Summary
There has been an increase in the number of published tuberculosis/HIV (TB/HIV) research findings in recent times. The potential impact of these findings on routine care has informed this review which aims at discussing current concepts and practices underpinning TB/HIV care and control. Any HIV infected person with a cough of any duration is currently considered a TB suspect. Preliminary results also show that the diagnostic yield of same day sputum samples (front loading) is comparable to two-day samples. Laboratory diagnosis is shifting from Ziehl-Neelsen (ZN) smear microscopy and solid culture to fluorescent microscopy, molecular tests and liquid culture. Concomitant TB/HIV therapy improves survival and WHO has recommended ART for all TB/HIV patients. Unless CD4 cell counts are less than 50 cells/µl, ART can be deferred until end of intensive phase. Evidence of survival benefit at high CD4 cell counts is still lacking. New TB drugs and treatment shortening studies are underway but so far no new TB drugs has been added to the current arsenal and treatment duration still remains six months or more. WHO has recommended the 31s (intensified TB case finding, isoniazid prophylaxis and infection control) for TB/HIV control in addition to effective therapy, Antiretroviral therapy and TB vaccines. There has been immense progress in TB/HIV research, however optimal management of HIV-Infected TB patients, will require further research and appropriate translation of emerging evidence to policy and practice.
Keywords: Tuberculosis, HIV, Diagnosis, Treatment, Control
Introduction
The burden of tuberculosis (TB) among human immunodeficiency virus (HIV) infected persons continues to represent a major public health problem. Of the 9.4 million new TB cases reported in 2009, 1.1 million were HIV infected of which 380, 000 died (WHO Global Tuberculosis control report, 2010). Antiretroviral therapy (ART) reduces person and population risk of TB by 90% and 60% respectively (Lawn S et al., 2005, Badri et al., 2002). Unfortunately only 37% of TB/HIV patients who qualified to receive ART were initiated on ART in 2009 (WHO Global Tuberculosis control report, 2010). There has been an increase in basic, clinical and public health TB/HIV research, shedding new light on diagnosis, treatment and control of TB/HIV; hence those involved in the field of TB/HIV need to constantly update their knowledge in this field. The purpose of this review is to describe, discuss and summarize current concepts and practices in the diagnosis, treatment and control of HIV associated tuberculosis.
Diagnosis of HIV associated tuberculosis
TB symptoms: One of the major advances in the clinical diagnosis of TB/HIV has been the demonstration in number of studies that TB/HIV patients with cough should be tested for TB irrespective of the duration of the cough (Kevin et al., 2010), Ngadaya et al., 2009). In a study that evaluated the performance of different combination of TB symptoms, it was found that the best symptom combination included two predictors; fever of any duration in the previous 4 weeks plus cough of any duration in the previous 4 weeks (91% sensitivity and 37% specificity) (Kevin et al., 2010). Similar findings were documented in a study in Tanzania that found no significant difference in prevalence of TB among patients with cough of three weeks duration compared with those with cough of less duration (12.7% compared to 8.7%)(Ngadaya et al., 2009). One major weakness with these approaches is their low specificity. In addition, their application in high burden TB countries with limited resources may create huge workload that most facilities cannot afford. They should be used in an algorithm leading to increased sensitivity and specificity (Getahun et al., 2011). In line with these findings some National TB control programs have developed and deployed intensified TB case finding forms in HIV clinics. The impact of these on TB case detection in HIV patients is yet to be systematically evaluated.
Sample Collection: In the current practice of spot-morning sputum sample collection, some patients who provide a first sputum sample fail to return to provide a second sample thus potentially missing out on the diagnostic opportunity. Because of this, the concept of front loading is being evaluated in which a patient provides two specimens in one visit: spotspot. In a study of 923 TB suspects, there was no statistically significant difference in number of smear positive patients diagnosed when samples were spot-morning or spot-extra spot:203, 22% and ,200, 21.7% respectively (Ramsay et al., 2009).
Laboratory diagnosis of HIV/TB: the major advances in laboratory diagnosis of TB/HIV have been due to the introduction of fluorescent microscopy especially the Light Emitting Diode (LED) technology, liquid TB cultures, line probe assays and more recently the GeneXpert. These developments have led to improved accuracy and short, turn-around-times (TAT).
