Abstract
Breast cancer is a common malignancy in females, which is considered as a systemic disease, whose treatment involves combined modality including systemic as well as local treatment. Recent studies have shown that breast cancer also expresses Sodium Iodide Symporter (NIS) gene, like in the thyroid, which is the factor responsible for the uptake of iodide by the thyroid, enabling radioiodine therapy of thyroid disorders. This study aimed to evaluate various radionuclide imaging characteristics, in vitro radioiodine uptake (RAIU) and evaluation of NIS expression by using Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR) to explore sodium iodide symporter (NIS) expression and iodine uptake in breast cancer and to explor e whether radioiodine can be used for the diagnosis and treatment of breast cancer. Ways of differential regulation of NIS expression in breast cancer has also been explored. Female patients with palpable breast lump and histologically proven infiltrating duct carcinoma were taken up for the study, which included 50 females of mean age 49 years. (range: 23–73 years). The patients were categorized into different groups, depending on the type of the study performed. The uptake patterns in various imaging modalities were analyzed and compared with invitro and RT-PCR studies. 68 % of breast cancer cases showed 99mTc-pertechnetate uptake at the initial images. This finding could partly be due to tumor vascularity, which is usually higher compared to the normal tissues. The uptake in the delayed imaging could be related to that due to NIS in the breast. Use of perchlorate or stable iodine did not alter the pertechnetate uptake pattern in breast tumor. Good correlation between 99mTc-pertechnetate and 99mTc-tetrofosmin uptake in breast cancer was demonstrated. In vitro radioactive iodine uptake in the breast tumor was significantly higher than that in the normal breast tissue. Only 42 % of breast tumor samples studied using RT-PCR showed NIS expression. Correlation between 99mTc-pertechnetate uptake and NIS expression could not be well established. Further studies with higher dose of radioiodine and/or mechanisms of differentially blocking the thyroid are required to assess the feasibility of radioiodine therapy for breast cancer.
Keywords: Breast carcinoma, Sodium iodide symporter, Radioiodine uptake, Reverse transcriptase-polymerase chain reaction (RT-PCR), 99mTc-pertechnetate
Introduction
Breast cancer is one of the most common malignant neoplasms in women throughout the world. Breast cancer is a systemic disease involving a complex spectrum of host-tumor interrelations and variations in locoregional therapy are unlikely to affect survival substantially [1]. Surgical treatment for breast cancer include lumpectomy/quadrantectomy/simple mastectomy/modified radical mastectomy, breast conservation therapy etc. Adjuvant therapy includes chemotherapy, radiotherapy and hormonal therapy. The choice of therapy depends on the stage of the disease at the time of diagnosis (operable breast cancer; locally advanced breast cancer and metastatic breast cancer) Biological therapy and bone marrow transplantation are new methods that are currently being tested in clinical trials. Often, two or more methods are used in combination [1].
The ability of thyroid follicular cells to concentrate iodide was first reported as early as 1915 [2]. The thyroid gland was found to be capable of concentrating iodide by a factor of 20–40 with respect to its concentration in the plasma under physiological conditions. Hence the existence of a thyroid iodide transporter was inferred, and some of its properties were elucidated over the years [2]. The ability of the thyroid to accumulate iodide via NIS has long provided the basis for diagnostic scintigraphic imaging of the thyroid with radio-iodine and has served as an effective means for the treatment of hyperthyroidism and benign thyroid diseases. In addition, the evidence of the important role of iodine transport in thyroid cancer cells provided the basis for the use of radioiodine for the diagnosis and treatment of thyroid cancer [3]. In addition, 99mTc-pertechnetate has also been commonly used to image the thyroid gland. It is transported to thyrocytes by the same mechanism that transports and concentrates radioiodine in the thyroid gland [4].
The ability of cancerous thyroid cells to actively transport iodide via NIS provides a unique and effective delivery system to detect and target these cells for destruction with therapeutic doses of radioiodine, largely without harming other tissues. Therefore, it seems feasible that radioiodine could be a diagnostic and therapeutic tool for the detection and destruction of other cancers in which NIS is functionally expressed [5]. NIS mRNA is detected in many non-thyroid tissues, including the salivary glands, stomach, thymus, and breast. This is consistent with the finding that uptake of radioiodine or 99mTc-pertechnetate is almost always found in the salivary glands, stomach and lactating breast [6].
Physiologically, mammary gland epithelial cells express mammary gland NIS (mgNIS) only during late gestation and lactation, after the culmination of intense glandular proliferation and differentiation. Therefore, it seems possible that mgNIS could be expressed in cancer, as it also involves a proliferative process, although abnormal [7].
The detection and characterization of mgNIS suggests that radioiodine might serve as an agent in the diagnosis and treatment of breast cancer. Long before the cloning of NIS and identification of mgNIS it was known that breast atypia and malignancy reveal increased radioiodine uptake, and breast cancers can be detected by radioiodine/99mTc scintigraphy [8].
The aim of this study was to evaluate various radionuclide imaging characteristics to explore sodium iodide symporter (NIS) expression and iodine uptake in breast cancer and to explore whether radioiodine can be used for the diagnosis and treatment of breast cancer. Ways of differential regulation of NIS expression in breast cancer has also been explored. In vitro radioiodine uptake (RAIU) in the tumor and normal breast tissue specimen and evaluation of NIS expression by using Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR) were also undertaken.
Materials and Methods
Female patients with palpable breast lump and histologically proven Infiltrating Duct Carcinoma were taken up for the study. The study included 50 females of mean age 49 years. (range: 23–73 years). The patients were categorized into different groups, depending on the type of the study performed (Table 1).
Table 1.
Categorization and number of patients that were examined with each specific modality
| Group | No. of cases | TcO4− scans | TcO4− with Perchlorate | Tetrofos-min cases | Iodine cases | |
|---|---|---|---|---|---|---|
| 1 | 99mTcO4−only | 18 | 34 | |||
| 2 | 99mTcO4− + (oral KClO4−) | 7 | 13 | |||
| 3 | 99mTcO4− + 99mTc-Tetrofosmin + (oral KclO4−) | 3 | 9 | |||
| 4 | 99mTcO4− + Tetro + 131I + (oral KclO4−) | 3 | 22 | |||
| 5 | 99mTcO4− + Tetro + 131I (stable I2) | 3 | ||||
| 6 | 131I only (25 μCi) (stable I2) | 3 | ||||
| 7 | 131I only (100 μCi) (stable I2) | 13 | ||||
| Total | 50 | |||||
Protocol for Pertechnetate Scintimammography
18 patients underwent 99mTc-pertechnetate scintimammography. 10 mCi of 99mTc-pertechnetate (99mTcO4−) was injected intravenously. 99mTcO4−, which is widely used for imaging of the thyroid gland, is a very safe radionuclide for imaging. It has a short half-life of 6 h, low gamma energy of 140 keV and low thyroid uptake (0.2 to 3 % only). The whole body absorbed dose is only 0. 01 rads/mCi. The absorbed dose in the thyroid is 0. 13 rads/mCi.
