Abstract
The history of entomopathogenic nematology is briefly reviewed. Topic selections include early descriptions of members of Steinernema and Heterorhabditis, how only morphology was originally used to distinguish between the species; descriptions of the symbiotic bacteria and elucidating their role in the nematode- insect complex, including antibiotic properties, phase variants, and impeding host defense responses. Other topics include early solutions regarding production, storage, field applications and the first commercial sales of entomopathogenic nematodes in North America. Later studies centered on how the nematodes locate insect hosts, their effects on non-target organisms and susceptibility of the infective juveniles to soil microbes. While the goals of early workers was to increase the efficacy of entomopathogenic nematodes for pest control, the increasing use of Heterorhabditis and Photorhabdus as genetic models in molecular biology is noted.
Keywords: History, entomopathogenic nematodes, Heterorhabditis spp., Steinernema spp
Entomopathogenic nematodes of the families Steinernematidae and Heterorhabditidae are symbiotically associated with bacteria in the genera Xenorhabdus and Photorhabdus, respectively. When an infective juvenile enters the body cavity of a susceptible host, the bacteria are released, multiply and host death occurs within two days, hence the term, entomopathogenic. The nematodes develop and reproduce within the insect cadaver, feeding on the symbiotic bacteria and degraded host tissues.
The first entomopathogenic nematode was described by Steiner as Aplectana kraussei (now Steinernema kraussei) in 1923 and at that time was considered no more than a curiosity whose systematic position was problematic. The systematic position of the second entomopathogenic nematode, Neoaplectana glaseri Steiner (1929) from material isolated by Glaser and Fox (1930), was still not certain and Steiner placed it in the family Oxyuridae. The early history of this nematode will be discussed later but after its use against the Japanese beetle, it ceased to be of interest and all cultures were lost.
It was not until Jaroslav Weiser (1955) (Fig. 1) described a European population of Neoaplectana carpocapsae from codling moth larvae and Dutky and Hough (1955) isolated the DD-136 strain of an undescribed steinernematid from codling moth larvae in Eastern North America that serious studies on the pathogenicity and life history of entomopathogenic nematodes began. In 1965, cultures of S. carpocapsae were obtained from Weiser and using morphology and hybridization studies, it was shown that the Czechoslovakian strain of S. carpocapsae and the North American DD-136 nematode were conspecific (Poinar, 1967).
Fig. 1.
Jaroslav Weiser described Steinerma carpocapsae in 1955. At the International Colloquium of Insect Pathology and Microbial Control in Wageningen, the Netherlands in 1966. Photo by G. Poinar.
The symbiotic bacterium (under the name, Achromobacter nematophilus) associated with S. carpocapsae was described by Poinar and Thomas (1965). The location of the bacteria in the infective stage juveniles using light microscopy and later electron microscopy was then demonstrated (Poinar,1966; Poinar and Leutenegger,1968). The role of the bacterium in the development of the nematode and death of the host was elucidated (Poinar and Thomas, 1966, 1967) and the bacterium was later transferred to a new genus, Xenorhabdus (Thomas and Poinar, 1979) (Fig. 2).
Fig. 2.
Gerard Thomas (left) and George Poinar at UC Berkeley described the symbiotic bacterium associated with Steinernema carpocapsae in 1965 and Heterorhabditis bacteriophora in 1979. They also revealed the significance of the bacteria in the development of the nematodes. Photo by Roberta Poinar.
At first, morphology of the male tail or features of the infective stage juveniles alone could be used to differentiate between the various species of Steinernema (Poinar, 1986; Wright, 1990). Also, using the biological species concept, which is well suited for Steinernema, cross-breeding could be performed to determine specific or intra-specific status of the numerous geographical strains that were discovered (Poinar and Veremtschuk, 1970; Poinar, 1986, 1990). As more isolates were unearthed, (there are now some 36 species of Steinernema (Stock and Hunt, 2005), measurements between them overlapped and genomic analysis also was used to determine their specific status (Liu et al., 2000; Ciche, 2007).
The genus Heterorhabditis was described in 1976 and the symbiotic bacterium of H. bacteriophora was characterized as Xenorhabditis luminescence in 1979 (Poinar, 1976; Thomas and Poinar, 1979). The fascinating character of the symbiotic bacteria of Heterorhabditis spp. was its ability to fluoresce, so much so that the entire infected insect cadaver glowed in the dark (Fig.3) and light even could be detected in a single infective stage (Poinar et al., 1980a). This bacterial species was later transferred to the genus Photorhabdus (Boemare et al., 1993). The location of the bacterial cells in the infective stage was shown with electron microscopy (Poinar et al., 1977) (Fig. 4) and aspects of its behavior were elucidated by Milstead (1977). As with Steinernema, there are many geographic species and strains of Heterorhabditis (Poinar, 1990; Stock and Hunt, 2005) and the global distribution of both Heterorhabditis and Steinernema indicates that their lineages were present when all land masses were combined as the Pangaea supercontinent. It is interesting to note that a genetic analysis showed that Heterorhabditis is a sister group to the vertebrate-parasitic strongylids and that both groups arose independently from the free-living Rhabditis group (Kiontke et al., 2007). The obligate association of Heterorhabditis with a unique genus of luminescent symbiotic bacteria, its ability to enter the body of healthy insects, the alternating of sexual and hermaphroditic generations and the unique morphology of the parasitic adults explains why this genus was assigned family status. One unique feature of the infective juveniles of Heterorhabditis that is lacking in Steinernema and other rhabditids is the presence of a dorsal “hook” on the tip of the head. This structure allows the infectives to enter the body cavity through the outer tegument of potential hosts, as well as through the trachea and gut wall (Bedding and Molyneux, 1982; Poinar and Georgis, 1990). The ancient age of the Heterorhabditis clade was recently shown when a 100 million year old fossil (Proheterorhabditis burmanicus) was discovered in Early Cretaceous Burmese amber (Poinar, 2011).
Fig. 3.

One character that distinguished Heterorhabditis from other rhabditids was its ability to transmit a luminescent bacterium. Here are cadavers of the wax moth glowing in the dark after being infected with Heterorhabditis bacteriophora 48 hours earlier. Photo by G. Thomas.
Fig. 4.