TB Microscopy: Laboratory diagnosis of TB has largely depended on the detection of the mycobacteria by microscopy in sputum specimens and cultivation of mycobacteria from sputum and other tissues. The light microscope and Ziehl-Neelsen staining (and its modifications) have been in use for more than a century and has been central in the fight against tuberculosis. Light microscopy has a good specificity but variable and low sensitivity which is worse in HIV-infected TB patients (Cattamanchi et al., 2009). To improve sensitivity of light microscopy, concentration methods have been developed. Concentration methods improve ZN yield by 13% and should be encouraged where appropriate (Cattamanchi et al., 2009). However, this approach can only be performed at relatively well equipped laboratories-where there is electricity and centrifuge- limiting its usefulness in peripheral clinics where the need is highest.
Fluorescent Microscopy (FM) is an improvement on the light microscope. FM has been demonstrated to be superior to light microscopy and ZN (Steingart 2006). In the developed world this has virtually replaced the light microscope. However, the cost and need for electricity has led to limited application of fluorescent microscopy. Because of this the Foundation for Innovation of New Diagnostics (FIND) has been supporting the developments of newer diagnostics for TB. It has supported the development of a new fluorescent microcopy using Light Emitting Diodes (LED) which can use ordinary batteries and do not need the use of a dark room (WHO, 2010). The LED has a sensitivity comparable to that of standard FM and it is also cheaper. Currently, there are efforts to roll out this technology to high-burden countries.
TB Culture: Traditionally MTB is grown on solid media such as the Lowenstein Jensen (LJ) media. There are various solid media methods in use but generally it takes up to 8 weeks for a diagnosis to be made or excluded. A number of new liquid methods have been in use in the last decade. The Mycobacteria Growth Indicator Tube (MGIT) is one such method. It comes in manual and automated forms. These methods markedly reduce the time required for culture with results reported as early as 3 days depending on the bacillary load (Gil-Setas et al., 2004, Williams-Bouyer et al., 2000), Muyoyeta et al., 2009). Given the high mortality in HIV/TB patients these methods are preferred to solid methods. Their major limitation is cost, and higher contamination rates. Their utility in monitoring treatment is also not yet well established. Based on the fact that MTB grows faster in liquid media with characteristic features, a highly sensitive phenotypic culture method called mycobacteria observation drug susceptibility (MODS) has been developed. It combines culture and drug susceptibility testing if drugs are added to the media; it is faster and has potential for use in resource limited settings (Moore, 2006).
Molecular detection of TB and TB drug resistance: the principle of these tests is to detect genes specific to MTB and mutations commonly associated with drug resistance to diagnose TB and drug resistance respectively. Cepheid GeneXpert MTB/RIF Assay is an automated commercial system for identification of Mycobacteria tuberculosis complex and mutations of the genes responsible for rifampicin resistance (the rpoB gene). It utilizes real-time PCR for detection of the MTB and resistance to rifampicin within 2 hours (ref for details). A multi-country study by Boehmes et al has shown that this is highly sensitive and specific (Boehme et al., 2010).
Molecular Line probe assays available for diagnosis of Multidrug resistance TB include those by HAIN Lifesciences (GenoType® MTB-DRplus and GenoType ® MTBDRsl) and by Innogenetics (INNO-LiPA). These tests are particularly useful when sputum smears are positive and perform poorly when the smear is negative (Boehme 2010). Degradation of mycobacteria tuberculosis (MTB) generates a cell wall component called lipoarabinomanann (LAM). It has been shown in a number of studies that this molecule appears in urine and is a marker of active disease. Its sensitivity is slightly above that of sputum microscopy and its specificity as comparable to microscopy (Mutetwa et al., 2009). Clinically if it's proven to be useful it will have an advantage over sputum microscopy because of ease of performing it and due to the fact that generally a urine sample may be easier to obtain.
Bronchoscopy: On account of the fact that many HIV patients have smear negative TB, invasive procedures like bronchoscopy and bronchioalveolar lavage (BAL) for TB testing have gained importance in TB diagnosis. In one study 20/83 patients who had sputum smear negative TB were found to have active TB when TB microscopy was performed on BAL (Worodria et al., 2003). Bronchoscopy has the advantage of diagnosing other chest conditions that occur in advanced HIV such as pneumocystitis jiroveci pneumonia (PJP) and Kaposi's sarcoma. Where this service is available HIV patients with smear negative microscopy for MTB, bronchoscopy and BAL examination for TB and other infections should be considered.