Anterior and lateral images of the chest and neck were acquired at 5 min, 60 min and 120 min post injection. The images were acquired using a large field of view (FOV) Gamma Camera (ELSCINT) with low energy all purpose (LEAP) collimator and 20 % symmetric window centered around 140 keV photopeak. The anterior images were acquired without zoom, and the lateral images were acquired with a zoom factor of 1.5. The images were visually analyzed for the evidence and pattern of pertechnetate uptake at the tumor site, with the opposite breast, which was considered as the control, for comparison. The pattern of tracer uptake/retention at various time intervals (5 min, 60 min, and 120 min) were also analyzed.
Scan Analysis
99mTcO4− - pertechnetate uptake pattern in the breast lesion was divided into 4 categories:
Focal uptake : focal uptake distinctly visualized (Figs. 1 and 2)
Faint focal uptake : focal uptake, slightly more than background activity (Fig. 3)
Diffuse uptake : diffusely increased tracer uptake in the normal breast (Fig. 2)
- No focal uptake : no abnormal focal/diffuse uptake seen
-
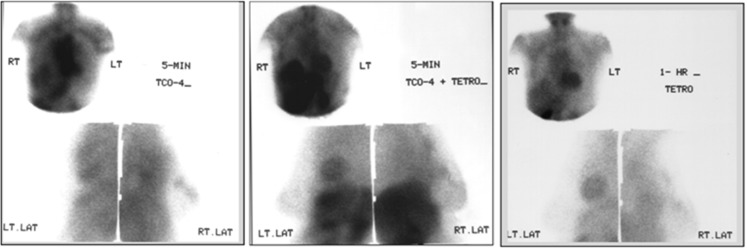
13 patients were pre-treated with 500 mg of potassium perchlorate (KClO4−) 20 min prior to the 99mTcO4− injection to block the pertechnetate uptake in the thyroid and in the stomach. The imaging protocol was the same as for those who were not given potassium perchlorate (Fig. 4).In order to correlate the specificity of pertechnetate uptake in the tumor, a few cases were evaluated by using 99mTc-tetrofosmin as well. Nine patients had undergone both pertechnetate and tetrofosmin scans (Fig. 5). All of these cases were given 500 mg potassium perchlorate orally, 20 min prior to pertechnetate injection. These patients had an initial scan with 5 mCi of pertechnetate. Anterior and lateral views were acquired at 5 min and 1 h. These patients had a second injection with 10 mCi of 99mTc -tetrofosmin and the same imaging protocol was followed at 5 min and 1 h. These cases were evaluated for the pattern of tetrofosmin uptake and washout.
- Three patients had undergone pertechnetate, tetrofosmin and radioiodine scans. 25 μCi of 131I was administered orally and the neck scan and uptake and chest scan were performed at 2 h and 24 h, to look for the thyroidal uptake of radioiodine and the uptake by the breast tumor tissue. The thyroidal radioiodine uptake was measured using thyroid spectrometer and the scans were performed using a rectilinear scanner. The whole body absorbed dose with 131I is only 0. 047 rads/100 μCi. The thyroid absorbed dose (with 15 % uptake) is 78 rads/100 μCi
- Additionally 3 patients had undergone pertechnetate, tetrofosmin and radioiodine scans, who were pretreated with 100 mg of stable iodine, starting 24 h prior to the radionuclide injection.
- In three patients, only radioiodine scan was performed with evaluation of neck uptake and neck and chest scans. They were not pre-treated with either pertechnetate or stable iodine.
- In an additional 13 cases, radioiodine scan was performed after administering 100 μCi of 131I. These patients were pretreated with 100 mg of stable iodine for 2 days, starting 24 h prior to the study. Two hours and 24 h neck uptake and neck & chest scans were done.
-
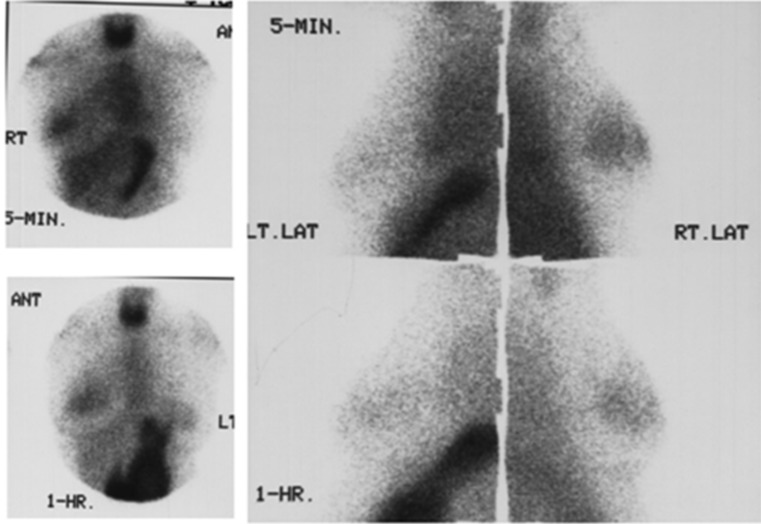
Fig. 1.
99mTc04-scintimammography (group I): the tumor shows focal uptake in the right breast mass at 5 minutes (upper panel). The uptake remains focal even at 1 hour of imaging (lower panel). Uptake in the thyroid and stomach are seen
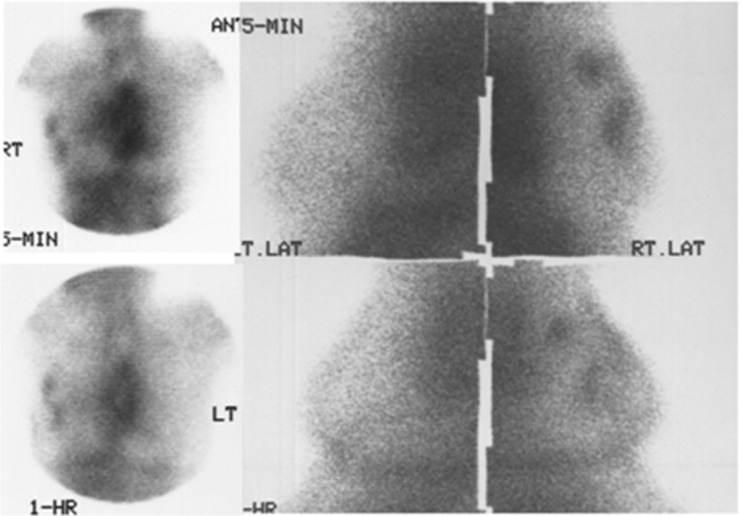
Fig. 2.
