Abstract
Background
Hypertension is a toxicity of antiangiogenic therapies and a possible biomarker that identifies patients with superior cancer outcomes. Understanding its mechanism will aid in treatment and could lead to the development of other biomarkers for predicting toxicity and anticancer efficacy. Recent evidence implicates nitric oxide (NO) suppression and endothelin-1 (ET-1) stimulation as potential mechanisms leading to antiangiogenic therapy-induced hypertension. The aim of this study was to evaluate the effects of regorafenib, a novel broad-spectrum kinase inhibitor with activity against multiple targets, including vascular endothelial growth factor receptor 2 inhibition, on NO and ET-1 levels.
Methods
Regorafenib was administered to 32 subjects with gastrointestinal stromal tumor on a 3-week-on, 1-week-off basis. Plasma levels of NO and ET-1 were measured at baseline, 2, 4, and 6 weeks of therapy. Data analysis was by Wilcoxon rank-sum and paired t-tests.
Results
Twenty subjects (63%) developed regorafenib-induced hypertension. Two weeks after starting regorafenib therapy, plasma ET-1 levels increased (25% increase, P < 0.05) and NO was suppressed (20% decrease, P < 0.05). These normalized after 1-week washout but ET-1 rose again by 30% (P < 0.05) and NO fell by 50% (P < 0.05) after restarting regorafenib.
Conclusions
These findings indicate that regorafenib induces a coordinated and reversible suppression of NO and stimulation of ET-1. Whether NO and ET-1 might predict therapeutic efficacy in these patients requires further study.
American Journal of Hypertension, advance online publication 12 July 2012. doi:10.1038/ajh.2012.97
Keywords: antiangiogenic therapy, blood pressure, endothelin-1, hypertension, nitric oxide
Antiangiogenic therapies target the vascular endothelial signaling pathway (VEGF) among other proangiogenic pathways,1 and they are effective for many solid tumors.2–5 Hypertension is a common, occasionally dose-limiting toxicity of antiangiogenic therapies.6–10 Importantly, emerging clinical evidence suggests that the development of antiangiogenic therapy-induced hypertension may be related to superior outcomes in select populations.9,11–13 However, whether this association can be exploited for clinical benefit remains unclear and how best to identify patients with treatment-induced hypertension is an open question. Indeed, the utility of blood pressure as a predictive biomarker is limited by variation in office-based blood pressure measurement and the early institution of antihypertensive medications. Surrogate biomarkers of hypertension might overcome these limitations.
Preclinical and clinical evidence implicates inhibition of VEGF-mediated regulation of vascular tone in the etiology of antiangiogenic therapy-induced hypertension. Specifically, blockade of the VEGF-signaling pathway reduces vasodilatory nitric oxide (NO), and increases vasoconstrictive endothelin-1 (ET-1).14–20 VEGF stimulates endothelial NO synthase via the phosphatidylinositol 3-kinase-Akt pathway.21 However, how antiangiogenic therapies modulate ET-1 release is less clear. NO suppresses ET-1 release by inhibiting preproendothelin synthesis22 and ET-receptor antagonist attenuates the effects of NOS inhibition,23 suggesting an interaction between these two pathways. Whether ET-1 stimulation by antiangiogenic therapies might be a consequence of NO inhibition remains to be elucidated. Regardless of the specific mechanism, circulating levels of these vasoactive factors might represent surrogate biomarkers for antiangiogenic therapy-induced hypertension that could be used to predict tumor response.
Antiangiogenic therapies currently available include anti-VEGF monoclonal antibodies, multitargeted tyrosine kinase inhibitors with activity against the VEGF receptor (VEGFR) and VEGF Trap.24 Of note, tyrosine kinase inhibitors target not only the VEGF receptor, but also other kinase receptors including platelet-derived growth factor receptor and epithelial growth factor receptor.24 The use of these broad-spectrum agents enhances their therapeutic usefulness by expanding the actions to oncogenic targets as well. However, whether individual antiangiogenic therapies with different kinase activity spectrums also have different effects on the NO and ET-1 pathways is uncertain. Given the expanding use and wide variety of tyrosine kinase inhibitors with antiangiogenic activity, there is a need to evaluate the effects of individual antiangiogenic therapies on these pathways.