Diagnosis of Latent TB infection (LTBI): Another area where significant advances have occurred is LTBI diagnosis. For several decades, LTBI diagnosis has been done by the tuberculin skin test (TST). Two newer blood tests for latent TB have been developed: Quantiferon TB Gold (QFT-G, Cellestis, Carnegie, Australia) and TB SPOT (TSPOT.TB, Oxford Immunotec, Oxford, UK, collectively referred to Interferon Gamma Release Assays (IGRA) ( Lalvani A et al., 2009). Contrary to earlier studies that had indicated that these test are more sensitive and specific in the diagnosis of LTBI in HIV infected persons, a recent meta-analysis has shown that the accuracy is not superior to TST (Cattamanchi et al., 2011). Despite this finding these tests have the advantage of not requiring a second visit to establish the results and should be used if cost allows.
Clinicians must all recognize that although there is a battery of new TB diagnostics, not all of them will be available in their practice. This will be in most cases, because of cost. The guiding principle should therefore be doing the best for your patients by maximizing the usage of the tests available in your laboratory. Costs of these new diagnostic will go down with time as their use becomes more frequent. This fact therefore requires clinicians to update themselves even with those tests that are not available in their settings.
Treatment of HIV associated tuberculosis: Many observational and intervention studies have been conducted to evaluate the efficacy of concomitant TB/HIV therapy as well as its safety and timing (Table 1). For example, a study in South Africa showed all-cause mortality reductions of >50% (Abdool Karim et al., 2010). On the basis of findings from these studies, the WHO 2010 report recommended initiation of ART in all HIV positive patients who have active TB (WHO ART guidelines, 2010). These guidelines further recommend that ART should be initiated as soon as anti-tuberculosis Therapy (ATT) is tolerated but preferably within 2–8 weeks. The aim for these guidelines is to prevent the high mortality associated with TB/HIV and for prevention purposes. It should however be noted that the evidence for these guidelines is still weak since they were based on studies insufficiently powered to provide direct evidence for patients in the higher CD4 categories. There are a number of ongoing studies (STRIDE and TBHAART) that are expected to provide missing information on optimal timing (Table 3).
Table 1.
Managing Drug Interactions with Protease Inhibitors and Rifamycins (Adapted from Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. January 10, 2011; 1–166)
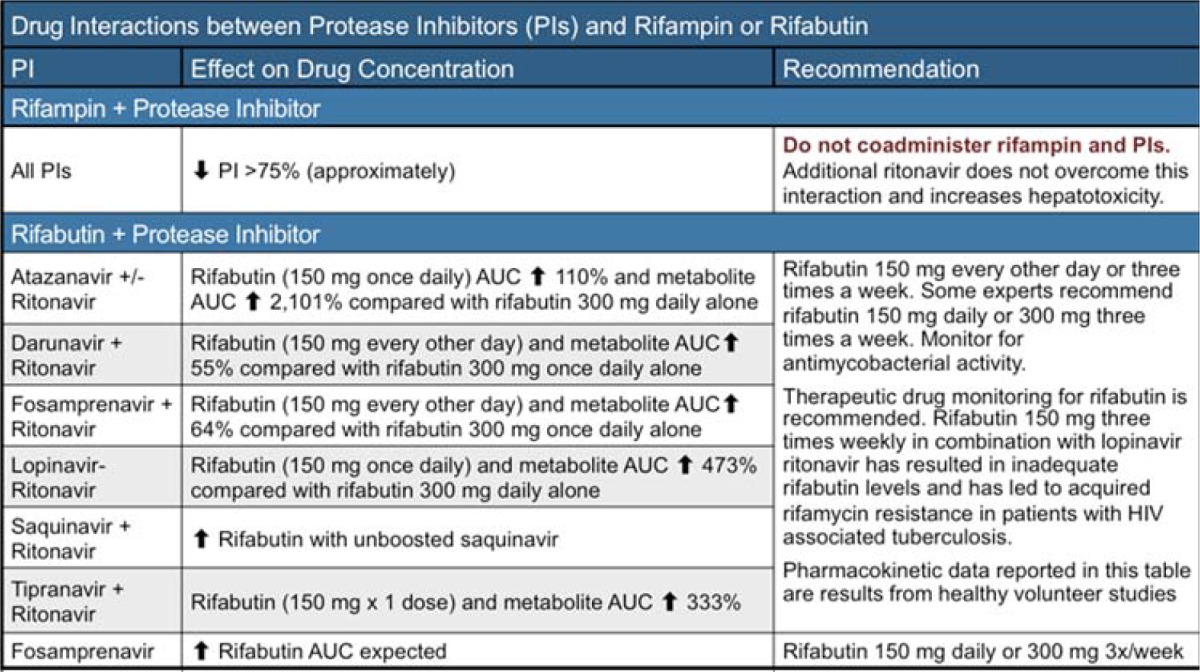 |
Table 3.
