Abstract
Two classes of hepatitis C antiviral agents currently exist, ie, direct-acting antivirals and host-targeting antivirals. Direct-acting antivirals target viral proteins including NS3/NS4A protease, NS5B polymerase and NS5A protein, while host-targeting antivirals target various host proteins critical for replication of the hepatitis C virus (HCV). Alisporivir is the most advanced host-targeting antiviral in clinical development. Alisporivir blocks HCV replication by neutralizing the peptidyl-prolyl isomerase activity of the abundant host cytosolic protein, cyclophilin A. Due to its unique mechanism of antiviral action, alisporivir is pangenotypic, provides a high barrier for development of viral resistance, and does not permit cross-resistance to direct-acting antivirals. Alisporivir has an excellent pharmacokinetic and safety profile. Phase I and II clinical studies have demonstrated that alisporivir causes a dramatic reduction in viral loads in HCV-infected patients. Alisporivir was shown to be highly potent in treatment-naïve and treatment-experienced patients with genotype 1 as well as in those with genotypes 2 or 3. Low viral breakthrough rates were observed and the most frequent clinical and laboratory adverse events associated with alisporivir in combination with pegylated interferon-alpha and ribavirin were similar to those associated with pegylated interferon-alpha and ribavirin used alone. A laboratory abnormality observed in some patients receiving alisporivir is hyperbilirubinemia, which is related to transporter inhibition and not to liver toxicity. The most recent clinical results suggest that alisporivir plus other direct-acting antivirals should provide a successful treatment option for difficult-to-treat populations, such as nonresponders to prior interferon-alpha therapy and patients with cirrhosis. In conclusion, alisporivir represents an attractive candidate component of future interferon-free regimens.
Keywords: alisporivir, cyclophilins, cyclophilin inhibitors, hepatitis C virus, treatment
Introduction
The hepatitis C virus (HCV) is the major causative agent of acute and chronic liver disease, which is associated with a high risk of hepatocellular carcinoma and liver cirrhosis.1 Nearly 200 million people worldwide (3% of the population), including 4–5 million in the US, are chronically infected with HCV, and four million new infections occur every year.2,3 In the developed world, HCV accounts for two thirds of all cases of liver cancer and transplants and, in the US, about 12,000 people are estimated to die from HCV each year.4,5 Weekly injection of pegylated interferon-alpha (IFNα) together with daily administration of the nucleoside analog, ribavirin, has greatly enhanced the percentage of chronically HCV-infected patients able to reach a sustained antiviral response, defined as a clearance of HCV RNA from blood 24 weeks after treatment termination.6,7 However, the combination of pegylated IFNα and ribavirin has a success rate of only about 50% in patients with genotype 1 or 4 and has severe side effects.8–10 Not only is genotype 1 the most prevalent HCV genotype in Europe, North and South America, China, and Japan, it is also the most difficult to treat.11 The recent approval of the direct-acting antiviral protease inhibitors, boceprevir and telaprevir, improved efficacy to a sustained antiviral response of 75% in patients with genotype 1 when used in combination with pegylated IFNα and ribavirin, but this does not apply to other genotypes. Therefore, there is an urgent need for development of new potent pangenotypic anti-HCV agents in order to improve, shorten, simplify, and decrease the cost of management of HCV. Cyclophilin inhibitors represent an attractive class of such potent pangenotypic anti-HCV agents.
Discovery of alisporivir
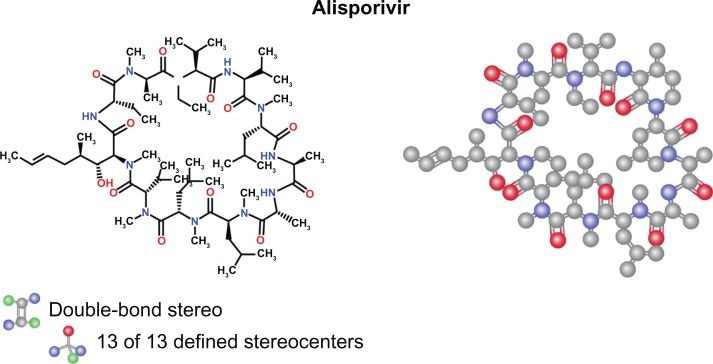
The first cyclophilin inhibitor shown to have in vitro anti-HCV activity was cyclosporine A, an immunosuppressant drug widely used in organ transplantation to prevent rejection.12 Cyclosporine A was first reported to be clinically effective against HCV when a combination of cyclosporine A and IFNα was found to be more effective than IFNα alone.13,14 Subsequent in vitro studies demonstrated that cyclosporine A prevents both HCV RNA replication and HCV protein production in hepatoma cell lines.15–18 Cyclosporine A, by binding to its intracellular receptor, cyclophilin A, forms a ternary complex with calcineurin that triggers a reduction in activity of the immune system by interfering with the activity and growth of T cells.12 This led several companies to synthesize a second generation of cyclophilin inhibitors devoid of immunosuppressive activity, including the cyclosporine A derivatives, NIM-811, alisporivir (previously called Debio-025), and SCY-635.19–21 The chemical structure of alisporivir is shown in Figure 1. Alisporivir, which is synthesized from cyclosporine A, differs from parent cyclic undecapeptide cyclosporine A, with sarcosine replaced by Me-alanine at position 3, with leucine replaced by valine at position 4, and with the nitrogen being N-ethylated instead of N-methylated (Figure 1). These modifications enhance the binding affinity of alisporivir for cyclophilins while abolishing its binding to calcineurin and thus immunosuppressive activity.
Figure 1.
Structure of alisporivir.
Notes: The chemical name of alisporivir is cyclo[L-alanyl-D-alanyl-N-methyl-L-leucyl-N-methyl-L-leucyl-N-methyl-L-valyl- (2S,3R,4R,6E)-3-hydroxy-4-methyl-2-(methylamino)-6-octenoyl-(2S)-2-aminobutanoyl- N-methyl-D-alanyl-N-ethyl-L-valyl-L-valyl-N-methyl-L-leucyl]. The molecular formula of alisporivir is C63H113 N11O12 and its molecular weight is 1216.6.
