, Florida, host status, nematode reproduction, Portulaca oleracea, root-knot nematodes, Solanum americanum
Sandy soils and subtropical environmental conditions in many agricultural production areas of Florida make nematode control extremely challenging. High value Florida vegetable crops susceptible to several Meloidogyne spp. include tomato, pepper, eggplant, and many cucurbits. Production of high-value vegetable crops in Florida occurs during the fall and spring seasons, with two cropping cycles possible for most vegetables in southern regions of the state. Each of these crops remains highly dependent on chemicals for nematode and weed control, and each crop presents challenges with regard to production system, region, and nematode and weed species combinations. Chemicals currently available for combined nematode and weed control in vegetable crops in Florida include methyl isothiocynate-generators, such as metam sodium (Vapam®) or metam potassium (K-Pam®) and Paladin™ + chloropicrin (dimethyl disulfide 79:21 DMDS:chloropicrin). Previous research with these products has shown that Paladin can substitute for methyl bromide and provide good control of root-knot nematodes (Kokalis-Burelle et al., 2010). The resurgence of root-knot nematode populations in soil following treatment with fumigants including methyl bromide (Kokalis-Burelle et al., 2010), indicates that sustainable nematode control under Florida conditions may not be achieved with the use of broad-spectrum biocides.
The phase-out of methyl bromide for soil fumigation (Rosskopf et al., 2005; Zasada et al., 2010) and the observed resurgence of soil nematode populations following many chemical soil treatments (Kokalis-Burelle et al., 2010) necessitate a greater understanding of nematode ecology and nematode:weed interactions. There is a high probability that weeds which are uncontrolled by alternative chemical treatments and other strategies will serve as hosts for the three most common species of root-knot nematodes found in Florida, Meloidogyne arenaria, M. incognita, and M. javanica. Also of great concern are several less commonly detected species of Meloidogyne which are emerging as important pathogens (Kaur et al., 2007). Greenhouse trials were conducted to determine the host status of several weeds common to Florida production regions and to assess their susceptibility and ability to enhance reproduction of the three primary species of root-knot nematodes which occur most commonly in those regions, M. arenaria, M. incognita, and M. javanica.
Materials and Methods
Weeds tested: Weeds were selected based on their prevalence, the importance of their control in Florida agricultural production, and availability of viable seed. Although generally considered a cover crop in southern regions of the U.S., Aeschynomene americana (American jointvetch) commonly becomes naturalized in southeastern Florida, and for the purposes of these studies will be considered a weed. Locally, a dense population of A. americana became established after a weed-fallow experimental field was disked and planted to bedded ornamental crops. Other weeds evaluated were Amaranthus retroflexus (redroot pigweed), Cyperus esculentus (yellow nutsedge), Eleusine indica (goosegrass), Portulaca oleracea (common purslane), and Solanum americanum (American black nightshade). Amaranthus retroflexus, E. indica, and P. oleracea seed and C. esculentus tubers were purchased from Azlin Seed Service (Leland, MS). Aeschynomene americana seed were collected from the experimental field population described above and increased in the greenhouse for use in these experiments. Solanum lycopersicum (‘Rutgers’ tomato, Totally Tomatoes, Randolph, WI) was included as a root-knot nematode susceptible control in all experiments, and for comparison of weed susceptibility to a known host of all three nematode species tested.
Plant propagation: Germination and growth rates were predetermined for all weed seed used in this experiment. Seed were planted to simultaneously obtain seedlings of the same growth stage at the time of nematode inoculation. Seed were planted into 128 cell flats containing a mixture of washed builder’s grade sand and steamed Fafard® germination mix (Conrad Fafard Inc., Agawam, MA) at a ratio of 400 dm3 of Fafard mix to 91 dm3 sand. This mix of sand and Fafard germination mix will be referred to as soil, and was used to conduct all experiments. Two 15 cm-dia pots were nested together with Kimwipes (Kimwipes® EX-L, Kimberly-Clark Corp., Roswell, GA) placed between the pots to eliminate loss of the soil during watering. Pots were then filled with approximately 1500 cm3 of the soil mixture. When plants reached the 1-2 true leaf stage, they were transplanted into the 15 cm pots, watered daily, and fertilized once a week with 20-10-20 at 250 ppm nitrogen (Peters Professional 20-10-20 GP, Scotts-Sierra Horticultural Products Co., Marysville, OH). Plant foliage was trimmed weekly to avoid contact between plants. Plants were separated on the greenhouse bench by 6 mm thick polycarbonate dividers with each plant in a grid square measuring 30 cm × 30 cm × 30 cm to avoid cross contamination of nematodes during watering. Plants were sprayed at label rates on an as-needed basis to control powdery mildew (Bayleton®, triadimefon, Bayer CropScience; Cabrio®, pyraclostrobin, BASF Corp., Research Triangle Park, NC), mites (Horticultural oil, Abamectin), aphids (M-Pede, propylene glycol:potassium hydroxide, Dow AgroSciences, Indianapolis, IN), thrips and whitefly (Safari®, Dinotefuran, N-methyl-N’-nitro-N”- [(tetrahydro-3-furanyl)methyl] guanidine, Valent U.S.A. Corp.,;Talstar®, bifenthrin, FMC Corp. Philadelphia, PA).
