Abstract
Purpose
To assess methods for determination of exercise intensity, and to investigate practice variation with respect to the contents, volume and intensity of exercise training programs in Dutch cardiac rehabilitation (CR) centres.
Methods
A paper questionnaire was sent to all Dutch CR centres, consisting of 85 questions for patients with an acute coronary syndrome (ACS) or after coronary revascularisation (Group 1) and for patients with chronic heart failure (CHF, Group 2).
Results
CR professionals from 45 centres completed the questionnaires (58 %). Symptom-limited exercise testing was used to determine exercise capacity in 76 % and 64 % of the CR centres in group 1 and group 2, respectively; in these centres, a percentage of the maximum heart rate was the most frequently used exercise parameter (65 % and 56 %, respectively). All CR centres applied aerobic training and the majority applied strength training (64 % in group 1 and 92 % in group 2, respectively). There was a considerable variation in training intensity for both aerobic and strength training, as well as in training volume (1–20 h and 1–18 h respectively).
Conclusion
Among Dutch CR centres, considerable variation exists in methods for determination of exercise intensity. In addition, there is no uniformity in training volume and intensity.
Keywords: Cardiac rehabilitation, Exercise, Physical training
Introduction
Cardiovascular disease is one of the most important causes of morbidity and mortality, accounting for approximately 40,000 annual deaths and 365,000 hospital admissions for a cardiovascular disease in the Netherlands [1]. In 2003, annual healthcare costs for cardiovascular diseases were estimated to be more than €1.2 billion [2]. It is expected that this economic burden will increase over the next decades due to ageing of our population. Therefore, it is of crucial importance that new therapeutic strategies are not only aimed at improving the health status of cardiovascular patients but also at prevention of future cardiac events and hospitalisation. As such, exercise-based cardiac rehabilitation (ECR) after an acute coronary syndrome (ACS), coronary revascularisation and in patients with chronic heart failure (CHF) has been shown to be a highly effective treatment, improving exercise capacity and quality of life, while reducing the risk of recurrent cardiovascular events and mortality [3, 4]. Therefore, exercise training in these patients is now recommended in both national and international guidelines [4–7].
An important requirement for ECR programs to be safe and effective is an exact determination of exercise intensity using parameters derived from a symptom-limited exercise test [8]. From a safety perspective, exercise intensity should not be too high as it has been postulated that strenuous exercise increases platelet activation [9, 10]. Especially in high-risk CR populations, strenuous exercise could potentially lead to an increased risk of adverse cardiovascular events during exercise training. On the other hand, it has been shown that low exercise intensity programs result in less improvement in exercise capacity than moderate to high intensity programs both in post-ACS and post-revascularisation patients as well as in CHF patients [10–14]. In addition, low exercise intensity is related to lower therapy adherence as compared with higher exercise intensity [15, 16]. Therefore, to avoid low and excessively high training intensities, assessment of the individual exercise capacity is preferable in patients entering CR. Currently, symptom-limited exercise testing prior to a CR program is recommended by the European Association of Cardiovascular Prevention and Rehabilitation (EACPR) for post-ACS/post-revascularisation patients, using either maximal heart rate or maximal workload to determine training intensity [5]. For CHF patients entering a CR program, it is strongly recommended to perform a symptom-limited exercise test (SET) with gas exchange analysis to determine peak oxygen uptake, because of superior assessment of exercise capacity and prognosis in this patient category [4].
To our knowledge, no studies have addressed the actual contents and prescription methods of exercise training programs in Dutch CR centres. Knowledge on this issue, however, is mandatory to develop strategies and interventions to improve quality of exercise-based CR in the Netherlands. Therefore, the purpose of this study is to investigate methods for determination of exercise intensity and to assess practice variation with respect to the contents, intensity and volume of ECR programs in Dutch CR centres.
Methods
A paper-based questionnaire was sent to all physical therapists coordinating exercise-based CR programs in Dutch CR centres, after they had been contacted by telephone. If the questionnaire was not returned or fully answered within 2 weeks, a reminder was sent by email. If necessary they were contacted again by telephone 2 weeks later.