Preclinical studies
Alisporivir clears HCV replicon cells when used alone or in combination with direct-acting antivirals, such as protease or polymerase inhibitors.22 The combination of alisporivir with IFNα resulted in additive antiviral effects.22 Resistant replicon cells, selected by cell passage in the presence of increasing concentrations of alisporivir, remained sensitive to protease or polymerase inhibitors and IFNα.22 Alisporivir exerts its antiviral activity against wild-type HCV, as well as HCV replicons resistant to protease or polymerase inhibitors.22 The emergence of variants resistant to protease inhibitors is restrained by the presence of alisporivir.22 Alisporivir was potent against HCV in a chimeric mouse model and its antiviral effect was enhanced in combination with IFNα.23 At a nM range, alisporivir inhibits the replication of replicons derived from genotypes 1a, 1b, 2a, and 3, as well as the infectivity of whole viruses (genotype 2a JFH-1).20,24,25
Pharmacokinetics and metabolism
A high loading dose of alisporivir may help to achieve adequate drug levels rapidly in plasma. Specifically, peak plasma levels of alisporivir were already reached 2 hours post administration of single 50–1600 mg doses, while a steady-state drug concentration was achieved after 14 days of treatment.26 Once-daily administration of alisporivir was chosen in the Phase II studies based on an estimated terminal half-life of approximately 100 hours.26 Additional pharmacokinetic studies confirmed rapid absorption of alisporivir (<2 hours) and high plasma accumulation after 4 weeks of treatment.27 Alisporivir, orally administered as soft gel capsules, is metabolized and oxidized by the very abundant liver cytochrome P450 isoenzyme, 3A4, and is ultimately eliminated in the bile.
Clinical studies
The results and status of multiple Phase I and II studies, which tested alisporivir for safety and efficacy in HCV patients, are described in the following sections.
Phase I DEB-025-103 study
Alisporivir was the first oral nonimmunosuppressive cyclophilin inhibitor to enter into clinical trials. Proof-of-concept was established for alisporivir when administered as monotherapy to patients coinfected with human immunodeficiency virus-1 and HCV.26 Specifically, a 14-day, double-blind, placebo-controlled study using 1200 mg oral doses of alisporivir twice daily resulted in a maximal drop in HCV RNA levels of 3.63 log10 copies/mL.26 In 15 of 16 subjects treated with alisporivir, the HCV viral load decreased by more than 2 log, meaning a decrease of more than 99% in viral load. In three patients, the virus became undetectable after 8–15 days. All three HCV genotypes (1, 3, and 4) identified in the study responded well to the dose administered and no patient developed viral breakthrough during treatment, suggesting that alisporivir has a high barrier to resistance selection.26 Viral rebound occurred only after termination of treatment.26 The most commonly reported adverse events were abdominal pain, feeling hot, vomiting, fatigue, and pyrexia; however, none of these were considered dose-limiting. Transient hyperbilirubinemia led to discontinuation of treatment in a few patients.26 Alisporivir was found to be more efficacious in patients infected with genotype 3 than in patients infected with genotype 1 or 4.26
Phase II studies
DEB-025-HCV-203
The efficacy of alisporivir in combination with pegylated IFNα was evaluated in a 29-day Phase II study.28 This study included 90 treatment-naïve patients with chronic HCV infection who were randomized into five cohorts. Four cohorts received placebo or alisporivir 200, 600, or 1000 mg in combination with pegylated IFNα. The last cohort received monotherapy of alisporivir 1000 mg. To reach high drug plasma levels rapidly, patients received alisporivir twice daily for a week, and then once daily for the next 3 weeks. At the end of the study (day 29), the viral load drop was 3.56 log10 copies/mL in patients treated with pegylated IFNα and placebo; 3.30 log10 copies/mL in patients treated with alisporivir 200 mg and pegylated IFNα; and 2.87 log10 copies/mL in patients treated with alisporivir 1000 mg alone. More profound viral load drops were found in patients treated with alisporivir 600 mg/1000 mg and pegylated IFNα (5.07 log10 and 5.09 log10 copies/mL, respectively). Confirming the Phase I results above, the antiviral activity of alisporivir monotherapy was more dramatic in patients infected with genotype 2 or 3 than in patients infected with genotype 1 or 4. Headache, nausea, fatigue, and hyperbilirubinemia were the most frequent adverse events in patients treated with the highest dose of alisporivir (1000 mg). Importantly, the frequency of these adverse events was diminished in patients treated with lower doses of alisporivir. Remarkably, four patients who did not receive pegylated IFNα and ribavirin treatment remained with undetectable viral RNA even after 28–116 weeks at the end of the 29 days of treatment with alisporivir. This finding is critical because it suggests that short-term treatment (4 weeks) with alisporivir suffices to reach a sustained antiviral response.28
DEB-025-HCV-205
The DEB-025-HCV-205 (ESSENTIAL) study evaluated the safety and efficacy of alisporivir combined with pegylated IFNα and ribavirin in treatment-naïve patients infected with genotype 1.29 The first endpoint was to evaluate the sustained virologic response at 24 weeks post-treatment (SVR24) in 288 patients randomized into four arms. All patients received pegylated IFNα and ribavirin. In arm 1, patients received placebo once daily (n = 73) for 48 weeks. In arms 2, 3, and 4, all patients received alisporivir twice daily at a dose of 600 mg (1200 mg total daily dose) as part of a loading dose strategy. At the end of the first week, all patients in arms 2, 3, and 4 were subsequently treated with alisporivir 600 mg once daily alone. In arm 2, patients received alisporivir combined with pegylated IFNα and ribavirin for 48 weeks (n = 72). In arm 3, the duration of treatment with alisporivir with pegylated IFNα and ribavirin (n = 71) was determined using response-guided therapy. In arm 3, patients who developed a rapid virologic response qualified to receive a shortened 24-week treatment, whereas patients without a rapid virologic response continued treatment for 48 weeks. In arm 4, patients received alisporivir with pegylated IFNα and ribavirin (n = 72) for 24 weeks. An assessment was conducted in all patients 24 weeks after treatment cessation.
The frequencies of serious adverse events and discontinuations were similar in all arms through the 24-week and 48-week treatment periods. An increased frequency of laboratory abnormalities, including total bilirubin, was found in alisporivir-treated patients. However, hyperbilirubinemia was mainly observed during the first week when alisporivir was administered twice daily, and total bilirubin levels returned to pretreatment levels at week 12.
SVR24 was improved in all arms when alisporivir was administered, ie, 55% in arm 1 (pegylated IFNα and ribavirin), 76% (statistically significant) in arm 2 (48 weeks of alisporivir), 69% in arm 3 (24 or 48 weeks of response-guided treatment with alisporivir), and 53% in arm 4 (24 weeks of alisporivir). Alisporivir improved the sustained antiviral response rate up to 100% in patients with the CC IL28B allele and up to 73% in patients with the TT IL28B allele compared with 17% in the pegylated IFNα and ribavirin arm. The frequency of viral breakthrough was low (about 2.8%) in patients who received alisporivir. Together, these data suggest that 48 weeks of treatment with alisporivir 600 mg, pegylated IFNα, and ribavirin significantly improves the SVR24 in treatment-naïve patients infected with genotype 1.