Nematode inoculation: Nematode inoculum was extracted from pure cultures of M. arenaria, M. incognita, and M. javanica, maintained in the greenhouse on tomato (Solanum lycopersicum, ‘Rutgers’). Root-knot nematode species identifications were confirmed based on their esterase phenotypes (PhastSystem,™ GE Healthcare) before inoculation of experimental plants. Meloidogyne spp. eggs were extracted from tomato roots using a modified NaOCl method (Hussey and Barker, 1973). The final concentration of eggs, collected on a 25 μm-pore sieve, was adjusted to 1000 eggs/ml in order to represent nematode inoculum levels that may be encountered in a field environment. Plants were inoculated with nematode eggs by pipetting 1 ml of egg suspension into a 2 cm deep impression in the soil approximately 1.5-2.0 cm from the plant stem. Inoculation sites were covered by pressing the soil mixture into place, and plants were lightly watered. Experiments were maintained in the greenhouse for 8 weeks, established in early- and mid- June 2010 and evaluated in early- and mid- August 2010, with a two week interval between repeated experiments. Greenhouse temperatures during the experimental period were 28-32° C (day) and 23-27° C (night).
Plant evaluation: At the end of all experiments, total fresh root weight was recorded and roots were evaluated for galling and root condition. Following galling and root condition evaluations, roots were dissected and divided into two equivalent portions, one for extraction of J2 and the second for extraction of eggs. Nematode J2 were extracted from half of the root system, as well as from 100 cm3 of the soil using Baermann funnels. Root and soil samples remained in extraction funnels for approximately 60 hr, after which funnels were drained and nematodes were identified and counted microscopically. Anatomical alterations of the root system were used as a general indicator of root disease and were assessed using a subjective root condition scale of 0 to 5, with 0 representing healthy roots with no apparent signs of disease and 5 representing completely diseased and degraded or dead roots. Root galling was assessed using a root gall index based on a scale of 0 to 10, with zero representing no galls and 10 representing severe (100%) galling (Bridge and Page, 1980). Nematode eggs were extracted from the second half of the root system as described above for the nematode inoculation procedure. Eggs were counted using an inverted microscope. Nematode reproduction was quantified and expressed as the number of eggs extracted per gram of fresh root tissue.
Statistical analysis: All experiments were conducted twice during the summer of 2010 using a completely randomized design. Data from both experiments were subjected to a T test and subsequently combined when no differences were found between tests. Data were analyzed according to standard statistical procedures including SAS general linear model (GLM) and least significant difference (LSD) procedures (SAS 9.2, Cary, NC). Unless otherwise stated, effects and differences were considered significant at p < 0.05.
Results
Experimental results are presented by nematode species with results of both tests for each species combined. Solanum americanum was not available in sufficient quantities for testing with M. javanica, however, results are presented for tests of S. americanum susceptibility to M. arenaria and M. incognita.
