Abstract
Objective
Retinol binding protein 4 (RBP4), a specific carrier for retinol in the blood, is a novel adipokine that has been implicated in the pathophysiology of insulin resistance, and its gene expression has been associated with adipose tissue inflammation. Recently, proteomic profiling of amniotic fluid (AF) from women with preterm labor (PTL) revealed over-expression of RBP4 in those who delivered preterm. The aim of this study was to determine whether RBP4 is present in AF, and if its concentrations change with gestational age, in the presence of labor, and intra-amniotic infection/inflammation (IAI) in patients with spontaneous PTL
Study design
This cross-sectional study included pregnant women in the following groups: 1) mid-trimester (n=30); 2) term not in labor (n=31); 3) term in labor (n=30); 4) spontaneous PTL without IAI who delivered at term (n=60); 5) PTL without IAI who delivered preterm (n=64); and 6) PTL with IAI (n=56). RBP4 concentrations in AF were determined by ELISA. Non-parametric statistics were used for analyses.
Results
1) RBP4 was detected in all AF samples; 2) among patients with PTL, women with IAI had a higher median AF RBP4 concentration than those without IAI who delivered preterm (1268.9 ng/mL, interquartile range (IQR) 900.3-1970.1 vs. 815.8 ng/mL, IQR 592.4-1098.1; p<0.001) and at term (828.7 ng/mL, IQR 499.7-1119.6; p<0.001); 3) the median AF RBP4 concentration was higher in women in the midtrimester than in those at term not in labor (1861.1 ng/mL, IQR 1486.2-2034.3 vs. 766.1 ng/mL, IQR 608.5-1154.1; p<0.0001; 4) the median AF RBP4 concentration did not differ significantly between patients with PTL without IAI who delivered preterm and those who delivered at term (p=0.7); and 5) among women at term, the median AF RBP4 concentrations was not significantly different between those in labor and those not in labor (p=0.4).
Conclusions
Retinol binding protein 4 is a physiologic constituent of the amniotic fluid. Among patients with PTL, the median AF concentration of immunoreactive RBP4 is elevated in pregnancies complicated by IAI. These results suggest that RBP4 may participate in the host response against intra-amniotic infection/inflammation.
Keywords: pregnancy, preterm delivery, preterm labor, microbial invasion of the amniotic cavity, RBP4, chorioamnionitis, adipokines
INTRODUCTION
Adipokines are adipocyte-derived cytokines that provide a link between obesity, insulin resistance and obesity-related inflammatory disorders.[1] This family of biologically active molecules includes peptides and proteins such as leptin (the first adipokine to be discovered[2]), adiponectin, visfatin and resistin, as well as other cytokines and chemokines (i.e., tumor necrosis factor (TNF), interleukin (IL)-6, IL-1 and monocyte chemotactic protein (MCP)-1) which were described to be secreted by adipose tissue.[1] Retinol binding protein 4 (RBP4),[3] has recently been added to the rapidly expanding list of adipokines.[4-6]
Retinol-binding protein 4 belongs to the lipocalin family and is a specific carrier for retinol (vitamin A) in the blood. Until recently, its only function was thought to be the delivery of retinol to peripheral tissues. However, in 2005 Yang et al[3] rekindled interest in this molecule by describing RBP4 as a novel adipokine. Using global gene expression analysis in an adipocye-specific Glut4 knockout mouse model, RBP4 was found to be over-expressed in adipose tissue, and implicated in the pathophysiology of insulin resistance.[3] Indeed, increased circulating RBP4 concentrations have been reported in patients with diabetes.[3] Moreover, high plasma concentrations of RBP4 precede the development of overt diabetes.[7,8] In contrast, other investigators, in contrast, have not established an association between plasma RBP4 concentrations and insulin resistance.[9,10] Furthermore, emerging evidence suggests that RBP4 plays a role in the inflammatory response. Indeed, in non-diabetic human subjects, RBP4 gene expression was found to be strongly associated with inflammatory markers (i.e., CD68 and MCP-1) of adipose tissue.[10]
Recently, our group has demonstrated that increased amniotic fluid concentrations of adipokines, such as visfatin[11] and resistin,[12] are associated with intra-amniotic infection/inflammation (IAI). Furthermore, proteomic profiling of the amniotic fluid of patients with spontaneous preterm labor (PTL) revealed that RBP4 is over-expressed in patients who delivered preterm, compared to those with an episode of PTL who delivered at term.[13] While this increased expression was noted regardless of the presence or absence of IAI, it was more profound in microbial invasion of the amniotic cavity.[13]
Thus, the aim of this study was to determine whether the amniotic fluid concentrations of RBP4 change with advancing gestational age, spontaneous labor at term, and in the presence of intra-amniotic infection/inflammation in patients with spontaneous preterm labor and intact membranes.
MATERIALS AND METHODS
Study design and population
A cross-sectional study was conducted by searching our clinical database and bank of biological specimens, and included pregnant women in the following groups: 1) women in the mid-trimester of pregnancy (14-18 weeks) who underwent amniocentesis for genetic indications and delivered a normal neonate at term (n=30); 2) normal pregnant women at term with (n=30) and without spontaneous labor (n=31); 3) patients with an episode of spontaneous PTL and intact membranes who were classified into: a) PTL without IAI who delivered at term (n=60); b) PTL without IAI who delivered preterm (n=64); and c) PTL with IAI (n=56).
All participants provided written informed consent prior to the collection of amniotic fluid. The collection of amniotic fluid and its utilization for research purposes was approved by the Institutional Review Boards of the participating institutions and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD/NIH/DHHS). Many of these samples have been used previously to study the biology of inflammation, hemostasis, angiogenesis regulation, and growth factor concentrations in normal pregnant women and those with pregnancy complications.