CDEB025A2211
The CDEB025A2211 (VITAL-1) study evaluated the safety and efficacy of alisporivir administered either alone, with pegylated IFNα, or with pegylated IFNα and ribavirin in 385 treatment-naïve patients infected with genotype 2 or 3 randomized to one of five arms.30 In arm 1, patients (n = 83) received alisporivir 1000 mg alone once daily. In arm 2, patients (n = 84) received alisporivir 600 mg once daily with ribavirin. In arm 3, patients (n = 94) received alisporivir 800 mg once daily with ribavirin. In arm 4, patients (n = 84) received alisporivir 600 mg once daily with pegylated IFNα. In arm 5, patients (n = 40) received pegylated IFNα and ribavirin. All patients received alisporivir 600 mg twice daily for the first week, after which they were randomized. The rapid virologic response rate was analyzed in all treatment arms at week 4 to determine whether a patient should remain on the original treatment or switch at week 6 to alisporivir 600 mg once daily with pegylated IFNα and ribavirin through to the end of treatment at week 24.
Alisporivir was well tolerated in all arms through the 24-week treatment period. A few patients (six of 260) who received alisporivir developed hyperbilirubinemia, which was detected at week one and maintained until week 24. This transient and reversible event was due to alisporivir-mediated block of the uptake of bilirubin by transporters OATP1B1 and OATP1B3, leading to elevated indirect bilirubin, and efflux by transporter multidrug resistance-associated protein 2, also called canalicular multispecific organic anion transporter 1, leading to elevated direct bilirubin.
Alisporivir improved the sustained virologic response at 12 weeks post-treatment (SVR12) in all arms compared with pegylated IFNα and ribavirin. The SVR12 value was 81%, 83%, 81%, 77%, and 58% for arms 1, 2, 3, 4, and 5, respectively, and the rapid virologic response rate value was 29%, 37%, and 42% for arms 1, 2, and 3, respectively, suggesting that alisporivir is an attractive strategy as monotherapy or in combination with ribavirin for treatment-naïve patients infected with genotype 2 or 3, who demonstrated a rapid virologic response 4 weeks post treatment. Patients who did not achieve a rapid virologic response switched at week 6 to a triple therapy until the end of treatment, and the SVR12 value was 94%, 92%, and 96% for arms 1, 2, and 3, respectively, suggesting that postponed supplementation of alisporivir with pegylated IFNα and ribavirin facilitates HCV clearance in patients infected with genotype 2 or 3 who do not achieve a rapid virologic response.
New results from the VITAL-1 study presented at the 63rd Annual Meeting of the American Association for the Study of Liver Diseases revealed that alisporivir improved SVR24 rates in all arms compared with pegylated IFNα and ribavirin.31 Specifically, SVR24 rates were 80%, 85%, 81%, 80%, and 58% in arms 1, 2, 3, 4, and 5, respectively. Relapse rates were diminished in alispo rivir-treated patients (11%, 8%, 6%, and 10% in arms 1, 2, 3 and 4, respectively) compared with those treated with pegylated IFNα and ribavirin (25% in arm 5). The difference in rapid virologic response rate between patients with the CC IL28 allele and those with the non-CC IL28 allele was overcome by adequate alisporivir exposure. Alisporivir exposure, low baseline viral load, and ribavirin dose were the most important parameters predicting high rapid virologic response. Interestingly, a comparative analysis of expression of IFN-stimulated genes between baseline and week 4 indicated downregulation of IFN-stimulated genes by IFN-free alisporivir treatment compared with strong upregulation of IFN-stimulated genes by IFN-containing regimens.31 The safety profile of the IFN-free alisporivir treatment was markedly superior to that of regimens containing IFN. The final results of the VITAL-1 study demonstrated that alisporivir combined with ribavirin achieves high rates of sustained HCV clearance (SVR24) as an IFN-free or an IFN-addon regimen in treatment-naïve patients infected with genotype 2 or 3 with early HCV clearance, and with low viral breakthrough or post-treatment relapse.
CDEB025A2210
The CDEB025A2210 (FUNDAMENTAL) study evaluated the safety and efficacy of alisporivir in combination with pegylated IFNα and ribavirin in 461 patients infected with genotype 1 who relapsed or did not respond to prior treatment with pegylated IFNα and ribavirin.32 In arm 1, patients (n = 121) received alisporivir 600 mg once daily. In arm 2, patients (n = 117) received 800 mg alisporivir 800 mg once daily. In arm 3, patients (n = 109) received alisporivir 400 mg twice daily. In arm 4, patients (n = 114) received placebo once or twice daily. All patients received pegylated IFNα and ribavirin. In arms 1 and 2, patients received alisporivir 600 mg twice daily for a week, after which they were randomized to their respective alisporivir dose. The total duration of treatment for all arms was 48 weeks, with intended assessment of SVR12 and SVR24.
A primary endpoint was comparison of the percentage of patients who achieved a complete early virologic response (<25 copies/mL) at week 12.31 Rapid virologic response rates were also quantified at week 4 in all patients. Alisporivir was well tolerated during the first 12 weeks of treatment. The frequencies of serious adverse events and discontinuations were comparable among all arms. The most frequent adverse events were nausea, neutropenia, and insomnia. Alisporivir achieved an improvement in complete early virologic response of 46.4%, 61.1%, 71.3%, and 32.7% in arms 1, 2, 3, and 4, respectively. Administration of alisporivir 400 mg twice daily increased the percentage of patients who achieved a complete early virologic response compared with the 800 mg once daily dose, and mediated higher viral drops over time in comparison with arms 1 and 2 as well as arm 4 (pegylated IFNα and ribavirin). Alisporivir improved the proportion of relapsers who achieved a complete early virologic response to 62.5%, 77.6%, 72.9%, and 51.9% in arms 1, 2, 3, and 4, respectively. Alisporivir significantly improved the percentage of nonresponders who achieved complete early virologic response (a 47.5% complete early virologic response in arm 2 and a 70% complete early virologic response in arm 3 compared with 14.3% in arm 4 which received pegylated IFNα and ribavirin). The 400 mg twice daily dose (arm 3) was extremely efficacious in the null responders who achieved a 69.7% complete early virologic response compared with 11.1% for those on pegylated IFNα and ribavirin. Viral breakthrough was low across all groups. Together these data suggest that addition of alisporivir to pegylated IFNα and ribavirin represents an attractive treatment option for difficult-to-treat patients.