List and status ongoing TB/HIV treatment clinical trials
| Study title | Country/design and sample size |
CD4 cell count /TB diagnosis eligibility |
Timing | ART /TB regimen |
Primary outcome |
Study status and expected completion |
|
An evaluation of the impact of early initiation of HAART on TB treatment outcomes for TB patients co-infected with HIV (TBHAART) |
Uganda, Tanzania Zambia, South Africa. Randomized, Double blind and placebo controlled 1800 to be enrolled |
220–350 >350 Cultured confirmed TB |
2 weeks vs.6 months |
CBV/EFV WHO 4-FDC |
Composite: Death, failure and recurrence at 12 months |
2013, 1405 patients enrolled. Recruitment continues |
| A Strategy Study of Immediate Versus Deferred Initiation of Antiretroviral Therapy for HIV Infected Persons Treated for Tuberculosis With CD4 Less Than 200 Cells/mm3 |
Randomized open label active controlled 800 patients enrolled |
CD4<200cells/ uL TB: Confirmed or probable |
2 weeks vs. 8–12 weeks |
ART: Truvada/EF V AT drugs: standard TB drugs |
Survival without AIDS progression at 8 weeks |
2013 Enrollment completed |
|
Initiation of a Once Daily Regimen of Tenofovir, Lamivudine and Efavirenz After 4 Weeks Versus 12 Weeks of Tuberculosis Treatment in HIV-1 Infected Patients (Time Study) |
Thailand Randomized open label uncontrolled with parallel assignment 210 patients to be enrolled |
CD4<350 cells/uL TB: Possible Probable or culture confirmed |
4–12 weeks after start of TB treatment |
TDF/3TC/E FV |
Composite endpoint of death, hospitalization and adverse events at 48,96, and 144 weeks |
2013 And still recruiting participants |
Key: CBV/EFV:=Combivir, Efavirez, TDF= Tenofovir, 3TC=Lamivudine, FDC= Fixed Dose Combination anti-tuberculosis drugs
Anti-TB therapy (ATT) regimens to be used remain the same but TB/HIV patients should be placed on regimens with rifampicin in the continuation phase. A recent meta-analysis has however shown an increased risk of recurrences with this approach (Khan et al., 2009) but this finding has not yet lead to a policy change on this matter. Initiating ART in patients on TB treatment presents a number of clinical and public health challenges. Clinical challenges include overlapping toxicities, drug-drug interactions, pill burden and adherence and TB-IRIS. The choice ART regimen for special patients populations such as pregnant TB/HIV patients may also be challenging because safety of ART in pregnancy. While it is easy to find data on survival, safety events are not consistently reported in most studies. It's therefore difficult to ascertain whether concomitant therapy is associated with more adverse events. The key adverse events that require careful evaluations include drug induced liver injury (DILI), cutaneous drug reactions (CDR) and TB-IRIS. The SAPIT trial reported about a two fold increase in the occurrence of Grade 4 Liver Enzyme Elevation (LEE) in concomitant therapy and TB-IRIS was three times more common (Abdool Karim et al., 2010). Dean et al reported 17% CDR which led to treatment interruption in 68% of the cases (Dean GL et al., 2002). Preliminary results also show that starting ART during the intensive phase of TB therapy (after 2 months) does not decrease mortality but increases adverse events, drug switches and TB-IRIS (Havlir et al., 2011, Abdool Karim et al., 2011) The rest of the findings are shown in Table 2 below. From the public health perspective the New WHO guidelines recommending ART initiation at ≤350 cells/mm3 has increased the number of patients in need of ART from about 10.1 million (≤ 200cells/mm3) to 14.6 million (WHO UNAIDS UNICEF progress report, 2010). It remains to be documented if resource limited countries will be able to cope with these numbers.