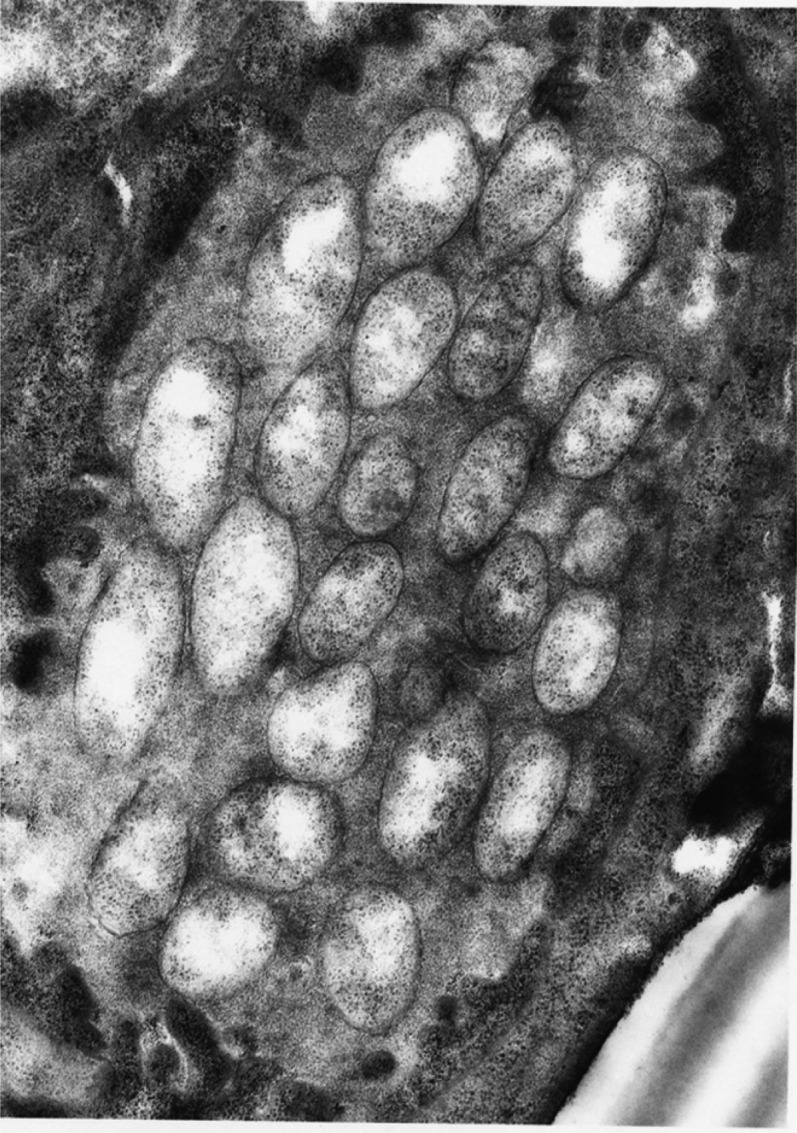
Electron micrograph of Photorhabdus luminescens in the anterior portion of the intestine of the infective stage of Heterorhabditis bacteriophora. Photo by Roberta Poinar.
It is now evident that all Steinernema species have mutualistic associations with species of Xenorhabdus while all Heterorhabditis species have symbiotic associations with Photorhabdus species (Akhurst and Boemare, 1990; Boemare, 2002). The elucidation of the symbiosis between entomopathogenic nematodes and their associated bacteria was a major turning point in the development of the nematodes as commercial biological control agents.
Dutky (1959) first noticed the antibiotic properties of the bacterium associated with S. carpocapsae, which explained how it could destroy foreign bacteria that invaded the insect cadaver containing the developing nematodes. Since then several antibiotics, including xenorhabdins, xenocaumacins, hydroxystilbenes, indole derivatives, and anthraquinone derivatives, were recovered from cultures of Xenorhabdus and Photorhabdus (Webster et al., 2002).
Akhurst (1980) discovered that in culture, Xenorhabdus existed in two (or more) genetically identical phase variants that differed in colony morphology, colony color and antimicrobial activity. The primary phase, which is carried by naturally occurring infective stages, provided maximum nematode growth and antibiotic production. But the primary phase would suddenly revert to a secondary phase, which was much less supportive of nematode growth and had limited antibody production. This shift was a major hindrance in the commercial production of the nematodes. The cause of this sudden phase shift was unknown until a bacteriophage of Heterorhabditis was discovered that only attacked the primary phase. Shifting to the secondary phase would then be a survival response of the Xenorhabdus primaries (Poinar et al., 1980b; Poinar et al., 1989).
Insects have a barrage of defense reactions to invading parasites and the most significant against nematodes is melanization and encapsulation. Normally, the bacteria will kill the host before a lethal response can be effected. However, in some experimental hosts such as mosquitoes, a rapid melanization reaction will kill the infectives before they can release their symbiotic bacteria (Bronskill,1962; Welch and Bronskill, 1962). Also, if the infectives of S. carpocapsae lack cells of their symbiotic bacterium, the developing stages can be encapsulated and killed, even in larvae of Galleria mellonella, which is the most common host used for raising entomopathogenic nematodes (Poinar, 1969).
There were also natural enemies of the infective stages to consider, especially protozoa and fungi. When caterpillars already infected with microsporidians were later invaded by S. carpocapsae, the protozoan infections were transferred to the nematodes (Veremchuk and Issi, 1970). Naturally occurring populations of entomopathogenic nematodes can also be infected with microsporidians (Poinar, 1988). Infective stages of entomopathogenic nematodes are also susceptible to infection by several common soil fungi (Poinar and Jansson, 1986a, 1986b) showing that before applying the nematodes to soils rich in humus, it is prudent to make a survey of nematophagous fungi that might be present.
While the first entomopathogenic nematode, S. kraussei Steiner, was described in 1923, the biocontrol potential of these nematodes was first investigated by Glaser and his colleagues who investigated S. glaseri to control populations of the Japanese beetle that had recently invaded New Jersey. Growth of the nematodes was tested on different types of artificial media for mass production (Fig. 5). While S. glaseri is associated with a symbiotic bacterium, Glaser and his group were unaware of its presence and this bacterium became lost during the sterilization processes when the nematodes were transferred to artificial media. It was fortunate for the New Jersey team that S. glaseri is one of the most catholic nematodes in the genus regarding the ability to develop on other bacteria as well as yeast in the absence of its symbiont. While production is much lower than with the natural-occurring symbiotic bacterium, the nematodes can still kill invade, destroy and reproduce inside insects (Poinar, 1969). Mass-production on artificial media was achieved and large-scale field releases of S. glaseri were conducted with the nematodes dispersed from a motor driven tank (Glaser, 1932; Glaser and Farrell, 1935). Details of the field trails are summarized in Poinar (1979). The symbiotic bacterium was later discovered in a population of S. glaseri from North Carolina (Poinar and Brooks, 1977; Poinar, 1978).
Fig. 5.
Rudolf Glaser (right) and co-worker examine culture plates with S. glaseri in their New Jersey laboratory in the early 1930s.
Living insects were used to produce the first entomopathogenic nematodes for field testing. Nutrilite Corporation in Lakeview, CA used larvae of the wax moth, Galleria mellonella to produce Biotrol NCS-DD-136 in 1970 for experimental use. In 1981, “The Nematode Farm” in Berkeley produced several entomopathogenic nematodes (S. carpocapsae, S. glaseri and H. bacteriophora) in Galleria mellonella for commercial use against garden pests. Also in 1981, BR Supply in Exeter, CA raised S. carpocapsae on crickets and packaged a product called Neocide for use against the carpenter worm. In 1982, Biosys, in Palo Alto, CA, (previously established as “The California Nematode Laboratories” in Emeryville, CA, and for a brief period “Biosis” in Palo Alto, CA), was the first to use a fermentation process to mass produce Steinernema spp. and their commercial products, BioSafe, BioVector, etc., were aimed at lawn and garden insects. In 1983, Biotechnology Australia produced nematodes on particles of sponge impregnated with an artificial diet, based on a method developed earlier by Bedding (1981). This technique used polyether polyurethane sponge as a three-dimensional support that allowed the nematodes to move through the matrix and provide air exchange. Their product, Otinem, was aimed at black vine weevils in Australia and Europe. Commercial production of entomopathogenic nematodes in liquid culture was later perfected by a team of researchers led by Friedman (1990) at Biosys Inc. A number of additional smaller companies, some as cottage industries, appeared in the mid- 80s.