Regorafenib (BAY-73-4506) is a novel broad-spectrum kinase inhibitor with activity against multiple targets including VEGFR1, 2, 3, platelet-derived growth factor receptor, KIT, TIE2, RET, FGFR1, RAF, and p38 MAPK.25 In this study, we prospectively investigated whether regorafenib induces changes in circulating levels of NO and ET-1 in patients with metastatic and/or unresectable gastrointestinal stromal tumor (GIST). Secondary aims were to explore whether NO suppression and ET-1 stimulation were associated with high blood pressure and better tumor response (i.e., clinical benefit).
Methods
Study population. Plasma samples were collected from subjects enrolled in a single arm, open label, multicenter phase II study evaluating regorafenib in subjects with metastatic and/or unresectable GIST (NCT0106878769).26 Of the 34 subjects enrolled in this phase II trial, 32 subjects had plasma collected and were included in this analysis.
The inclusion criterion for the study required a histologically confirmed diagnosis of metastatic and/or unresectable GIST with prior therapeutic failure (defined as progression of disease or intolerance) to imatinib and disease progression on sunitinib. Patients may have received any number of prior therapies, including kinase inhibitors, with the exception of prior sorafenib. Key exclusion criteria were use of unapproved tyrosine kinase inhibitors or investigational agents within 2 weeks before study entry, uncontrolled hypertension at baseline (defined as systolic blood pressure >140 mm Hg, diastolic blood pressure >90 mm Hg) despite optimal medical management, persistent grade 3 proteinuria (defined as >3.5 g of proteins in 24 h), clinically significant arrhythmias or cardiac disease, or renal failure requiring dialysis.26
All patients received oral regorafenib on a daily basis, with a starting daily dose of 160 mg, following a 3-week-on drug, 1-week-off drug regime per cycle. Regorafenib dose was adjusted for toxicity.
Data collection. Plasma samples collected at baseline, cycle 1 day 15 (2 weeks), cycle 2 day 1 (at 4 weeks, after a 1-week washout period) and cycle 2 day 15 (6 weeks) were evaluated. Plasma samples were collected in EDTA-containing tubes, centrifuged for 5 min at 1,500g within 30 min of collection and stored at –80 °C until analysis.
Baseline clinical information was collected by chart review, including age, sex, body mass index, previous history of hypertension, antihypertensive medications, diabetes diagnosis, use of statins and estimated glomerular filtration rate (eGFR) by the modification of diet in renal disease formula.27 Blood pressures measurements, heart rate, eGFR, addition of new antihypertensive medications and/or dose changes at all time points of plasma collection were also obtained by chart review.
All patients provided written informed consent to collect plasma samples for research use. The study was approved by the Dana Farber Cancer Institute Institutional Review Board.
Measurement of biomarkers. The NO pathway was evaluated in plasma by measuring NO metabolites (nitrates and nitrites). ET-1 levels were directly measured in plasma. All markers were evaluated using commercially available kits. NO metabolites were measured by using a colorimetric assay (Parameter; R&D Systems, Minneapolis, MN) and ET-1 by chemiluminescent ELISA (QuantiGlo; R&D Systems). Inter- and intra-assay coefficients of variations for all assays were <15%.