A subsequent interim assessment was conducted at week 24.33 The complete early virologic response values were 48.2%, 61.1%, 72.5%, and 35.5% in arms 1, 2, 3 and 4, respectively, and rapid virologic response values were 20.9%, 25.0%, 40.4%, and 7.3% in arms 1, 2, 3 and 4, respectively. All alisporivir treatment groups maintained higher virologic response rates compared with those treated with pegylated IFNα and ribavirin, and the patients who received the 400 mg twice daily dose (arm 3) showed the highest viral drop at week 24. Specifically, VR24 values were 64.5%, 70.4%, 73.4%, and 31.8% in arms 1, 2, 3, and 4, respectively. Alisporivir improved VR24 (70.8%–79.2%) in relapsers compared with pegylated IFNα and ribavirin (55.6%). The 400 mg twice daily alisporivir dose (arm 3) improved VR24 (75.4%) in nonresponders compared with pegylated IFNα and ribavirin (8.9%). Importantly, the 400 mg twice daily dose improved VR24 (70.6%) in the most difficult-to-treat null responders compared with pegylated IFNα and ribavirin (5.6%). The benefit of alisporivir was observed in all groups independently of cirrhosis status or the IL28 allele. The overall viral breakthrough rates observed were 11.8%, 7.3%, 1.8%, and 4.5% in arms 1, 2, 3, and 4, respectively. Viral breakthroughs were higher in nonresponders compared with relapsers. The 400 mg twice daily dose of alisporivir clearly had the lowest viral breakthrough rate in all patient populations, which correlated with higher plasma trough levels of the compound. Resistance analysis of these viral breakthroughs is currently ongoing.
Serious adverse events were more frequent in alisporivir-treated patients than in pegylated IFNα-treated and ribavirin-treated patients, with the greatest incidence (11.1%) in patients treated with the 400 mg twice daily dose. One case of transient pancreatitis was reported. The most frequent clinical and laboratory adverse events were neutropenia, anemia, hyperbilirubinemia, and thrombocytopenia. Hyperbilirubinemia was transient and reversible and not associated with liver toxicity. Table 1 summarizes the results of the CDEB025A2211,VITAL-1, and FUNDAMENTAL studies.
Table 1.
Summary of results of the VITAL-1 and FUNDAMENTAL studies
| Study | Treatment | Results |
|---|---|---|
| VITAL-1 | Arm 1: Alisporivir 1,000 mg qda–c Arm 2: Alisporivir 600 mg qda–c plus ribavirin Arm 3: Alisporivir 800 mg qda–c plus delayed addition of IFN and ribavirin Arm 4: Alisporivir 600 mg qda plus IFN and ribavirin |
Alisporivir exposure, low baseline viral load and ribavirin dose were the most important parameters to predict high RVR. The safety profile of the IFN-free alisporivir treatment was markedly superior to that of IFN-containing regimens. The final results of the study demonstrated that alisporivir combined with ribavirin achieves high rates of sustained HCV clearance (SVR24) as IFN-free or IFN-add-on regimen in treatment-naïve genotype 2- or 3-infected patients with early HCV clearance, with low viral breakthrough or post-treatment relapse. All alisporivir treatment groups maintained higher virological response rates compared to IFN and ribavirin, and patients, who received the 400 mg bid dose, exhibited the highest viral drop. Alisporivir improved VR24 in relapsers compared to IFN and ribavirin. The 400 mg bid alisporivir dose improved VR24 in nonresponders compared to IFN and ribavirin. The 400 mg bid dose improved VR24 in the most difficult-to-treat null responders compared to IFN and ribavirin. The benefit of alisporivir was observed in all groups independently of the cirrhosis status or the IL28 allele. Viral breakthroughs were higher in nonresponders compared to relapsers. The 400 mg bid of alisporivir had the lowest viral breakthrough rate in all patient populations, which correlated with the higher Ctrough level of the compound it provided. Serious AEs were more frequent in alisporivir-treated patients compared to IFN and ribavirin-treated patients, with the greatest incidence in 400 mg bid dose-treated patients. The most frequent clinical and laboratory AEs were neutropenia, anemia, hyperbilirunemia and thrombocytopenia. Hyperbilirunemia was transient and reversible and not associated with liver toxicity. |
| FUNDAMENTAL | Arm 1: Alisporivir 600 mg qdd Arm 2: Alisporivir 800 mg qdd Arm 3: Alisporivir 400 mg bid Arm 4: Placebo qd or bid |
Notes:
All patients received 7 days of alisporivir 600 mg bid. On day 8 patients initiated their randomized dose of alisporivir;
patients exhibiting RVR (<25 IU/mL) received their originally randomized regimen throughout the remainder of the 24-week treatment period;
patients with viral load ≥ 25 lU/mL at week 4 switched treatment at week 6 to triple therapy containing alisporivir 600 mg qd plus IFN and ribavirin for the remaining 18 weeks;
patients in arms 1 and 2 received 7 days of loading dose treatment with alisporivir 600 mg bid. On day 8 patients initiated treatment with their randomized dose of alisporivir.
Abbreviations: mg, milligram; qd, once daily; bid, twice daily; IFN, interferon; RVR, rapid virologic response; HCV, hepatitis C virus; SVR24, sustained virologic response after 24 weeks; VR24, virologic response after 24 weeks; IL28, interleukin 28; AEs, adverse events; IU/mL, international units/milliliter.
Mechanism of action of alisporivir
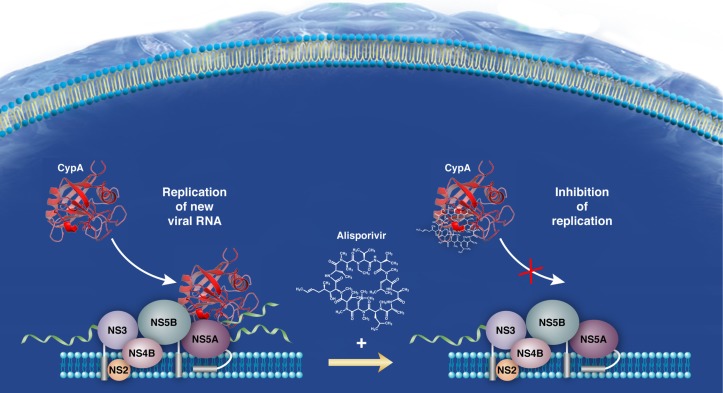
The anti-HCV mechanism of action of alisporivir remain to be unraveled. Importantly, a number of critical discoveries have helped to guide the basic research field to understand better the mechanism of action of alisporivir as well as other cyclophilin inhibitors. A fundamental finding was that the intracellular host cytosolic protein, cyclophilin A, governs HCV replication via its peptidyl-prolyl isomerase hydrophobic pocket.34,35 Another was that cyclophilin inhibitors, including alisporivir, inhibit the peptidyl-prolyl isomerase activity of cyclophilin A by binding directly to the enzymatic pocket of cyclophilin A.36,37 An additional key finding, which helped our understanding of the mechanism of action of alisporivir, was the recent discovery of the viral ligand for cyclophilin A, ie, the HCV nonstructural 5A (NS5A) protein.38–42 Importantly, alisporivir blocks cyclophilin A-NS5A interactions in a dose-dependent manner.40,41,43 This block by alisporivir was observed for NS5A proteins derived from all genotypes tested so far.40 Cyclophilin A binds to prolines located in the domain II of NS5A.38,41,44 Altogether, these in vitro data suggest that cyclophilin A-NS5A contacts are vital for HCV replication and that disrupting them is an attractive mechanism of action for alisporivir (Figure 2). It is important to note that the cellular function of cyclophilin A remains unknown. The unique function attributed to cyclophilin A is its peptidyl-prolyl isomerase activity. This folding activity can mediate or influence many cellular functions, including protein transport, protein stability, and protein–protein interactions.