One of the serious problems in the commercialization of entomopathogenic nematodes, aside from mass production, was storage under conditions that maintained high viability together with high infectivity. Refrigeration was a suitable method but not practical for retailers and growers who wanted to sell or apply the nematodes over a period of several weeks or even days. Studies were then initiated on the possibility of desiccating the nematodes so they could be stored at room temperatures. Simons and Poinar (1973) discovered that if the infective stages of S. carpocapsae were desiccated slowly, they entered a partial anhydrobiosis and could be quickly revised with water and still retain their infectivity. Based on these findings, Bedding (1988) later developed a “clay sandwich” formulation in which nematodes were placed in layers of clay to remove surface water and induce partial anhydrobiosis. Anhydrobiosis was further used to enhance the ambient storage stability of entomopathogenic nematodes (Grewal, 2000). Scientists at Biosys (see Georgis, 1990) developed an alginate formulation in which sheets of calcium alginate spread over plastic screens were used to entrap and maintain nematodes. Bedding and Butler (1994) reported a formulation in which the nematode slurry was mixed with a powder of anhydrous polyacrylamide to achieve a water activity between 0.800 and 0.995. Capinera and Hibbard (1987) developed a granular formulation in which nematodes were partially encapsulated in lucerne meal and wheat flour. Later Connick et al. (1993) described an extruded granule in which nematodes were distributed throughout a wheat gluten matrix. This “pesta” formulation included a filter and humectant to enhance nematode survival.
A major leap in the development of nematode formulations was reported by Silver et al. (1995) who developed a water dispersible granular formulation in which the nematodes were encased in 10-20 mm diameter granules consisting of a mixture of various types of silica, clay, cellulose, lignin, and starches. With this formulation, the shelf-life of commercially produced S. carpocapsae was extended to 7 months at ambient temperatures (Gaugler et al., 2000). Applications against aerial pests also posed a problem since if the nematodes desiccated too rapidly, their effectiveness was greatly reduced. Webster and Bronskill (1968) used an evaporation retardant to prolong the life of the nematodes used against foliar pests.
The infective stages of entomopathogenic nematodes can be easily applied using conventional pesticide application equipment, however, applying the nematodes simultaneously with other agents saves labor costs. Rao et al. (1975) showed that S. carpocapsae can be tank mixed with certain insecticides and in their trials against the corn rootworm, Poinar et al. (1983) applied the infective stages of S. carpocapsae with liquid fertilizer. These initial results were followed by a series of comprehensive studies on the effects of combining the infective stages of Steinernema and Heterorhabditis with pesticides (Rovesti and Deseo, 1989,1990, 1991). As compatibility information is critical for implementation of nematodes in integrated pest management systems, a comprehensive review was published by Koppenhöfer and Grewal (2005). Kaya and Nelson (1985) investigated the application of infective stages in alginate gels for increased persistence and this practice was commercialized in the application of nematodes to tree trunks. Since application problems were often experienced, Lello et al. (1996) and Fife et al. (2003, 2004, 2005) systematically evaluated the influence of droplet size, pressure differentials, hydraulic nozzles, contraction flow fields and agitation, on nematode viability and virulence. Masson et al. (1999) proposed the potential utility of spinning disc technology for the application of nematodes against foliar pests. Reed et al. (1986) were the first to apply nematodes through tickle irrigation, Wright et al. (1993) through center-pivot irrigation, and Cabanilas and Raulston (1996) through furrow irrigation systems. Subsurface application of nematodes with an adapted seed-driller was reported by Shetlar (1993). Bari (1992) developed a method of soaking plant cuttings in nematode suspensions to control the artichoke plume moth while Pye and Pye (1985) proposed a root dip method to economize nematode application rates. A slow release formulation using an absorbent gel was used to apply nematodes in citrus (Georgis, 1990) and a similar formulation (tea bag) was used in oilseed rape (Menzler-Hokkanen and Hokkanen, 2004). Infected insect cadavers can also serve as slow release systems for nematodes (Jansson and Lecrone, 1994) and Shapiro-Illan et al (2001) improved this method by for application by formulating nematode infected cadavers with powered starch to reduce their stickiness.
Miller (1989) developed a pathogenicity assessment technique to determine the virulence of commercially produced S. carpocapsae. This method was later called the one-on-one Galleria mellonella bioassay (Converse and Miller, 1999). Grewal et al. (1999) developed the sand-well method, which is suitable for routine quality assessment of most entomopathogenic nematode species at low concentrations. Many other methods serve as indicators of infectiousness, pathogenicity or the general quality of the nematodes (see Grunder et al., 2005). Gaugler et al. (2000) assessed the quality of commercially produced nematodes, thus raising awareness about the importance of effective quality control during commercialization. Georgis et al., (2006) discussed the success and failures of entomopathogenic nematodes used as biological control agents.
Attention then turned to the behavior of the infective stages and how they were able to locate insect hosts. Reed and Wallace (1965) described three types of movement of the infective stages of S. carpocapsae; gliding, bridging and leaping. The infectives used a gliding motion to reach the soil surface. The bridging motion involved the nematodes standing on their tails and waving their anterior ends (nictating). Such behavior had already been noted in free-living rhabditids that had phoretic relationships with insects. The amazing leaping behavior consisted of the infectives coiling their bodies around a water droplet (which produced a tension force), suddenly releasing the droplet by uncoiling and being propelled horizontally across the substrate by the tension force. The actual leap was too rapid to be followed with the naked eye. It is obvious that various clues are used by the infective stages to locate their hosts. Byers and Poinar (1982) showed that S. carpocapsae infectives could locate hosts by minute temperature gradients while Lewis et al. (1992) showed S. carpocapsae and S. glaseri responded to long-range volatile cues.
Differences in the vertical and horizontal dispersal of Steinernema infectives were determined to be related to host-finding behavior by Moyle and Kaya (1981). These authors noted that while S. carpocapsae did not shift its position significantly from the site of application, S. glaseri moved horizontally long distances. Georgis and Poinar (1983a, 1983b) showed how soil texture affected the distribution and infectivity of S. carpocapsae and S. glaseri, respectively and Poinar and Hom (1986) studied the survival and horizontal movement of the infective stages of S. carpocapsae. Vertical migration of Heterorhabditis spp. in soil was investigated by Georgis and Poinar (1983c).
A series of studies conducted in Gaugler’s laboratory described two host finding behaviors of the infective stages; ambushers and cruisers. Nematodes using the ambush type of foraging are better adapted at locating highly mobile hosts on the soil surface while nematode species that use the cruiser type are more adapted for sedentary hosts in the soil. Gaugler and Campbell (1991) proposed that the ambushing type of host-finding behavior may explain the limited movement of some entomopathogenic nematode species in the soil. Grewal et al. (1994a, 1994b) demonstrated that S. feltiae and S. riobrave use an intermediate type of foraging behavior between the ambushing and cruising exhibited by S. carpocapsae and S. glaseri, respectively. These studies showed researchers that it was possible to match the host-finding behavior of nematodes with the life history parameters of target pests.