99mTc04- scintimammography (group I): Focal uptake in the left breast mass at 5 min (upper panel). One-hour image shows diffuse pattern of uptake in the left breast. The opposite normal breast also shows diffuse uptake at 1 hour (lower panel). Uptake in the thyroid and stomach are seen
Fig. 3.
99mTc04- scintimammography (group I): faint focal uptake in the left breast mass at 5 minutes (upper panel). One hour image (lower panel) shows diffuse pattern of uptake in the left breast. Uptake in the thyroid and stomach are seen
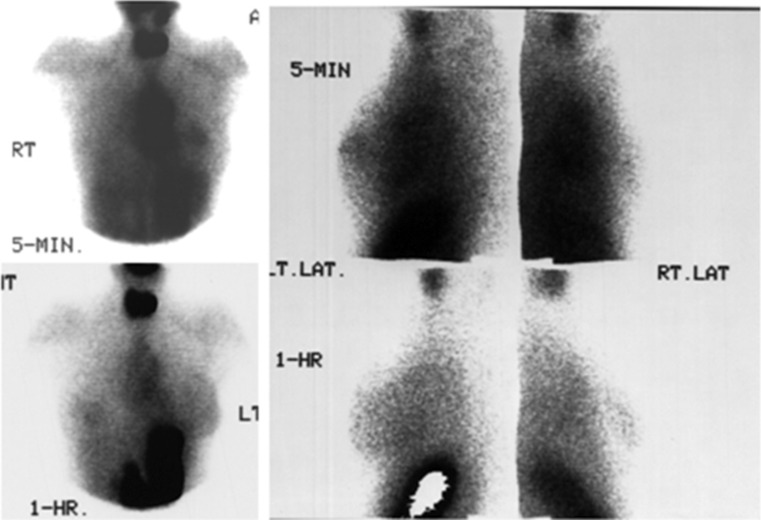
Fig. 4.
99mTc04- scintimammograpfly with pretreatment with perchlorate (group II): The two lesions in the right breast show focal pertechnetate uptake at 5 minutes (upper panel) and one hour of imaging (lower panel). The opposite normal breast does not show tracer uptake. Uptake in the thyroid and stomach are blocked by the use of potassium perchlorate
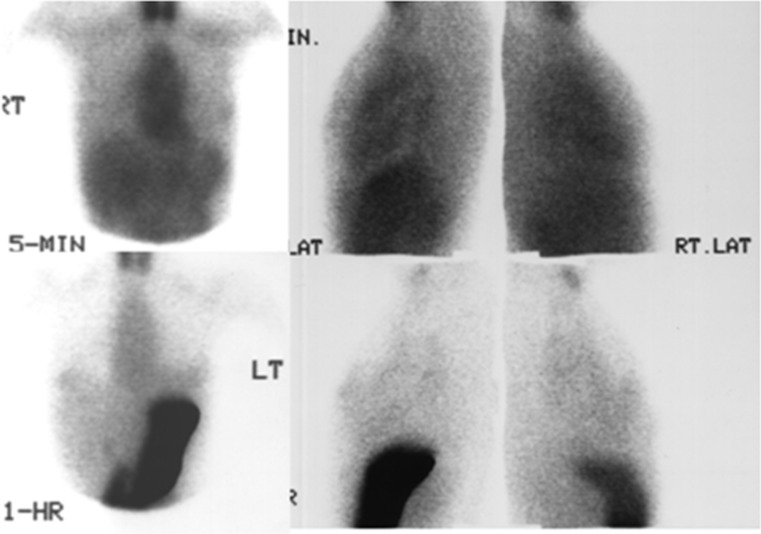
Fig. 5.
99mTc04- scintimammography & 99mTc-Tetrofosmin (group III): Focal uptake in the right breast mass with pertechnetate at 5 minutes (left panel) and with tetrofosmin 5 minutes post injection (middle panel). The delayed 1 hour image (right panel) shows washout of tetrofosmin from the breast mass
Of all the aforementioned patients of different groups, 19 underwent modified radical mastectomy (MRM), and two breast conservation therapy (BCT). Surgical samples from the tumor and normal breast tissue were collected from the patients who underwent MRM, in order to estimate in vitro radioiodine uptake (RAIU) in the specimen and evaluation of NIS expression by using Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR).
Protocol for in Vitro Iodine Uptake Study
The breast tissue obtained at surgery was cleansed and cut into small pieces (50–100 mg each). The tissue was placed in a plastic vial with IMDM (2 ml) containing Na125I (Amersham, UK) 10 × 105 cpm/vial. The vials were incubated in a shaker water bath at 37 °C. At the end of 1 h, the tissue was washed 3 times with 0. 02 M PBS pH 7.2, blotted and the radioactive uptake was counted in a multi gamma counter (Packard, USA). Results were expressed as cpm x 105/gram of the tissue. The uptake of the normal breast tissue and tumor tissue was carried out separately, and the results were compared using paired ‘t’ test.
Protocol for RT-PCR of NIS
The breast tissue (tumor as well as normal breast tissue separately) was homogenized in 2 ml of trizol (Gibco, BRL, USA) in a polytron homogeniser and total RNA was extracted according to the manufacturer’s protocol. The quantity and quality of RNA was determined by measuring the absorbance at 260 nm and 280 nm and by electrophoresis on 1 % agarose gel.
One microgram of total RNA was used for preparation of cDNA using the enzyme M-MULV reverse transcriptase (MBI Fermentas, USA) and the NIS antisense primer. The reaction was carried out at 37 °C for 2 h. The cDNA thus prepared was amplified by PCR using NIS specific primers. (GENSET, USA). The PCR product (10 μl) was electrophoresed on 1.5 % agarose gel. A 100 base pair DNA ladder was used for comparison. The gel was stained with ethidium bromide and the results were recorded using the gel documentation system. The NIS specific band was obtained at 454 bp.