Blood pressure measurement and hypertension definition. Single oscillometric blood pressure measurements taken at each visit day were recorded. Regorafenib-induced hypertension was defined as recurrent or persistent systolic blood pressure >140 mm Hg and/or diastolic blood pressure >90 mm Hg, requiring medical intervention, or having increases in blood pressure <20 mm Hg associated with symptoms, if previous blood pressure was within normal limits. This definition includes all subjects developing grade 2 or higher hypertension as per the Common Terminology Criteria for Adverse Events Version 4.0.28
Assessment of tumor response. Tumor response was assessed by clinical benefit defined by the composite of complete response, partial response, and stable disease lasting ≥16 weeks per RECIST 1.129 as a measure of disease control. Briefly, response is defined relative to the sum diameters of target lesions at baseline. Complete response refers to the disappearance of all target lesions as well as a reduction to <10 mm in short axis of any pathological lymph nodes. On the other hand, partial response refers to a decrease of at least 30% in the sum of diameters of target lesions relative to baseline. Finally, stable disease refers to the clinical situation where the increase or shrinkage in target lesions is not sufficient to qualify for progressive disease or partial response, respectively.29
Statistical analysis. Descriptive statistics were employed to define the study group. Continuous variables were evaluated using means and standard deviations for normally distributed data and medians and interquartile ranges for non-normal data. Categorical variables were summarized using percentages. We evaluated whether regorafenib induces NO suppression and ET-1 stimulation by comparing biomarkers levels at different time points relative to baseline by using Wilcoxon rank-sum tests. We evaluated whether NO suppression and ET-1 activation were associated with mean blood pressure (MAP) rise by using Spearman's correlation tests. Specifically, we compared changes in NO and ET-1 from baseline to 2 weeks (ΔNO0–2wks and ΔET0–2wks) to mean arterial pressure (MAP) from baseline to 2 weeks (ΔMAP0–2wks) as well as changes in NO and ET-1 from baseline to 6 weeks (ΔNO0–6wks and ΔET0–6wks) to MAP from baseline to 6 weeks (ΔMAP0–6wks). We evaluated whether NO suppression and ET-1 activation were associated with clinical benefit by comparing absolute changes in NO and ET-1 from baseline to 6 weeks (ΔNO0–6wks and ΔET0–6wks) among subjects who achieved clinical benefit and those who did not achieve clinical benefit by using Wilcoxon rank-sum test. Statistical significance was set at <0.05. All analyses were performed using GraphPad Prism5 and SAS v9.2.
Results
Subject characteristics
Thirty-two subjects were evaluated in this study. The median age of the participants was 56 years, 60% were male and 88% were white. All patients had an eGFR >60 ml/min/1.73 m2 at study entry (median 88 ml/min/1.73 m2). Sixty five percent of the subjects had a diagnosis of hypertension at baseline (Table 1).
Table 1.
Clinical characteristics at baseline (n = 32)

Biomarkers
Circulating NO levels decreased by 20% 2 weeks after regorafenib was started (P < 0.05) but returned to baseline 1-week off therapy. Two weeks later, after resuming regorafenib, systemic NO fell by 50% compared with baseline (Figure 1a) (P < 0.05). Systemic levels of ET-1 rose by 25% at 2 weeks while on regorafenib (P < 0.05) and by 30% as compared with baseline at 6 weeks (P < 0.05). ET-1 also returned to baseline after the washout period (Figure 1b). Plasma samples were collected in 31, 30, 31, and 28 of 32 subjects at baseline, 2, 4, and 6 weeks, respectively.
Figure 1. Variations in circulating levels of (a) nitric oxide (NO) and (b) endothelin-1 (ET-1) in patients with gastrointestinal stromal tumor on regorafenib at 0, 2, 4, and 6 weeks after starting therapy. Wilcoxon rank-sum test. *P < 0.05; †P < 0.05 to 4 weeks.

Relationship between blood pressure and biomarkers
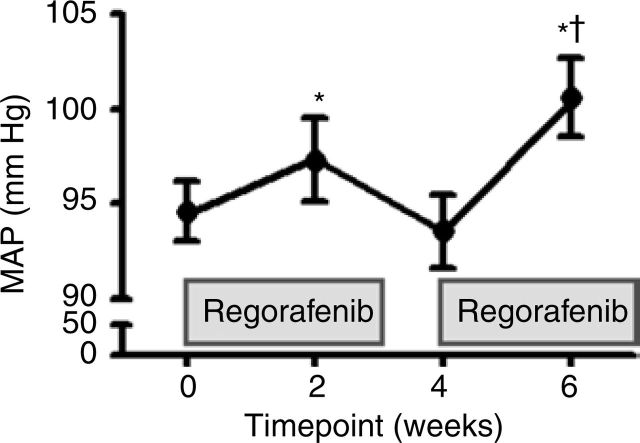
Sixty-three percent of subjects developed regorafenib-induced hypertension at any point during the phase II study. MAP increased by 4 mm Hg two weeks after regorafenib therapy was started (P < 0.05). During the washout period, MAP returned to baseline levels. At 6 weeks, the MAP was increased by 6 mm Hg (P < 0.05) (Figure 2). Within the first 2 weeks, 8 patients (25%) required increasing doses of antihypertensive medications or addition of new antihypertensive medication, which were started before the first blood pressure measurement was documented in our study. By 6 weeks, 4 (13%) additional subjects required the addition or increase of antihypertensive medications. No changes in heart rate or eGFR were observed while patients were on or off regorafenib (Table 2). No correlations were observed between increasing MAP and the plasma levels of NO or ET-1.