The questionnaire consisted of 85 questions, using both multiple-choice and open-ended response formats. Questions addressed the following issues:
Responder characteristics (credentials, job title)
Function and education of prescribers of exercise training programs
Patient population characteristics (diagnosis categories)
Program characteristics (training modalities such as aerobic, strength)
Methods to determine exercise capacity and training intensity (e.g. using parameters derived from a symptom-limited exercise test, a 6-min walk/shuttle test, Borg scores or clinical judgement)
Training volume (i.e. training frequency, duration of training sessions and total duration of the training program)
Prescribed exercise intensities.
Program characteristics and exercise prescription methods were assessed separately for two patient categories:
Group 1: patients with an ACS (ST-elevated myocardial infarction, non-ST-elevated myocardial infarction or unstable angina) and patients that underwent PCI or CABG.
Group 2: patients with CHF.
Before starting the survey, the questionnaire was pilot tested by two physical therapists in two different CR centres. They judged the questionnaire as being relevant and clear with some minor comments. After adjustment of the questionnaire, it was sent to the participating CR centres. The full questionnaire can be downloaded from the following website: http://www.amc.nl/web/Research/Departments/Overview/Medical-Informatics-KIK-1/Medical-Informatics-KIK/List-of-Technical-Reports.htm
Statistical analysis
Data were analysed using descriptive statistics (frequency counts, percentages, medians). Practice variation in program characteristics and prescription methods was assessed by calculating medians and ranges.
Results
Questionnaires were sent to all centres offering outpatient CR programs (n = 78). From these, 45 centres (58 %) eventually returned completed questionnaires. The 45 responding centres consisted of 4 specialised rehabilitation clinics (57 %), 4 university hospitals (80 %) and 37 non-university hospitals (56 %). The 33 non-responding centres consisted of 3 specialised rehabilitation clinics (43 %), 1 university hospital (20 %) and 29 non-university hospitals (44 %). All responding centres (n = 45) offered ECR programs for post-ACS and post-revascularisation patients (group 1). Among the responding centres, 39 (87 %) offered ECR for CHF patients (group 2).
Exercise intensity, duration and modalities were determined by physical therapists in 42 CR centres (93 %) for group 1 and in 37 centres for group 2 (95 %). Other healthcare professionals prescribing exercise programs included cardiologists (in 4 and 3 CR centres for group 1 and group 2, respectively), specialised nurses (in 5 CR centres for both groups), exercise physiologists (in 2 CR centres for both groups), a rehabilitation physician (in 1 CR centre for group 1 only) and an exercise physiologist (in 2 centres for both groups). In 6 CR centres (13 %) the contents of the training program were determined during a multidisciplinary team meeting.
Training modalities
All centres applied aerobic training in both patient categories. Interval training was applied in 24 of these centres for group 1 (62 %) and in 34 of these centres for group 2 (87 %). All centres used a cycle ergometer; a treadmill was used in 29 and 25 centres respectively. Other less often used training devices included a rowing ergometer (15 and 7 centres respectively), a cross trainer (4 and 0 centres respectively) and a step trainer (1 and 0 centres respectively). Strength training was applied in 29 (64 %) and 36 (92 %) of the centres in group 1 and group 2 respectively. Respiratory training was applied in 5 (11 %) and 4 (10 %) of the CR centres, respectively.