Results
A total of 50 patients were studied for testing this hypothesis. The mean age of patients included in the study was 49 years. (Range: 23–73 years). The site of the breast cancer was in the left in 27 cases, and in the right in 23 cases.
34 patients had undergone pertechnetate scintimammography. Of these, 16 cases had been pretreated with 500 mg of potassium perchlorate 20 min before the tracer injection. 18 cases had 99mTc-pertechnetate chest and neck scan without pretreatment with perchlorate. Nine patients were evaluated using 99mTc-tetrofosmin scintimammography also. Three patients were pretreated with stable iodine (100 mg) 24 h prior to scintimammography. Scintimammography using radioiodine (131I) was performed in 22 cases. 25 μCi was given in 9 cases and 100 μCi in 13 cases. All the latter subgroup were also pre-treated with stable iodine.
Tumor Size & Histology
The tumor size was T2 (2–5 cm) in 11 cases, T3 (> 5 cm) in 29 cases, and T4 (chest wall/skin involvement) in 10 cases (Table 2).
Table 2.
showing distribution of cases according to Tumor size and the population who underwent pertechnetate scintigraphy with and without perchlorate intervention
| Tumor size | Total No. of cases | Pertechnetate scintigraphy | Pertechnetate scintigraphy + perchlorate |
|---|---|---|---|
| T2 | 11 | 7 | 2 |
| T3 | 29 | 9 | 12 |
| T4 | 10 | 2 | 2 |
| Total | 50 | 18 | 16 |
All the patients were histologically proven to have Infiltrating Duct Carcinoma (Table 3).
Table 3.
Categorization and number of patients that were examined with each specific modality with respect to tumor size
| Group | T 2 | T 3 | T 4 | Total | |
|---|---|---|---|---|---|
| 1 | 99mTcO4− only | 7 | 9 | 2 | 18 |
| 2 | 99mTcO4− + oral KclO4− | 1 | 5 | 1 | 7 |
| 3 | 99mTcO4− + 99mTc-Tetro + oral KclO4− | 1 | 2 | — | 3 |
| 4 | 99mTcO4− + Tetro + 131I + oral KclO4− | — | 3 | — | 3 |
| 5 | 99mTcO4− + Tetro + 131I (stable I2− 100 mg per day for 2 days, starting 24 h prior to the study) | — | 2 | 1 | 3 |
| 6 | 131I only (25 μCi) (stable I2) | — | 2 | 1 | 3 |
| 7 | 131I only (100 μCi) (stable I2) | 2 | 6 | 5 | 13 |
| Total | 11 | 29 | 10 | 50 | |
Scan Findings
34 patients had undergone pertechnetate scintimammography. 18 cases had pertechnetate chest and neck scan done. 16 cases were pretreated with 500 mg of potassium perchlorate, 20 min before the tracer injection. 19 patients were pretreated with stable iodine (100 mg), starting 24 h prior to the study.
Of the 34 cases, 22 lesions showed focal tracer uptake. Only faint uptake was seen in 6 lesions and 2 showed no pertechnetate uptake. Diffuse uptake was seen in 4 cases.
Pattern of Tracer Uptake with Respect to Time
Of the 22 lesions, where the initial 5 min uptake was focal, it remained focal in only 8 cases, up to a follow up time of 2 h. The uptake pattern in the lesion became more diffuse in 11 cases (Fig. 2). In 3 cases, delayed imaging could not be performed.
4 out of 6 cases, which showed focal faint uptake, continued to show focal faint uptake at 1 and 2 h. 2/6 lesions became diffuse at delayed imaging.
In 4 cases, where the uptake was diffuse, it continued to be diffuse up to 2 h of imaging.
The 2 lesions with no uptake at 2 h were persistently showing no uptake even at 2 h of imaging (Table 4).
Table 4.
Pattern of tracer uptake in the tumor lesions with respect to time of Image Acquisition
| Time | Focal uptake | Faint focal uptake | Diffuse uptake | No uptake | Total | |||
|---|---|---|---|---|---|---|---|---|
| 5 min. | 22 | 6 | 4 | 2 | ||||
| focal | diffuse | not done | faint focal | diffuse | ||||
| 1 h | 8 | 11 | 3 | 4 | 2 | 4 | 2 | |
| 2 h | 8 | 11 | 3 | 4 | 2 | 4 | 2 | |
| Total | 22 | 6 | 4 | 2 | 34 | |||
Uptake Pattern in the Normal Breast
The normal breast in 31/34 case in whom pertechnetate scintigraphy was performed, showed no abnormal radiotracer uptake at 5 min. 3 normal breasts showed diffusely increased tracer uptake. 15/31 of the cases which showed no tracer uptake, was persistently showing no uptake, even at delayed imaging at 1 and 2 h. 13 of these 31 cases showed a diffuse pattern of increased uptake (Fig. 2) at delayed imaging. In 3 cases, delayed imaging could not be done. 3 normal breasts showed diffuse uptake at 5 min, which continued to persist at a later time (Table 5).
Table 5.
Pattern of pertechnetate uptake in the normal breast with respect to time of Image Acquisition
| No uptake | Diffuse uptake | Total | |||
|---|---|---|---|---|---|
| 5 min. | 31 | 3 | |||
| No uptake | Diffuse | Not done | |||
| 1 h | 15 | 13 | 3 | 3 | |
| 2 h | 15 | 13 | 3 | 3 | |
| Total | 31 | 3 | 34 | ||
Group 1: Only Pertechnetate Scan
18 patients were subjected to 99mTc- pertechnetate scintimammography.
Of these 18 patients, the breast lesion was seen at 5 min as focal uptake in 12 cases. Four showed only faint focal uptake and no uptake was seen in 2 cases. Out of the 12 lesions with focal uptake at 5 min, only 4 continued to persist as focal uptake. In 6 cases, the pattern of uptake had changed to generalized increased uptake (diffuse uptake) in the glandular tissue of the breast. The 2 cases which was not showing uptake at 5 min was not showing uptake even at imaging at 1 or 2 h. In 2 cases delayed imaging could not be done.
The opposite normal breast showed no uptake (only background activity) in 15 cases. In 3 cases, the contralateral breast showed diffusely increased activity in the 5th minute images. Diffuse uptake was seen in 7 normal breasts in delayed imaging. 4 of the normal breasts which showed no uptake at the 5th minute, showed diffusely increased uptake in the glandular tissue in delayed imaging. In 2 cases, delayed pattern could not be acquired. In one case, where the lesion was not showing the uptake, the corresponding opposite breast also was not showing tracer uptake (Tables 6 and 7).