Poinar (1979) summarized early reports on challenging non-insect invertebrates and vertebrates with S. carpocapsae and Georgis et al. (1991) later demonstrated the safety of entomopathogenic nematodes to soil invertebrates. Aside from the negative report of S. carpocapsae killing adult honey bees (Hackett and Poinar, 1973), these nematodes have a minimal effect on non-target invertebrates. However, apart from their use for controlling a wide range of insects, the ability of entomopathogenic nematodes to infect some non-insects groups was a bonus. Samish and Glazer (1991) discovered that entomopathogenic nematodes are capable of killing engorged female cattle ticks. Ticks of the genera Amblyomma, Argas, Boophilus, Dermacenteor, Hylomma, and Rhipicephalus have now been found to be susceptible to entomopathogenic nematodes (Glazer et al., 2005). Although the infective nematodes can invade and kill ticks and thus have potential for their control, there is no evidence of nematode reproduction in these arachnids.
Some insect vectors of human diseases are also susceptible to entomopathogenic nematodes. The susceptibility of fleas was first demonstrated with the cat flea, Ctenocephalides felis (Silverman et al., 1982). Biosys developed a biocontrol product with S. carpocapsae for the control of fleas in home lawns as a part of an integrated control program (Manweiler, 1994). Susceptibility of the body louse Pediculus humanus humanus to entomopathogenic nematodes was first demonstrated by Weiss et al. (1993). They reported that S. carpocapsae and S. glaseri caused over 85% mortality of female lice within 24 h. Doucet et al. (1998) showed that the head louse P. humanus capitis also is susceptible to entomopathogenic nematodes and H. bacteriophora was most effective of those tested. Larvae of phlebotomine flies, the vectors of leishmaniasis, are also susceptible to infection by Steinernema and Heterorhabditis (Poinar et al., 1993).
There are some reports of vertebrate infections by entomopathogenic nematodes. The infectives of S. carpocapsae were able to kill tadpoles of the Antillan toad, Bufo marinus (Kermarrec and Mauléon, 1985) and Heterorhabditis and Steinernema infectives caused mortality of frog tadpoles (Poinar and Thomas, 1988). While runoff could carry the infectives into standing water where tadpoles occurred, the likelihood of them making contact with amphibian larvae would be minimal.
Although natural populations of entomopathogenic nematodes are well-adapted to their native habitat and hosts through natural selection, additional useful traits could be established in their genome to make them even more efficient against other hosts in different environments. Poinar (1991) discussed some desirable traits that could be introduced into nematodes through recombinant DNA technology such as microinjection, gene transplantation, mutagenesis and selective breeding. Already in 1980, Burman and Pye had developed a temperature- selective strain of S. carcopasae whose infectives gravitate toward the temperature at which they underwent development. Fodor et al (1989) were able to obtain mutants of S. carpocapsae that were resistant to anthelminthics. Gaugler et al. (1989) demonstrated the use of selective breeding for enhanced host-finding in S. carpocapsae. Hashmi et al. (1995) used microinjection to produce a transgenic strain of H. bacteriophora that incorporated a heat shock protein gene from Caenorhabditis elegans and Sandhu et al. (2006) reported expressed-sequenced tags (ESTs) of H. bacteriophora. Although the benefits of genetic selection were demonstrated, the only field application of a selected strain was the Kapow strain of S. carpocapsae created by Jim Lindegren. This strain was developed by using the first juveniles to leave the host to infect subsequent hosts. The infectives of the Kapow strain were more active than the source strain and were used in field trials against navel orangeworms in California almond orchards (Agudelo-Silva et al., 1987). Genetic manipulation of the symbiotic bacteria is also feasible and studies have been undertaken to examine the genome of Photorhabdus luminescens (Duchaud et al., 2003).
This brief synopsis of the history of entomopathogenic nematodes necessitated a certain brevity, which did not allow us to include the works of many others who contributed to this field. For instance, the “International Symposium on Entomopathogenic nematodes” in Biological Control that Harry Kaya and Randy Gaugler organized at Asilomar, CA in 1989 (Fig. 6) provided a chance for researchers around the world to discuss common problems. It is easy to forget the many past hurdles that had to be overcome in order to reach where we are today. One crucial obstacle was obtaining government registration of not just the nematodes, but also their symbiotic bacteria. Educating the public by making it clear that entomopathogenic nematodes would not infect plants and humans was another early issue. There are still many ways in which the productivity and use of entomopathogenic nematodes can be improved (Grewal et al., 2005), however an interesting sidelight regarding Heterorhabditis and its associated bacterium is their increasing use as genetic models in molecular biology (Ciche, 2007; Duchaud et al., 2003) (Fig. 7).
Fig. 6.
Harry Kaya (left) and Randy Gaugler at the “International Symposium on Entomopathogenic nematodes in Biological Control” that they organized in Asilomar, CA, August 20-22, 1989. Photo by G. Poinar.
Fig. 7.
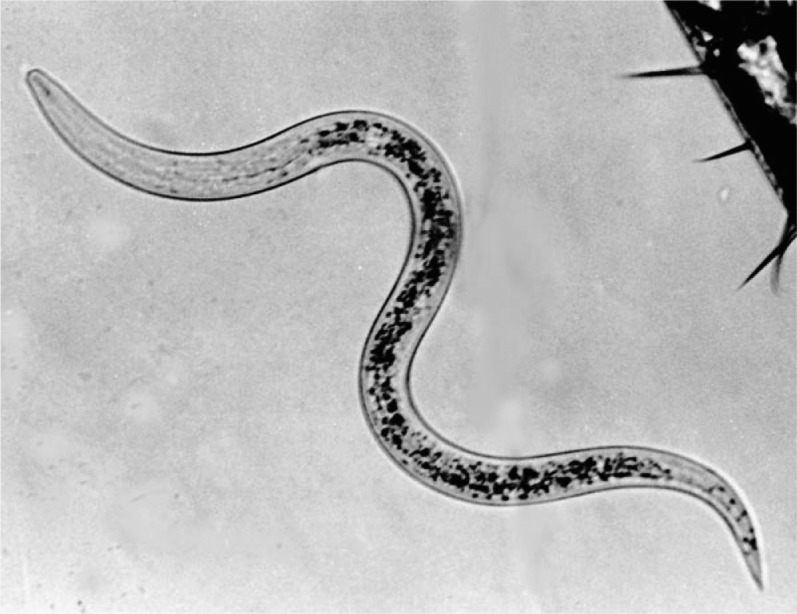
Infective stage of the HP88 strain of Heterorhabditis bacteriophora. Aside from their role as biocontrol agents, members of this genus and their associated bacteria are also serving as genetic models in molecular biology. Photo by G. Poinar.
Acknowledgments
The senior author thanks Roberta Poinar for comments on earlier drafts of this work.