Table 2.
Summary of results from completed TB/HIV treatment studies
| Author, Date, setting |
Design | Timing | No patients | CD4cells/µl | Outcome, Mortality | |
| ART | No ART | |||||
|
Francois Xavier Black, 2010 Cambodia |
RCT | 2 weeks vs. 2 months |
332 | 329 | all <200 | 30% (59/332, 90/329) mortality reduction |
|
AS Karim 2010, South Africa |
RCT | 1–3 vs. 6 months |
271 | 137 | Median 150(77–245) | 50% mortality reduction (5.1.Vs.12.1/100pys) |
|
Somsak Akksilp, 2007, Thailand |
Prospective cohort |
93 vs. 0–170 days |
71 | 219 | Median 53 (1–873) | 7% vs. 43% mortality RR 0.2, 95% CI 0.1.0.4, ARR 36, NNT 3 |
|
Payam T, 2009, Iran |
Retrospective cohort |
2 vs. 8 weeks | 22 | 47 | Mean 204+/− 184.6 |
27.7% vs. 4.5% |
|
Westreich Daniel, 2009, South Africa |
Retrospective cohort |
1 vs. 2–3 Months |
330 | 320 | not available | HR of 2.04 for 1 month vs. 1.32 for 2–3 months |
|
Velasco Maria, 2009 , Spain |
Retrospective Cohort |
HAART vs. no HAART |
140 | 173 | Median 173(60–293) | 9.3% vs. 19.7%, p=0.011 |
|
Manosuthi, W, 2006, Thailand |
Cohort | 6 months vs. early initiation |
411 | 592 | Range of 19–161 | HR 2.7 95% CI , 1.2–6.1, P 0.018, |
|
Bunthoueun E, 2009, Cambodia |
Analysis of program data |
Compares TB mortality before and after ART introduction in the program settings |
Mortality decreased from 37% in pre-HAART to 18% HAART era |
|||
|
Sao H, 2009, Tanzania |
RCT | 2 weeks vs. no ART |
35 | 35 | Median 329( 329–384) |
2 deaths in early ART vs. 1 in delayed |
|
Sanguanwonge N, 2008, Thailand |
Analysis of program data |
compares TB mortality before and after ART introduction in the program |
626 | 643 | Median 54, 1–1169 | 46% mortality pre-ART vs. 11% post ART ( RR 0.24 CI 0.19.0.30) |
|
Dean GL 2002, London |
Prospective Cohort |
2 vs.delay up to 14 months |
85 | 103 | Median 90 (30–180) | 3 vs. 13 deaths |
Drug-drug interactions (DDI): Following in the foot steps of drug toxicity and TBIRIS is the challenge of drug- drug interactions. Most DDIs hinge around rifamycins which are potent inducers of hepatic cytochrome P450 (CYP450) and uridine diphosphate glucuronosyltransferase (UGT) 1A1 enzymes (Burman WJ et al 1999). This induction can potentially lower blood levels of other drugs that utilize this pathway for metabolism, including many antiretroviral medications. Firstly induction of CYP450 reduces blood concentrations of NNRTIs and protease Inhibitors (PIs). Among the NNRTIs the most affected is nevirapine. For this reason the use of nevirapine is not recommended in combination with rifampicin. It has long been shown that efavirenz is not significantly affected by rifamycins and is currently the drug of choice for use in combination with antituberculous drugs (WHO ART guidelines, 2010). Earlier on it was thought that higher doses of efavirenz (800mg) instead of 600mg are necessary to compensate for the reductions with rifamycins but recent data indicate that this is not necessary (Manosuthi et al 2006). New NNRTIs etravirine and rilpivirine are currently in use. Experience and data with the use of these drugs in combination with rifamycins is limited and their use should be avoided.
