Abstract
Langerhans Cells (LC) are dendritic cell (DC) that resides in the epidermis of skin. Paul Langerhans originally observed and named this epinonymous cell more than 140 years ago1. Their network-like distribution and dendritic processes that extended up into the stratum corneum convinced him that they represented peripheral nerve cells. It was not determined until almost 100 years later that LC are, in fact, bone marrow-derived and function as skin-resident antigen presenting cells (APC)2, 3. Many studies have shown that LC are highly immunostimulatory. Recently, data has begun to accumulate suggesting LC have immunoregulatory properties. This review will focus on the participation of Langerhans cells in the development and regulation of adaptive immune responses.
The Langerhans Cell Paradigm
The earliest insights into LC function come from in vitro experiments using LC isolated from mouse skin. Antigen-pulsed LC-enriched epidermal cells induced a proliferative response in immune T cells and outstanding immunostimulatory capacities in the mixed leukocyte reaction (MLR) assay4, 5. LC were also shown to allow for efficient CTL induction6. Studies using LC-like cells derived in vitro from bone marrow or hematopoietic stem cells showed similar results7–9
The most commonly used assay of LC function in vivo is contact hypersensitivity (CHS) to haptens that are applied directly onto the skin. Mice are sensitized by application of the hapten to their abdomens. The degree of ear swelling after application of the same hapten to the ear several days later measures the degree of the anti-hapten effector response. A major challenge to the study of LC was how to separate the functional effects of antigen presentation of LC from other APC populations. A variety of methods including UV-irradiation, pretreatment with topical steroid preparations, and tape-stripping of the epidermis were employed to reduce/eliminate LC. When mice were sensitized on skin in which LC had been eliminated, CHS responses were reduced10–12. In addition, mice sensitized on their ears which have a high density of LC developed enhanced CHS compared with mice sensitized on their tails that have low LC density13. Thus, it was concluded that LC are required for optimal immune responses to cutaneous antigen.
More recently, Merad and colleagues discovered that although LC are initially derived from hematopoetic stem cells, they are not replaced by donor-derived LC in bone-marrow chimeras14. In the setting of graft-versus-host disease, the presence of donor-derived LC was required for disease suggesting that LC are important for presentation of skin antigen to T cells15. In addition, LC pulsed with ovalbumin or LC isolated from mice in which the keratinocytes express an immunodominant OVA peptide, were able to acquire antigen and cross-present to CD8 T cells16, 17.
In concert with this functional data, it was observed first in guinea pig and then in mouse and human skin that LC acquire antigen in the skin and migrate to T cell areas of skin-draining LN18–22. This also occurs in absence of inflammation23. These data (and many more not discussed here) evolved into what has been coined the “LC paradigm”24. This concept involves 3 parts to the LC life cycle (reviewed in 25, 26). In steady state, immature LC are highly endocytic and form a dense network in the epidermis where they constantly scan the environment by extending and retracting their dendrites and are ideally positioned to detect any pathogen breaching the skin barrier. After activation by microbial products and or inflammatory cytokines, LC detach from keratinocytes by down-regulating E-cadherin migrate to skin-draining LN in a CCR-7 dependent process. During migration LC undergo a process of maturation and acquire potent immuno-stimulatory properties. They process antigen acquired in the skin and present it in the context of MHC class I/II to naïve T-cells. By increasing surface expression of co-stimulatory molecules and secreting inflammatory cytokines they provide the second and third signals for T-cell activation and differentiation. Thus, by the time LC arrive in the LN, they have acquired the surface phenotype of a ‘functionally’ mature DC capable of activating naïve T cells and thereby initiating an adaptive immune response specifically tailored to fight off the invading cutaneous pathogen.
The LC paradigm has been expanded to include LC that migrate to LN carrying self antigens in steady state in the absence of activating signals. Targeted delivery of antigen in the immunological steady state, via the endocytic receptor CD205 expressed on immature DC (including LC), failed to induce immunity and instead established CD4 and CD8 T-cell tolerance possibly through the induction of regulatory T cells27–29. This steady state presentation of self antigens has been proposed to eliminate self-reactive T-cells and provide a mechanism of peripheral tolerance30.
LC paradigm, however, does not explain all aspects of skin adaptive immunity. Grabbe et al. used topical steroids to deplete LC and found that CHS responses were increased if LC were depleted during the challenge phase31. Later, using HSV skin infections models it was noted that during infection of the vaginal epithelium with HSV2 and the skin with HSV1, antigen was acquired by CD11b+ submucosal and CD8+ DC, respectively32, 33. LC did not appear to participate in the development of T responses. An important caveat, however, is that HSV infection is lytic and induces apoptosis in DC34. These apoptotic bodies may travel as free material to LN where they can be acquired by LN-resident CD8+ DC. Antigens from non-lytic viruses such as lentiviruses are directly presented by skin derived DC and not CD8α+ DC35.