Table 6.
Pattern of pertechnetate uptake in the breast lesions with respect to time of Image Acquisition
| Ca breast | Total | Normal breast | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Focal uptake | Faint focal uptake | No uptake | No uptake | Diffuse uptake | |||||||
| 5 min | 12 | 4 | 2 | 18 | 15 | 3 | |||||
| focal | diffuse | Not done | faint focal | diffuse | no uptake | diffuse | not done | ||||
| 1 h. | 4 | 6 | 2 | 2 | 2 | 2 | 18 | 9 | 4 | 2 | 3 |
| 2 h. | 4 | 6 | 2 | 2 | 2 | 2 | 18 | 9 | 4 | 2 | 3 |
| Total | 12 | 4 | 2 | 18 | 15 | 3 | |||||
Table 7.
Results of Pertechnetate scintigraphy according to Tumor size
| Tumor size | Timing of scintigraphy | Pattern of uptake | ||||
|---|---|---|---|---|---|---|
| focal | Faint focal | diffuse | No uptake | Total | ||
| T2 | 5 min. | 4 | 2 | 1 | 7 | |
| 1 h | 2 | 1 | 3 | 1 | ||
| T 3 | 5 min. | 6 | 2 | 1 | 9 | |
| 1 h | 3 | 6 | ||||
| T 4 | 5 min. | 2 | 2 | |||
| 1 h | 1 | 1 | ||||
| 18 | ||||||
Group 2: 99mTc-Pertechnetate with Oral Potassium Perchlorate
13 patients were evaluated with scintimammography using pertechnetate with pre-treatment with oral perchlorate in order to block the uptake in the thyroid and stomach and to look for any change in the uptake pattern in the breast lesions and in normal breast. Out of the 13 patients, 9 showed focal uptake and 3 showed diffuse uptake on the lesion side. One lesion did not show pertechnetate uptake at all. Out of the 9 with focal tracer concentration, only 4 were persistently showing focal uptake in the delayed images as well. Delayed images showed diffuse uptake pattern in 8 cases. There was no uptake in the contralateral normal breast in 11 of the 13 cases. 2 normal breasts showed diffuse pattern of uptake. 6 of the normal breasts showed diffuse uptake on delayed imaging (Table 8 and 9).
Table 8.
Pattern of pertechnetate uptake in the breast lesions with perchlorate intervention with respect to time of Image Acquisition
| Ca breast | Total | Normal breast | ||||
|---|---|---|---|---|---|---|
| Focal | Diffuse uptake | No uptake | No uptake | Diffuse uptake | ||
| 5 min. | 9 | 3 | 1 | 13 | 11 | 2 |
| 1 h. | 4 | 8 | 1 | 13 | 7 | 6 |
| 2 h. | 2 | 5 | 7 | 3 | 4 | |
Table 9.
Results of Pertechnetate scintigraphy with perchlorate intervention according to tumor size
| Tumor size | Timing of scintigraphy | Pattern of uptake | ||||
|---|---|---|---|---|---|---|
| focal | Faint focal | diffuse | No uptake | Total | ||
| T2 | 5 min. | 2 | 2 | |||
| 1 h | 2 | |||||
| T 3 | 5 min. | 5 | 1 | 2 | 4 | 12 |
| 1 h | 2 | 7 | 3 | |||
| T 4 | 5 min. | 1 | 1 | 2 | ||
| 1 h | 1 | 1 | ||||
| 16 | ||||||
In 2 of these cases, RT-PCR/invitro studies were done. In one of the positive RT-PCR cases, the pertechnetate uptake was focal, where as in one, the lesion side was showing diffuse uptake. The in vitro radioiodine uptake was 7.3 % in the diffuse uptake case and 4.18 in the case where the uptake was focal.
Group 3: 99mTc-Pertechnetate and 99mTc-Tetrofosmin
9 cases were evaluated with both 99mTc-pertechnetate and 99mTc-tetrofosmin scintimammography, to check for the specificity of pertechnetate uptake in breast tumors. Of these 9 cases, where 99mTc-tetrofosmin and 99mTc-pertechnetate scans sere performed, 1 lesion did not show uptake with either tracer. All the other 8 cases showed focal uptake with both pertechnetate and tetrofosmin. Of the 8 cases (8/9) where pertechnetate was positive at 5 min, 5 lesions continued to show focal uptake and 3 became diffuse at 1 h time. In 8/9 cases where tetrofosmin was positive, only 4 cases showed retention of activity at 2 h and 4 cases showed tracer washout.
In the four cases where the lesion was showing persistent tetrofosmin uptake at 2 h, 3 were showing persistent pertechnetate uptake in the delayed images. [focal pertechnetate uptake at 1 h is indicative of tetrofosmin retention in 75 % of cases]
None of the normal side was showing pertechnetate uptake at 5 min. Three of the normal breasts showed diffuse uptake at 1 h. 8/9 normal breasts showed no tetrofosmin uptake. 1 case showed diffusely increased uptake. The pattern was the same even after 2 h.
In the normal breast where diffuse tetrofosmin uptake was seen, pertechnetate was showing no uptake at 5 min, but showed diffuse uptake at 1 h (Table 10).
Table 10.
Comparison of Pattern of tracer uptake in the breast lesions with pertechnetate and tetrofosmin scintigraphy with respect to time of Image Acquisition
| Ca breast | Total | Normal breast | ||||
|---|---|---|---|---|---|---|
| Focal uptake | Diffuse uptake | No uptake | No uptake | Diffuse uptake | ||
| 99mTc -Pertechnetate scan | ||||||
| 5 min. | 8 | — | 1 | 9 | 9 | — |
| 1 h. | 5 | 3 | 1 | 9 | 6 | 3 |
| 99mTc-Tetrofosmin scan | ||||||
| 5 min. | 8 | - | 1 | 9 | 8 | 1 |
| 2 h. | 4 | - | 5 | 9 | 8 | 1 |
Group 4: 99mTc-Pertechnetate, 99mTc-Tetrofosmin and 131I
3 patients were evaluated using the three scintigraphic procedures.
All the lesions showed focal pertechnetate and tetrofosmin uptakes. No radioiodine concentration was seen either in the lesion side or the normal breast in any of these patients. The neck RAIU was 15 %, 17 % and 7 % in these cases. All the patients were clinically euthyroid.