Definitions
Patients were considered to have a normal pregnancy outcome if they did not have obstetrical complications and delivered at term (≥37 weeks) a healthy neonate with appropriate birthweight for gestational age [14,15]. Spontaneous preterm labor was defined by the presence of regular uterine contractions (occurring at a frequency of at least two every 10 minutes) associated with cervical change that required hospitalization before 37 completed weeks of gestation. Preterm delivery was defined as delivery before 37 completed weeks of gestation. Intra-amniotic infection was defined as a positive amniotic fluid culture for micro-organisms. Intra-amniotic inflammation was diagnosed by an amniotic fluid IL-6 concentration ≥2.6 ng/mL.[16] Histologic chorioamnionitis was diagnosed based on the presence of inflammatory cells in the chorionic plate and/or chorioamniotic membranes, and acute funisitis was diagnosed by the presence of neutrophils in the wall of the umbilical vessels and/or Wharton's jelly, using the criteria previously described.[17,18]
Amniotic fluid sample collection
Amniotic fluid samples were obtained by transabdominal amniocenteses performed for genetic indication, evaluation of microbial status of the amniotic cavity, and/or assessment of fetal lung maturity in patients approaching term. Women at term not in labor underwent amniocentesis for the assessment of fetal lung maturity prior to cesarean section. Women at term in labor consisted of women who were suspected to have PTL because of uncertain dates and had an amniocentesis for the assessment of microbial invasion of the amniotic cavity and fetal lung maturity. These patients were retrospectively considered to be in labor at term if all the following criteria were met: (1) spontaneous labor; (2) delivery within 24 hours of amniocentesis; (3) analysis of amniotic fluid consistent with maturity; (4) birthweight >2500 grams; and (5) absence of complications of prematurity and physical examination of the newborn by pediatricians consistent with a term neonate. A sample of amniotic fluid was transported to the laboratory in a sterile capped syringe and cultured for aerobic/anaerobic bacteria and genital Mycoplasmas. White blood cell (WBC) count, glucose concentration and Gram-stain were also performed shortly after collection as previously described.[19-21] The results of these tests were used for subsequent clinical management. Amniotic fluid IL-6 concentrations were employed only for research purposes. Amniotic fluid not required for clinical assessment was centrifuged for 10 minutes at 4°C, and the supernatant was aliquoted and stored at –70°C until analysis. Mid-trimester samples were not evaluated for markers of infection at the time of retrieval, however, all samples have subsequently been found to have an amniotic fluid IL-6 concentration <2.6 ng/mL.
Placenta, umbilical cord and chorioamniotic membranes were collected in patients with spontaneous PTL and intact membranes who delivered within 72 hours of amniocentesis, and the presence or absence of histologic chorioamnionitis and/or funisitis was assessed. The 72 hours time period was chosen in order to preserve a meaningful temporal relationship between amniotic fluid RBP4 concentrations and placental histopathologic findings.
Determination of retinol binding protein 4 concentration in amniotic fluid
Amniotic fluid concentration of retinol binding protein 4 was determined by sensitive enzyme-linked immunoassays (Millipore Corporation, St. Charles, MO, USA). The RBP4 immunoassay was validated for human amniotic fluid in our laboratory, prior to the conduction of this study. Validation included spike and recovery experiments which produced parallel curves indicating that amniotic fluid constituents did not interfere with antigen-antibody binding in this assay. Immunoassays were carried out according to the manufacturer's recommendations. Amniotic fluid samples were incubated in duplicate wells of the micro titer plates, which had been pre-coated with antibodies specific for the analyte (RBP4). During this incubation, the analyte present in the standards or amniotic fluid samples was bound by the immobilized antibodies in the respective assay plates. After repeated washing and aspiration to remove all unbound substances, an enzyme-linked polyclonal antibody specific for the analyte was added to the wells of the assay plates. Unbound enzyme conjugate was removed by repeated washing and a substrate solution was added to the wells of the assay plates, with color developing in proportion to the amount of the analyte bound in the initial step. Color development was stopped with the addition of an acid solution, and the intensity of color was read using a programmable spectrophotometer (SpectraMax M2, Molecular Devices, Sunnyvale, CA, USA). The concentrations of RBP4 in amniotic fluid samples were determined by interpolation from individual standard curves. The calculated inter- and intra-assay coefficients of variation for RBP4 immunoassays in our laboratory were 6.98% and 6.05%, respectively. The sensitivity was calculated to be 0.09 ng/mL.
Statistical analysis
Shapiro-Wilk and Kolmogorov-Smirnov tests were used to test for normal distribution of the data. Since amniotic fluid RBP4 concentrations were not normally distributed, non parametric tests were used for analyses. Correlations between continuous variables were assessed by the Spearman's rank correlation test. Comparisons between proportions were performed with Chi-square test. Kruskal-Wallis with post-hoc test (Mann-Whitney U tests) was used for continuous variables. Among patients with PTL and intact membranes, a receiver-operating characteristic (ROC) curve analysis was performed to determine amniotic fluid RBP4 concentration cutoff for identification of patients who had IAI. A multivariable logistic regression model (stepwise) was utilized to examine the association between the RBP4 concentrations, maternal age, pre-pregnancy body mass index (BMI), smoking status, gestational age at amniocentesis, and the presence of IAI. A p-value of <0.05 was considered statistically significant. Statistical analysis was performed with SPSS package version 12 (SPSS Inc, Chicago, IL, USA).
RESULTS
Demographic and clinical characteristics of the study population
Table I displays the demographic and clinical characteristics of patients in the midtrimester, term not in labor and term in labor groups. Table II presents the demographic and clinical characteristics of patients with spontaneous PTL and intact membranes. Among patients with PTL, those with IAI had a significantly lower median gestational age at amniocentesis than both patients without IAI who delivered preterm and those who delivered at term (Table II).
Table 1.
Demographic and clinical characteristics of women with normal pregnancies in the mid-trimester and at term, with and without spontaneous labor.
| Mid-trimester (n=30) | pa | Term not in labor (n = 31) | pb | Term in labor (n = 30) | pc | |
|---|---|---|---|---|---|---|
| Maternal age (years)† | 36 (35-38) | <0.001 | 28 (21.5-33) | <0.001 | 22 (19-26.2) | <0.001 |
| GA at amniocentesis (weeks)† | 16.2 (16-17) | <0.001 | 38.5 (37.9-39.6) | NS | 38.4 (38-39.2) | <0.001 |
| GA at delivery (weeks) | 39 (38-40) | NS | 38.5 (37.9-39.6) | NS | 38.4 (38-39.2) | NS |
| Birth weight (grams) | 3346 (3218-3671) | NS | 3200 (3015-3634) | NS | 3295 (3092-3510) | NS |
Values expressed as median (interquartile range)
GA: gestational age; NS: not significant.
p between mid-trimester and term not in labor
p between term not in labor and term in labor
p mid-trimester and term in labor
p<0.001, Kruskal-Wallis test
Table 2.