Rifamycins have significant DDI with protease inhibitors (PIs) Table 1. Rifampicin and rifabutin are the commonest rifamycins in common use in the management of TB/HIV. Rifampicin reduces blood concentrations of PIs while PIs increase blood concentrations of rifabutin. There are several situations in which PIs may be necessary in patients with active TB. Firstly PIs are the backbone of second line ART. The commonest indication of second line ART is ART failure. Ideally patients will first experience virological failure followed by immunological failure and subsequently clinical failure. In resource limited settings viral load and immunological follow up is sometimes not possible and hence in many patients the initial sign of ART failure will be a clinical event such as TB. Secondly some patients develop toxicity to first line ART and require PIs in their regimen. One option would to be to delay ART until the end of intensive phase of TB treatment at which point patients would continue TB therapy with isoniazid and ethambutol. It has however been shown that this approach is less effective than one in which the continuation regimen contains rifampicin for the entire treatment period. The second approach is to use rifabutin, a rifamycin that has been shown to be less affected by PIs (Brainard et al 2011). This approach has been hindered by the unavailability of rifabutin. There is however light at the end of tunnel since price reduction of rifabutin is starting to happen. We must further note that despite this development to our knowledge rifabutin is not yet on the list of antituberculous drugs procured by national TB control programs.
Pregnant women represent a patient population that needs special considerations for TB/HIV management. Efavirenz the recommended NNRTI for use in patients on TB treatment is contraindicated in the first and second trimester of pregnancy for fear of teratogenicity. There are reports that didanosine and stavudine have increased risk of toxicity in pregnant women (Sarner and Fakoya, 2002). Women with CD4 > 250 have also been reported to have increased risk of nevirapine related hepatitis (Leith et al., 2005). PIs serum levels are also reported to be reduced in later stages of pregnancy. In settings where rifabutin is not readily available to allow use of PIs options include the use of triple nucleosides or nucleosides and a nucleiotide. These approaches are a last resort since triple nucleoside regimens are inferior to HAART in as far as ART efficacy is concerned.
Cotrimoxazole prophylaxis DDI (CPT): Majority of TB/HIV patients will be on CPT while taking TB drugs and ART. There are overlapping toxicities to be expected. CDR can be caused by TB drugs, NNRTI and cotrimoxazole. Zidivudine, a backbone of most first line regimens causes bone marrow suppression commonly manifesting as neutropenia, thrombocytopenia and anemia (Moore et al., 2004). Cotrimoxazole is also known to cause bone marrow suppression (Raoul Moh et al., 2005).
The approach for severe CDR is to discontinue all the drugs and manage the reaction. Re-introduction should be by challenge starting with the most likely drug to have caused the reaction. In cases of TB drugs, ART and CPT, this approach is challenging but since most patients will have been on CPT long before TB and ART drugs, the approach is to consider the reaction to be due to the newly introduced drugs. As study done in Cote d'Voire has however shown that neutropenia occurs in patients who have been on CPT in whom zidovudine is introduced and this is reversed when CPT is stopped in most patients without requiring discontinuation of zidovudine (Raoul Moh et al., 2005) suggesting an independent role of contromoxazole in bone marrow suppression.
Tuberculosis Inflammatory Reconstitution Syndrome (TB-IRIS): TB-IRIS can be categorized into 2 main clinical entities: Paradoxical and unmasking TB-IRIS. The former denotes worsening or development of new TB symptoms upon initiation of ART in HIV-infected TB patients on TB Therapy or on initiation of TB treatment alone; while the later is TB diagnosed in a patient who at the start of ART did not have clinically apparent TB (Meintjes et al., 2008). Since its recognition as a major problem in ART, a number of large TB-IRIS studies have been concluded. It has become apparent that TB-IRIS is not as common as earlier thought. A recent cohort study from Uganda has found that only 3.8% of the 219 patients enrolled developed unmasking TBIRIS (Worodria et al., 2011). Another development in our understanding of TB-IRIS has been the publication of the first clinical trial in the management of TB-IRIS. In this trial steroids reduced hospital stay, symptoms and chest radiographic findings (Meintjes et al., 2010). Although a lot of research has been conducted to find biological markers and hence a diagnostic test for TB-IRIS, no such is in clinical use to date. Therefore, TB-IRIS remains a clinical diagnosis.