Training volume
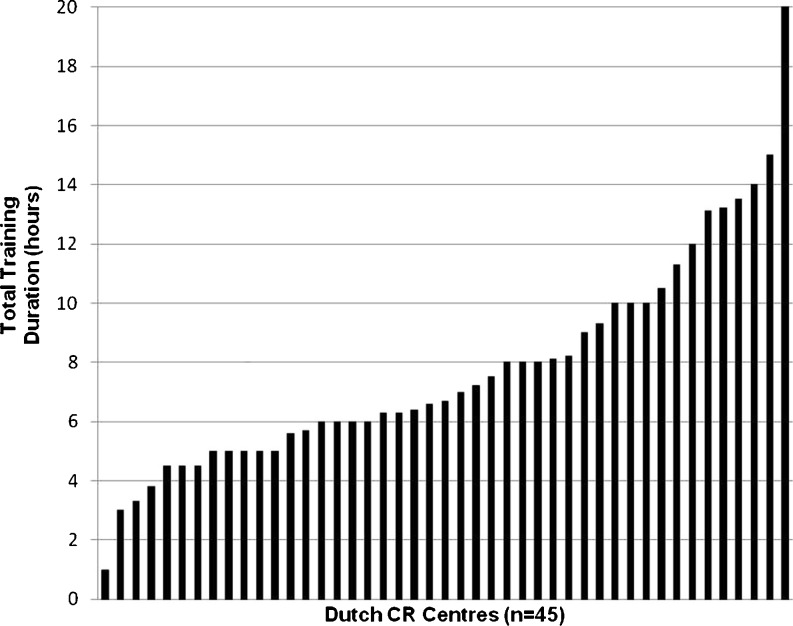
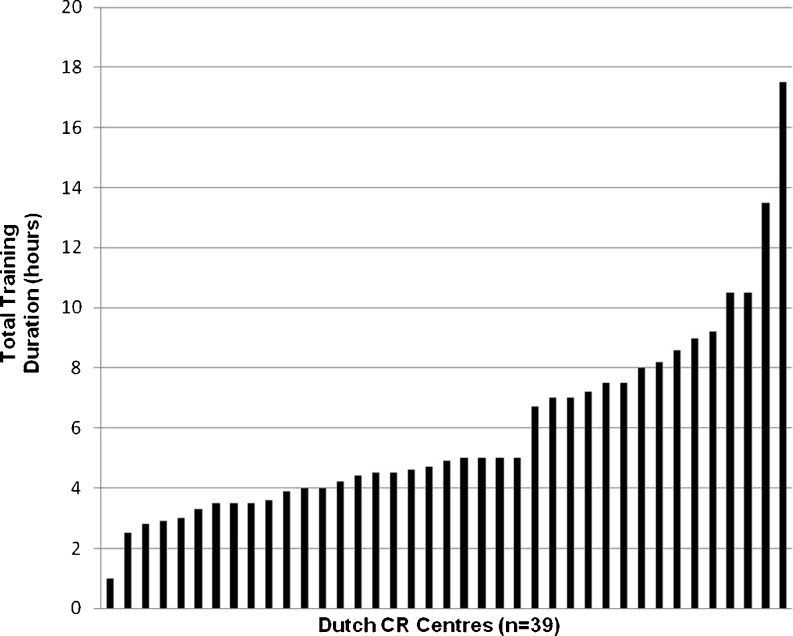
The median duration of training sessions was 30 min (range 3–75 min) in group 1 and 24 min (range 3–45 min) in group 2. The median training frequency was 2.3 and 2.1 sessions per week (range 1–3) for group 1 and 2 respectively. Median total training duration for group 1 was 6.7 h (range 1–20 h), and 4.9 h (range 1–17.5 h) for group 2 (Figs. 1 and 2).
Fig. 1.
Variation in total aerobic training duration. This figure shows the total duration of aerobic exercise training for group 1 patients given by the CR centres. The centres are shown from left to right in increasing training duration
Fig. 2.
Variation in total aerobic raining duration. This figure shows the total duration of aerobic exercise training for group 2 patients given by the CR centres. The centres are shown from left to right in increasing training duration
Exercise intensity
Aerobic training
For group 1, an SET was used to assess exercise capacity in 34 CR centres (76 %) and for group 2 in 25 centres (64 %). From these centres, 3 (8 %) used additional respiratory gas exchange analysis (measuring peak VO2), only for group 2 patients.