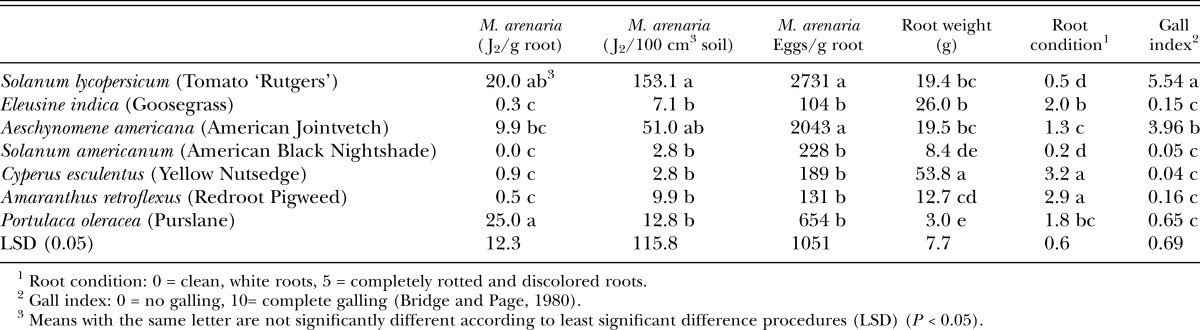
Susceptibility to Meloidogyne arenaria: High numbers of M. arenaria J2 were isolated from both roots and soil of the control, S. lycopersicum, which also had high levels of eggs/g of root tissue and moderate galling (Table 1). Results for A. americana were similar to S. lycopersicum but lower numbers of nematodes were isolated from roots and soil, with less root galling and a lower number of eggs/g root. However, P. oleracea had high numbers of M. arenaria J2 isolated from roots and relatively low numbers found in soil, and very low levels of galling (Table 1). Although the number of eggs/g root was lower than for S. lycopersicum and A. americana, the egg values for P. oleracea were not reflected by corresponding moderate values of root galling index for this weed.
Table 1.
Root-knot nematode juveniles (J2) in roots and soil, eggs per gram of root, plant root weight, plant root condition, and nematode gall index values for both experiments on weed susceptibility to Meloidogyne arenaria.
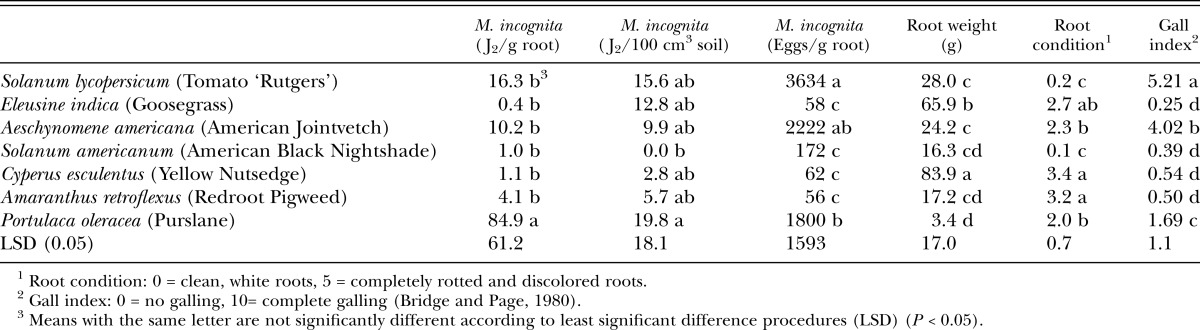
Susceptibility to Meloidogyne incognita: Results of host susceptibility to M. incognita (Table 2) were very similar to those for M. arenaria. Based on the severity of root galling, commonly relied on as an indication of nematode infestation, E. indica, S. americanum, C. esculentus, and A. retroflexus were significantly less susceptible to infestation by M. incognita than the positive control S. lycopersicum, as well as A. americana, and P. oleracea (Table 2). Of the three susceptible plant hosts, P. oleracea produced the lowest level of galling. Again, although P. oleracea had a relatively low level of root galling, the highest number of M. incognita J2 /g fresh root tissue were isolated from P. oleracea roots, and a moderate number of J2 were found in the soil (Table 2). Also, very high numbers of M. incognita eggs were isolated from P. oleracea roots, similar to the number isolated from the more symptomatic roots of A. americana.
Table 2.
Root-knot nematode juveniles (J2) in roots and soil, eggs per gram of root, plant root weight, plant root condition, and nematode gall index values for both experiments on weed susceptibility to Meloidogyne incognita.