LC are immuno-stimulatory
Despite considerable progress, much of the understanding of LC function has been derived either from in vitro assays or from complex, indirect assays such as bone-marrow chimerism or depletion of LC by topical agents that have manifold effects on the immune system beyond LC depletion. The absence of mice with a selective LC deficiency was a major impediment. In 2005, 2 groups reported the generation of mice in which LC could be inducible ablated22, 36. These mice were developed by “knocking-in” the gene for the primate diphtheria toxin receptor (DTR) into the LC-specific gene, langerin. For simplicity these mice will be referred to as muLangerin-DTR mice for the remainder of this review. Mice lack a high-affinity receptor for diphtheria toxin (DT) and are therefore relatively resistant to DT37. Expression of the primate DTR in LC makes these cells sensitive to DT and allows their inducible depletion in vivo after exogenous introduction of DT. In both muLangerin-DTR lines, LC are efficiently depleted by DT in 1–2 days which is followed by a slow repopulation starting approximately 2–3 weeks after depletion36, 38.
The consequence of LC ablation was examined by both groups using CHS. Surprisingly, one group, Bennett et al., observed decreased CHS while the other group, Kissenpfennig et al., did not observe any effect of LC ablation22, 36. Although this was initially quite confusing, it appears that differences in CHS experimental technique accounts for the different results. In particular, the timing of DT administration can affect the outcome38, 39. Kissenpfennig et al., administered DT on day -3 and +1 while Bennett et al, gave DT on day -3 only. CHS in the mice made by Kissenpfennig is greatly diminished if DT is administered only on day -3. Thus, although both muLangerin-DTR lines have not been tested side-by-side, it appear quite likely that there are no intrinsic differences between the two mouse lines and that CHS responses are diminished by DT treatment. Importantly, the elimination of LC affects sensitization and not challenge36. Similarly, after epicutaneous protein immunization, the proliferation of adoptively transferred CD4 and CD8 transgenic T cells were diminished in muLangerin-DTR mice treated with DT39, 40. Thus, it appeared that LC were indeed immunostimulatory and cross present antigen to CD8 T cells.
Revision to LC Paradigm: Are LC are functionally redundant?
It was recognized relatively soon after the discovery of Langerin that it is not a specific marker for LC. Blood-derived CD8+ DC in the thymus, lung, spleen, cutaneous LN (CLN) and mesenteric LN also express intermediate-low levels of Langerin that depends somewhat on genetic background22, 41–44. The ablation of CD8+ DC complicates the interpretation of functional assays in muLangerin-DTR mice. Although the percentage of CD8+ DC that express Langerin is relatively small on the C57BL/6 background44, Langerin+ CD8+ DC do cross-present antigen efficiently45, 46.
Recently, four groups described the existence of a novel Langerin+ DC subset in the dermis that is independent of epidermal LC38, 47–49. It had been assumed that Langerin+ DC in the dermis represented LC in the process of migrating from the epidermis into lymphatics. In fact, Langerin+ dermal DC (dDC) are quite distinct from epidermal LC. Although Langerin+ dDC are phenotypically very similar to LC in expression levels of maturation and co-stimulatory molecules, they can be distinguished from LC using CD103 (Integrin αIEL chain), CD11b (Integrin alpha M) and the adhesion molecule Ep-CAM (gp40)38. Langerin+ dDC are CD11blo, CD103+, Ep-CAMlo, while LC are CD11bhi, CD103neg and Ep-CAMhi. Unlike LC which are long-lived, radio-resistant and appear in LN 3–4 days after stimulation, Langerin+ dDC are short-lived, radio-sensitive and appear in LN within 2 days of stimulation38, 48, 49.
Since both Langerin+ dDC and LC are ablated in muLangerin-DTR mice, the function of each cell type cannot be distinguished. Langerin+ dDC, however are short-lived cells and repopulate much more quickly than LC after DT treatment. Two weeks after DT, LC are still largely absent from the epidermis, but Langerin+ dDC have repopulated to approximately 50% of their steady-state levels. This allows a window during which LC are selectively absent to assess function of LC. As discussed above, CHS responses are greatly diminished when DT is administered on day -1 prior to sensitization. Mice sensitized 7 or 13 days after DT administration, at a time when only the epidermal LC remain absent, CHS responses have returned to normal38. From these studies, it appears that dermal Langerin+ DC but not LC are critical for the development of efficient CHS responses. One exception is CHS using low dose hapten which was reduced even 28 days after DT-injection50. In addition, CD8 responses after epicutaneous immunization were decreased if immunization occurred 1 day after DT administration40, 51 but were normal 7 or 13 days after DT administration51. These findings were confirmed using bone-marrow chimera experiments in which either radio-resistant DC (i.e. LC) or radio-sensitive DC could present antigen after epicutaneous immunization39. Epidermal LC were not required for CD8 responses. Finally, in a model of HSV recurrence, cross-presentation of viral antigen was primarily carried out by Langerin+ dDC and by other migratory or LN-resident DC to a much lesser extent46. Thus for CHS and cross-presentation of epidermal antigen, LC appear to be redundant and the pro-inflammatory role ascribed to LC in the Langerhans Cell paradigm is performed by Langerin+ dDC instead.