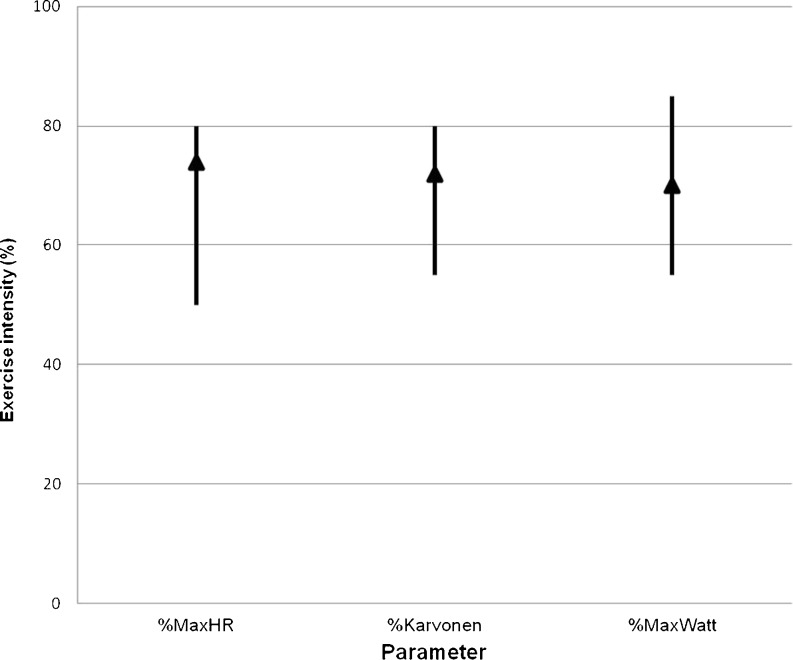
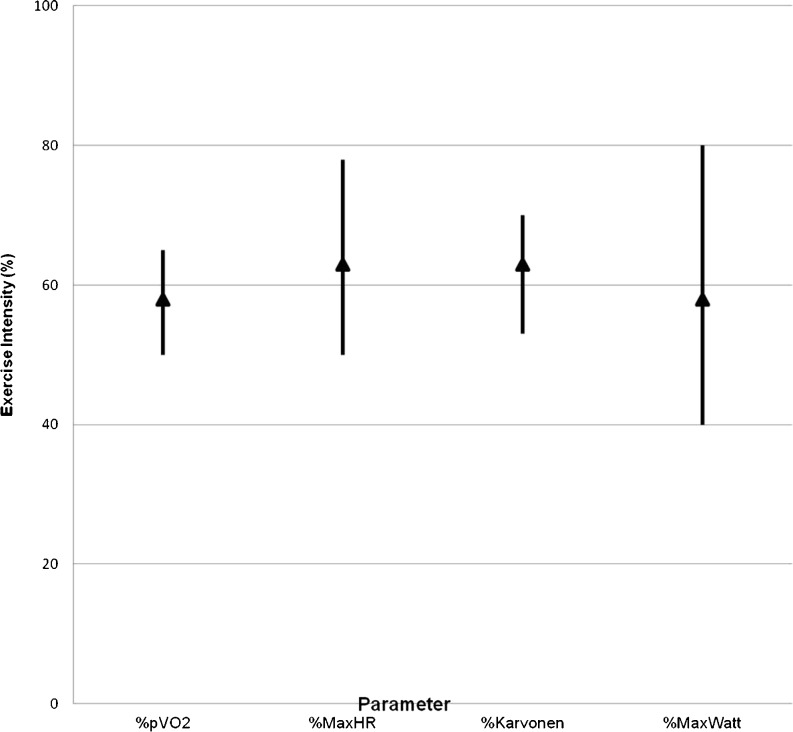
Parameters used to determine exercise intensity are shown in Table 1. Forty CR centres (89 %) used more than one parameter to determine exercise intensity. In centres using an SET, a percentage of maximum heart rate was the most frequently used exercise parameter. In centres not using an SET, the Borg score was the most frequently used method for determination of exercise intensity. Figures 3 and 4 show the range in aerobic exercise intensity for centres using parameters derived from an SET.
Table 1.
Used parameters to determine exercise intensity determination in aerobic training
| Aerobic intensity exercise determination | Group I | Group II |
|---|---|---|
| SET | N = 34 | N = 25 |
| %Maximal HR | 22(65 %) | 14(56 %) |
| Karvonen | 14(41 %) | 9(36 %) |
| Workload | 11(32 %) | 7(28 %) |
| pVO2 | 0(−) | 3(12 %) |
| Borg score | 28(82 %) | 19(76 %) |
| No SET | N = 11 | N = 14 |
| % Estimated Max HR | 4(36 %) | 6(43 %) |
| Clinical judgement | 6(55 %) | 9(64 %) |
| Borg score | 9(82 %) | 12(85 %) |
SET Symptom limited/maximal exercise testing; HR Heart rate; pVO2 peak oxygen uptake
Fig. 3.
Used aerobic exercise intensity. This figure shows the ranges and means in used aerobic training intensity for different parameters in group 1 patients
Fig. 4.