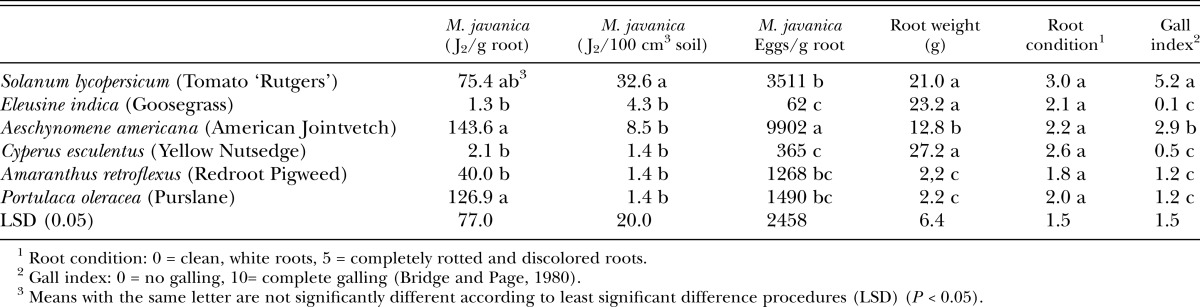
Susceptibility to Meloidogyne javanica: Results for M. javanica were similar to those for M. arenaria and M. incognita but, overall higher numbers of J2 and eggs were isolated from roots for M. javanica (Table 3). As with M. arenaria, A. americana and P. oleracea were highly susceptible to invasion of roots by J2 of M. javanica. As with both M. incognita and M. arenaria, galling on P. oleracea was lower than that seen on both S. lycopersicum and A. americana. Additionally, based on the number of J2 in roots and egg production, A. retroflexus appears to be more highly susceptible to M. javanica when compared with M. incognita and M. arenaria (Table 3).
Table 3.
Root-knot nematode juveniles (J2) in roots and soil, eggs per gram of root, plant root weight, plant root condition, and nematode gall index values for both experiments on weed susceptibility to Meloidogyne javanica.
Discussion
The importance of determining the host status of common and emerging weeds to predominant species of root-knot nematodes cannot be overstated. Weed species must be evaluated, not only for their ability to compete with crops for resources such as water, light, and fertilizer, but also for their ability to harbor pests and pathogens including nematodes. A more thorough understanding of the potential interactions of weeds and nematodes is critical in order to adequately assess the potential impact of weeds left uncontrolled. Work conducted by Schroeder et al. (2004) exemplifies the important implications of the weed:nematode interaction. In studies conducted with root-knot nematodes infecting purple and yellow nutsedge, tuber number and size were increased in plants infected by root-knot nematodes, increasing the potential for crop loss in an unexpected way.
Several excellent survey studies have been published on root-knot nematodes infecting weeds in Florida (Myers et al., 2004; Kaur et al., 2007; Brito et al., 2008; Rich et al., 2008) and elsewhere (Mani and Hinai, 1996; Belair and Benoit, 1996). These studies provide a large data base for weed hosts of many common and emerging Meloidogyne spp. found in Florida and worldwide. Determining the potential of specific weeds to serve as either reservoirs for low levels of nematode survival, or as hosts that greatly enhance nematode reproduction is particularly important. Unfortunately, controlled inoculation studies to assess levels of nematode reproduction independent of visible symptoms are not as common as surveys. Also, when controlled inoculation studies have been undertaken, a wide variety of methods have been used to assess and calculate nematode reproduction and host status.
Although nematode reproductive factor (Rf) is considered a quantitative measure that could be compared across studies, the methods used for calculating Rf are not always consistent. In our study, nematode egg production was assessed using half of the entire root system, with the second half used for assessment of nematode J2 in roots. The final nematode population densities (Pf) were expressed as nematode life stages per gram of root, without calculating the reproductive factor (Rf) (Pf/Pi), which requires the values of the final nematode densities for the entire root system. These Pf values were standardized by a unit of measure (number of nematode eggs/g root) and provide an immediate representation of the population levels that a weed is able to maintain in the field.