Are LC immuno-regulatory?
In addition to the muLangerin-DTR mice developed by two groups discussed above, our group used an alternative approach to develop mice that lack LC. Instead of inserting the DTR gene into the endogenous langerin gene, Bacterial Artificial Chromosome (BAC) transgenic mice were engineered in which the gene for sub-unit A of diphtheria toxin (DTA) was inserted into the 3’ UTR of the human langerin gene (huLangerin-DTA)52. Since DTA is expressed coordinately with Langerin, the resulting huLangerin-DTA mice have a constitutive and durable absence of LC. Interestingly, unlike in the muLangerin-DTR mice, only epidermal LC and no other Langerin+ DC are ablated in the DTA mice38. This presumably reflects altered regulation of expression by human langerin promoter compared with the endogenous mouse promoter driving DTR expression in the muLangerin-DTR mice. Thus huLangerin-DTA mice can be used to examine the functional consequence of LC ablation without the complication of also eliminating Langerin+ dDC.
CHS responses to several haptens at multiple doses are increased in huLangerin-DTA mice compared with control mice52, 53. This increase is due to the absence of LC during sensitization and not challenge52. In addition to CHS, skin-graft rejection was also enhanced by the absence of LC54. H-Y mismatches grafts (i.e. male→female) on the FVB background are normally maintained indefinitely. The absence of LC in the donor tissue led to brisk rejection. Finally, T cell responses after tick bites are also exaggerated by the absence of LC55. Thus, rather than having an immunostimulatory or redundant function, these data suggest that LC are regulatory DC.
There are many potential mechanisms for LC-mediated suppression. Since CHS and skin-graft rejection are T cell mediated, most of our work has focused on T cell responses. LC could potentially directly inhibit proliferation/function of CD4 or CD8 T cells, alter Th differentiation, and/or enhance Treg or other regulatory T subset. Analysis of antigen-specific T cells after hapten treatment revealed exaggerated numbers of both CD4 and CD8 T cells in huLangerin-DTA mice but cytokine expression by CD4 and CD8 T cells was unaltered. Number and homeostasis of Treg was also unaffected. Thus, LC appear to inhibit anti-hapten responses by reducing the numbers of antigen-specific T cells without affecting the nature of the response53.
We have also engineered huLangerin-Cre mice that express Cre only in LC and not in other Langerin-expressing cells56. These mice have been bred to MHC-II-flox mice to generate a LC-specific “knock-out” of MHC-II. As in huLangerin-DTA mice, CHS responses and numbers of hapten-specific CD4 and CD8 T cells were increased in these mice. Interestingly, expression of FasL by LC, a marker of CD40L-dependent LC activation, is absent in Langerin-Cre MHC-II-flox mice. Thus, direct cognate interaction between LC and CD4 cells appears to be required for LC activation and suppression of CHS. Indeed LC-T cell interaction is also required in vitro for LC-mediated suppression57. CHS was also enhanced in Langerin-Cre IL-10-flox mice demonstrating a non-redundant role for LC-derived IL-10 in the suppression of CHS. Taken together these data suggest the following model of LC suppression of anti-hapten responses (Figure 1): LC arrive in the skin-draining LN 3–4 days after hapten application, which is well after skin-resident Langerin+ dDC (and quite likely other dDC) have transported haptenated antigens to the LN and initiated adaptive responses. Upon cognate interaction with CD4 cells LC become fully activated presumably via CD40-CD40L interaction and secrete IL-10 which inhibits hapten-specific T cells. In the absence of LC (i.e. huLangerin-DTA) or activated LC (i.e. huLangerin-Cre MHC-II-flox) LC-derived IL-10 is absent. This results in a failure to suppress expansion of hapten-specific cells thereby leading to the development of exaggerated CHS. Other effectors which we have not yet examined such as LC-derived TGFβ, indoleamine 2,3-dioxygenase (IDO) and FasL mediated apoptosis could certainly also participate in this process.
Fig.1. Proposed model of immune response regulation by skin DC in CHS.