Figure 2.
Model for the mechanism of action of alisporivir.
The absolute need for direct contact between cyclophilin A and NS5A to allow robust HCV replication remains to be elucidated. The most interesting finding for a function of cyclophilin A in HCV replication is that cyclophilin A specifically promotes NS5A binding to viral RNA.45 An attractive model is that cis-trans isomerization of NS5A proline bonds by cyclophilin A enhances both NS5A binding to viral RNA and HCV replication. Given that NS5A binds NS5B directly, one cannot exclude the possibility that cyclophilin A, by interacting with NS5A, also modulates the polymerase activity of NS5B and therefore viral replication.46 Previous work has demonstrated that NS5A possesses the ability to counteract the innate response to permit establishment of HCV replication.47 A very recent study demonstrated that cyclophilin A binds both NS5A and the IFN-regulatory factors, especially IRF9, and that alisporivir blocks all these interactions.48 Thus, one can envisage that contact between cyclophilin A and NS5A and/or specific IFN-regulatory factors modulates the innate response. Therefore, if the main antiviral mechanism of action of alisporivir is disruption of cyclophilin A-NS5A interactions, it could regulate multiple phases of the HCV replication cycle and even the pathogenesis of HCV.
HCV resistance to alisporivir
HCV exists in every patient as a large population of quasi-species due to its high replication rate (about 1012 viruses per day) and high mutation frequency (about one per viral genome produced). Theoretically, all resistance “mutations” are already pre-existing prior to antiviral treatment, albeit at very low percentages, but can be quickly enriched under drug selection pressure. To date, a single mutation can confer high-level resistance for all direct-acting antivirals discovered. Except for nucleos(t)ide NS5B polymerase inhibitors, resistance develops quickly both in vitro and in patients. Therefore, it is likely that it may take at least three direct-acting antivirals to suppress resistance completely in an IFN-free regimen. A complementary or alternative approach is to target host factors essential for viral replication that may present a higher genetic barrier to resistance. The host-targeting antiviral, alisporivir, is such a candidate and may serve as a critical component for future IFN-free regimens.
In vitro studies have shown that alisporivir-resistant replicons can be selected under drug selection pressure. However, the development of resistance is a long process (>3 weeks) compared with development of resistance to direct-acting antiviral agents (about one week), suggesting that alisporivir raises a high barrier to development of resistance.49 Attempts to increase the alisporivir concentration or level of resistance further were unsuccessful. Replicons cleared instead of becoming more resistant.22 Mutations, which emerged in alisporivir-resistant replicons were mainly located in the domain II of NS5A, which is the binding locus of cyclophilin A.40,41,43,44 Individual and combinational analyses of NS5A mutations emerging during alisporivir selection revealed that D320E constantly arose in alisporivir-resistant replicons (genotype 1a and 1b), but by itself only reduced susceptibility to alisporivir by approximately 2–3-fold. Note that the mutations occur only in the viral NS5A protein and not in the host cyclophilin A protein. NS5A mutations did not significantly affect the fitness of resistant replicons. Additional in vitro studies suggest that it is not a single mutation in the domain II of NS5A (ie, D320E), but rather a combination of multiple mutations (ie, D320E/Y321N), that is required to render HCV resistant to alisporivir.40,41,43 This again suggests that alisporivir is associated with a high genetic barrier for development of viral resistance.
Importantly, very low viral breakthroughs occurred in alisporivir-treated patients.49 Viral breakthrough was observed only in patients with the non-CC IL28B allele in the ESSENTIAL study, and was associated with a reduction in dose or cessation of pegylated IFNα and ribavirin, or with low drug exposure. The D320E mutation was detected in some but not all patients who had a viral breakthrough. Phenotypic analysis suggested that the D320E substitution causes only a modest (about three-fold) increase of the EC50 to alisporivir, and that D320E alone is not sufficient to cause viral breakthrough. In the VITAL-1study, multiple NS5A amino acid changes were identified. Some of the NS5A mutations collectively contributed to a mild to moderate fold increase in EC50 to alisporivir (<17-fold). These results further suggest that, in contrast with direct-acting antivirals, multiple mutations (rather than a single one) are required to confer a significant level of resistance to alisporivir. Importantly, alisporivir-resistant clones selected from patients remained sensitive to direct-acting antivirals.49 Altogether, these in vivo data strongly suggest that alisporivir has a high barrier to the development to viral resistance.
Future of alisporivir in HCV therapies
Triple therapy approved by the US Food and Drug Administration, which consists of the first-generation direct-acting antivirals, ie, the protease inhibitors, boceprevir and telaprevir, combined with pegylated IFNα and ribavirin, increases the risk of rash and anemia in HCV patients compared with pegylated IFNα and ribavirin. Phase III studies with the second-generation direct-acting antivirals demonstrate improved safety profiles, but adverse events associated with pegylated IFNα and ribavirin have remained. Because of this major treatment limitation, a novel therapeutic strategy has recently emerged. This strategy consists of an IFN-free treatment that would offer high efficacy, and at the same time, a low incidence of adverse events. Two classes of inhibitors could currently be part of this IFN-free treatment. The first class consists of direct-acting antivirals, which comprise a large number of inhibitors, including the NS3/4A protease inhibitors, NS5B polymerase inhibitors, and NS5A inhibitors. The second class of inhibitors consists of host-targeting antivirals, including the cyclophilin inhibitors and several immunomodulators such as monoclonal antibodies, novel IFN formulations, vaccines, and RNA interference and immunomodulatory therapies.