The concept of determining the Rf for root-knot nematodes in host range studies is well established. However, the current classification of a good-host or non-host based on Rf criteria is sometimes uninformative. Reproductive factor is defined and the ratio of final nematode population to initial population (Pf/Pi). The term population is left open for interpretation and often refers to the numbers of eggs and/or J2 in soil, roots, or both. Under these criteria, an Rf value of less than 1.0 indicates that a plant is a non-host, as no increase occurred in nematode numbers isolated at the end of the study compared with the number introduced at the beginning of the study. However, this system results in Rf values as high as 610 as not differing from values of 5.0, while an Rf value of 5.0 is significantly different from a an Rf of 0.5 (Kauer et al., 2007). While technically correct, these data, if presented alone as GH (good host, (Rf ≥ 1), PH (poor host, 0.1 < Rf < 1), or NH (non-host, Rf ≤ 0.1), are insufficient to assess the potential of nematodes to reproduce on a host. These data on the reproductive factors should be supplemented by information on the number of eggs detected in the roots of a given tested plant as reported by Kaur et al (2007) who included data on the number of root-knot nematode eggs extracted per gram of root in each of their experiments. The data presented in this way, standardized to a unit of measure (number of nematode eggs/g root) are extremely useful in assessing the ability of a plant to maintain the nematode populations.
Of the weeds tested here, A. retroflexus was the only weed species common to studies by Kaur et al., (2007), and our results are similar to theirs with respect to M. arenaria and M. incognita egg production, which was less for A. retroflexus than for the susceptible host, Rutgers tomato. However, our results for M. javanica differed from those of Kaur et al (2007). Although lower, the numbers of M. javanica eggs isolated from roots of A. retroflexus in our studies were statistically similar to those isolated from Rutgers tomato. These differences may be due to the different isolates of M. javanica used, or differences in sources for A. retroflexus seed.
Aeschynomene americana is recommended as an annual grazing crop or fallow season cover crop in the southeastern U.S (USDA, NRCS, 2006), Numerous field, microplot, and greenhouse studies have been performed in the southeastern U.S. evaluating the host status of A. americana to several Meloidogyne spp. with various results reported (Rhoades, 1980; Taylor et al., 1985; Rhoades and Forbes, 1986; Rodríguez-Kábana et al., 1988a, 1988b, 1989; Kinloch and Dunavin, 1993; McSorley et al., 1994a, 1994b, 1994c; McSorley and Dickson, 1995; McSorley, 1999). In general, A. americana appears to reduce populations of Meloidogyne spp. in soil when planted as a summer cover crop, but when a susceptible host crop is planted following A. americana, soil populations of Meloidogyne spp. rebound, and galling has been reported both on A. americana (Rhoades, 1980; Taylor et al., 1985; McSorley et al., 1994a) and the subsequent susceptible crop (Rhoades and Forbes, 1986; Rodríguez-Kábana et al., 1989; McSorley et al., 1994b). In our greenhouse trials, performed during the summer in southeastern Florida using seed propagated from wild A. americana, we found significant galling and nematode egg production for all three Meloidogyne spp. tested on A. americana roots.
In other studies involving a different species of root knot nematode, Meloidogyne hapla, Belair and Benoit (1996) did not find A. retroflexus or P. oleracea to be important hosts in Quebec. However, Bélair and Benoit (1996) used fresh root weight and number of galls and eggs per entire root system to compare the susceptibility of 32 weed species to M. hapla. In those studies Pf/Pi or Rf was calculated using the total number of J2 and eggs extracted from soil and roots for each pot in the study. This method requires further calculation and knowledge of the volume of pots used for the studies and the weight of the root systems, in order to standardize their results for comparison. Although the method used for calculating Rf was somewhat ambiguous, Bélair and Benoit (1996) do importantly note that Pf/Pi values are highly dependent on the length of time during which an experiment is conducted, and that this additional knowledge is necessary in order to make comparisons of results for Rf among different research trials. In order to avoid misleading data either in favor or against infection levels and host status, experiments should be run for the period required to complete one generation life cycle under the specific environmental conditions of the experiment.
In studies similar to those presented here, Tedford and Fortnum (1988) found both M. arenaria and M. incognita to reproduce to a moderate level (egg mass index of 2.2 for each nematode species on a scale of 0 to 5) on P. oleracea while producing low gall index values of 0.5 and 0.2 respectively on a scale of 0 to 10. In these studies, reproductive ratings (R) were calculated by dividing the average egg-mass index for each weed species by the average egg-mass index for PD 4 tobacco, a known host of both species (Tedford and Fortnum, 1988). Based on those criteria, P. oleracea was determined to be a moderate host for both M. arenaria and M. incognita with reproductive rating values of 0.8 and 0.6 respectively, and root gall index values (on a scale of 0 to 10) of 0.5 and 0.2 respectively. Mani and Hinai (1996) reported P. oleracea to be an important host of M. incognita in several regions in the Sultanate of Oman, while it was not a host of M. javanica. Their results were based on gall index values of 4.0 on a scale of 0 to 5 for M. incognita on P. oleracea. No evaluation of egg production was performed by Mani and Hinai (1996).