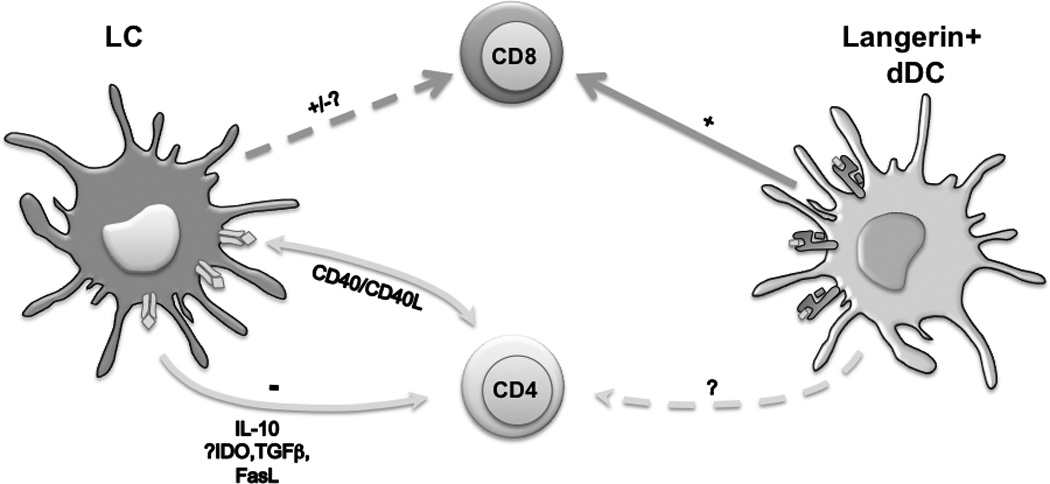
Antigen acquired in the skin is initially presented to naïve/memory T cells by Langerin+ dDC leading to a productive response. Other DC subtypes not depicted (i.e. Langerin- dDC, inflammatory DC) likely also participate. LC arrive in the LN later than other skin migratory DC. After cognate interaction with CD4 T cells and CD40 ligation, LC secrete IL-10 which inhibits the expansion of both CD4 and CD8 cells.
A key question remains unanswered. Why was enhanced CHS not observed in muLangerin-DTR mice at time points after DT administration when LC remain absent but Langerin+ dDC have largely returned? Since LC migrate to skin-draining LN in the steady-state and have been proposed to participate in the maintenance of peripheral tolerance, it is possible that the absence of this process in huLangerin-DTA mice accounts for the observed exaggerated responses. Alternatively, the still-decreased numbers of Langerin+ dDC or a functional impairment that occurs during their repopulation could reduce the pro-inflammatory capacity of Langerin+ dDC. As a result, the immuno-regulatory function of LC would be masked and CHS response appear unchanged. Preliminary data from a newly generated line of mice in which only epidermal LC are ablated by DT (huLangerin-DTR) favors the latter hypothesis (unpublished observations).
It is important to note that the immunosuppressive effect of LC has only been carefully studied in CHS which is predominantly a Th1/Th17 response. Even though levels of the Th2 cytokines, IL-4 and IL-13, are unchanged after sensitization with hapten in the absence of LC, LC may be specialized to promote Th2 responses in other settings53. Indeed, selective targeting of antigen to LC using a gene gun led to the production of Th2-related antibody isotypes58. In addition, LC stimulated in vitro promoted the development of Th2 cells59, 60 and topical application of vitamin D analog appears to selectively activate LC to promote Th2-type responses61. Thus, presentation by LC in the absence of other APC may promote Th2-type responses. If so, one function of LC may be to promote appropriate Th2-type responses against epidermal mites or parasites.
The late arrival of LC in the skin-draining LC and the importance of LC-derived IL-10 also raises the possibility that LC have evolved to limit effector responses against epithelial antigens. This function would presumably have evolved to prevent deleterious immune responses against commensal organisms and innocuous environmental antigens. It may also participate in the pathogenesis of psoriasis62 and cutaneous lupus63. Antigen found exclusively in the epithelium would be presented by LC leading either to no response or the development of tolerance. In the case of an invasive pathogen which penetrate below the epidermis, antigen is present in both the epidermis and in the dermis. In this situation antigen is acquired by dermal DC responsible for the generation of effector responses and by LC which inhibit that response with the balance of the two determining the ultimate response.
Final Thoughts
In the past few years there has been considerable progress understanding LC function. Despite the sometime contradictory results from LC-deficient mice, they have been proven quite helpful in examining LC function in isolation from other DC subtypes. In vivo, mouse LC are not required for adaptive immune responses but appear to suppress the response. While the mechanism has been partially defined for CHS, their role in other types of immune responses remains to be examined. One theme that is becoming increasingly clear is that LC behavior is quite dependent on the methods used to assay their function. Seemingly small differences in technique can give opposite results. This is evident in the disparate results obtained from LC-deficient mice mice. In addition, LC assayed in vitro behave quite differently than in vivo. This raises the exciting possibility that the environmental niche could affect LC function perhaps via signals from keratinocytes (e.g. vitamin D, RANK-L; TSLP, TGFβ)64–67 and/or neurons (e.g. CGRP, PACAP, VIP etc.)60, 68–71. We anticipate that further improvements to experimental systems will help address these questions in the future.