Used aerobic exercise intensity. This figure shows the ranges and means in used aerobic training intensity for different parameters in group 2 patients
Strength training
For group 1 the intensity of strength training was determined using a percentage of maximum strength (% max strength) in 10 centres (38 %); Borg score was used in 13 centres (50 %) and clinical judgement in 16 centres (62 %). For group 2 the % max strength method was used in 18 centres (53 %), Borg score in 19 centres (56 %) and clinical judgement in 9 centres (26 %).
In centres using % max strength to determine training intensity, the median exercise intensity was 63 % (range: 50–85 %) for group 1 and 53 % (range: 40–85 %) for group 2.
Discussion
This study shows that a considerable variation exists in methods for determination of exercise intensity in Dutch CR centres, with a substantial number of CR centres not using SET to determine exercise intensity. Even when SET was used, there was a wide variety in used exercise parameters, as well as in actually applied training intensity and training volume.
To our knowledge, the present survey is the first to evaluate the actual content of ECR programs in Dutch CR centres. Data from other countries are scarce. Whereas numerous studies have investigated the uptake of exercise-based CR only a few studies evaluated the actual content and exercise prescription methods of ECR programs. In a study among Japanese CR centres, it was shown that only 10 % of the patients eligible for CR underwent an ECR program based on exercise testing [17]. In line with these findings, a study in Northern Ireland performed by Bradley et al. showed that only 12.5 % (1 out of 8) CR centres used SET on a regular basis and 50 % (4 out of 8) used SET sometimes [18]. Four of these centres also reported that if SET was performed the outcome measurements of the test did not reach the ECR program prescriber. In contrast with these studies, Deturk et al. reported that in New York State 90 % of the CR centres used an SET to determine training intensity [19]. As outlined by the authors of the former studies, this discrepancy may be explained by a lack of proper facilities and financial resources in Japan and Ireland.
As mentioned, an exact determination of training intensity by using SET is important for ECR programs to be safe and effective [5]. In fact, in line with the EACPR recommendations, the Netherlands Society for Cardiology (NVVC) has developed a clinical algorithm for assessment of patient needs for CR [20, 21], which recommends SET for all patients entering outpatient CR, including gas exchange analysis for CHF patients. Clearly, our study shows that these recommendations are not well implemented in Dutch CR centres.
Many barriers for implementing practice guidelines into care practice have been reported in the literature [22–24]. Often, a distinction is made between internal and external barriers. Internal barriers are related to the professional’s attitude towards and knowledge of the guidelines. Considering this, there have been clear recommendations for cardiologists, but physical therapists might not be aware of these guidelines. There have also been guidelines for physical therapists in the past but these were less concrete in their recommendations concerning ECR. A new guideline with concrete recommendations for physical therapists appeared in 2012, but it was not yet available during our study. Furthermore, prescribers of ECR might not always be capable of translating the results of an SET into a training prescription. Another internal barrier for non-adherence could be that prescribers of ECR disagree with parts of the recommendations.
External barriers are not directly related to the functioning of a professional but either related to the complexity of guidelines themselves, to the patient (who may, for example, refuse a certain therapy), to the organisation in which the professional works, or to other environmental factors [24, 25]. Bradley suggested that the poor implementation of recommendations for ECR programs in Northern Ireland was due to a lack of facilities and to high costs [18]. Also in the Netherlands, lack of resources and budget ceilings sometimes hamper innovation in health care.
Strategies for achieving better guideline implementation should be attuned to the specific barriers that are obstructing it [26]. For instance, Goud et al. showed that computerised decision support systems improve guideline implementation if the key barrier is knowledge of professionals [25]. Future studies should identify key barriers in the implementation of ECR guidelines and consider the use of decision support systems for physical therapists.
There are several limitations to the present study. Despite attempts to persuade CR centres to participate, 33 centres (42 %) did not complete the questionnaire. Physical therapists reported that this was predominantly caused by a lack of time. Although organisation characteristics are comparable (moreover non-university hospitals) we cannot exclude that there was a response bias in our results. Another limitation is that all results are centre based and not patient based. As mentioned earlier, physical therapists reported great variance in the exercise intensity used in individual CR centres. In addition, there may be considerable variation in exercise intensity between individual patients. Patient-based characteristics of ET programs could give better insight into the reasons for individual differences in exercise intensity and prescription method. For this reason we are currently planning a follow-up study where patient-level measurements will be conducted.