Demographic and clinical characteristics among women presenting with spontaneous preterm labor (PTL) and intact membranes.
| Spontaneous PTL and intact membranes | ||||||
|---|---|---|---|---|---|---|
| Without IAI, term delivery (n=60) | pa | without IAI, preterm delivery (n = 64) | pb | with IAI, preterm delivery (n = 56) | pc | |
| Maternal age (years) | 23 (18.5-30) | NS | 23 (20-30) | NS | 23 (20-28.2) | NS |
| GA at amniocentesis (weeks)† | 32 (30.4-34) | NS | 32.2 (30.1-33.4) | <0.001 | 28.4 (25.1-33.2) | 0.001 |
| GA at delivery (weeks)†† | 39 (38.1-39.9) | <0.001 | 34.8 (33.7-35.8) | <0.001 | 29.5 (25.4-33.4) | <0.001 |
| Birth weight (grams)†† | 3240 (2972-3622) | <0.001 | 2480 (2025-2780) | <0.001 | 1425 (765-2185) | <0.001 |
Values expressed as median (interquartile range)
GA: gestational age; PTL: preterm labor; IAI: intra-amniotic infection/inflammation; NS: not significant.
p between PTL without IAI who delivered at term and PTL without IAI who delivered preterm
p between PTL without IAI who delivered preterm and PTL with IAI who delivered preterm
p between PTL without IAI who delivered at term and PTL with IAI who delivered preterm
p=0.001
p<0.001; Kruskal-Wallis test
Amniotic fluid RBP4 in normal pregnancies
Retinol-binding protein 4 was detected in all amniotic fluid samples (n=271). The median amniotic fluid RBP4 concentration in the mid-trimester was higher than that of women at term not in labor (1861.1 ng/mL, interquartile range (IQR) 1486.2-2034.3 vs. 766.1 ng/mL, IQR 608.5-1154.1; p<0.0001, Figure 1). Among women at term, the median amniotic fluid RBP4 concentration did not differ significantly between women with spontaneous labor and those not in labor (term in labor: 836.8 ng/mL, IQR 589.5-1112.8; p=0.4, Figure 1).
Figure 1.
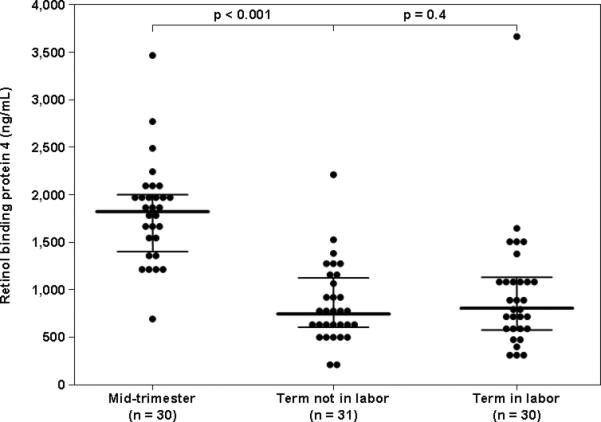
Amniotic fluid concentrations of retinol-binding protein 4 (RBP4) in normal pregnancies in the mid-trimester and at term, in labor and not in labor. The median amniotic fluid RBP4 concentration was higher in women in the mid-trimester than in those at term not in labor [1861.1 ng/mL, interquartile range (IQR) 1486.2-2034.3 vs. 766.1 ng/mL, IQR 608.5-1154.1; p<0.0001]. Among women at term, the median amniotic fluid RBP4 concentration did not differ significantly between women in labor and those not in labor (Term not in labor: 766.1 ng/mL, IQR 608.5-1154.1 vs. term in labor: 836.8 ng/mL, IQR 589.5-1112.8; p=0.4). The black lines represent the median and IQR.
Amniotic fluid RBP4 in spontaneous PTL and intact membranes
Among patients with PTL, the median amniotic fluid RBP4 concentration differed significantly between the three study groups (Kruskal-Wallis, p<0.001). Women with IAI had a higher median amniotic fluid RBP4 concentration than those with PTL without IAI who delivered preterm (1268.9 ng/mL, IQR 900.3-1970.1 vs. 815.8 ng/mL, IQR 592.4-1098.1; p<0.001) and those with PTL without IAI who delivered at term (828.7 ng/mL, IQR 499.7-1119.6; p<0.001, Figure 2). The median amniotic fluid RBP4 concentration did not differ significantly between patients with PTL without IAI who delivered preterm and those without IAI who delivered at term (p=0.7, Figure 2).
Figure 2.
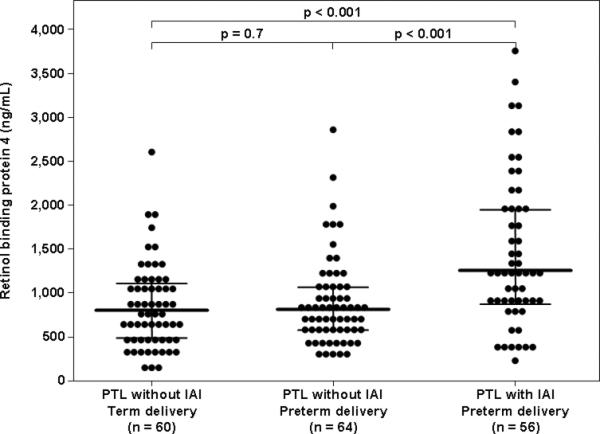
Amniotic fluid concentration of retinol-binding protein 4 (RBP4) among women with spontaneous preterm labor and intact membranes (PTL). The median amniotic fluid RBP4 concentration was higher in patients with intra-amniotic infection/inflammation (IAI) than in women with PTL without IAI who delivered preterm [1268.9 ng/mL, interquartile range (IQR) 900.3-1970.1 vs. 815.8 ng/mL, IQR 592.4-1098.1; p<0.001], and than those with PTL without IAI who delivered at term (828.7 ng/mL, IQR 499.7-1119.6; p<0.001)). Among women with PTL without IAI, there was no difference in the median amniotic fluid concentration of RBP4 between those who delivered preterm and those who delivered at term (p=0.7). The black lines represent the median and IQR.
The ROC curve of amniotic fluid RBP4 concentration for the identification of IAI among patients with PTL with intact membranes is displayed in Figure 3 (area under the curve (AUC) 0.71, p<0.001). A cutoff value for amniotic fluid RBP4 concentration of ≥1149.8 ng/mL had a sensitivity of 58.9% and a specificity of 79.8% for the identification of IAI in patients with PTL and intact membranes. In a backward stepwise multivariable logistic regression model including IAI as the dependent variable and maternal age, pre-pregnancy BMI, smoking status, gestational age at amniocentesis and amniotic fluid RBP4 concentration as covariates, only an amniotic fluid RBP4 concentration ≥1149.8 ng/mL and pre-pregnancy BMI were independently associated with the presence of IAI (Table 3).
Figure 3.