Our quantification of M. arenaria M. incognita, and M. javanica J2 in soil and roots, egg production, and galling, are a standardized method of quantitatively assessing root-knot nematode host status. Based on these results, there is high potential for both P. oleracea and A. americana to harbor and increase populations of M. arenaria, M. incognita, and M. javanica where these weeds occur in southern Florida agricultural production regions. These findings are particularly important because P. oleracea would initially appear to be only a mildly susceptible host to all three Meloidogyne spp. tested if only root galling symptoms were used as criteria. Additionally, the use of A. americana as a summer cover crop in some regions may also contribute to population level increase. Root galling on P. oleracea, as in other plants with fleshy roots due to presence of storage cortical or vascular parenchyma, was not obvious and should not be used as an index to express nematode root parasitization. In addition, a greater effort may need to be made to control P. oleracea, as it serves as host to other important crop pathogens (French-Monar et al, 2006). Further research is needed to more clearly identify susceptibility of A. americana accessions to important species of Meloidogyne occurring in subtropical regions.
In this experiment we chose to use the number of eggs extracted per gram of root tissue to determine the impact of the weed species on the three root-knot nematode species tested. This enabled an assessment of the impact of weeds on nematode populations and reproduction, and allowed the determination that some weeds which produce small root systems and relatively inconspicuous galling, such as P. oleracea, may have a disproportionate impact on root-knot nematode populations by harboring large numbers of nematodes and nematode eggs in relatively small root systems. Our studies are limited with respect to the number of weeds and nematode species assessed due to the labor intensive nature of the assessments. However, the studies presented here provide important data on the ability of these three important species of Meloidogyne to reproduce on several prevalent weed hosts, and provides new information on A. americana as a host for the three species of Meloidogyne tested, and on P. oleracea as a weed species capable of supporting high levels of nematode reproduction for all three Meloidogyne species, while exhibiting only mild to moderate symptoms of nematode infestation.
Acknowledgments
Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the United States Department of Agriculture. The authors would like to acknowledge Jackie Markle for her technical assistance.
Literature Cited
- Belair G, Benoit DL. Host susceptibility of 32 common weeds to Meloidogyne hapla in organic soils of southwestern Quebec. Journal of Nematology. 1996;28(4S):643–647. [PMC free article] [PubMed] [Google Scholar]
- Bridge J, Page SLJ. Estimation of root-knot infestation levels in roots using a rating chart. Tropical Pest Management. 1980;26:296–298. [Google Scholar]
- Brito JA, Kaur R, Cetintas R, Stanley JD, Mendes ML, McAvoy EJ, Powers TO, Dickson DW. Identification and characterization of Meloidogyne spp. infecting horticultural and agronomic crops, and weeds in Florida. Nematology. 2008;10:757–766. [Google Scholar]
- French-Monar RD, Jones JB, Roberts PD. Characterization of Phytophthora capsici associated with roots of weeds on Florida vegetable farms. Plant Disease. 2006;90:345–350. doi: 10.1094/PD-90-0345. [DOI] [PubMed] [Google Scholar]
- Hussey RS, Barker KR. A comparison of methods of collecting inocula for Meloidogyne spp., including a new technique. Plant Disease Reporter. 1973;57:1025–1028. [Google Scholar]
- Kaur RJ, Brito JA, Rich JR. Host suitability of selected weed species to five Meloidogyne species. Nematropica. 2007;37:107–120. [Google Scholar]
- Kinloch RA, Dunavin LS. Summer cropping effects on the abundance of Meloidogyne arenaria race 2 and subsequent soybean yield. Supplement to the Journal of Nematology. 1993;25(4S):806–808. [PMC free article] [PubMed] [Google Scholar]