Conclusion
This study shows that considerable variation exists in methods for determination of exercise intensity between Dutch CR centres, with a substantial number of CR centres not using SET to determine exercise intensity. In addition, there is no uniformity in training volume and intensity. Therefore, future studies should focus on developing implementation strategies to fill the gap between knowledge and practice in order to improve the quality of exercise training programs in the Netherlands.

Footnotes
The questions can be answered after the article has been published in print. You have to log in to: www.cvoi.nl.
References
- 1.Vaartjes I, van Dis I, Visseren FLJ, et al. Hart- en vaatziekten in Nederland 2010, cijfers over leefstijl- en risicofactoren, ziekte en sterfte. Den Haag: Nederlandse Hartstichting; 2010. [Google Scholar]
- 2.Leal J, Luengo-Fernández R, Gray A, et al. Economic burden of cardiovascular diseases in the enlarged European union. Eur Heart J. 2006;27(13):1610–1619. doi: 10.1093/eurheartj/ehi733. [DOI] [PubMed] [Google Scholar]
- 3.Müller-Riemenschneider F, Meinhard C, Damm K, et al. Effectiveness of nonpharmacological secondary prevention of coronary heart disease. Eur J Cardiovasc Prev Rehabil. 2010;17(6):688–700. doi: 10.1097/HJR.0b013e32833a1c95. [DOI] [PubMed] [Google Scholar]
- 4.Davies EJ, Moxham T, Rees K, et al. Exercise based rehabilitation for heart failure. Cochrane Database of Systematic Reviews 2010. [DOI] [PubMed]
- 5.Leon AS, Franklin BA, Costa F, et al. Cardiac rehabilitation and secondary prevention of coronary heart disease: an American heart association scientific statement from the council on clinical cardiology (subcommittee on exercise, cardiac rehabilitation, and prevention) and the council on nutrition, physical activity, and metabolism (subcommittee on physical activity), in collaboration with the American association of cardiovascular and pulmonary rehabilitation. Circulation. 2005;111(3):369–376. doi: 10.1161/01.CIR.0000151788.08740.5C. [DOI] [PubMed] [Google Scholar]
- 6.Piepoli MF, Corrà U, Benzer W, et al. Secondary prevention through cardiac rehabilitation: from knowledge to implementation. A position paper from the cardiac rehabilitation section of the European association of cardiovascular prevention and rehabilitation. Eur J Cardiovasc Prev Rehabil. 2010;17(1):1–17. doi: 10.1097/HJR.0b013e3283313592. [DOI] [PubMed] [Google Scholar]
- 7.Revalidatiecommissie Nederlandse Vereniging Voor Cardiologie/Nederlandse Hartstichting. Multisiciplinaire Richtlijn Hartrevalidatie 2011. Utrecht: Nederlandse Vereniging Voor Cardiologie 2011.; 2011.
- 8.Hansen D, Stevens A, Eijnde BO, et al. Endurance exercise intensity determination in the rehabilitation of coronary artery disease patients a critical Re-appraisal of current evidence. Sports Med. 2012;42(1):11–30. doi: 10.2165/11595460-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 9.Bartsch P. Platelet activation with exercise and risk of cardiac events. Lancet. 1999;354(9192):1747–1748. doi: 10.1016/S0140-6736(99)90259-3. [DOI] [PubMed] [Google Scholar]
- 10.Lee JY, Jensen BE, Oberman A, et al. Adherence in the training levels comparison trial. Med Sci Sports Exerc. 1996;28(1):47–52. doi: 10.1097/00005768-199601000-00013. [DOI] [PubMed] [Google Scholar]
- 11.Amundsen BH, Rognmo O, Hatlen-Rebhan G, et al. High intensity aerobic exercise improves diastolic function in coronary artery disease. Scand Cardiovasc J. 2008;42(2):110–117. doi: 10.1080/14017430701744477. [DOI] [PubMed] [Google Scholar]