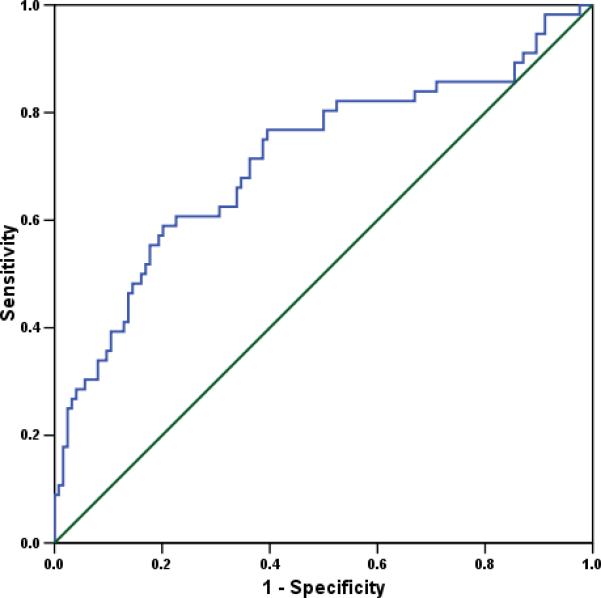
The receiver-operating characteristic (ROC) curve of amniotic fluid RBP4 concentration for the identification of IAI among patients with PTL with intact membranes (area under the curve (AUC) 0.71, p<0.001). Derived from this ROC curve, a cutoff value for amniotic fluid RBP4 concentration of ≥1149.8 ng/mL had a sensitivity of 58.9% and a specificity of 79.8% for the identification of IAI in patients with PTL and intact membranes.
Table 3.
A backward stepwise multivariable logistic regression model including IAI as the dependent variable and maternal age, pre-pregnancy BMI, smoking status and gestational age at amniocentesis and amniotic fluid RBP4 concentration ≥1149.8 ng/mL as covariates.
| Odds ratio | 95% CI | p | |
|---|---|---|---|
| Maternal age | 0.94 | 0.88-1.01 | 0.08 |
| Pre-pregnancy BMI | 1.17 | 1.05-1.30 | 0.004 |
| Smoking status | 1.79 | 0.63-5.09 | 0.3 |
| GA at amniocentesis | 0.91 | 0.77-1.09 | 0.3 |
| Amniotic fluid RBP4 concentration ≥1149.8 ng/mL | 4.6 | 1.91-11.05 | 0.001 |
CI: confidence interval; BMI: body mass index; GA: gestational age; RBP4: retinol binding protein 4
Among patients with PTL and intact membranes, the amniotic fluid RBP4 concentrations correlated significantly with amniotic fluid WBC count (r=0.20, p=0.007) and IL-6 concentrations (r=0.37, p<0.001), but not with amniotic fluid glucose concentrations (r=-0.36, p=0.6).
Amniotic fluid concentration of RBP4 and placental inflammation
Among patients with spontaneous PTL and intact membranes with IAI, 75% (42/56) delivered within 72 hours of amniocentesis, and placental histopathologic results were available from 74% (31/42) of those patients. The median amniotic fluid RBP4 concentration was significantly higher in patients with IAI who had histologic chorioamnionitis and/or funisitis than in those without these placental lesions (1901.0 ng/mL, IQR 1107.3-2492.0 vs. 968.2 ng/mL, IQR 634.7-1396.7; p=0.01, Figure 4).
Figure 4.
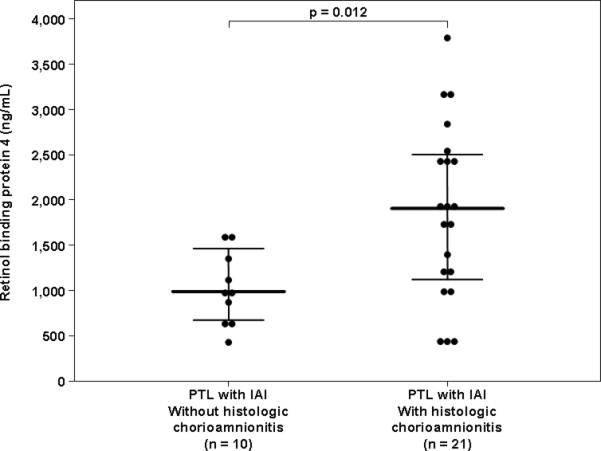
Amniotic fluid concentrations of retinol-binding protein 4 (RBP4) among women with spontaneous preterm labor (PTL) and intra-amniotic infection/inflammation (IAI) who delivered within 72 h of amniocentesis and have placental histopathologic diagnosis: The median amniotic fluid RBP4 concentration was significantly higher in patients with IAI and histologic chorioamnionitis than in women with IAI without histologic chorioamnionitis [1901.0 ng/mL, interquartile range (IQR) 1107.3-2492.0 vs. 968.2 ng/mL, IQR 634.7-1396.7; p=0.01]. The black lines represent the median and IQR.
DISCUSSION
Principal findings of this study
1) Retinol binding protein 4 was detected in all amniotic fluid samples; 2) Intra-amniotic infection/inflammation in patients with PTL was associated with a higher median amniotic fluid RBP4 than those without IAI; 3) The median amniotic fluid RBP4 concentration was higher in the mid-trimester (14-18 weeks) than at term; 4) Spontaneous labor at term was not associated with changes in the median amniotic fluid RBP4 concentration.
Retinol binding protein 4: an old carrier of retinol and a novel adipokine
Retinol binding protein, first described by Kanai, Raz and Goodman in 1968,[22] is the specific carrier of retinol in the blood, belongs to the lipocalin (transporters for small hydrophobic molecules) family, and has a molecular weight of approximately 21-22 kDa.[22] Retinol binding protein 4 is a plasma protein,[23] while RBP1, RBP2,[24] RBP5 and RBP7 are cellular proteins, and RBP3 is the interstitial form.[25]
Recently, RBP4, previously thought to play a role only in the delivery of retinol from the liver to peripheral tissues, has been classified as a novel adipokine implicated in the pathophysiology of insulin resistance.[3] Several lines of evidence support this view: 1) the expression of the gene encoding RBP4 is increased in the adipose tissue of mice with adipocyte-specific ablation of GLUT4;[3] 2) circulating RBP4 concentrations are significantly increased in humans with diabetes;[3] 3) patients who are destined to develop type 2 diabetes mellitus have higher plasma/serum RBP4 concentrations before the development of overt disease;[7,8] and 4) a high concentration of circulating RBP4 is an independent factor contributing to acute insulin response in obese subjects.[26] Of note, other studies have not found an association between RBP4 and insulin resistance.[9,10] In addition, among non-diabetic men, the serum concentrations of RBP4 are not significantly different between lean, overweight and obese subjects, and circulating RBP4 concentrations are not associated with age, BMI, waist-to-hip ratio, or metabolic parameters, including insulin sensitivity.[26] In summary, despite the fact that the role of RBP4 in diabetes and insulin resistance is controversial,[6] there is a growing body of evidence that supports the association between RBP4, metabolic complications and obesity-related disorders.