Figure 2. Mean arterial pressure (MAP) in patients with gastrointestinal stromal tumor treated with regorafenib at 0, 2, 4, and 6 weeks after starting therapy. Paired t-test. *P < 0.05; †P < 0.05 to 4 weeks.
Table 2.
Clinical characteristics of patients at baseline and 2, 4, and 6 weeks after starting regorafenib

Relationship between tumor response and biomarkers
Since hypertension has been reported to be an efficacy biomarker in some patients treated with antiangiogenic therapy, we assessed whether there were any correlations between changes in biomarker levels and clinical response. Data on clinical benefit was available for 27 subjects. Twenty-three subjects (85%) achieved clinical benefit and only 4 subjects (15%) did not achieve clinical benefit. There was no statistically significant association for either biomarker, although there was a trend towards a greater decrease in plasma NO and increase in ET-1 at 6 weeks in those who did achieve clinical benefit (ΔNO0–6wks: –15µmol/l vs. –8µmol/l, P = 0.24, ΔET0–6wks: 1 pg/ml vs. –0.04 pg/ml, P = 0.27). Of note, this analysis was underpowered. The power to detect differences with this number of patients was 28%; if the ratio between respondents and nonrespondents remains the same, we would need 101 patients to have 80% power to detect a significant relationship between biomarker level and clinical response.
Discussion
This study shows that administration of regorafenib, a novel broad-spectrum kinase inhibitor, induces a coordinated and rapidly reversible suppression of NO and stimulation of ET-1 in patients with metastatic and/or unresectable GIST. These pathways have been suggested as the major mediators of antiangiogenic therapy-induced hypertension, primarily via VEGF pathway blockade.14 For patients treated with regorafenib, these pathways represent rational targets for antihypertensive therapy. Given that drug-related hypertension has been associated with better clinical outcomes,10–13 circulating levels of these factors are also potential candidates for biomarkers of therapeutic efficacy.
Whether all antiangiogenic therapies cause increased ET-1 is an open question. Sunitinib is another broad-spectrum tyrosine kinase inhibitor that induces both hypertension and increased in ET-1,16 and ET-1 receptor blockade abrogates sunitinib-induced blood pressure rise in preclinical models.17,18,30 By contrast, in subjects treated with sorafenib, no changes in plasma ET-1 levels were reported,31 although baseline values were measured during the first two weeks of therapy, and our data here suggests that ET-1 levels rise quickly. Whether the specific tyrosine kinase inhibitory profile determines the effect of a given antiangiogenic agent on ET-1 and hypertension or whether these are universal properties of this class remains to be seen. Since ET-1 is under consideration as a biomarker of efficacy, this is an important question.32
In contrast to our results, NO bioavailability was not suppressed in a study of sunitinib-induced hypertension in swine.18 However, in a rodent model, anti-VEGF receptor 2 antibody treatment led to both suppressed NO synthase expression in kidney and caused hypertension.15,19 Also, in human subjects treated with vandetanib, an antiangiogenic agent with different tyrosine kinase spectrum, a small decrease in circulating NO levels was reported. In our study, we observed NO suppression by as much as 50% by week 6, which is the largest decrease in circulating NO yet reported in association with antiangiogenic therapy. Together, these findings suggest that biomarker changes may vary according to the individual antiangiogenic drug administered.
Similar to other antiangiogenic therapies, regorafenib caused hypertension with 63% of the subjects developing hypertension at some point during our study. However, the rise in blood pressure observed at 2 and 6 weeks of regorafenib therapy was mild and, as in other studies,16,19 no correlation was observed between blood pressure changes and NO suppression or ET-1 increases. This may reflect the early treatment of hypertension based on home monitoring in our study since most patients who developed hypertension were treated before the office visits at 2 and 6 weeks.