Literature Cited
- Agudelo-Silva F, Lindegren JE, Valero KA. Persistence of Neoaplectana carpocapsae (Kapow selection) infectives in almonds under field conditions. Florida Entomologist. 1987;70:288–291. [Google Scholar]
- Akhurst RJ. Morphological and functional dimorphism in Xenorhabditis spp., bacteria symbiotically associated with the insect pathogenic nematodes Neoaplectana and Heterorhabditis. Journal of General Microbiology. 1980;121:303–309. [Google Scholar]
- Akhurst RJ, Boemare NE. 1990 Biology and Taxonomy of Xenorhabdus, Pp. 75–90 in R. Gaugler, and H. K. Kaya, eds. Entomopathogenic nematodes in Biological Control. Boca Raton: CRC Press. [Google Scholar]
- Bari MA. Disinfestation of artichoke stumps with entomopathogenic nematode against the artichoke plume moth. Proceedings of the International Congress of Entomology, Beijing, China. 1992;19:319. [Google Scholar]
- Bedding RA. Low cost in vitro mass production of Neoaplectana and Heterorhabditis species (Nematoda) for field control of insect pests. Nematologica. 1981;27:109–114. [Google Scholar]
- Bedding RA. 1988. Storing third stage infective nematode juveniles by mixing with clay, placing between layers of clay or contacting with adsorbent. International Patent WO 88/08668. [Google Scholar]
- Bedding RA, Akhurst RJ. A simple baiting technique for the detection of insect parasitic rhabditid nematodes in soil. Nematologica. 1975;21:109–110. [Google Scholar]
- Bedding RA, Molyneux AS. Penetration of insect cuticle by infective juveniles of Heterorhabditis spp. (Heterorhabditidae: Nematoda) Nematologica. 1982;28:354–359. [Google Scholar]
- Bedding RA, Butler KL. 1994. Storage and transport of entomopathogenic nematodes. Australian Patent No. 608852. [Google Scholar]
- Boemare NE. 2002. Biology, taxonomy and systematics of Photorhabdus and Xenorhabdus. Pp. 35–56 in R. Gaugler, ed. Entomopathogenic Nematology. Wallingford, UK: CABI Publishing. [Google Scholar]
- Boemare NE, Akhurst RJ, Mourant RG. DNA relatedness between Xenorhabdus spp. (Enterobacteriaceae), symbiotic bacteria of entomopathogenic nematodes, and a proposal to transfer Xenorhabdus luminescens to a new genus, Photorhabdus gen. nov. International Journal of Systematic Bacteriology. 1993;43:249–255. [Google Scholar]
- Bronskill JF. Encapsulation of rhabditoid nematodes in mosquitoes. Canadian Journal of Zoology. 1962;40:1269–1275. [Google Scholar]
- Burman M, Pye AE. Neoaplectana carpocapsae: Movements of nematode populations on a thermal gradient. Experimental Parasitology. 1980;48:258–265. doi: 10.1016/0014-4894(80)90122-8. [DOI] [PubMed] [Google Scholar]
- Byers JA, Poinar GO., Jr Location of insect hosts by the nematode, Neoaplectana carpocapsae, in response to temperature. Behaviour. 1982;79:1–10. [Google Scholar]
- Cabanillas HE, Raulston JR. Effects of furrow irrigation on the distribution and infectivity of Steinernema riobravis against corn earworm in corn. Fundamental and Applied Nematology. 1996;19:273–281. [Google Scholar]
- Capinera JL, Hibbard BE. Bait formulations of chemical and microbial insecticides for suppression of crop-feeding grasshoppers. Journal of Agricultural Entomology. 1987;4:337–344. [Google Scholar]
- Ciche T. 2007. The biology and genome of Heterorhabditis bacteriophora. The biology and genome of Heterorhabditis bacteriophora (February 20, 2007), WormBook, ed. The C. elegans Research Community, WormBook, Doi/10.1895/wormbook.1.135.1, http://www.wormbook.org.
- Connick WJ, Nickle WR, Vinyard BT. Pesta: new granular formulations for Steinernema carpocapsae. Journal of Nematology. 1993;25:198–203. [PMC free article] [PubMed] [Google Scholar]
- Converse V, Miller RW. Development of the one-on-one quality assessment assay for entomopathogenic nematodes. Journal of Invertebrate Pathology. 1999;74:143–148. doi: 10.1006/jipa.1999.4867. [DOI] [PubMed] [Google Scholar]
- Doucet MA, Miranda MB, Bertolotti MA. Infectivity of entomogenous nematodes (Steinernematidae and Heterorhabditidae) to Pediculus humanus capitis De Geer (Anoplura: Pediculidae) Fundamental and Applied Nematology. 1998;21:13–16. [Google Scholar]
- Duchaud E, Rusniok C, Frangeul L, Buchrieser C, Givaudan A, Taourit S, Bocs S, Boursaux-Eude C, Chandler M, Charles JF. The genome sequence of the entomopathogenic bacterium Photorhabdus luminescens. Nature Biotechnology. 2003;21:1307–1313. doi: 10.1038/nbt886. [DOI] [PubMed] [Google Scholar]
- Dutky SR. Insect Microbiology. Advances in Applied Microbiology. 1959;1:175–200. doi: 10.1016/s0065-2164(08)70479-9. [DOI] [PubMed] [Google Scholar]
- Dutky SR, Hough WS. Note on a parasitic nematode from codling moth larvae, Carpocapsae pomonella. Proceedings of the Entomological Society of Washington. 1955;57:244. [Google Scholar]
- Fife JP, Derksen RC, Ozkan HE, Grewal PS. Effects of pressure differentials on the viability and infectivity of entomopathogenic nematodes. Biological Control, 2003;27:65–72. [Google Scholar]
- Fife JP, Derksen RC, Ozkan HE, Grewal PS, Chalmers JJ, Krause CR. Evaluation of a contraction flow field on hydrodynamic damage to entomopathogenic nematodes- a biological pest control agent. Biotechnology and Bioengineering. 2004;86:96–107. doi: 10.1002/bit.10879. [DOI] [PubMed] [Google Scholar]
- Fife JP, Ozkan HE, Derksen RC, Grewal PS, Krause CR. Viability of a biological pest control agent through hydraulic nozzles. Transactions of the American Society of Agricultural Engineers. 2005;48:45–54. [Google Scholar]
- Fodor A, Séringer G, Georgis R. Application of insect pathogenic nematodes (Neoaplectana carpocapsae and Heterorhabditis spp.) against Colorado potato beetle (Leptinotarsa decemlineata) larvae: Small plot experiments and laboratory trials. Novenyved. 1989;25:215. [Google Scholar]
- Friedman MJ. 1990. Commercial production and development. Pp. 153–172 in R. Gaugler, and H. K. Kaya, eds. Entomopathogenic nematodes in Biological Control. Boca Raton, CRC Press. [Google Scholar]
- Gaugler R, Campbell JF. Selection for enhanced host-finding of scarab larvae (Coleoptera: Scarabaeidae) in an entomopathogenic nematode. Environmental Entomology. 1991;20:700–706. [Google Scholar]