In addition to their suggested role in insulin resistance, adipocytokines have also been implicated in the pathophysiology of inflammatory disorders such as asthma,[27-29] inflammatory bowel disease,[30-32] rheumatoid arthritis,[33,34] as well as obesity.[3,6,11,35-44] Moreover, some adipocytokines, such as resistin[12,38,45,46] and visfatin,[11,47,48] have an immunoregulatory effect on the innate immune responses, while others (i.e. leptin) have been shown to regulate both the innate[49-52] and the adaptive limbs of the immune system.[53,54] Likewise, RBP4[1,6,12] has also been linked to inflammation. Indeed, RBP4 gene expression has been found to be strongly associated with inflammatory markers (i.e., CD68 and MCP-1) of adipose tissue in non-diabetic human subjects.[10]
Amniotic fluid RBP4 concentrations in normal pregnancies
Retinol binding protein 4 was detectable in the amniotic fluid of all patients included in this study, suggesting that RBP4 is a physiologic component of human amniotic fluid. Moreover, RBP4 concentrations were higher in the mid-trimester than at term. These findings raise a question regarding the source of this protein in the amniotic fluid. RBP in amniotic fluid can be of maternal or fetal origin. In the rat fetal circulation, RBP and retinol concentrations increase in parallel from 11 to 14 days of gestation, and this has been suggested to result from the transplacental transport of retinol bound to RBP.[55] Between 16 and 20 days of gestation, rat fetal concentrations of RBP increase significantly and this has been attributed to fetal synthesis of RBP.[55] Based on these results, it has been suggested that maternal RBP-retinol can cross the placenta.[55,56] However, Quadro et al,[57] using retinol binding protein-deficient (RBP -/-) mice and mice that express human RBP on the RBP-/- background, demonstrated that neither maternal nor fetal RBP crosses the placenta. Thus, amniotic fluid RBP can be derived from either the fetus or membranes. Indeed, animal studies have demonstrated that RBP is synthesized by the amnion, chorion and allantoic sac in the bovine[58] as well as by the yolk sac and fetal liver in the rat.[59] Furthermore, the latter report suggested that retinol transport to the fetus may involve RBP synthesized in the yolk sac and fetal tissues.[59]
The finding of higher mid-trimester concentrations of RBP4 in amniotic fluid compared to term gestation is novel. This decrease of RBP4 concentration with advancing gestational age is similar to the pattern of alpha feto-protein, a fetus specific protein. The concentration of the latter decreases significantly from the second trimester of pregnancy to term gestation.[60,61] Thus, this finding further supports the concept of a fetal origin of amniotic fluid RBP4.
Our finding of a higher mid-trimester concentration of RBP4 in amniotic fluid compared to term is in apparent discrepancy with two previous reports by Sklan et al,[56,59] which describe the amniotic fluid concentrations of RBP4 at different gestational ages. In the rat, the amniotic fluid concentrations of RBP (n=12, radioimmunoassay) did not change significantly along gestation.[59] Similarly, no significant differences were observed in the RBP concentrations determined by radial immunodiffusion in amniotic fluid obtained from a total of 10 patients at 16 to 18 and 37 to 42 weeks of gestation.[56] The small number of subjects and the different assays employed may account for this apparent inconsistency.
Amniotic fluid RBP4 concentration is increased in women with preterm labor and intra-amniotic infection/inflammation
The increased RBP4 concentration in the amniotic fluid of women with PTL and IAI reported herein is novel. This finding confirms a recent report from our group[13] in which, using proteomic profiling of the amniotic fluid of patients with preterm labor, as a discovery tool, over-expression of RBP4 was observed in patients who delivered preterm compared to those with an episode of PTL who delivered at term, regardless of the presence of intra-amniotic infection/inflammation. RBP4 expression, however, was more profound in infected amniotic fluid.[13] Of note, we could not demonstrate a significant difference in the amniotic fluid concentration of RBP4 between patients with PTL without IAI who delivered preterm or at term. Moreover, the lack of difference in the presence of term labor does not support a role for RBP4 in term or preterm parturition but, rather, in infection/inflammation.
The association between amniotic fluid RBP4 concentrations and IAI in patients with spontaneous PTL and intact membranes is consistent with recent reports from our group concerning other adipokines such as visfatin,[11] resistin,[12] and adiponectin,[62] in which IAI was associated with increased amniotic fluid adipokine concentrations. Moreover, the amniotic fluid concentration of RBP4 was independently associated with IAI in patients with PTL and correlated with other indices of intra-amniotic inflammation such as amniotic fluid WBC count (r=0.20, p=0.007) and IL-6 concentrations (r=0.37, p<0.001). In addition, the median amniotic fluid RBP4 concentration was higher among patients with IAI and histologic chorioamnionitis and/or funisitis than among those with IAI without these placental lesions.
The cross-sectional nature of this study does not allow us to discern a cause and effect relationship between amniotic fluid RBP4 and infection/inflammation. However, given the newly described role of RBP4 in the pathogenesis of insulin resistance, it is tempting to postulate that the higher RBP4 concentrations in amniotic fluid are related to the increased fetal demand for energy in cases of intra-amniotic infection/inflammation. In humans, insulin maintains normal glucose levels in the blood by binding to its receptor on the cell membrane, stimulating glucose uptake into muscle and fat cells through the GLUT4 transporter, as well as inhibition of glucose production in the liver. Yang et al.[3] demonstrated that RBP4 may be the link between GLUT4 suppression and insulin resistance. Elevated concentrations of RBP4 impair insulin signaling in the muscle, inhibits glucose uptake, and interfere with insulin-mediated suppression of glucose production in the liver, causing elevation of blood glucose concentrations.[63] Collectively, these findings may point to a role for RBP4 in the fetal inflammatory response to acute infection.
In conclusion, this study demonstrates that among patients with PTL, the amniotic fluid concentration of immunoreactive RBP4 is elevated in pregnancies complicated with IAI and, to a greater extent, in cases with histological chorioamnionitis. These results suggest that the newly classified adipokine, RBP4, participates in the host response against intrauterine infection/inflammation.
Acknowledgment
This research was supported (in part) by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS.