There are no reliable biomarkers to predict response in patients treated with antiangiogenic therapies and circulating biomarkers of antiangiogenic therapy-induced hypertension are attractive biomarkers for several reasons. Office-based blood pressure measurement is subject to variation due to “white coat hypertension,” pain and other factors, and while ambulatory blood pressure monitoring overcomes this limitation, it is not practical.6 Measurement of a biomarker, or several of them, might allow for more accurate readout of appropriate in vivo VEGF blockade. Furthermore, patients that develop antiangiogenic therapy-induced hypertension are typically treated aggressively with antihypertensive medications to reduce their blood pressure,33 thus reducing the utility of blood pressure as a predictive marker. Circulating biomarkers may not be subject to this limitation. Finally, it is unclear which is more predictive of clinical outcomes: hypertension itself (defined as blood pressure >140/90 mm Hg), an individual patient's absolute blood pressure rise (even if they do not develop hypertension), or a change at the tumor level for which hypertension is a surrogate marker.13 The measurement of biomarker changes induced by antiangiogenic therapy might offer an alternative to evaluate predictive efficacy.
In this study, we explored whether NO suppression and ET-1 stimulation were associated with clinical benefit. We did not observe a statistically significant association for either biomarker, although there was a trend towards a greater decrease in plasma NO and increase in ET-1 at 6 weeks in those who achieved clinical benefit. Notably, our ability to find an association between biomarker levels and clinical benefit was limited by the large proportion of patients achieving clinical benefit compared to those who did not, a conclusion supported by our power analysis. Future prospective studies that are larger will be required to evaluate the usefulness of these biomarkers in predicting tumor response.
This study has other limitations. First these patients were all tolerant to and previously failed to sunitinib therapy, making our population of patients a selected population, which might potentially limit the generalization of our conclusions to other patients receiving antiangiogenic therapy. We also lacked dietary information and dietary nitrate content could affect could affect plasma levels of NO metabolites. The reversibility of NO suppression off regorafenib makes this possibility less likely; however, it cannot be excluded.
In conclusion, regorafenib induces rapid and reversible changes in both NO and ET-1 in patients with GIST. These findings support continued investigation of these biomarkers in larger studies, in order to assess their predictive efficacy for cancer outcomes. Whether these changes are observed in other antiangiogenic therapies with broad-spectrum tyrosine kinase activity also requires further evaluation.
Acknowledgments
This work was supported by the National Institutes of Health Training Grant T32-DK007726 -27S1 (to N.D.G) and DK088923 (to B.D.H.) and by a Merit Review from the Department of Veterans Affairs (to M.C.H.), and Bayer (to S.G.).
Diclosure
Nilka de Jesús-González, none. Emily Robinson, none. Radostin Penchev, none. Michael Heinrich, research funding: Novartis, AROG Pharmaceuticals, Bayer (Clinical Trials), equity interest, MolecularMD. Margaret von Mehren, none. William Tap, none. Qian Wang, none. George Demetri, research funding, Novartis, Pfizer, Glaxo-Smith-Kline, Bayer, Johnson and Johnson, Bristol-Myers Squibb, Infinity Pharmaceuticals, consultant, Novartis, Pfizer, Glaxo-Smith-Kline, Bayer, Johnson and Johnson, Infinity Pharmaceuticals, honoraria, Novartis, Pfizer. Suzanne George, none. Benjamin D. Humphreys, none.