Literature cited
- 1.Langerhans P. Uber die Nerven der Menschlichen Haut. Virchows Arch. 1868;44:325–327. [Google Scholar]
- 2.Frelinger JG, Hood L, Hill S, Frelinger JA. Mouse epidermal Ia molecules have a bone marrow origin. Nature. 1979;282:321–323. doi: 10.1038/282321a0. [DOI] [PubMed] [Google Scholar]
- 3.Katz SI, Tamaki K, Sachs DH. Epidermal Langerhans cells are derived from cells originating in bone marrow. Nature. 1979;282:324–326. doi: 10.1038/282324a0. [DOI] [PubMed] [Google Scholar]
- 4.Stingl G, Katz SI, Clement L, Green I, Shevach EM. Immunologic functions of Ia-bearing epidermal Langerhans cells. J Immunol. 1978;121:2005, 2013. [PubMed] [Google Scholar]
- 5.Schuler G, Steinman RM. Murine epidermal Langerhans cells mature into potent immunostimulatory dendritic cells in vitro. J Exp Med. 1985;161:526–546. doi: 10.1084/jem.161.3.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pehamberger H, Stingl LA, Pogantsch S, Steiner G, Wolff K, Stingl G. Epidermal cell-induced generation of cytotoxic T-lymphocyte responses against alloantigens or TNP-modified syngeneic cells: requirement for Ia-positive Langerhans cells. J Invest Dermatol. 1983;81:208–211. doi: 10.1111/1523-1747.ep12517984. [DOI] [PubMed] [Google Scholar]
- 7.Caux C, Dezutter-Dambuyant C, Schmitt D, Banchereau J. GM-CSF and TNF-alpha cooperate in the generation of dendritic Langerhans cells. Nature. 1992;360:258–261. doi: 10.1038/360258a0. [DOI] [PubMed] [Google Scholar]
- 8.Herbst B, Kohler G, Mackensen A, Veelken H, Kulmburg P, Rosenthal FM, et al. In vitro differentiation of CD34+ hematopoietic progenitor cells toward distinct dendritic cell subsets of the birbeck granule and MIIC-positive Langerhans cell and the interdigitating dendritic cell type. Blood. 1996;88:2541–2548. [PubMed] [Google Scholar]
- 9.Ratzinger G, Baggers J, de Cos MA, Yuan J, Dao T, Reagan JL, et al. Mature human Langerhans cells derived from CD34+ hematopoietic progenitors stimulate greater cytolytic T lymphocyte activity in the absence of bioactive IL-12p70, by either single peptide presentation or cross-priming, than do dermal-interstitial or monocyte-derived dendritic cells. J Immunol. 2004;173:2780–2791. doi: 10.4049/jimmunol.173.4.2780. [DOI] [PubMed] [Google Scholar]
- 10.Streilein JW, Bergstresser PR. Langerhans cell function dictates induction of contact hypersensitivity or unresponsiveness to DNFB in Syrian hamsters. J Invest Dermatol. 1981;77:272–277. doi: 10.1111/1523-1747.ep12482453. [DOI] [PubMed] [Google Scholar]
- 11.Streilein JW, Lonsberry LW, Bergstresser PR. Depletion of epidermal langerhans cells and Ia immunogenicity from tape-stripped mouse skin. J Exp Med. 1982;155:863–871. doi: 10.1084/jem.155.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Romani N, Ebner S, Tripp CH, Flacher V, Koch F, Stoitzner P. Epidermal Langerhans cells--changing views on their function in vivo. Immunol Lett. 2006;106:119–125. doi: 10.1016/j.imlet.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 13.Toews GB, Bergstresser PR, Streilein JW. Epidermal Langerhans cell density determines whether contact hypersensitivity or unresponsiveness follows skin painting with DNFB. J Immunol. 1980;124:445–453. [PubMed] [Google Scholar]
- 14.Merad M, Manz MG, Karsunky H, Wagers A, Peters W, Charo I, et al. Langerhans cells renew in the skin throughout life under steady-state conditions. Nat Immunol. 2002;3:1135–1141. doi: 10.1038/ni852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Merad M, Hoffmann P, Ranheim E, Slaymaker S, Manz MG, Lira SA, et al. Depletion of host Langerhans cells before transplantation of donor alloreactive T cells prevents skin graft-versus-host disease. Nat Med. 2004;10:510–517. doi: 10.1038/nm1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stoitzner P, Tripp CH, Eberhart A, Price KM, Jung JY, Bursch L, et al. Langerhans cells cross-present antigen derived from skin. Proc Natl Acad Sci U S A. 2006;103:7783–7788. doi: 10.1073/pnas.0509307103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mayerova D, Parke EA, Bursch LS, Odumade OA, Hogquist KA. Langerhans cells activate naive self-antigen-specific CD8 T cells in the steady state. Immunity. 2004;21:391–400. doi: 10.1016/j.immuni.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 18.Silberberg-Sinakin I, Thorbecke GJ, Baer RL, Rosenthal SA, Berezowsky V. Antigen-bearing langerhans cells in skin, dermal lymphatics and in lymph nodes. Cell Immunol. 1976;25:137–151. doi: 10.1016/0008-8749(76)90105-2. [DOI] [PubMed] [Google Scholar]