- 12.Amundsen BH, Rognmo Ø, Hatlen-Rebhan G, et al. Training level comparison study: effect of high and low intensity training on ventilatory threshold in men with coronary artery disease. J Cardiopulm Rehabil. 1996;16(4):227–232. doi: 10.1097/00008483-199607000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Moholdt TT, Amundsen BH, Rustad LA, et al. Aerobic interval training versus continuous moderate exercise after coronary artery bypass surgery: a randomized study of cardiovascular effects and quality of life. Am Heart J. 2009;158(6):1031–1037. doi: 10.1016/j.ahj.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Rognmo Ø, Hetland E, Helgerud J, et al. High intensity aerobic interval exercise is superior to moderate intensity exercise for increasing aerobic capacity in patients with coronary artery disease. Eur J Cardiovasc Prev Rehabil. 2004;11(3):216–222. doi: 10.1097/01.hjr.0000131677.96762.0c. [DOI] [PubMed] [Google Scholar]
- 15.Cadroy Y, Pillard F, Sakariassen KS, et al. Strenuous but not moderate exercise increases the thrombotic tendency in healthy sedentary male volunteers. J Appl Physiol. 2002;93(3):829–833. doi: 10.1152/japplphysiol.00206.2002. [DOI] [PubMed] [Google Scholar]
- 16.Perri MG, Anton SD, Durning PE, et al. Adherence to exercise prescriptions: effects of prescribing moderate versus higher levels of intensity and frequency. Health Psychol. 2002;21(5):452–458. doi: 10.1037/0278-6133.21.5.452. [DOI] [PubMed] [Google Scholar]
- 17.Goto Y, Saito M, Iwasaka T, et al. Poor implementation of cardiac rehabilitation despite broad dissemination of coronary interventions for acute myocardial infarction in Japan: a nationwide survey. Circ J. 2007;71:173–179. doi: 10.1253/circj.71.173. [DOI] [PubMed] [Google Scholar]
- 18.Bradley JM, Wallace ES, McCoy PM, et al. A survey of exercise based cardiac rehabilitation services in Northern Ireland. Ulster Med J. 1997;66(2):100–106. [PMC free article] [PubMed] [Google Scholar]
- 19.Deturk WE, Scott LB. Physical therapists as providers of care: exercise prescriptions and resultant outcomes in cardiac and pulmonary rehabilitation programs in New York State. Cardiopulm Phys Ther J. 2008;19(2):35–43. [PMC free article] [PubMed] [Google Scholar]
- 20.Kemps HM, van Engen-Verheul MM, Kraaijenhagen RA, et al. Improving guideline adherence for cardiac rehabilitation in the Netherlands. Neth Heart J. 2011;19:285–289. doi: 10.1007/s12471-011-0104-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Engen-Verheul MM, Kemps HM, de Keizer NF, et al. Revision of the Dutch clinical algorithm for assessing patient needs in cardiac rehabilitation based on identified implementation problems. Eur J Prev Cardiol. 2012;19(3):504–514. doi: 10.1177/1741826711408148. [DOI] [PubMed] [Google Scholar]
- 22.Grol R, Grimshaw J, et al. From best evidence to best practice: effective implementation of change in patients’ care. Lancet. 2003;362(9391):1225–1230. doi: 10.1016/S0140-6736(03)14546-1. [DOI] [PubMed] [Google Scholar]
- 23.Fleuren M, Wiefferink K, Paulussen T, et al. Determinants of innovation within health care organizations. Int J Qual Health Care. 2004;16(2):107–123. doi: 10.1093/intqhc/mzh030. [DOI] [PubMed] [Google Scholar]
- 24.Cabana MD, Rand CS, Powe NR, et al. Why Don't physicians follow clinical practice guidelines?: a framework for improvement. JAMA. 1999;282(15):1458–1465. doi: 10.1001/jama.282.15.1458. [DOI] [PubMed] [Google Scholar]
- 25.Goud R, van Engen-Verheul M, de Keizer NF, et al. The effect of computerized decision support on barriers to guideline implementation: a qualitative study in outpatient cardiac rehabilitation. Int J Med Inform. 2010;79(6):430–437. doi: 10.1016/j.ijmedinf.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 26.Grimshaw JM, Thomas RE, MacLennan G, et al. Effectiveness and efficiency of guideline dissemination and implementation strategies. Health Technol Assess. 2004;8(6):1–72. doi: 10.3310/hta8060. [DOI] [PubMed] [Google Scholar]