Group 5: 99mTc-Pertechnetate, 99mTc-Tetrofosmin and 131I (Blocking with Stable Iodine)
Three patients were treated with stable iodine 100 mg/day, starting 24 h prior to the study. In this group also, the finding was the same, both tracers (pertechnetate and tetrofosmin) showing focal uptake in the lesions. None of these 3 patients showed radioiodine uptake in the breast lesion. The neck RAIU at 24 h was only 1.9 %, 1.3 % & 2.1 % in these 3 cases.
Group 6: Only 131I (25 μCi)
3 cases were evaluated with only radioiodine scans. These were neither given stable iodine nor perchlorate. None of the cases showed abnormal uptake in the breast cancer side or in the normal breast. The neck uptake at 24 h in these cases was 29 %, 10 % and 18 % respectively.
Group 7: Only 131I (100 μCi)
Thirteen cases were studied using 100 μCi of radioiodine (131I). These patients were pre-treated with 100 mg of stable iodine for blocking thyroidal uptake. None of the patients showed radioiodine uptake in the breast lump. The mean thyroidal radioiodine uptake at 24 h was 1. 03 % (range: 0.34 % to 1.85 %)
In Vivo And In Vitro Studies
In 3 cases showing focal tracer uptake, the RT-PCR was positive in 2 cases. RT-PCR for NIS gene expression was negative in one case, which was showing focal tracer uptake. It was negative in the 2 cases, where the scan was showing only faint uptake. In one case showing diffuse pattern in scan, RT-PCR was negative. It was positive in 2 cases, which on scan was showing no uptake (Table 11).
Table 11.
Comparison of Pattern of tracer uptake in the breast lesions with RT-PCR results
| Scan findings | Focal uptake | 3 | RT-PCR | + ve | 2 | 3 | RAIU (ratio of Ca/normal) | 20, 3 |
| - ve | 1 | 5 | ||||||
| Faint focal uptake | 2 | RT-PCR | + ve | - | 2 | RAIU (ratio of Ca/normal) | — | |
| - ve | 2 | 15, 8 | ||||||
| Diffuse uptake | 1 | RT-PCR | + ve | 1 | 1 | RAIU (ratio of Ca/normal) | 7 | |
| - ve | - | |||||||
| No uptake | 2 | RT-PCR | + ve | 2 | 2 | RAIU (ratio of Ca/normal) | 4, 8 | |
| - ve | - | |||||||
| Total | 8 | 8 | ||||||
In Vitro RAIU
In vitro radioiodine uptake of the tumor tissue and normal adjacent breast tissue obtained at surgery were evaluated. The tissues were incubated with 125I and counts measured and were expressed as cpm per gram of tumor and/or cpm per gram of normal breast tissue. The uptake in both tumor and normal tissue were analyzed using the paired ‘t’ test.
17 cases were analyzed like this. The mean uptake in the breast tumor was 3. 16 × 105cpm/gram, and that of the normal breast tissues was 0. 37 × 105cpm/gram.
The difference in uptake was found to be significant. (p value = 0.03)
The in vitro radioiodine uptake values ranged from 3.0 % to 20. 3 %. In one of the RT-PCR positive cases, the uptake was 20.3 % and in one case, it was 3. 0 %. In 3 of the negative RT-PCR, the uptake values were 15. 4 %, 7. 4 % and 5.5 %.
In Vitro RAIU + RT-PCR
15 cases had undergone this analysis. The RT-PCR was positive for NIS gene expression in 7 cases. The mean radioactive iodine uptake ratio (tumor to normal tissue) was 10 (range 3 to 22) in this group. In 8 RT-PCR negative cases, the mean radioiodine uptake ratio was 7.5 (range 1.2 to 15.4) (Table 12)
Table 12.
The mean RAIU ratio and the RT-PCR results in the patient population
| No. | RAIU ratio (mean) (breast tumor to normal breast) | Range | |
|---|---|---|---|
| RT-PCR + VE | 7 | 10 | 3–22 |
| RT-PCR -VE | 8 | 7.5 | 1.2—15.4 |
| Total | 15 |
Only RT-PCR
This was done in 24 breast tumor samples. Was positive in 10 cases only (42 %) and was negative in the rest 14 cases (58 %).
Discussion
In our study, in the group of patients with only pertechnetate scans (group I) 12/18 showed focal uptake (67 %). 4/12 cases showed focal tracer retention at 2 h (33 %). 6/12 (50 %) cases showed diffuse uptake in the breast at 2 h. Most of breast cancer lesions (67 %) show focal tracer uptake immediately post tracer injection, but does not show tracer retention in majority of the cases (67 %). This finding could also be due to tumor vascularity, which is usually higher compared to the normal tissues. This implies that even if the lesion concentrates radiotracer, the therapeutic efficiency is in question due to the short retention time of the tracer in the lesion, which needs to be assessed further. In normal breasts also, 7/15 cases showed diffuse pattern of uptake at 2 h. This figure is almost the same as that of the lesion side. 6/12 (50 %) cases showed diffuse uptake in the breast in the lesion side. Thus, it may be assumed that the delayed uptake in the breast be considered non-specific. So, the pattern of uptake at 5 min post injection can be considered to represent specific uptake based on NIS expression.
16/18 cases showed focal uptake (12 + 4 faint). This increased uptake could be mediated by specific uptake in the breast cancer lesions, by way of NIS expression, due to increased vascularity of the lesion/increased trapping of the tracer in the tumor vasculature.
In their study, Cancroft et al. [8] had demonstrated pertechnetate uptake in the malignant breast lesions, significantly higher than in benign lesions. They had shown positive uptake in 4/6 cases, and all the 4 were found to have carcinoma. The 2 lesions, which were negative on pertechnetate scintimammography, were found to have benign lesions. The mechanism of pertechnetate concentration in breast Ca was not clear at that time. Now it is presumed that uptake of radioiodine and pertechnetate in the thyroid, lactating breast and Breast Cancer is mediated by NIS [7]. To confirm this we have made an attempt to correlate the scan findings with the NIS expression in the tumor tissue by way of RT-PCR. In addition, the tissues have been subjected for in vitro radioiodine uptake and compared with the RAIU of normal breast tissue. We have studied patients who were proven to have malignancy, and have shown pertechnetate uptake in malignant breast lesions in 12/18 cases (67 %).