- Gaugler R, Campbell JF, McGuire TR. Selection for host-finding in Steinernema feltiae. Journal of Invertebrate Pathology. 1989;54:363–372. [Google Scholar]
- Gaugler R, Grewal P, Kaya H, Smith-Fiola D. Quality assessment of commercially produced entomopathogenic nematodes. Biological Control. 2000;17:100–109. [Google Scholar]
- Georgis R. 1990. Formulation and application technology. Pp. 173–191 in R. Gaugler, and H. K. Kaya, eds. Entomopathogenic Nematodes in Biological Control. Boca Raton: CRC Press. [Google Scholar]
- Georgis R, Poinar GO., Jr Effect of soil texture on the distribution and infectivity of Neoaplectana carpocapsae (Nematoda: Steinernematidae) 1983a Journal of Nematology 15:308–311. [PMC free article] [PubMed] [Google Scholar]
- Georgis R, Poinar GO., Jr Effect of soil texture on the distribution and infectivity of Neoaplectana glaseri (Nematoda: Steinernematidae) Journal of Nematology. 1983b;15:329–332. [PMC free article] [PubMed] [Google Scholar]
- Georgis R, Poinar GO., Jr . 1983c. Vertical migration of Heterorhabditis bacteriophora and H. heliothidis (Nematoda: Heterorhabditidae). Journal of Nematology 15:652–654. [Google Scholar]
- Georgis R, Kaya HK, Gaugler R. Effect of steinernematid and heterorhabditid nematodes (Rhabditida: Steinernematidae and Heterorhabditidae) on non-target arthropods. Environmental Entomology. 1991;20:815–822. [Google Scholar]
- Georgis R, Koppenhöfer AM, Lacey LA, Bélair G, Duncan LW, Grewal PS, Samish M, Tan L, Torr P, van Tol RWHM. Successes and failures in the use of parasitic nematodes for pest control. Biological Control. 2006;38:103–123. [Google Scholar]
- Glaser RW. Studies on Neoaplectana glaseri, a nematode parasite of the Japanese beetle (Popillia japonica) New Jersey Department of Agriculture, Circular. 1932;211:1–34. [Google Scholar]
- Glaser RW, Fox H. A nematode parasite of the Japanese beetle (Popillia japonica Newm.) Science. 1930;71:16–17. doi: 10.1126/science.71.1827.16-b. [DOI] [PubMed] [Google Scholar]
- Glaser RW, Farrell CC. Field experiments with the Japanese beetle and its nematode parasite. Journal of the New York Entomological Society. 1935;43:345–371. [Google Scholar]
- Grewal PS. Enhanced ambient storage stability of an entomopathogenic nematode through anhydrobiosis. Pest Management Science. 2000;56:401–406. [Google Scholar]
- Grewal PS, Selvan S, Gaugler R. Thermal adaptation of entomopathogenic nematodes: niche breadth for infection, establishment, and reproduction. Journal of Thermal Biology. 1994a;19:245–253. [Google Scholar]
- Grewal PS, Lewis EE, Campbell JF, Gaugler R. Host finding behavior as a predictor of foraging strategy for entomopathogenic nematodes. Parasitology. 1994b;108:207–215. [Google Scholar]
- Grewal PS, Converse V, Georgis R. Influence of production and bioassay methods on infectivity of two ambush foragers (Nematoda: Steinernematidae) Journal of Invertebrate Pathology. 1999;73:40–44. doi: 10.1006/jipa.1998.4803. [DOI] [PubMed] [Google Scholar]
- Grewal PS, Ehlers RU, Shapiro-Illan DI. 2005. Critical issues and research needs for expanding the use of nematodes in biocontrol. Pg. 479–489 in P. S. Grewal, R. U. Ehlers, and D. Shapiro-Ilan, eds. Nematodes as Biocontrol Agents. Wallingford, UK: CABI Publishing. [Google Scholar]
- Grunder JM, Ehlers R-U, Jung K. 2005. Quality Control of Entomopathogenic Nematodes. COST Action 819, Agrowscope Faw, Wadenswil, Switzerland. [Google Scholar]
- Hackett KJ, Poinar GO., Jr The ability of Neoaplectana carpocapsae Weiser (Steinernematidae: Rhabditoidea) to infect adult honeybees (Apis mellifera, Apidae: Hymenoptera) American Bee Journal. 1973;113:100. [Google Scholar]
- Hashmi S, Hasnmi G, Gaugler R. Genetic transformation of an entomopathogenic nematode by microinjection. Journal of Invertebrate Pathology. 1995;66:293–296. doi: 10.1006/jipa.1995.1103. [DOI] [PubMed] [Google Scholar]
- Jansson RK, Lecrone SH. Application methods for entomopathogenic nematodes (Rhabditida: Heterorhabditidae) aqueous suspensions versus infected cadavers. Florida Entomologist. 1994;77:281–284. [Google Scholar]
- Kaya HK, Nelson CE. Encapsulation of steinernematod and heterorhabditid nematodes with calcium alginate; a new approach for insect control and other applications. Environmental Entomology. 1985;14:572–574. [Google Scholar]
- Kermarrec A, Mauléon H. Potential noxiousness of the entomogenous nematode Neoaplectana carpocapsae Weiser to the Antillan toad Bufo marinus L. Mededelingen van de Faculteit Landbouwwetenschappen, Rijksuniversiteit (Gent) 1985;50:831–838. [Google Scholar]
- Kiontke K, Barrière A, Kolotuev I, Podbilewicz B, Sommer R, Fitch DHA, Félix M-A. Trends, Stasis, and Drift in the evolution of nematode vulva development. Current Biology. 2007;17:1925–1937. doi: 10.1016/j.cub.2007.10.061. [DOI] [PubMed] [Google Scholar]
- Koppenhöfer AM, Grewal PS. 2005. Interactions with other biological control agents and agrochemicals. Pg. 363–381 in P. S. Grewal, R. U. Ehlers, and D. Shapiro-Ilan, eds. Nematodes as Biocontrol Agents. Wallingford, UK, CABI Publishing. [Google Scholar]
- Lello ER, Patel MN, Matthews GA, Wright DJ. Application technology for entomopathogenic nematodes against foliar pests. Crop Protection. 1996;15:567–574. [Google Scholar]
- Lewis EE, Gaugler R, Harrison R. Entomopathogenic nematode host finding: response to host contact cues by cruise and ambush foragers. Parasitology. 1992;105:309–319. [Google Scholar]
- Liu J, Poinar GO, Jr, Berry RE. Control of insect pests with entomopathogenic nematodes: The impact of molecular biology and phylogenetic reconstruction. Annual Review of Entomology. 2000;45:287–306. doi: 10.1146/annurev.ento.45.1.287. [DOI] [PubMed] [Google Scholar]
- Manweiler SA. 1994. Development of the first cat flea biological control product employing the entomopathogenic nematode Steinernema carpocapsae. Pp.1005–1012 in Proceedings of the Brighton Crop Protection Conference: Pest and Diseases, Farnham, Surrey: British Crop Protection Council. [Google Scholar]