- 19.Henri S, Vremec D, Kamath A, Waithman J, Williams S, Benoist C, et al. The dendritic cell populations of mouse lymph nodes. J Immunol. 2001;167:741–748. doi: 10.4049/jimmunol.167.2.741. [DOI] [PubMed] [Google Scholar]
- 20.Geissmann F, Dieu-Nosjean MC, Dezutter C, Valladeau J, Kayal S, Leborgne M, et al. Accumulation of immature Langerhans cells in human lymph nodes draining chronically inflamed skin. J Exp Med. 2002;196:417–430. doi: 10.1084/jem.20020018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stoitzner P, Holzmann S, McLellan AD, Ivarsson L, Stossel H, Kapp M, et al. Visualization and characterization of migratory Langerhans cells in murine skin and lymph nodes by antibodies against Langerin/CD207. J Invest Dermatol. 2003;120:266–274. doi: 10.1046/j.1523-1747.2003.12042.x. [DOI] [PubMed] [Google Scholar]
- 22.Kissenpfennig A, Henri S, Dubois B, Laplace-Builhe C, Perrin P, Romani N, et al. Dynamics and Function of Langerhans Cells In Vivo Dermal Dendritic Cells Colonize Lymph Node AreasDistinct from Slower Migrating Langerhans Cells. Immunity. 2005;22:643–654. doi: 10.1016/j.immuni.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 23.Hemmi H, Yoshino M, Yamazaki H, Naito M, Iyoda T, Omatsu Y, et al. Skin antigens in the steady state are trafficked to regional lymph nodes by transforming growth factor-beta1-dependent cells. Int Immunol. 2001;13:695–704. doi: 10.1093/intimm/13.5.695. [DOI] [PubMed] [Google Scholar]
- 24.Wilson NS, Villadangos JA. Lymphoid organ dendritic cells: beyond the Langerhans cells paradigm. Immunol Cell Biol. 2004;82:91–98. doi: 10.1111/j.1440-1711.2004.01216.x. [DOI] [PubMed] [Google Scholar]
- 25.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 26.Romani N, Clausen BE, Stoitzner P. Langerhans cells and more: langerin-expressing dendritic cell subsets in the skin. Immunological Reviews. 2010;234 doi: 10.1111/j.0105-2896.2009.00886.x. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamazaki S, Iyoda T, Tarbell K, Olson K, Velinzon K, Inaba K, et al. Direct expansion of functional CD25+ CD4+ regulatory T cells by antigen-processing dendritic cells. J Exp Med. 2003;198:235–247. doi: 10.1084/jem.20030422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bonifaz L, Bonnyay D, Mahnke K, Rivera M, Nussenzweig MC, Steinman RM. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J Exp Med. 2002;196:1627–1638. doi: 10.1084/jem.20021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hawiger D, Inaba K, Dorsett Y, Guo M, Mahnke K, Rivera M, et al. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J Exp Med. 2001;194:769–779. doi: 10.1084/jem.194.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steinman RM, Nussenzweig MC. Avoiding horror autotoxicus: the importance of dendritic cells in peripheral T cell tolerance. Proc Natl Acad Sci U S A. 2002;99:351–358. doi: 10.1073/pnas.231606698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grabbe S, Steinbrink K, Steinert M, Luger TA, Schwarz T. Removal of the majority of epidermal Langerhans cells by topical or systemic steroid application enhances the effector phase of murine contact hypersensitivity. J Immunol. 1995;155:4207–4217. [PubMed] [Google Scholar]
- 32.Allan RS, Smith CM, Belz GT, van Lint AL, Wakim LM, Heath WR, et al. Epidermal viral immunity induced by CD8alpha+ dendritic cells but not by Langerhans cells. Science. 2003;301:1925–1928. doi: 10.1126/science.1087576. [DOI] [PubMed] [Google Scholar]
- 33.Zhao X, Deak E, Soderberg K, Linehan M, Spezzano D, Zhu J, et al. Vaginal submucosal dendritic cells, but not Langerhans cells, induce protective Th1 responses to herpes simplex virus-2. J Exp Med. 2003;197:153–162. doi: 10.1084/jem.20021109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bosnjak L, Miranda-Saksena M, Koelle DM, Boadle RA, Jones CA, Cunningham AL. Herpes simplex virus infection of human dendritic cells induces apoptosis and allows cross-presentation via uninfected dendritic cells. J Immunol. 2005;174:2220–2227. doi: 10.4049/jimmunol.174.4.2220. [DOI] [PubMed] [Google Scholar]
- 35.He Y, Zhang J, Donahue C, Falo LD., Jr Skin-derived dendritic cells induce potent CD8(+) T cell immunity in recombinant lentivector-mediated genetic immunization. Immunity. 2006;24:643–656. doi: 10.1016/j.immuni.2006.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bennett CL, van Rijn E, Jung S, Inaba K, Steinman RM, Kapsenberg ML, et al. Inducible ablation of mouse Langerhans cells diminishes but fails to abrogate contact hypersensitivity. J Cell Biol. 2005;169:569–576. doi: 10.1083/jcb.200501071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saito M, Iwawaki T, Taya C, Yonekawa H, Noda M, Inui Y, et al. Diphtheria toxin receptor-mediated conditional and targeted cell ablation in transgenic mice. Nat Biotechnol. 2001;19:746–750. doi: 10.1038/90795. [DOI] [PubMed] [Google Scholar]