Rudolfo L et al. [9]. studied 36 volunteers with palpable breast lesions. 6 out of these patients were proved to have carcinoma. 5/6 (83 %) of these had a positive pertechnetate mammographic study. In their study, DH Moon et al. [10]. showed significantly increased uptake in 4 proven carcinoma cases, and these were associated with higher levels of NIS expression. They could demonstrate increased pertechnetate uptake in breast cancer lesions in only 16 % (4/25) of cases. Our study showed scan positivity in 67 % of cases. Kilbane et al. [11]. demonstrated NIS expression by RT-PCR in 2 of 2 fibroadenomata and 6 of 7 breast carcinoma mRNA isolates. They also showed that the tissue iodide content of breast carcinomas was significantly lower than that in remote normal tissue from the tumor-bearing breast or in fibroadenomata. Tazeby et al. [7]. demonstrated by immunohistochemistry that more than 80 % of human breast cancer samples expressed hNIS protein compared with none of the normal breast tissues. They also demonstrated specific active transport of pertechnetate in mammary adenocarcinoma in transgenic mice bearing Ras or Neu tumors.
Richman SD et al. [12] demonstrated good correlation between malignancy and positive scintigraphy using 99mTcO4 in 16 cases of breast carcinoma (88 % accuracy). But the false positive rate was also high (28 %). This limits the use of 99mTcO4 as an aid to differential diagnosis of breast masses. In the group of patients pre-treated with potassium perchlorate (no = 13), 9/13 showed focal uptake (69 %). 3/13 showed diffuse uptake, which may be considered non-specific. In the group not given perchlorate, only 1/18 (5.5 %) showed diffuse uptake. In the perchlorate group, 3/13 (23 %) showed non-specific uptake.
There was no significant change in the percentage of cases showing focal pertechnetate uptake following perchlorate administration—12/18 cases (67 %) in the pertechnetate only group versus 9/13 cases (69 %) in the perchlorate group. (Stomach and thyroid uptake was blocked in all by the use of perchlorate in these cases).
Tazeby et al. [7]. had demonstrated inhibition of pertechnetate concentration in thyroid, stomach and lactating mammary gland in animal studies. Das BK et al. [13]. studied the effect of perchlorate in the pertechnetate uptake in breast cancer and concluded that pertechnetate uptake is significantly suppressed by perchlorate in the thyroid and normal breast, but not in tumor tissue of the breast and suggested a different mechanism of handling of potassium perchlorate by NIS in tumor tissue. They also opined that the inhibition of pertechnetate uptake by perchlorate in mice tumor reported recently is not applicable to human breast tumor.
Cancroft et al. [8]. in 1974 had evaluated pertechnetate scintimammography after oral administration of perchlorate. They had reported that 4/6 cancer cases showed pertechnetate uptake, suggesting that NIS expression is not impaired in presence of perchlorate.
This suggests that the use of perchlorate selectively block the thyroidal and stomach uptake of pertechnetate, and also radioiodine, but does not inhibit the uptake in breast tumor tissues expressing NIS. This is a favorable finding, in considering radioiodine treatment for NIS expressing breast tumors, by reducing the dose to the normal thyroid. However, further studies are required to clarify this issue and to assess methods of selectively blocking the thyroidal NIS expression to consider Radionuclide therapy for NIS expressing breast cancer (primary as well as metastatic disease).
In our group of patients given stable iodine prior to the study, (no. = 3) no change in the pattern of pertechnetate uptake in the breast lesion was seen. All the 3 cases showed focal tracer uptake at 5 min. The radioactive iodine uptake (RAIU) of the thyroid was significantly lower in this group of patients. This suggests that the thyroidal uptake may be blocked by the use of stable iodine, whereas uptake pattern in breast cancer could not be assessed. This, again, may help in differentially blocking the thyroidal uptake of therapeutic doses of radionuclides, and thereby preferentially targeting the breast cancer. The effect of stable iodine on the uptake in breast cancer could not be assessed in our study, since none of the cases were showing radioiodine uptake, probably the dose of radioiodine (131I) used might have been too low. The dosimetric considerations prevent the use of higher doses of 131I for imaging. Also the amount of tracer available for breast tumor uptake after thyroidal trapping is still reduced. Blocking the thyroid may help in more tracer available for the breast uptake. Whether the stable iodine will block uptake in the breast cancer also need to be evaluated.
We have studied 13 cases with palpable breast tumor by using 100 μCi of 131 I. These patients were pre-treated with stable iodine. None of the cases showed radioiodine uptake in the breast tumor. RAIU (neck) was blocked by the use of stable iodine (mean 1. 03 %). Whether the use of stable iodine blocks NIS mediated radioiodine iodine uptake in the breast tumor is not known. There was no change in the pattern of pertechnetate uptake in 3 cases, who were given stable iodine.
However, the number of cases studied is limited and further evaluation in a large number of cases is required to arrive at a definite conclusion. Scanning with higher doses of 123I may be an alternative.
Since the thyroidal radioiodine uptake is under TSH control, Eltroxin supplementation for TSH suppression and hence, reduced radioiodine uptake in the thyroid should be explored as a potential way of differentially regulating the NIS expression in the thyroid and in the breast cancer.
Nakamoto et al. [14]., in their study, suggested that inducing cancer cells to express functionally active NIS will enable those cells to accumulate iodide from plasma in vivo and may lead to radioiodine cancer treatment. In their study, physiologic uptake in the thyroid and stomach was also very high and pointed out that it could be a limiting factor in clinical use. They suggested that total thyroidectomy and supplementation of thyroidal hormones would be acceptable if the usefulness of radioiodine concentrator gene therapy for patients with multiple metastasis could be guaranteed.
Zuckier LS, N. Carrasco et al. [15], concurred with Nakamoto et al. [14] that 131I iodide therapy may have a promising role to play in breast cancer. They also suggested that use of endogenous mgNIS represent a more readily attainable means of delivery of radioiodine, and because thyroid NIS and mgNIS are differentially regulated, it should be possible to adequately decrease thyroid iodide uptake without affecting breast cancer uptake through suppression of pituitary thyroid-stimulating hormone release. Therefore, Zuckier LS et al. differed from Nakamoto et al. that, when treating breast carcinoma using endogenous mgNIS, there may be no need for thyroidectomy.
To increase the specificity of pertechnetae uptake in the malignant breast lesion, a correlation with 99mTc-tetrofosmin uptake in these cases were assessed. In 9 cases assessed in this way, 8 cases showed focal uptake in the lesion with pertechnetate as well as tetrofosmin, thereby supporting the idea that pertechnetate uptake in breast cancer lesion is specific and can be considered as a screening test for malignancy, making use of the NIS expression in breast cancer. The high sensitivity and specificity of 99mTc-tetrofosmin scintimammography has been assessed in several studies.