Recent Phase II studies presented at the 63rd Annual Meeting of the American Association for the Study of Liver Diseases provided proof-of-concept that direct-acting antiviral combination therapies can provide high efficacy in the absence of pegylated IFNα and ribavirin. For example, the ELECTRON study, which combined the polymerase inhibitor, sofosbuvir (GS-7977), the NS5A inhibitor, GS-5885, and ribavirin, showed high SVR12 rates in treatment-naïve patients infected with genotype 1.50 Similarly, the combination of GS-7977 and daclatasvir (BMS-790062), an NS5A inhibitor, showed a high sustained antiviral response (90%–100%) in treatment-naïve patients infected with genotype 1, 2, or 3, even in the absence of ribavirin.51 Together, these two studies suggest that the combination of a polymerase inhibitor and an NS5A inhibitor is an attractive direct-acting antiviral combination therapy for treatment-naïve HCV patients. Another Phase II study, which combined daclatasvir, the protease inhibitor asunaprevir (BMS-650032), and the polymerase inhibitor BMS-791325, without ribavirin, showed a high SVR12 rate (94%) in treatment-naïve patients infected with genotype 1a.52 An additional study, which combined the protease inhibitor ABT-450, the NS5A inhibitor ABT-267, and the polymerase inhibitor ABT-333, without ribavirin, showed a high SVR12 rate in treatment-naïve patients infected with genotype 1a (83%) or 1b (96%). Importantly, the same triple therapy plus ribavirin showed a high SVR12 rate in null responders (89% and 100% for genotype 1a and 1b patients, respectively).53 Although some of these recent Phase II studies had low numbers of enrolled patients, overall they provide proof-of-concept that IFN-free regimens represent the future for an HCV therapy or cure.
Although the combination of alisporivir and ribavirin showed high efficacy in treatment-naïve patients infected with genotype 2 or 3, it is likely that alisporivir will have to be combined with one or even two direct-acting antivirals to achieve a high sustained antiviral response in treatment-naïve patients infected with all genotypes, relapsers, and nonresponders.30,31 Alisporivir can be combined with either a second-generation protease inhibitor, a polymerase inhibitor, and/or an NS5A inhibitor to be part of a potent IFN-free regimen. Multiple combination studies should be conducted (at least in vitro) to identify the drug combination with the best synergistic and pan-genotypic antiviral effects. An interesting combination could be alisporivir and an NS5A inhibitor because alisporivir has been shown to block contact between cyclophilin A and the domain II of NS5A, and NS5A inhibitors were shown to target the domain I of NS5A.54,55 This dual mechanism of antiviral action raises the appealing possibility that use of these two classes of inhibitors, which act on two distinct domains of NS5A, is the best drug combination for alisporivir. Nevertheless, this hypothesis remains to be demonstrated in patients.
Conclusion
Alisporivir is a novel host-targeting antiviral agent with a mechanism of action that differs from those of all existing direct-acting antivirals. Because it targets the host protein cyclophilin A, alisporivir is pan-genotypic (demonstrated with genotypes 1, 2, 3, and 4). Alisporivir in combination with pegylated IFNα and ribavirin has demonstrated high efficacy in treatment-naïve patients, relapsers, and nonresponders. An IFN-free alisporivir treatment combined with ribavirin is highly efficacious in treatment-naïve patients infected with genotype 2 or 3. In contrast with the first-generation direct-acting antivirals, boceprevir and telaprevir, alisporivir can be administered daily, a feature that should improve adherence. Importantly, alisporivir is associated with a very low viral breakthrough rate, and no selection of resistant variants has been observed. This high genetic barrier and lack of cross-resistance to direct-acting antiviral agents makes alisporivir an excellent candidate as a drug rescue regimen for patients who have not responded to boceprevir or telaprevir combined with pegylated IFNα and ribavirin. Given that the HCV therapy field is moving very quickly towards IFN-free and ribavirin-free regimens, alisporivir with all its distinctive features as described in this review represents a perfect candidate for combination with specific direct-acting antiviral agents.
Acknowledgments
We acknowledge financial support from the US Public Health Service (AI087470 to PAG).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Dienstag JL, McHutchison JG. American Gastroenterological Association technical review on the management of hepatitis C. Gastroenterology. 2006;130:231–264. doi: 10.1053/j.gastro.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 2.Alter MJ. Epidemiology of hepatitis C virus infection. World J Gastroenterol. 2007;13:2436–2441. doi: 10.3748/wjg.v13.i17.2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soriano V, Madejon A, Vispo E, et al. Emerging drugs for hepatitis C. Expert Opin Emerg Drugs. 2008;13:1–19. doi: 10.1517/14728214.13.1.1. [DOI] [PubMed] [Google Scholar]
- 4.Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis. 2005;5:558–567. doi: 10.1016/S1473-3099(05)70216-4. [DOI] [PubMed] [Google Scholar]
- 5.Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144:705–714. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- 6.Sy T, Jamal MM. Epidemiology of hepatitis C virus (HCV) infection. Int J Med Sci. 2006;3:41–46. doi: 10.7150/ijms.3.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tong MJ, Reddy KR, Lee WM, et al. Treatment of chronic hepatitis C with consensus interferon: a multicenter, randomized, controlled trial. Consensus Interferon Study Group. Hepatology. 1997;26:747–754. doi: 10.1002/hep.510260330. [DOI] [PubMed] [Google Scholar]
- 8.Cross TJ, Antoniades CG, Harrison PM. Current and future management of chronic hepatitis C infection. Postgrad Med J. 2008;84:172–176. doi: 10.1136/pgmj.2008.068205. [DOI] [PubMed] [Google Scholar]
- 9.Simmonds P, Bukh J, Combet C, et al. Consensus proposals for a unified system of nomenclature of hepatitis C virus genotypes. Hepatology. 2005;42:962–973. doi: 10.1002/hep.20819. [DOI] [PubMed] [Google Scholar]