References
- 1.Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature 2005;438:967–974 [DOI] [PubMed] [Google Scholar]
- 2.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004;350:2335–2342 [DOI] [PubMed] [Google Scholar]
- 3.Yang JC, Haworth L, Sherry RM, Hwu P, Schwartzentruber DJ, Topalian SL, Steinberg SM, Chen HX, Rosenberg SA. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med 2003;349:427–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Demetri GD, van Oosterom AT, Garrett CR, Blackstein ME, Shah MH, Verweij J, McArthur G, Judson IR, Heinrich MC, Morgan JA, Desai J, Fletcher CD, George S, Bello CL, Huang X, Baum CM, Casali PG. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet 2006;368:1329–1338 [DOI] [PubMed] [Google Scholar]
- 5.Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Oudard S, Negrier S, Szczylik C, Pili R, Bjarnason GA, Garcia-del-Muro X, Sosman JA, Solska E, Wilding G, Thompson JA, Kim ST, Chen I, Huang X, Figlin RA. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol 2009;27:3584–3590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maitland ML, Kasza KE, Karrison T, Moshier K, Sit L, Black HR, Undevia SD, Stadler WM, Elliott WJ, Ratain MJ. Ambulatory monitoring detects sorafenib-induced blood pressure elevations on the first day of treatment. Clin Cancer Res 2009;15:6250–6257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robinson ES, Matulonis UA, Ivy P, Berlin ST, Tyburski K, Penson RT, Humphreys BD. Rapid development of hypertension and proteinuria with cediranib, an oral vascular endothelial growth factor receptor inhibitor. Clin J Am Soc Nephrol 2010;5:477–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu X, Stergiopoulos K, Wu S. Risk of hypertension and renal dysfunction with an angiogenesis inhibitor sunitinib: systematic review and meta-analysis. Acta Oncol 2009;48:9–17 [DOI] [PubMed] [Google Scholar]
- 9.Humphreys BD, Atkins MB. Rapid development of hypertension by sorafenib: toxicity or target? Clin Cancer Res 2009;15:5947–5949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ranpura V, Pulipati B, Chu D, Zhu X, Wu S. Increased risk of high-grade hypertension with bevacizumab in cancer patients: a meta-analysis. Am J Hypertens 2010;23:460–468 [DOI] [PubMed] [Google Scholar]
- 11.Rixe O, Billemont B, Izzedine H. Hypertension as a predictive factor of Sunitinib activity. Ann Oncol 2007;18:1117. [DOI] [PubMed] [Google Scholar]
- 12.Scartozzi M, Galizia E, Chiorrini S, Giampieri R, Berardi R, Pierantoni C, Cascinu S. Arterial hypertension correlates with clinical outcome in colorectal cancer patients treated with first-line bevacizumab. Ann Oncol 2009;20:227–230 [DOI] [PubMed] [Google Scholar]
- 13.Rini BI, Cohen DP, Lu DR, Chen I, Hariharan S, Gore ME, Figlin RA, Baum MS, Motzer RJ. Hypertension as a biomarker of efficacy in patients with metastatic renal cell carcinoma treated with sunitinib. J Natl Cancer Inst 2011;103:763–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robinson ES, Khankin EV, Karumanchi SA, Humphreys BD. Hypertension induced by vascular endothelial growth factor signaling pathway inhibition: mechanisms and potential use as a biomarker. Semin Nephrol 2010;30:591–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Facemire CS, Nixon AB, Griffiths R, Hurwitz H, Coffman TM. Vascular endothelial growth factor receptor 2 controls blood pressure by regulating nitric oxide synthase expression. Hypertension 2009;54:652–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kappers MH, van Esch JH, Sluiter W, Sleijfer S, Danser AH, van den Meiracker AH. Hypertension induced by the tyrosine kinase inhibitor sunitinib is associated with increased circulating endothelin-1 levels. Hypertension 2010;56:675–681 [DOI] [PubMed] [Google Scholar]
- 17.Kappers MH, Smedts FM, Horn T, van Esch JH, Sleijfer S, Leijten F, Wesseling S, Strevens H, Jan Danser AH, van den Meiracker AH. The vascular endothelial growth factor receptor inhibitor sunitinib causes a preeclampsia-like syndrome with activation of the endothelin system. Hypertension 2011;58:295–302 [DOI] [PubMed] [Google Scholar]