Horne T et al. [16]. conducted scintimammography with both 99mTc-tetrofosmin and 99m Tc-MIBI in 35 patients and found sensitivity and specificity for 99mTc-tetrofosmin as 90 % and 80 %, and that for 99m Tc-MIBI as 89.4 % and 80 % respectively.
Ortapamuk H et al. [17]. had demonstrated the sensitivity and specificity of 99m Tc-tetrofosmin in the diagnosis of malignant breast masses as 95 % and 96 % respectively.
The cases we have studied with pertechnetate scintimammography were all proven to have malignancy (infiltrating duct carcinoma Gr.III) and only 21/31 cases have shown focal pertechnetate uptake (68 %). It can be inferred that NIS expression is present in this 68 % of cases. This value is in concurrence with the findings by Tazeby et al. [7]. that more than 80 % of breast cancer expresses NIS.
But, when the tumor tissue was analyzed for NIS expression by RT-PCR (different set of patients), only 42 % of the samples showed NIS expression. None of the normal breast tissues studied expressed NIS gene.
DH Moon et al. [10]. found mRNA expression of hNIS in both breast cancer tissue as well as normal breast tissue. They showed that the expressions were higher in tumors with positive uptakes on pertechnetate scintimammography and suggested that pertechnetate scintimammography may predict hNIS mRNA expression in human breast tumor tissues.
Kilbane et al. [11] had demonstrated hNIS expression in 6/7 cases of breast cancer using RT-PCR.
Conclusion
99mTc-pertechnetate scintimammography can be performed to assess the expression of NIS in breast cancers. In our study, 68 % of breast cancer cases showed positive study. Delayed imaging resulted in non-specific uptake in the breast, which raises the possibility of tumor vascularity playing a role in the uptake. Use of perchlorate or stable iodine did not alter the early pertechnetate uptake pattern in breast tumor. Good correlation between 99mTc-pertechnetate and 99mTc-tetrofosmin uptake in breast cancer was demonstrated. In vitro radioactive iodine uptake in the breast tumor was significantly higher than that in the normal breast tissue. Only 42 % of breast tumor samples studied using RT-PCR showed NIS expression. Correlation between 99mTc-pertechnetate uptake and NIS expression could not be established. Further studies with higher dose of radioiodine and/or mechanisms of differentially blocking the thyroid are required to assess the feasibility of radioiodine therapy for breast cancer.
References
- 1.Richard G. Margolese et al.: Neoplasms of the Breast, Chapter 118; Text book of Cancer Medicine, 5th Edition (an Official Publication of the American Cancer Society) July 2000.
- 2.Carrasco N. Iodide transport in the thyroid gland. Biochim Biophys Acta. 1993;1154:65–82. doi: 10.1016/0304-4157(93)90017-I. [DOI] [PubMed] [Google Scholar]
- 3.Dai G, et al. Cloning and characterization of the thyroid iodide transporter. Nature. 1996;379:458–460. doi: 10.1038/379458a0. [DOI] [PubMed] [Google Scholar]
- 4.Chung JK. Sodium iodide symporter: its role in nuclear medicine. J Nucl Med. 2002;43:1188–1200. [PubMed] [Google Scholar]
- 5.Mazaferri EL. In: Carcinoma of the follicular epithelium. In the thyroid: A fundamental and clinical text. Braverman LE, Utiger RD, editors. Raven: Lippincott; 2000. pp. 904–930. [Google Scholar]
- 6.Filetti S, et al. Sodium iodide symporter: a key transport system in thyroid cancer cell metabolism. Eur J Endocrinol. 1999;141(5):443–457. doi: 10.1530/eje.0.1410443. [DOI] [PubMed] [Google Scholar]
- 7.Tazeby UH, et al. The mammary gland iodide transporter is expressed during lactation and in breast cancer. Nat Med. 2000;6(8):871–878. doi: 10.1038/78630. [DOI] [PubMed] [Google Scholar]
- 8.Goldsmith SJ, et al. 99mTc-pertechnatate scintigraphy as an aid in the diagnosis of breast masses. Radiology. 1973;106:441–444. doi: 10.1148/106.2.441. [DOI] [PubMed] [Google Scholar]
- 9.Rudolfo L, et al. Experimental pertechnetate mammography. Radiology. 1974;111:657–661. doi: 10.1148/111.3.657. [DOI] [PubMed] [Google Scholar]
- 10.Moon DH, et al. Correlation between 99mTc-pertechnetate uptakes and expression of human sodium iodide symporter gene in breast tumor tissues. Nucl Med Biol. 2001;28:829–834. doi: 10.1016/S0969-8051(01)00243-8. [DOI] [PubMed] [Google Scholar]
- 11.Kilbane, et al. Tissue iodine content and serum mediated 125I uptake-blocking activity in breast cancer. J Clin Endocrinol Metab. 2000;85:1245–1250. doi: 10.1210/jc.85.3.1245. [DOI] [PubMed] [Google Scholar]
- 12.Richman SD, et al. Breast scintigraphy with 99mTc-pertechnetate and 67Ga-citrate. J Nucl Med. 1975;16(4):293–9. [PubMed] [Google Scholar]
- 13.Das BK, et al. Scintigraphic study of the effect of potassium perchlorate on expression of sodium iodide symporter (NIS) in human breast cancer: Scientific papers, Proceedings of the SNM 49th annual meeting. J Nucl Med. 2002;43(5):120. [Google Scholar]
- 14.Nakamoto Y, et al. Establishment and characterization of a breast cancer cell line expressing Na+/I− symporters for radioiodide concentrator gene therapy. J Nucl Med. 2000;41:1898–1904. [PubMed] [Google Scholar]
- 15.Zuckier LS, Carrasco N, et al. The endogenous mammary gland Na+/I− symporter may mediate effective radioiodide therapy in breast cancer. J Nucl Med. 2001;42(6):987–8. [PubMed] [Google Scholar]
- 16.Horne T, et al. 99mTc(m)-tetrofosmin scintimammography for detecting breast cancer: a comparative study with 99Tc(m)-MIBI. Nucl Med Commun. 2001;22(7):807–11. doi: 10.1097/00006231-200107000-00012. [DOI] [PubMed] [Google Scholar]
- 17.Ortapamuk H, et al. Role of technetium tetrofosmin scintimammography in the diagnosis of malignant breast masses and axillary lymph node involvement: a comparative study with mammography and histopathology. Eur J Surg. 1999;165(12):1147–53. doi: 10.1080/110241599750007676. [DOI] [PubMed] [Google Scholar]