- 38.Bursch LS, Wang L, Igyarto B, Kissenpfennig A, Malissen B, Kaplan DH, et al. Identification of a novel population of Langerin+ dendritic cells. J Exp Med. 2007;204:3147–3156. doi: 10.1084/jem.20071966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang L, Jameson SC, Hogquist KA. Epidermal Langerhans cells are not required for UV-induced immunosuppression. J Immunol. 2009;183:5548–5553. doi: 10.4049/jimmunol.0900235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stoitzner P, Green LK, Jung JY, Price KM, Tripp CH, Malissen B, et al. Tumor immunotherapy by epicutaneous immunization requires langerhans cells. J Immunol. 2008;180:1991–1998. doi: 10.4049/jimmunol.180.3.1991. [DOI] [PubMed] [Google Scholar]
- 41.McLellan AD, Kapp M, Eggert A, Linden C, Bommhardt U, Brocker EB, et al. Anatomic location and T-cell stimulatory functions of mouse dendritic cell subsets defined by CD4 and CD8 expression. Blood. 2002;99:2084–2093. doi: 10.1182/blood.v99.6.2084. [DOI] [PubMed] [Google Scholar]
- 42.Sung SS, Fu SM, Rose CE, Jr, Gaskin F, Ju ST, Beaty SR. A major lung CD103 (alphaE)-beta7 integrin-positive epithelial dendritic cell population expressing Langerin and tight junction proteins. J Immunol. 2006;176:2161–2172. doi: 10.4049/jimmunol.176.4.2161. [DOI] [PubMed] [Google Scholar]
- 43.Douillard P, Stoitzner P, Tripp CH, Clair-Moninot V, Ait-Yahia S, McLellan AD, et al. Mouse lymphoid tissue contains distinct subsets of langerin/CD207 dendritic cells, only one of which represents epidermal-derived Langerhans cells. J Invest Dermatol. 2005;125:983–994. doi: 10.1111/j.0022-202X.2005.23951.x. [DOI] [PubMed] [Google Scholar]
- 44.Flacher V, Douillard P, Ait-Yahia S, Stoitzner P, Clair-Moninot V, Romani N, et al. Expression of langerin/CD207 reveals dendritic cell heterogeneity between inbred mouse strains. Immunology. 2008;123:339–347. doi: 10.1111/j.1365-2567.2007.02785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Farrand KJ, Dickgreber N, Stoitzner P, Ronchese F, Petersen TR, Hermans IF. Langerin+CD8alpha+ dendritic cells are critical for cross-priming and IL-12 production in response to systemic antigens. J Immunol. 2009;183:7732–7742. doi: 10.4049/jimmunol.0902707. [DOI] [PubMed] [Google Scholar]
- 46.Bedoui S, Whitney PG, Waithman J, Eidsmo L, Wakim L, Caminschi I, et al. Cross-presentation of viral and self antigens by skin-derived CD103+ dendritic cells. Nat Immunol. 2009;10:488–495. doi: 10.1038/ni.1724. [DOI] [PubMed] [Google Scholar]
- 47.Poulin LF, Henri S, de Bovis B, Devilard E, Kissenpfennig A, Malissen B. The dermis contains langerin+ dendritic cells that develop and function independently of epidermal Langerhans cells. J Exp Med. 2007;204:3119–3131. doi: 10.1084/jem.20071724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ginhoux F, Collin MP, Bogunovic M, Abel M, Leboeuf M, Helft J, et al. Blood-derived dermal langerin+ dendritic cells survey the skin in the steady state. J Exp Med. 2007;204:3133–3146. doi: 10.1084/jem.20071733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shklovskaya E, Roediger B, Fazekas de St Groth B. Epidermal and dermal dendritic cells display differential activation and migratory behavior while sharing the ability to stimulate CD4+ T cell proliferation in vivo. J Immunol. 2008;181:418–430. doi: 10.4049/jimmunol.181.1.418. [DOI] [PubMed] [Google Scholar]
- 50.Bennett CL, Noordegraaf M, Martina CA, Clausen BE. Langerhans cells are required for efficient presentation of topically applied hapten to T cells. J Immunol. 2007;179:6830–6835. doi: 10.4049/jimmunol.179.10.6830. [DOI] [PubMed] [Google Scholar]
- 51.Wang L, Bursch LS, Kissenpfennig A, Malissen B, Jameson SC, Hogquist KA. Langerin Expressing Cells Promote Skin Immune Responses under Defined Conditions. J Immunol. 2008;180:4722–4727. doi: 10.4049/jimmunol.180.7.4722. [DOI] [PubMed] [Google Scholar]