- Menzler-Hokkanen I, Hokkanen HMT. Developing entomopathogenic nematode delivery systems for biological control of oilseed rape pests. International organization for Biological and integrated control of noxious animals and plants, West Palaearctic Regional Section, Bulletin. 2004;28:19–22. [Google Scholar]
- Miller RW. Novel pathogenicity assessment technique for Steinernema and Heterorhabditis entomopathogenic nematodes. Journal of Nematology. 1989;21:574. [Google Scholar]
- Milstead JE. 1977. The life cycle and pathobiology of Heterorhabditis bacteriophora Poinar (Rhabditoidea: Nematoda) in its lepidopteran hosts. Ph.D. dissertation. University of California, Berkeley, 113 pp. [Google Scholar]
- Moyle PL, Kaya HK. Dispersal and infectivity of the entomogenous nematode, Neoaplectana carpocapsae Weiser (Rhabditida: Steinernematodae), in sand. Journal of Nematology. 1981;13:295–300. [PMC free article] [PubMed] [Google Scholar]
- Poinar GO., Jr The presence of Achromobacter Nematophilus Poinar and Thomas in the infective stage of a Neoaplectana sp. (Steinernematidae: Nematoda) Nematologica. 1966;12:105–108. [Google Scholar]
- Poinar GO., Jr Description and Taxonomic position of the DD-136 nematode (Steinernematidae, Rhabditoidea) and its relationship to Neoaplectana carpocapsae Weiser. Proceedings of the Helminthological Society of Washington. 1967;34:199–209. [Google Scholar]
- Poinar GO., Jr . 1969. Arthropod immunity to worms. Pg. 173–210 in G. J. Jackson, and R. Herman, eds. Immunity to Parasitic Animals. Vol. 1. New York: Appleton-Century Crofts Co. [Google Scholar]
- Poinar GO., Jr Description and biology of a new insect parasitic rhabditoid. Heterorhabditis bacteriophora n. gen., n. sp. (Heterorhabditidae, n. fam.) (Rhabditida [Oerley). Nematologica. 1976;21:463–470. [Google Scholar]
- Poinar GO., Jr Generation polymorphism in Neoaplectana glaseri Steiner (Steinernematidae: Nematoda), redescribed from Strigoderma arboricola (Fab.) (Scarabaeidae: Coleoptera) in North Carolina. Nematologica. 1978;24:105–114. [Google Scholar]
- Poinar GO., Jr . 1979. Nematodes for Biological Control of Insects. CRC Press, Boca Raton. 277 pp. [Google Scholar]
- Poinar GO., Jr Recognition of Neoaplectana species (Steinernematidae: Rhabditida) Proceedings of the Helminthological Society of Washington. 1986;53:121–129. [Google Scholar]
- Poinar GO., Jr A microsporidian parasite of Neoaplectana glaseri (Steinernematidae: Rhabditida) Revue de Nematologie. 1988;11:359–361. [Google Scholar]
- Poinar GO., Jr . 1991. Genetic engineering of nematodes for pest control. Pg. 77–93 in K. Maramorosch, ed. Biotechnology for Biological Control of Pests and Vectors. Boca Raton: CRC Press. [Google Scholar]
- Poinar GO., Jr . 1990. Taxonomy and biology of Steinernematidae and Heterorhabditidae. Pg. 23–61 in R. Gaugler and H. K. Kaya, eds. Entomopathogenic Nematodes in Biological Control. Boca Raton: CRC Press. [Google Scholar]
- Poinar GO., Jr . 2011. The Evolutionary History of nematodes. Brill, Leiden, 439 pp. [Google Scholar]
- Poinar GO, Jr, Thomas GM. A new bacterium, Achromobacter Nematophilus sp. nov. (Achromobactericeae Eubacteriales) associated with a nematode. International Bulletin of Bacterial Nomenclature and Taxonomy. 1965;15:249–252. [Google Scholar]
- Poinar GO, Jr, Thomas GM. Significance of Achromobacter nematophilus (Achromobactericeae Eubacteriales) in the development of the nematode, DD-136 (Neoaplectana sp. Steinernematidae) Parasitology. 1966;56:385–390. doi: 10.1017/s0031182000070980. [DOI] [PubMed] [Google Scholar]
- Poinar GO, Jr, Thomas GM. The Nature of Achromobacter nematophilus as an insect pathogen. Journal of Invertebrate Pathology. 1967;9:510–514. [Google Scholar]
- Poinar GO, Jr, Leutenegger R. Anatomy of the infective and normal third stage juveniles of Neoaplectana carpocapsae Weiser. Journal of Parasitology. 1968;54:340–350. [PubMed] [Google Scholar]
- Poinar GO, Jr, Veremtshuk GV. A new strain of entomopathogenic nematodes and geographical distribution of Neoaplectana carpocapsae Weiser (Rhabditida, Steinernematidae) Zoological Jhurnal. 1970;49:966–969. [Google Scholar]
- Poinar GO, Jr, Brooks WM. Recovery of the entomogenous nematode, Neoaplectana glaseri Steiner from a native insect in North Carolina. International Research Communications System Medical Science. 1977;5:473. [Google Scholar]
- Poinar GO, Jr, Hom A. Survival and horizontal movement of infective stage Neoaplectana carpocapsae in the field. Journal of Nematology. 1986;18:34–36. [PMC free article] [PubMed] [Google Scholar]
- Poinar GO, Jr, Jansson HB. Susceptibility of Neoaplectana spp. and Heterorhabditis heliothidis to the endoparasitic fungus Drechmeria coniospora. 1986a Journal of Nematology 18:225–230. [PMC free article] [PubMed] [Google Scholar]
- Poinar GO, Jr, Jansson H-B. Infection of Neoaplectana and Heterorhabditis (Rhabditida: Nematoda) with the predatory fungi, Monacrosporium ellipsosporum and Arthrobotrys oligospora (Moniliales: Deuteromycetes) Revue du Nematologie. 1986b;9:241–244. [Google Scholar]
- Poinar GO, Jr, Thomas GM. Infection of frog tadpoles (Amphibia) by insect parasitic nematodes (Rhabditida) Experientia. 1988;44:528–531. [Google Scholar]
- Poinar GO, Jr, Georgis R. Characterization and field application of Heterorhabditis bacteriophora strain HP88 (Heterorhabditidae: Rhabditida) Revue de Nématologie. 1990;13:387–393. [Google Scholar]
- Poinar GO, Jr, Thomas GM, Hess R. Characteristics of the specific bacterium associated with Heterorhabditis bacteriophora (Heterorhabditidae: Rhabditida) Nematologica. 1977;23:97–102. [Google Scholar]
- Poinar GO, Jr, Thomas G, Haygood M, Nealson KH. Growth and luminescence of the symbiotic bacteria associated with the terrestrial nematode, Heterorhabditis bacteriophora. Soil Biology and Biochemistry. 1980a;12:5–10. [Google Scholar]
- Poinar GO, Jr, Hess RT, Thomas G. Isolation of defective bacteriophages from Xenorhabdus spp. (Enterobacteriaceae) IRCS Medical Science. 1980b;8:141. [Google Scholar]
- Poinar GO, Jr, Evans JS, Schuster E. Field test of the entomogenous nematode, Neoaplectana carpocapsae, for control of corn rootworm larvae (Diabrotica sp., Coleoptera) Protection Ecology. 1983;5:337–342. [Google Scholar]