Treatment shortening and new ATT drugs studies: The HIV epidemic and the TB upsurge that followed it has lead to renewed interest in TB drugs development. A number of treatment shortening trials and studies looking at development of new compounds have been conducted. However none of these has seen any practical utility in routine TB case management. TB treatment is still largely dependent on drugs developed over 4 decades ago (Fox et al., 1999). Research has been conducted to re-investigate existing drugs. Higher doses of rifampicin have been found to have better bactericidal activity (Diacon et al., 2007). Fluoroquinolones are recommended for treatment of MDRTB but new methoxyfluoroquinolones (gatifloxacin and moxifloxacin) are being evaluated for treatment shortening of drug susceptibility when substituted for ethambutol or isoniazid (Conde et al., 2009, Burman et al., 2006). Several new drugs in clinical phases of evaluation for TB treatment include diarylquinolines (TMC-207), Nitroimidazoles (PA-824 and OPC-67683), oxazolidolinones (linezolid and PNU -100480), ethylenediamines (SQ-109), SQ-109. About 34 other compounds are in preclinical development (Lienhardt et al., 2010).
New and treatment shortening studies face 2 major challenges: available 6 months regimens are effective and therefore shortening trials have to be non-inferiority trials thus requiring large samples sizes hence expensive to conduct. TB therapy is by combination, therefore, new drugs have to be effective on their own however their clinical use can only be after they are integrated into combinations. Each drug can take up to 5 years to develop to clinical stage and it would theoretically take up to 20 years to develop a completely new TB regimen (Ginsberg et al., 2007).
TB control among HIV infected populations: Recent studies have enabled new approaches in the control of HIV associated tuberculosis. The WHO recommends ART and the three Is: Isoniazid prophylaxis therapy (IPT), Intensified case finding and Infection control (WHO TB infection control policy, 2009). Efforts to find an effective TB vaccine are also under way.
IPT: IPT significantly reduces the risk of active TB but has no effect on mortality and duration of protection is about 3 years (Akolo et al., 2010). There are, however, advances in the delivery of IPT. One of them is long duration IPT. A recent trial in Botswana has shown that IPT given for 36 months is safe and feasible and has a 92% risk reduction of active TB (Samandari et al., 2009). However, it is yet to be seen how this would be translated into routine policy and practice. There have been arguments that IPT may not been necessary since ART reduces TB risk and therefore with widespread use of ART, IPT will become less important. While ART reduces TB risk, HIV patients on ART continue to have increased risk of TB. In one study by Moore et al in Uganda, the incidence of TB among HIV patients on ART was three times higher than in the HIV negative group highlighting the continued need for other TB prevention methods along side ART for TB control among HIV positive persons (Moore et al 2007).
ART: It is now fairly clear that ART given to HIV patients without TB is a TB prevention strategy. This has followed observations that ART reduces TB incidence by about 80% (Badri et al., 2002, Lawn et al., 2005). However mathematical models have indicated that bigger impact on TB burden reduction will be seen if ART is initiated at high CD4 cell counts (Williams et al., 2003; Granich et al., 2009; Dodd et al., 2010). In a model by Granich et al., it has been shown that to achieve at least 50% TB reduction with ART over a 20 year period, the threshold for ART initiation would need to be shifted to CD4 cell counts > 500 cells/uL (Granich et al., 2009). However clinical data in support of these models is lacking.
Infection Control: TB Infection control gained attentions in the control of TB/HIV because HIV patients have increased risk of TB infection and once infected they rapidly progress to active TB without going through the latent phase of infection. About 30–40% of HIV patients will develop active TB in their lifetime if they do not receive ART or IPT (WHO TB infection control policy, 2009). In addition health care providers are also at an increased risk of TB (Kayanja et al., 2005).
All HIV and TB clinics should therefore have an infection control plan. Control measures should include personal protection, administrative controls and environmental measures. Administrative controls include (i) early identification and separation of infectious patients, (ii) controlling infectiousness by educating patients on proper cough etiquette and respiratory hygiene, and (iii) limiting time patients spend in health care facilities through decreasing hospital stays and increasing outpatient TB treatment. Environmental controls include (i) encouraging natural and mechanical ventilation and (ii) upper-room ultraviolet light. Personal measures include (i) staff use fit tested particulate respirators such as the N95 (ii) provision of surgical masks for patients to create a mechanical barrier to the spread of infectious droplet nuclei. Designated places for cough should be created within health facilities. Doors and windows should be opened at all times (WHO TB infection control policy, 2009). Where surgical masks are not available, pieces of cloth folded into a diamond can be used by patients. Large pieces of cloth should be avoided as these can spread aerosols upon unfolding them.