Reference List
- 1.Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat.Rev.Immunol. 2006;6:772–783. doi: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]
- 2.Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK, Friedman JM. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269:543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- 3.Yang Q, Graham TE, Mody N, Preitner F, Peroni OD, Zabolotny JM, Kotani K, Quadro L, Kahn BB. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature. 2005;436:356–362. doi: 10.1038/nature03711. [DOI] [PubMed] [Google Scholar]
- 4.Hida K, Wada J, Eguchi J, Zhang H, Baba M, Seida A, Hashimoto I, Okada T, Yasuhara A, Nakatsuka A, et al. Visceral adipose tissue-derived serine protease inhibitor: a unique insulin-sensitizing adipocytokine in obesity. Proc.Natl.Acad.Sci.U.S.A. 2005;102:10610–10615. doi: 10.1073/pnas.0504703102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Souza Batista CM, Yang RZ, Lee MJ, Glynn NM, Yu DZ, Pray J, Ndubuizu K, Patil S, Schwartz A, Kligman M, et al. Omentin plasma levels and gene expression are decreased in obesity. Diabetes. 2007;56:1655–1661. doi: 10.2337/db06-1506. [DOI] [PubMed] [Google Scholar]
- 6.Tilg H, Moschen AR. Role of adiponectin and PBEF/visfatin as regulators of inflammation: involvement in obesity-associated diseases. Clin.Sci.(Lond) 2008;114:275–288. doi: 10.1042/CS20070196. [DOI] [PubMed] [Google Scholar]
- 7.Cho YM, Youn BS, Lee H, Lee N, Min SS, Kwak SH, Lee HK, Park KS. Plasma retinol-binding protein-4 concentrations are elevated in human subjects with impaired glucose tolerance and type 2 diabetes. Diabetes Care. 2006;29:2457–2461. doi: 10.2337/dc06-0360. [DOI] [PubMed] [Google Scholar]
- 8.Graham TE, Yang Q, Bluher M, Hammarstedt A, Ciaraldi TP, Henry RR, Wason CJ, Oberbach A, Jansson PA, Smith U, et al. Retinol-binding protein 4 and insulin resistance in lean, obese, and diabetic subjects. N.Engl.J Med. 2006;354:2552–2563. doi: 10.1056/NEJMoa054862. [DOI] [PubMed] [Google Scholar]
- 9.Janke J, Engeli S, Boschmann M, Adams F, Bohnke J, Luft FC, Sharma AM, Jordan J. Retinol-binding protein 4 in human obesity. Diabetes. 2006;55:2805–2810. doi: 10.2337/db06-0616. [DOI] [PubMed] [Google Scholar]
- 10.Yao-Borengasser A, Varma V, Bodles AM, Rasouli N, Phanavanh B, Lee MJ, Starks T, Kern LM, Spencer HJ, III, Rashidi AA, et al. Retinol binding protein 4 expression in humans: relationship to insulin resistance, inflammation, and response to pioglitazone. J Clin.Endocrinol.Metab. 2007;92:2590–2597. doi: 10.1210/jc.2006-0816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mazaki-Tovi S, Romero R, Kusanovic JP, Erez O, Gotsch F, Mittal P, Than NG, Nhan-Chang CL, Hamill N, Vaisbuch E, et al. Visfatin/Pre-B cell colony-enhancing factor in amniotic fluid in normal pregnancy, spontaneous labor at term, preterm labor and prelabor rupture of membranes: an association with subclinical intrauterine infection in preterm parturition. J Perinat.Med. 2008;36:485–496. doi: 10.1515/JPM.2008.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kusanovic JP, Romero R, Mazaki-Tovi S, Chaiworapongsa T, Mittal P, Gotsch F, Erez O, Vaisbuch E, Edwin SS, Than NG, et al. Resistin in amniotic fluid and its association with intra-amniotic infection and inflammation. J Matern.Fetal Neonatal Med. 2008;21:902–916. doi: 10.1080/14767050802320357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bujold E, Romero R, Kusanovic JP, Erez O, Gotsch F, Chaiworapongsa T, Gomez R, Espinoza J, Vaisbuch E, Mee KY, et al. Proteomic profiling of amniotic fluid in preterm labor using two-dimensional liquid separation and mass spectrometry. J Matern.Fetal Neonatal Med. 2008;21:697–713. doi: 10.1080/14767050802053289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol. 1996;87:163–168. doi: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez RP, Gomez RM, Castro RS, Nien JK, Merino PO, Etchegaray AB, Carstens MR, Medina LH, Viviani PG, Rojas IT. [A national birth weight distribution curve according to gestational age in Chile from 1993 to 2000]. Rev.Med.Chil. 2004;132:1155–1165. doi: 10.4067/s0034-98872004001000001. [DOI] [PubMed] [Google Scholar]
- 16.Yoon BH, Romero R, Moon JB, Shim SS, Kim M, Kim G, Jun JK. Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Obstet.Gynecol. 2001;185:1130–1136. doi: 10.1067/mob.2001.117680. [DOI] [PubMed] [Google Scholar]
- 17.Pacora P, Chaiworapongsa T, Maymon E, Kim YM, Gomez R, Yoon BH, Ghezzi F, Berry SM, Qureshi F, Jacques SM, et al. Funisitis and chorionic vasculitis: the histological counterpart of the fetal inflammatory response syndrome. J Matern.Fetal Neonatal Med. 2002;11:18–25. doi: 10.1080/jmf.11.1.18.25. [DOI] [PubMed] [Google Scholar]
- 18.Redline RW. Inflammatory responses in the placenta and umbilical cord. Semin.Fetal Neonatal Med. 2006;11:296–301. doi: 10.1016/j.siny.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 19.Romero R, Emamian M, Quintero R, Wan M, Hobbins JC, Mazor M, Edberg S. The value and limitations of the Gram stain examination in the diagnosis of intraamniotic infection. Am J Obstet Gynecol. 1988;159:114–119. doi: 10.1016/0002-9378(88)90503-0. [DOI] [PubMed] [Google Scholar]
- 20.Romero R, Jimenez C, Lohda AK, Nores J, Hanaoka S, Avila C, Callahan R, Mazor M, Hobbins JC, Diamond MP. Amniotic fluid glucose concentration: a rapid and simple method for the detection of intraamniotic infection in preterm labor. Am J Obstet Gynecol. 1990;163:968–974. doi: 10.1016/0002-9378(90)91106-m. [DOI] [PubMed] [Google Scholar]