- 10.Manns MP, Wedemeyer H, Cornberg M. Treating viral hepatitis C: efficacy, side effects, and complications. Gut. 2006;55:1350–1359. doi: 10.1136/gut.2005.076646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Global Surveillance and Control of Hepatitis C. Report of a WHO Consultation organized in collaboration with the Viral Hepatitis Prevention Board, Antwerp, Belgium. J Viral Hepat. 1999;6:35–47. [PubMed] [Google Scholar]
- 12.Borel JF. History of the discovery of cyclosporin and of its early pharmacological development. Wien Klin Wochenschr. 2002;114:433–437. [PubMed] [Google Scholar]
- 13.Inoue K, Sekiyama K, Yamada M, Watanabe T, Yasuda H, Yoshiba M. Combined interferon alpha2b and cyclosporin A in the treatment of chronic hepatitis C: controlled trial. J Gastroenterol. 2003;38:567–572. doi: 10.1007/s00535-002-1104-5. [DOI] [PubMed] [Google Scholar]
- 14.Inoue K, Yoshiba M. Interferon combined with cyclosporine treatment as an effective countermeasure against hepatitis C virus recurrence in liver transplant patients with end-stage hepatitis C virus related disease. Transplant Proc. 2005;37:1233–1234. doi: 10.1016/j.transproceed.2004.11.041. [DOI] [PubMed] [Google Scholar]
- 15.Goto K, Watashi K, Murata T, et al. Evaluation of the anti-hepatitis C virus effects of cyclophilin inhibitors, cyclosporin A, and NIM811. Biochem Biophys Res Commun. 2006;343:879–884. doi: 10.1016/j.bbrc.2006.03.059. [DOI] [PubMed] [Google Scholar]
- 16.Ishii N, Watashi K, Hishiki T, et al. Diverse effects of cyclosporine on hepatitis C virus strain replication. J Virol. 2006;80:4510–4520. doi: 10.1128/JVI.80.9.4510-4520.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakagawa M, Sakamoto N, Enomoto N, et al. Specific inhibition of hepatitis C virus replication by cyclosporin A. Biochem Biophys Res Commun. 2004;313:42–47. doi: 10.1016/j.bbrc.2003.11.080. [DOI] [PubMed] [Google Scholar]
- 18.Watashi K, Hijikata M, Hosaka M, et al. Cyclosporin A suppresses replication of hepatitis C virus genome in cultured hepatocytes. Hepatology. 2003;38:1282–1288. doi: 10.1053/jhep.2003.50449. [DOI] [PubMed] [Google Scholar]
- 19.Ma S, Boerner JE, TiongYip C, et al. NIM811, a cyclophilin inhibitor, exhibits potent in vitro activity against hepatitis C virus alone or in combination with alpha interferon. Antimicrob Agents Chemother. 2006;50:2976–2982. doi: 10.1128/AAC.00310-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paeshuyse J, Kaul A, De Clercq E, et al. The non-immunosuppressive cyclosporin DEBIO-025 is a potent inhibitor of hepatitis C virus replication in vitro. Hepatology. 2006;43:761–770. doi: 10.1002/hep.21102. [DOI] [PubMed] [Google Scholar]
- 21.Hopkins S, Scorneaux B, Huang Z, et al. SCY-635, a novel nonimmunosuppressive analog of cyclosporine that exhibits potent inhibition of hepatitis C virus RNA replication in vitro. Antimicrob Agents Chemother. 2010;54:660–672. doi: 10.1128/AAC.00660-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coelmont L, Kaptein S, Paeshuyse J, et al. Debio 025, a cyclophilin binding molecule, is highly efficient in clearing hepatitis C virus (HCV) replicon-containing cells when used alone or in combination with specifically targeted antiviral therapy for HCV (STAT-C) inhibitors. Antimicrob Agents Chemother. 2009;53:967–976. doi: 10.1128/AAC.00939-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inoue K, Umehara T, Ruegg UT, et al. Evaluation of a cyclophilin inhibitor in hepatitis C virus-infected chimeric mice in vivo. Hepatology. 2007;45:921–928. doi: 10.1002/hep.21587. [DOI] [PubMed] [Google Scholar]
- 24.Grisé H, Frausto S, Logan T, Tang H. A conserved tandem cyclophilin-binding site in hepatitis C virus nonstructural protein 5A regulates alisporivir susceptibility. J Virol. 2012;86:4811–4822. doi: 10.1128/JVI.06641-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaul A, Stauffer S, Berger C, et al. Essential role of cyclophilin A for hepatitis C virus replication and virus production and possible link to polyprotein cleavage kinetics. PLoS Pathog. 2009;5:e1000546. doi: 10.1371/journal.ppat.1000546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flisiak R, Horban A, Gallay P, et al. The cyclophilin inhibitor Debio-025 shows potent anti-hepatitis C effect in patients coinfected with hepatitis C and human immunodeficiency virus. Hepatology. 2008;47:817–826. doi: 10.1002/hep.22131. [DOI] [PubMed] [Google Scholar]
- 27.Herrmann E, Kafer A, Flisiak R, Nicolas-Metral V, Zeuzem S, Crabbe R. PK-PD modeling of viral kinetics during treatment with Debio-025 plus pegylated interferon α-2a in treatment-naive HCV patients. J Hepatol. 2009;50(S1):S344. [Google Scholar]
- 28.Flisiak R, Feinman SV, Jablkowski M, et al. The cyclophilin inhibitor debio 025 combined with Peg-IFN2a significantly reduces viral load in treatment naïve hepatitis C patients. Hepatology. 2009;49:1460–1468. doi: 10.1002/hep.22835. [DOI] [PubMed] [Google Scholar]
- 29.Flisiak R, Pawlotsky JM, Crabbe R, et al. Once daily alisporivir (DEB025) plus peg-IFN-alfa-2a/ribavirin results in superior sustained virologic response (SVR24) in chronic hepatitis C genotype 1 treatment-naïve patients – the ESSENTIAL study. J Hepatol. 2011;55 Abstract 190. [Google Scholar]
- 30.Pawlotsky JM, Sarin SK, Foster GR, et al. Alisporivir plus ribavirin is highly effective as interferon-free or interferon-add-on regimen in previously untreated HCV-G2 or G3 patients: SVR12 results from VITAL-1 phase 2b study. J Hepatol. 2012;56 Abstract 1405. [Google Scholar]
- 31.Pawlotsky JM, Sarin SK, Foster GR, et al. Alisporivir plus ribavirin achieves high rates of sustained HCV clearance (SVR24) as interferon (IFN)-free or IFN-add-on regimen in treatment-naive patients with HCV GT2 or GT3: final results from VITAL-1study. Abstract 233 presented at the 63rd Annual Meeting of the American Association for the Study of Liver Diseases; Boston, MA. November 9–12, 2012. [Google Scholar]
- 32.Alberti M, Chuang WL, Flisiak R, et al. Alisporivir (ALV) plus peg-interferon/ribavirin (PR) in HCV G1 treatment-experienced patients achieves primary endpoint with superior efficacy at treatment week 12 compared to retreatment with PR. J Hepatol. 2012;56 Abstract 1406. [Google Scholar]
- 33.Davis G, Kao J, Alberti A, et al. Alisporivir (ALV) plus peg-interferon/ribavirin (P/R) achieves high on-treatment undetectable HCV RNA levels among the most difficult to treat HCV G1 patients. Results of a planned treatment week 24 interim analysis of a randomized, double blind, placebo controlled trial (FUNDAMENTAL study). Presented at the 63rd Annual Meeting of the American Association for the Study of Liver Diseases; Boston, MA. November 9–12, 2012. [Google Scholar]