- 18.Kappers MH, de Beer VJ, Zhou Z, Danser AH, Sleijfer S, Duncker DJ, van den Meiracker AH, Merkus D. Sunitinib-induced systemic vasoconstriction in swine is endothelin mediated and does not involve nitric oxide or oxidative stress. Hypertension 2012;59:151–157 [DOI] [PubMed] [Google Scholar]
- 19.Mayer EL, Dallabrida SM, Rupnick MA, Redline WM, Hannagan K, Ismail NS, Burstein HJ, Beckman JA. Contrary effects of the receptor tyrosine kinase inhibitor vandetanib on constitutive and flow-stimulated nitric oxide elaboration in humans. Hypertension 2011;58:85–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robinson ES, Khankin EV, Choueiri TK, Dhawan MS, Rogers MJ, Karumanchi SA, Humphreys BD. Suppression of the nitric oxide pathway in metastatic renal cell carcinoma patients receiving vascular endothelial growth factor-signaling inhibitors. Hypertension 2010;56:1131–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hood JD, Meininger CJ, Ziche M, Granger HJ. VEGF upregulates ecNOS message, protein, and NO production in human endothelial cells. Am J Physiol 1998;274:H1054–H1058 [DOI] [PubMed] [Google Scholar]
- 22.Boulanger C, Lüscher TF. Release of endothelin from the porcine aorta. Inhibition by endothelium-derived nitric oxide. J Clin Invest 1990;85:587–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fink J, Fan NY, Rosenfeld L, Stier CT., Jr Contribution of endothelin to the acute pressor response of L-NAME in stroke-prone spontaneously hypertensive rats. J Cardiovasc Pharmacol 1998;31:618–622 [DOI] [PubMed] [Google Scholar]
- 24.Heath VL, Bicknell R. Anticancer strategies involving the vasculature. Nat Rev Clin Oncol 2009;6:395–404 [DOI] [PubMed] [Google Scholar]
- 25.Wilhelm SM, Dumas J, Adnane L, Lynch M, Carter CA, Schütz G, Thierauch KH, Zopf D. Regorafenib (BAY 73-4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer 2011;129:245–255 [DOI] [PubMed] [Google Scholar]
- 26.NIH. Regorafenib in patients with metastatic and/or unresectable gastrointestinal stromal tumor <http://clinicaltrials.gov/ct2/show/NCT01068769>. Accessed 26 February 2012.
- 27.Levey AS, Coresh J, Greene T, Marsh J, Stevens LA, Kusek JW, Van Lente F; Chronic Kidney Disease Epidemiology Collaboration Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem 2007;53:766–772 [DOI] [PubMed] [Google Scholar]
- 28.NCI. Common terminology criteria for adverse events (CTCAE) v4.0. <http://evs.nci.nih.gov/ftp1/CTCAE/About.html>. [PubMed]
- 29.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–247 [DOI] [PubMed] [Google Scholar]
- 30.Banfor PN, Franklin PA, Segreti JA, Widomski DL, Davidsen SK, Albert DH, Cox BF, Fryer RM, Gintant GA. ETA receptor blockade with atrasentan prevents hypertension with the multitargeted tyrosine kinase inhibitor ABT-869 in telemetry-instrumented rats. J Cardiovasc Pharmacol 2009;53:173–178 [DOI] [PubMed] [Google Scholar]
- 31.Veronese ML, Mosenkis A, Flaherty KT, Gallagher M, Stevenson JP, Townsend RR, O'Dwyer PJ. Mechanisms of hypertension associated with BAY 43-9006. J Clin Oncol 2006;24:1363–1369 [DOI] [PubMed] [Google Scholar]
- 32.van den Meiracker AH, Danser AH, Sleijfer S, Kappers MH. Re: Hypertension as a biomarker of efficacy in patients with metastatic renal cell carcinoma treated with sunitinib. J Natl Cancer Inst 2011;103:1557; author reply 1558. [DOI] [PubMed] [Google Scholar]
- 33.Maitland ML, Bakris GL, Black HR, Chen HX, Durand JB, Elliott WJ, Ivy SP, Leier CV, Lindenfeld J, Liu G, Remick SC, Steingart R, Tang WH; Cardiovascular Toxicities Panel, Convened by the Angiogenesis Task Force of the National Cancer Institute Investigational Drug Steering Committee Initial assessment, surveillance, and management of blood pressure in patients receiving vascular endothelial growth factor signaling pathway inhibitors. J Natl Cancer Inst 2010;102:596–604 [DOI] [PMC free article] [PubMed] [Google Scholar]