- 21.Romero R, Quintero R, Nores J, Avila C, Mazor M, Hanaoka S, Hagay Z, Merchant L, Hobbins JC. Amniotic fluid white blood cell count: a rapid and simple test to diagnose microbial invasion of the amniotic cavity and predict preterm delivery. Am J Obstet Gynecol. 1991;165:821–830. doi: 10.1016/0002-9378(91)90423-o. [DOI] [PubMed] [Google Scholar]
- 22.Kanai M, Raz A, Goodman DS. Retinol-binding protein: the transport protein for vitamin A in human plasma. J Clin.Invest. 1968;47:2025–2044. doi: 10.1172/JCI105889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rocchi M, Covone A, Romeo G, Faraonio R, Colantuoni V. Regional mapping of RBP4 to 10q23----q24 and RBP1 to 3q21----q22 in man. Somat.Cell Mol.Genet. 1989;15:185–190. doi: 10.1007/BF01535081. [DOI] [PubMed] [Google Scholar]
- 24.Demmer LA, Birkenmeier EH, Sweetser DA, Levin MS, Zollman S, Sparkes RS, Mohandas T, Lusis AJ, Gordon JI. The cellular retinol binding protein II gene. Sequence analysis of the rat gene, chromosomal localization in mice and humans, and documentation of its close linkage to the cellular retinol binding protein gene. J Biol Chem. 1987;262:2458–2467. [PubMed] [Google Scholar]
- 25.Liou GI, Fong SL, Gosden J, van TP, Ledbetter DH, Christie S, Rout D, Bhattacharya S, Cook RG, Li Y, et al. Human interstitial retinol-binding protein (IRBP): cloning, partial sequence, and chromosomal localization. Somat.Cell Mol.Genet. 1987;13:315–323. doi: 10.1007/BF01534925. [DOI] [PubMed] [Google Scholar]
- 26.Broch M, Vendrell J, Ricart W, Richart C, Fernandez-Real JM. Circulating retinol-binding protein-4, insulin sensitivity, insulin secretion, and insulin disposition index in obese and nonobese subjects. Diabetes Care. 2007;30:1802–1806. doi: 10.2337/dc06-2034. [DOI] [PubMed] [Google Scholar]
- 27.Gurkan F, Atamer Y, Ece A, Kocyigit Y, Tuzun H, Mete N. Serum leptin levels in asthmatic children treated with an inhaled corticosteroid. Ann.Allergy Asthma Immunol. 2004;93:277–280. doi: 10.1016/S1081-1206(10)61501-3. [DOI] [PubMed] [Google Scholar]
- 28.Guler N, Kirerleri E, Ones U, Tamay Z, Salmayenli N, Darendeliler F. Leptin: does it have any role in childhood asthma? J Allergy Clin.Immunol. 2004;114:254–259. doi: 10.1016/j.jaci.2004.03.053. [DOI] [PubMed] [Google Scholar]
- 29.Shore SA, Schwartzman IN, Mellema MS, Flynt L, Imrich A, Johnston RA. Effect of leptin on allergic airway responses in mice. J Allergy Clin.Immunol. 2005;115:103–109. doi: 10.1016/j.jaci.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 30.Barbier M, Vidal H, Desreumaux P, Dubuquoy L, Bourreille A, Colombel JF, Cherbut C, Galmiche JP. Overexpression of leptin mRNA in mesenteric adipose tissue in inflammatory bowel diseases. Gastroenterol.Clin.Biol. 2003;27:987–991. [PubMed] [Google Scholar]
- 31.Siegmund B, Sennello JA, Lehr HA, Batra A, Fedke I, Zeitz M, Fantuzzi G. Development of intestinal inflammation in double IL-10- and leptin-deficient mice. J Leukoc.Biol. 2004;76:782–786. doi: 10.1189/jlb.0404239. [DOI] [PubMed] [Google Scholar]
- 32.Tuzun A, Uygun A, Yesilova Z, Ozel AM, Erdil A, Yaman H, Bagci S, Gulsen M, Karaeren N, Dagalp K. Leptin levels in the acute stage of ulcerative colitis. J Gastroenterol.Hepatol. 2004;19:429–432. doi: 10.1111/j.1440-1746.2003.03300.x. [DOI] [PubMed] [Google Scholar]
- 33.Schaffler A, Ehling A, Neumann E, Herfarth H, Tarner I, Scholmerich J, Muller-Ladner U, Gay S. Adipocytokines in synovial fluid. JAMA. 2003;290:1709–1710. doi: 10.1001/jama.290.13.1709-c. [DOI] [PubMed] [Google Scholar]
- 34.Bernotiene E, Palmer G, Talabot-Ayer D, Szalay-Quinodoz I, Aubert ML, Gabay C. Delayed resolution of acute inflammation during zymosan-induced arthritis in leptin-deficient mice. Arthritis Res.Ther. 2004;6:R256–R263. doi: 10.1186/ar1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang WS, Lee WJ, Funahashi T, Tanaka S, Matsuzawa Y, Chao CL, Chen CL, Tai TY, Chuang LM. Weight reduction increases plasma levels of an adipose-derived anti-inflammatory protein, adiponectin. J Clin.Endocrinol.Metab. 2001;86:3815–3819. doi: 10.1210/jcem.86.8.7741. [DOI] [PubMed] [Google Scholar]
- 36.Esposito K, Pontillo A, Di PC, Giugliano G, Masella M, Marfella R, Giugliano D. Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women: a randomized trial. JAMA. 2003;289:1799–1804. doi: 10.1001/jama.289.14.1799. [DOI] [PubMed] [Google Scholar]
- 37.Frayn KN. Obesity and metabolic disease: is adipose tissue the culprit? Proc.Nutr.Soc. 2005;64:7–13. doi: 10.1079/pns2004403. [DOI] [PubMed] [Google Scholar]
- 38.Bokarewa M, Nagaev I, Dahlberg L, Smith U, Tarkowski A. Resistin, an adipokine with potent proinflammatory properties. J Immunol. 2005;174:5789–5795. doi: 10.4049/jimmunol.174.9.5789. [DOI] [PubMed] [Google Scholar]
- 39.Klok MD, Jakobsdottir S, Drent ML. The role of leptin and ghrelin in the regulation of food intake and body weight in humans: a review. Obes.Rev. 2007;8:21–34. doi: 10.1111/j.1467-789X.2006.00270.x. [DOI] [PubMed] [Google Scholar]