- 34.Chatterji U, Bobardt MD, Selvarajah S, et al. The isomerase active site of cyclophilin A is critical for hepatitis C virus replication. J Biol Chem. 2009;284:16998–17005. doi: 10.1074/jbc.M109.007625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu Z, Yang F, Robotham JM, Tang H. Critical role of cyclophilin A and its prolyl-peptidyl isomerase activity in the structure and function of the hepatitis C virus replication complex. J Virol. 2009;83:6554–6565. doi: 10.1128/JVI.02550-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ke HM, Zydowsky LD, Liu J, Walsh CT. Crystal structure of recombinant human T-cell cyclophilin A at 2.5 A resolution. Proc Natl Acad Sci U S A. 1991;88:9483–9487. doi: 10.1073/pnas.88.21.9483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zydowsky LD, Etzkorn FA, Chang HY, et al. Active site mutants of human cyclophilin a separate peptidyl-prolyl isomerase activity from cyclosporin A binding and calcineurin inhibition. Protein Sci. 1992;1:1092–1099. doi: 10.1002/pro.5560010903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hanoulle X, Badillo A, Wieruszeski JM, et al. Hepatitis C virus NS5A protein is a substrate for the peptidyl-prolyl cis/trans isomerase activity of cyclophilins A and B. J Biol Chem. 2009;284:13589–13601. doi: 10.1074/jbc.M809244200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fernandes F, Ansari IU, Striker R. Cyclosporine inhibits A direct interaction between cyclophilins and hepatitis c NS5A. PLoS One. 2010;5:e9815. doi: 10.1371/journal.pone.0009815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chatterji U, Lim P, Bobardt MD, et al. HCV resistance to cyclosporin A does not correlate with a resistance of the NS5A-cyclophilin A interaction to cyclophilin inhibitors. J Hepatol. 2010;53:50–56. doi: 10.1016/j.jhep.2010.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coelmont L, Hanoulle X, Chatterji U, et al. DEB025 (alisporivir) inhibits hepatitis C virus replication by preventing a cyclophilin A induced cis-trans isomerisation in domain II of NS5A. PLoS One. 2010;5:e13687. doi: 10.1371/journal.pone.0013687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Waller H, Chatterji U, Gallay P, Parkinson T, Targett-Adams PJ. The use of alphalisa technology to detect interaction between hepatitis C virus-encoded ns5a and cyclophilin A. Virol Methods. 2010;165:202–210. doi: 10.1016/j.jviromet.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garcia-Rivera JA, Bobardt MD, Chatterji U, et al. Multiple mutations in HCV NS5A domain II are required to confer significant level of resistance to alisporivir. Antimicrob Agents Chemother. 2012;56:5113–5121. doi: 10.1128/AAC.00919-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang F, Robotham JM, Grise H, et al. A major determinant of cyclophilin dependence and cyclosporine susceptibility of hepatitis C virus identified by a genetic approach. PLoS Pathog. 2010;6:e1001118. doi: 10.1371/journal.ppat.1001118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Foster TL, Gallay P, Stonehouse NJ, Harris M. Cyclophilin A interacts with domain II of hepatitis C virus NS5A and stimulates RNA binding in an isomerase-dependent manner. J Virol. 2011;85:7460–7464. doi: 10.1128/JVI.00393-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shirota Y, Luo H, Qin W, et al. Hepatitis C virus (HCV) NS5A binds RNA-dependent RNA polymerase (Rdrp) NS5B and modulates RNA-dependent RNA polymerase activity. J Biol Chem. 2002;277:11149–11155. doi: 10.1074/jbc.M111392200. [DOI] [PubMed] [Google Scholar]
- 47.He Y, Staschke KA, Tan SL. HCV NS5A: A multifunctional regulator of cellular pathways and virus replication. In: Tan SL, editor. Hepatitis C Viruses: Genomes and Molecular Biology. Norfolk, UK: Horizon Bioscience; 2006. [PubMed] [Google Scholar]
- 48.Bobardt M, Hopkins S, Baugh J, et al. HCV NS5A and IRF9 compete for CypA binding. J Hepatol. 2013;58:16–23. doi: 10.1016/j.jhep.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tiongyip C, Jones CT, Tang Y, et al. Host targeting cyclophilin inhibitor alisporivir presents a high barrier to resistance with no cross-resistance to direct acting antivirals. Presented at the Sixth International Workshop on Hepatitis C, Resistance and New Compounds; Cambridge, MA. June 23–24, 2011. [Google Scholar]
- 50.Gane EJ, Stedman CA, Hyland RH, et al. Once daily sofosbuvir (GS-7977) plus ribavirin in patients with HCV genotypes 1, 2, and 3: the ELECTRON trial. Abstract 229 presented at the 63rd Annual Meeting of the American Association for the Study of Liver Diseases; Boston, MA. November 9–13, 2012. [Google Scholar]
- 51.Sulkowski MS, Gardiner DF, Rodriguez-Torres M, et al. High rate of sustained virologic response with the all-oral combination of daclatasvir (NS5A inhibitor) plus sofosbuvir (nucleotide NS5B inhibitor), with or without ribavirin, in treatment-naive patients chronically infected with HCV genotype 1, 2, or 3. Abstract LB-2 presented at the 63rd Annual Meeting of the American Association for the Study of Liver Diseases; Boston, MA. November 9–13, 2012. [Google Scholar]
- 52.Everson GT, Sims KD, Rodriguez-Torres M, et al. An interferon-free, ribavirin-free 12-week regimen of daclatasvir (DCV), asunaprevir (ASV), and BMS-791325 yielded SVR4 of 94% in treatment-naive patients with genotype (GT) 1 chronic hepatitis C virus (HCV) infection. Abstract LB-3 presented at the 63rd Annual Meeting of the American Association for the Study of Liver Diseases; Boston, MA. November 9–13, 2012. [Google Scholar]
- 53.Kowdley KV, Lawitz E, Poordad F, et al. A 12-week interferon-free treatment regimen with ABT-450/r, ABT-267, ABT-333 and ribavirin achieves SVR rates (observed data) of 99% in treatment-naive patients and 93% in prior null responders with HCV genotype 1 infection. Abstract LB-1 presented at the 63rd Annual Meeting of the American Association for the Study of Liver Diseases; Boston, MA. November 9–13, 2012. [Google Scholar]
- 54.Gao M, Nettles RE, Belema M, et al. Chemical genetics strategy identifies an HCV NS5A inhibitor with a potent clinical effect. Nature. 2010;465:96–100. doi: 10.1038/nature08960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lemm JA, O’Boyle D, 2nd, Liu M, et al. J Virol. 2010;84:482–491. doi: 10.1128/JVI.01360-09. [DOI] [PMC free article] [PubMed] [Google Scholar]