- Kokalis-Burelle N, Rosskopf EN, Albano JP, Holzinger J. Effects of Midas® on Nematodes in Commercial Floriculture Production in Florida. Journal of Nematology. 2010;42:17–21. [PMC free article] [PubMed] [Google Scholar]
- Mani A, Hinai MA. Host range and distribution of Meloidogyne incognita and M. javanica in the Sultanate of Oman. Nematropica. 1996;26:73–79. [Google Scholar]
- McSorley R. Host suitability of potential cover crops for root-knot nematodes. Supplement to the Journal of Nematology. 1999;31(4S):619–623. [PMC free article] [PubMed] [Google Scholar]
- McSorley R, Dickson DW. Effects of tropical rotation crops on Meloidogyne incognita and other plant-parasitic nematodes. Supplement to Journal of Nematology. 1995;27(4S):535–544. [PMC free article] [PubMed] [Google Scholar]
- McSorley R, Dickson DW, de Brito JA. Host status of selected tropical rotation crops to four populations of root-knot nematodes. Nematropica. 1994a;24:45–53. [Google Scholar]
- McSorley R, Dickson DW, de Brito JA, Hewlett TE, Frederick JJ. Effects of tropical rotation crops on Meloidogyne arenaria population densities and vegetable yields in microplots. Journal of Nematology. 1994b;26(2):175–181. [PMC free article] [PubMed] [Google Scholar]
- McSorley R, Dickson DW, de Brito JA, Hochmuth RC. Tropical rotation crops influence nematode densities and vegetable yields. Journal of Nematology. 1994c;26(3):308–314. [PMC free article] [PubMed] [Google Scholar]
- Myers L, Wang KH, McSorley R, Chase C. Investigations of weeds as reservoirs of plant-parasitic nematodes in agricultural systems in Northern Florida. Proceedings of 26th Annual Souther Conservation Tillage Conference for Sustainable Agriculture. North Carolina Agricultural Research Service Technical Bulletin TB-321. 2004:258–267. [Google Scholar]
- Rich JR, Brito JA, Kaur R, Ferrell JA. Weed species as hosts of Meloidogyne: A review. Nematropica. 2008;39:157–185. [Google Scholar]
- Rhoades HL. Relative susceptibility of Tagetes patula and Aeschynomene americana to plant nematodes in Florida. Nematropica. 1980;10:116–120. [Google Scholar]
- Rhoades HL, Forbes RB. Effects of fallow, cover crops, organic mulches, and fenamiphos on nematode populations, soil nutrients, and subsequent crop growth. Nematropica. 1986;16:141–151. [Google Scholar]
- Rodríguez-Kábana R, King PS, Robertson DG, Weaver CF. Potential of crops uncommon to Alabama for management of root-knot and soybean cyst nematodes. Annals of Applied Nematology. 1988a;2:116–120. [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Kábana R, King PS, Robertson DG, Weaver CF, Carden EL. New crops with potential for management of soybean nematodes. Nematropica. 1988b;18:45–52. [Google Scholar]
- Rodríguez-Kábana R, Robertson DG, Wells L, King PS, Weaver CF. Crops uncommon to Alabama for the management of Meloidogyne arenaria in peanut. Supplement to Journal of Nematology. 1989;21(4S):712–716. [PMC free article] [PubMed] [Google Scholar]
- Rosskopf EN, Chellemi DO, Kokalis-Burelle N, Church GT. Alternatives to methyl bromide: A Florida perspective. Online publication. 2005 doi:10.1094/PHP-2005-1027-01-RV. [Google Scholar]
- Schroeder J, Thomas SH, Murray LW. Root-knot nematodes affect annual and perennial weed interactions with chile pepper. Weed Science. 2004;52:28–46. [Google Scholar]
- Taylor SG, Baltensperger TD, Dunn RA. Interactions between six warm-season legumes and three species of root-knot nematodes. Journal of Nematology. 1985;17:367–370. [PMC free article] [PubMed] [Google Scholar]
- Tedford EC, Fortnum BA. Weed hosts of Meloidogyne arenaria and M. incognita common in tobacco fields in South Carolina. Journal of Nematology. 1988;20(2S):102–105. [PMC free article] [PubMed] [Google Scholar]
- USDA, NRCS. 2006 Plant Fact Sheet: Jointvetch, Aeschynomene americana L. USDA Florida Plant Materials Center, Brooksville, FL. [Google Scholar]
- Zasada IA, Halbrendt JM, Kokalis-Burelle N, LaMondia J, McKenry MV, Noling JW. Managing Nematodes Without Methyl Bromide. Annual Review of Phytopathology. 2010;48:15.1–15.18. doi: 10.1146/annurev-phyto-073009-114425. [DOI] [PubMed] [Google Scholar]