- Poinar GO, Jr, Hess RT, Lanier W, Kinney S, White JH. Preliminary observations of a bacteriophage infecting Xenorhabdus luminescens (Enterobacteriaceae) Experientia. 1989;45:191–192. doi: 10.1007/BF01954872. [DOI] [PubMed] [Google Scholar]
- Poinar GO, Jr, Ferro C, Morales A, Tesh RB. Anandranema phlebotophaga n.gen., n.sp. (Allantonematidae: Tylenchida), a new nematode parasite of phlebotomine sand flies (Psychodidae: Diptera) with notes on experimental infections of these insects with parasitic rhabditoids. Fundamental and Applied Nematology. 1993;16:11–16. [Google Scholar]
- Pye AE, Pye NL. Different applications of the insect parasitic nematode Neoaplectana carpocapsae to control the large pine weevil, Hylobius abietis. Nematologica. 1985;31:109–116. [Google Scholar]
- Rao PSP, Das PK, Pahdi G. Note on compatibility of DD-136 (Neoaplectana carpocapsae) Dutky, an insect parasitic nematode with some insecticides and fertilizers. Indian Journal of Agricultural Science. 1975;45:275–277. [Google Scholar]
- Reed EM, Wallace HR. Leaping locomotion by an insect-parasitic nematode. Nature. 1965;206:210–211. [Google Scholar]
- Reed DK, Reed G, Creighton CS. Introduction of entomogenous nematodes into trickle irrigation systems to control striped cucumber beetle (Coleoptera, Chrysomelidae) Journal of Economic Entomology. 1986;79:1330–1333. [Google Scholar]
- Rovesti L, Deseö KV. Effect of neem kernal extract on steinernematod and heterorhabditid nematodes. Nematologica. 1989;35:493–496. [Google Scholar]
- Rovesti L, Deseö KV. Compatibility of chemical pesticides with the entomopathogenice nematodes, Steinernema carpocapsae Weiser and Steinernema feltiae (Nematoda, Steinernematodae) Nematologica. 1990;36:237–245. [Google Scholar]
- Rovesti L, Deseö KV. Compatibility of pesticides with the entomopathogenic nematode, Heterorhabditis heliothidis. Nematologica. 1991;37:113–116. [Google Scholar]
- Samish M, Glazer I. Killing ticks with parasitic nematodes of insects. Journal of Invertebrate Pathology. 1991;58:281–282. doi: 10.1016/0022-2011(91)90075-2. [DOI] [PubMed] [Google Scholar]
- Sandhu SK, Jagdale GB, Hogenhout SA, Grewal PS. Comparative analysis of the expressed genome of the infective juvenile entomopathogenic nematode, Heterorhabditis bacteriophora. Molecular Biochemistry and Parasitology. 2006;145:239–244. doi: 10.1016/j.molbiopara.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Shetlar DJ. 1999. Application methods in different cropping systems. Pg. 31–36 in S. Polavarapu, ed. Procedings of the National Workshop on optimal use of insecticidal nematodes in pest management. Chatsworth, NJ: Rutgers University. [Google Scholar]
- Shapiro-Ilan DI, Lewis EE, Behle RW, McGuires MR. Formulation of entomopathogenic nematode-infected cadavers. Journal of Invertebrate Patholology. 2001;78:17–23. doi: 10.1006/jipa.2001.5030. [DOI] [PubMed] [Google Scholar]
- Silver SC, Dunlop DB, Grove DI. 1995. Granular formulation of biological entities with improved storage stability. International Patent No. WO 95/05077. [Google Scholar]
- Silverman J, Platzer EG, Rust MK. Infection of the cat flea, Ctenocephalides felis (Bouche) by Neoaplectana carpocapsae Weiser. Journal of Nematology. 1982;14:394–397. [PMC free article] [PubMed] [Google Scholar]
- Simons WR, Poinar FO., Jr The ability of Neoaplectana carpocapsae (Steinernematidae: Nematodea) to survive extended periods of desiccation. Journal of Insect Pathology. 1973;22:228–230. [Google Scholar]
- Steiner G. Aplectana kraussei n. sp., einer in der Blattwespe Lyda sp. parasitierende Nematodenform, nebst Bemerkungen über das Seitenorgan der parasitischen nematoden. Zentralblatt fuer Bakteriologie, Parasitenkunde, Infektionskrankheiten und Hygiene. 1923;59:14–18. [Google Scholar]
- Steiner G. Neoaplectana glaseri n. g., n. sp. (Oxyuridae) a new nemic parasite of the Japanese beetle (Popillia japonica Newm.) Journal of the Washington Academy of Science. 1929;19:436–440. [Google Scholar]
- Stock SP, Hunt DJ. 2005. Morphology and systematics of Nematodes used in Biocontrol. In Grewal, P. S., Ehlers, R-U., and Shapiro-Ilan, D. I. Nematodes as biocontrol agents. Wallingford: CABI Publishing. [Google Scholar]
- Thomas GM, Poinar GO., Jr Xenorhabdus gen. nov., a genus of entomopathogenic, nematophilic bacteria of the family Enterobacteriaceae. International Journal of Systematic Bacteriology. 1979;29:352–360. [Google Scholar]
- Veremchuk GV, Issi IV. The development of microsporidians of insects in the entomopathogenic nematode Neoaplectana carpocapsae (Nematoda: Steinernematodiae) Parazitologiya. 1970;4:3–7. [Google Scholar]
- Webster JM, Bronskill JF. Use of Gelgard M and an evaporation retardant to facilitate control of larch sawfly by a nematode-bacterium complex. Journal of Economic Entomology. 1968;61:1370–1371. [Google Scholar]
- Webster JM, Chen G, Hu K, Li J. 2002. Bacterial metabolites. Pp. 99–114 in R. Gaugler, ed. Entomopathogenic Nematology. Oxon, UK: CAB International. [Google Scholar]
- Weiser J. Neoaplectana carpocapsae n. sp. (Anguillata, Steinernematidae) novy cizopasnik housenek obalece jablecneho, Carpocapsae pomonella L. Vestnik Ceskoslovenske Spolecnosti Zoologicke. 1955;19:44–52. [Google Scholar]
- Weiss M, Glazer I, Mumcuoglu KY, Elking Y, Galun R. Infectivity of steinernematid and heterorhabditid nematodes for the human body louse Pediculus humanus hunanus (Anoplura: Pediculidae) Fundamental and Applied Nematology. 1993;16:489–493. [Google Scholar]
- Welch HE, Bronskill JF. Parasitism of mosquito larvae by the nematode, DD-136 (Nematoda: Neoaplectanidae) Canadian Journal of Zoology. 1962;40:1263–1268. [Google Scholar]
- Wright LC, Witkowski JF, Echtenkamp G, Georgis R. Efficacy and persistence of Steinernema carpocapsae (Rhabditida: Steinernematidae) applied through center-pivot irrigation system against larval corn rootworms (Coleoptera: Chrysomelidae) Journal of Economic Entomology. 1993;86:148–1354. [Google Scholar]
- Wright PJ. Morphological characterization of the entomogenous nematodes Steinernema spp. and Heterorhabditis spp. (Nematoda: Rhabditida) New Zealand of Zoology. 1990;17:577–586. [Google Scholar]