- 40.Nien JK, Mazaki-Tovi S, Romero R, Erez O, Kusanovic JP, Gotsch F, Pineles BL, Gomez R, Edwin S, Mazor M, et al. Plasma adiponectin concentrations in non-pregnant, normal and overweight pregnant women. J Perinat.Med. 2007;35:522–531. doi: 10.1515/JPM.2007.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nien JK, Mazaki-Tovi S, Romero R, Erez O, Kusanovic JP, Gotsch F, Pineles BL, Gomez R, Edwin S, Mazor M, et al. Adiponectin in severe preeclampsia. J Perinat.Med. 2007;35:503–512. doi: 10.1515/JPM.2007.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nien JK, Mazaki-Tovi S, Romero R, Kusanovic JP, Erez O, Gotsch F, Pineles BL, Friel LA, Espinoza J, Goncalves L, et al. Resistin: a hormone which induces insulin resistance is increased in normal pregnancy. J Perinat.Med. 2007;35:513–521. doi: 10.1515/JPM.2007.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mazaki-Tovi S, Romero R, Kusanovic JP, Vaisbuch E, Erez O, Than NG, Chaiworapongsa T, Nhan-Chang CL, Pacora P, Gotsch F, et al. Visfatin in human pregnancy: maternal gestational diabetes vis-a-vis neonatal birthweight. J Perinat.Med. 2009;37:218–231. doi: 10.1515/JPM.2009.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mazaki-Tovi S, Romero R, Kusanovic JP, Erez O, Vaisbuch E, Gotsch F, Mittal P, Than GN, Nhan-Chang C, Chaiworapongsa T, et al. Adiponectin multimers in maternal plasma. J Matern.Fetal Neonatal Med. 2008;21:796–815. doi: 10.1080/14767050802266881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Verma S, Li SH, Wang CH, Fedak PW, Li RK, Weisel RD, Mickle DA. Resistin promotes endothelial cell activation: further evidence of adipokine endothelial interaction. Circulation. 2003;108:736–740. doi: 10.1161/01.CIR.0000084503.91330.49. [DOI] [PubMed] [Google Scholar]
- 46.Silswal N, Singh AK, Aruna B, Mukhopadhyay S, Ghosh S, Ehtesham NZ. Human resistin stimulates the pro-inflammatory cytokines TNF-alpha and IL-12 in macrophages by NF-kappaB-dependent pathway. Biochem.Biophys.Res.Commun. 2005;334:1092–1101. doi: 10.1016/j.bbrc.2005.06.202. [DOI] [PubMed] [Google Scholar]
- 47.Ognjanovic S, Bryant-Greenwood GD. Pre-B-cell colony-enhancing factor, a novel cytokine of human fetal membranes. Am J Obstet Gynecol. 2002;187:1051–1058. doi: 10.1067/mob.2002.126295. [DOI] [PubMed] [Google Scholar]
- 48.Jia SH, Li Y, Parodo J, Kapus A, Fan L, Rotstein OD, Marshall JC. Pre-B cell colony-enhancing factor inhibits neutrophil apoptosis in experimental inflammation and clinical sepsis. J Clin.Invest. 2004;113:1318–1327. doi: 10.1172/JCI19930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gainsford T, Willson TA, Metcalf D, Handman E, McFarlane C, Ng A, Nicola NA, Alexander WS, Hilton DJ. Leptin can induce proliferation, differentiation, and functional activation of hemopoietic cells. Proc.Natl.Acad.Sci.U.S.A. 1996;93:14564–14568. doi: 10.1073/pnas.93.25.14564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tian Z, Sun R, Wei H, Gao B. Impaired natural killer (NK) cell activity in leptin receptor deficient mice: leptin as a critical regulator in NK cell development and activation. Biochem.Biophys.Res.Commun. 2002;298:297–302. doi: 10.1016/s0006-291x(02)02462-2. [DOI] [PubMed] [Google Scholar]
- 51.Matarese G, Moschos S, Mantzoros CS. Leptin in immunology. J Immunol. 2005;174:3137–3142. doi: 10.4049/jimmunol.174.6.3137. [DOI] [PubMed] [Google Scholar]
- 52.Zhao T, Hou M, Xia M, Wang Q, Zhu H, Xiao Y, Tang Z, Ma J, Ling W. Globular adiponectin decreases leptin-induced tumor necrosis factor-alpha expression by murine macrophages: involvement of cAMP-PKA and MAPK pathways. Cell Immunol. 2005;238:19–30. doi: 10.1016/j.cellimm.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 53.Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature. 1998;394:897–901. doi: 10.1038/29795. [DOI] [PubMed] [Google Scholar]
- 54.Howard JK, Lord GM, Matarese G, Vendetti S, Ghatei MA, Ritter MA, Lechler RI, Bloom SR. Leptin protects mice from starvation-induced lymphoid atrophy and increases thymic cellularity in ob/ob mice. J Clin.Invest. 1999;104:1051–1059. doi: 10.1172/JCI6762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Takahashi YI, Smith JE, Goodman DS. Vitamin A and retinol-binding protein metabolism during fetal development in the rat. Am J Physiol. 1977;233:E263–E272. doi: 10.1152/ajpendo.1977.233.4.E263. [DOI] [PubMed] [Google Scholar]
- 56.Sklan D, Shalit I, Lasebnik N, Spirer Z, Weisman Y. Retinol transport proteins and concentrations in human amniotic fluid, placenta, and fetal and maternal sera. Br.J Nutr. 1985;54:577–583. doi: 10.1079/bjn19850144. [DOI] [PubMed] [Google Scholar]
- 57.Quadro L, Hamberger L, Gottesman ME, Colantuoni V, Ramakrishnan R, Blaner WS. Transplacental delivery of retinoid: the role of retinol-binding protein and lipoprotein retinyl ester. Am J Physiol Endocrinol.Metab. 2004;286:E844–E851. doi: 10.1152/ajpendo.00556.2003. [DOI] [PubMed] [Google Scholar]
- 58.Liu KH, Baumbach GA, Gillevet PM, Godkin JD. Purification and characterization of bovine placental retinol-binding protein. Endocrinology. 1990;127:2696–2704. doi: 10.1210/endo-127-6-2696. [DOI] [PubMed] [Google Scholar]
- 59.Sklan D, Ross AC. Synthesis of retinol-binding protein and transthyretin in yolk sac and fetus in the rat. J Nutr. 1987;117:436–442. doi: 10.1093/jn/117.3.436. [DOI] [PubMed] [Google Scholar]
- 60.Seppala M, Ruoslahti E. Alpha fetoprotein in amniotic fluid: an index of gestational age. Am J Obstet Gynecol. 1972;114:595–598. doi: 10.1016/0002-9378(72)90834-4. [DOI] [PubMed] [Google Scholar]
- 61.Seppala M, Ruoslahti E. Alpha-fetoprotein in antenatal diagnosis. Lancet. 1973;1:155. doi: 10.1016/s0140-6736(73)90232-8. [DOI] [PubMed] [Google Scholar]
- 62.Mazaki-Tovi S, Vaisbuch E, Kusanovic JP, Erez O, Mittal P, Chaiworapongsa T, Than NG, Kim SK, Nhan-Chang C, Jodicke C, et al. A Role For Adiponectin in Intra-Amniotic Infection. Reprod.Sci. 2009;16:220A. [Google Scholar]
- 63.Muoio DM, Newgard CB. Metabolism: A is for adipokine. Nature. 2005;436:337–338. doi: 10.1038/436337a. [DOI] [PubMed] [Google Scholar]