- 52.Kaplan DH, Jenison MC, Saeland S, Shlomchik WD, Shlomchik MJ. Epidermal langerhans cell-deficient mice develop enhanced contact hypersensitivity. Immunity. 2005;23:611–620. doi: 10.1016/j.immuni.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 53.Igyarto BZ, Jenison MC, Dudda JC, Roers A, Muller W, Koni PA, et al. Langerhans cells suppress contact hypersensitivity responses via cognate CD4 interaction and langerhans cell-derived IL-10. J Immunol. 2009;183:5085–5093. doi: 10.4049/jimmunol.0901884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Obhrai JS, Oberbarnscheidt M, Zhang N, Mueller DL, Shlomchik WD, Lakkis FG, et al. Langerhans cells are not required for efficient skin graft rejection. J Invest Dermatol. 2008;128:1950–1955. doi: 10.1038/jid.2008.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vesely DL, Fish D, Shlomchik MJ, Kaplan DH, Bockenstedt LK. Langerhans cell deficiency impairs Ixodes scapularis suppression of Th1 responses in mice. Infect Immun. 2009 doi: 10.1128/IAI.00030-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kaplan DH, Li MO, Jenison MC, Shlomchik WD, Flavell RA, Shlomchik MJ. Autocrine/paracrine TGFbeta1 is required for the development of epidermal Langerhans cells. J Exp Med. 2007;204:2545–2552. doi: 10.1084/jem.20071401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Imai Y, Hayashi N, Yasuda K, Tsutsui H, Mizutani H, Nakanishi K. Freshly isolated Langerhans cells negatively regulate naive T cell activation in response to peptide antigen through cell-to-cell contact. J Dermatol Sci. 2008;51:19–29. doi: 10.1016/j.jdermsci.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 58.Nagao K, Ginhoux F, Leitner WW, Motegi S, Bennett CL, Clausen BE, et al. Murine epidermal Langerhans cells and langerin-expressing dermal dendritic cells are unrelated and exhibit distinct functions. Proc Natl Acad Sci U S A. 2009;106:3312–3317. doi: 10.1073/pnas.0807126106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Klechevsky E, Morita R, Liu M, Cao Y, Coquery S, Thompson-Snipes L, et al. Functional specializations of human epidermal Langerhans cells and CD14+ dermal dendritic cells. Immunity. 2008;29:497–510. doi: 10.1016/j.immuni.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ding W, Stohl LL, Wagner JA, Granstein RD. Calcitonin gene-related peptide biases Langerhans cells toward Th2-type immunity. J Immunol. 2008;181:6020–6026. doi: 10.4049/jimmunol.181.9.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Elentner A, Finke D, Schmuth M, Chappaz S, Ebner S, Malissen B, et al. Langerhans cells are critical in the development of atopic dermatitis-like inflammation and symptoms in mice. J Cell Mol Med. 2009 doi: 10.1111/j.1582-4934.2009.00797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cumberbatch M, Singh M, Dearman RJ, Young HS, Kimber I, Griffiths CE. Impaired Langerhans cell migration in psoriasis. J Exp Med. 2006;203:953–960. doi: 10.1084/jem.20052367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Eriksson AU, Singh RR. Cutting edge: migration of langerhans dendritic cells is impaired in autoimmune dermatitis. J Immunol. 2008;181:7468–7472. doi: 10.4049/jimmunol.181.11.7468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Soumelis V, Reche PA, Kanzler H, Yuan W, Edward G, Homey B, et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol. 2002;3:673–680. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- 65.Loser K, Mehling A, Loeser S, Apelt J, Kuhn A, Grabbe S, et al. Epidermal RANKL controls regulatory T-cell numbers via activation of dendritic cells. Nat Med. 2006;12:1372–1379. doi: 10.1038/nm1518. [DOI] [PubMed] [Google Scholar]
- 66.Ebner S, Nguyen VA, Forstner M, Wang YH, Wolfram D, Liu YJ, et al. Thymic stromal lymphopoietin converts human epidermal Langerhans cells into antigen-presenting cells that induce proallergic T cells. J Allergy Clin Immunol. 2007;119:982–990. doi: 10.1016/j.jaci.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 67.Ghoreishi M, Bach P, Obst J, Komba M, Fleet JC, Dutz JP. Expansion of antigen-specific regulatory T cells with the topical vitamin d analog calcipotriol. J Immunol. 2009;182:6071–6078. doi: 10.4049/jimmunol.0804064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hosoi J, Murphy GF, Egan CL, Lerner EA, Grabbe S, Asahina A, et al. Regulation of Langerhans cell function by nerves containing calcitonin gene-related peptide. Nature. 1993;363:159–163. doi: 10.1038/363159a0. [DOI] [PubMed] [Google Scholar]
- 69.Kodali S, Ding W, Huang J, Seiffert K, Wagner JA, Granstein RD. Vasoactive intestinal peptide modulates Langerhans cell immune function. J Immunol. 2004;173:6082–6088. doi: 10.4049/jimmunol.173.10.6082. [DOI] [PubMed] [Google Scholar]
- 70.Seiffert K, Granstein RD. Neuroendocrine regulation of skin dendritic cells. Ann N Y Acad Sci. 2006;1088:195–206. doi: 10.1196/annals.1366.011. [DOI] [PubMed] [Google Scholar]
- 71.Ding W, Wagner JA, Granstein RD. CGRP, PACAP, and VIP modulate Langerhans cell function by inhibiting NF-kappaB activation. J Invest Dermatol. 2007;127:2357–2367. doi: 10.1038/sj.jid.5700858. [DOI] [PubMed] [Google Scholar]