TB vaccines: The global TB control plan recognizes TB vaccines as one of the new strategies that should be evaluated for TB control (Stop TB Partnership: Global plan to stop TB 2011-15). Despite this, no new TB vaccine has completed development. Introduced in 1921, BCG remains the only TB vaccines in practice to date. It generally protects against severe forms of TB in the first 10 years of life and its efficacy is about 50% (Colditz et al., 1994). Candidate TB vaccines can be categorized into those that are aimed at preventing TB infection among those never infected with TB (pre-exposure vaccines) and those that can prevent reactivation of latent TB(post exposure vaccines). Most new TB vaccines are focused on boosting BCG but development of new TB vaccines (replacement vaccines) is also ongoing . Booster vaccines are preferred because they will preserve the safety and low cost advantages of BCG.
Some of the new candidate vaccines have gone into phase II trials in developed countries and in some parts of Africa (Black et al., 2002). The most promising of them include rBCG30, a BCG strain over expressing the immunodominant M. tuberculosis antigen 85B, and rBCGΔUreC:Hly, which is deficient in urease and expresses listeriolysin (Grode L et al 2005). These vaccines are designed to improve antigen presentation and hence produce a stronger T cell response. Other BCG boosting vaccines have used viral vectors that express immune-dominant M. tuberculosis antigens for the initiation of strong lymphocyte responses. This group includes Modified Vaccinia Ankara (MVA85A) that that incorporates MTB subunit 85A into the vaccinia virus and AERAS 402 and AdAg85A that employ adenovirus (McShane H et al 2004).
Fusion proteins of immune-dominant antigens from the MTB have also been used to elicit a strong immune response. For example one called hybrid-1 contains antigens 85B and ESAT-6 (Dietrich J et al 2006). Few innovations have gone into post exposure vaccines. Mycobacterium vaccae (derived from heat killed environmental saprophytes) and semi purified mycobacterium fragments RUTI are among the most promising (Vilaplana C et al 2010, Von Reyn CF et al 2010). These vaccines can be used alongside chemotherapy that eliminates the active organisms while the vaccine elicited immune response mops the remaining pathogens through the strong immune response so elicited
Conclusion: Great advances have been made in the field of diagnoses, treatment and overall management of HIV-associated TB in the immediate past period. The control of TB is however still challenged by poor case detection in spite of major advances in diagnostics. Advances in treatment seem to lag behind the major leaps in diagnostic research, resulting in less than optimal control for HIV-associated TB. Preventive vaccines, immunomodulation as an adjunct to treatment including effective treatment shortening strategies are urgently needed. Health system capacity in spite of advancements need to keep pace with advances in major intervention for care and support of HIV-infected TB patients, to enable the realization of the major public health goal of optimal TB/HIV control and the potential for TB elimination.
Table 4.
Summary of results from published studies on Safety of TB/HIV treatment
| Author, Journal, Date, Setting | Parameters | On ART | Not on ART |
| Abdool Karim, Salim, NEJM ,2010, South Africa | |||
| LEE | 8.4% | 3.6% | |
| Grade 4 LEE | 3.96% | 1.9% | |
| CDR | 0.7% | 1.9% | |
| TBIRIS | 12.4% | 3.8% | |
| Tabarsi Payam, J IAS ,2009, Iran | |||
| LEE | 14.5% | Did not significantly differ | |
| CDR | 7.7% | ||
| TBIRIS | 13% | ||
|
Sao Humphrey, AIDS Research and Human retroviruses, 2009, Tanzania |
No specific report but generally reports no difference in death and grade 3 or 4 AE in the two arms |
||
| Dean GL 2002, AIDS, London | CDR | CDR: 17% developed rash, in 5/29 rash due to ART and 68% had treatment interruption due to rash (18 TB treatments and 6 ART). |
|
| LEE/DILI | 6% developed DILI | ||
| Overall simultaneous TB/HIV therapy associated with severe adverse events (1.88, 95% CI, 1.03+/−3.42) |
|||
Key: LEE: Liver Enzyme Elevation, AE: Adverse Event, DILI: Drug Induced Liver Injury, CDR: Cutaneous Drug Reaction, TBIRIS: TB Immune Reconstitution Inflammatory Syndrome
List of abbreviations
- ART
antiretroviral therapy
- ATT
anti-tuberculosis Therapy
- BAL
bronchioalveolar lavage
- CDR
cutaneous drug reactions TB-IRIS
- DDI
Drug-Drug interactions
- DILI
drug induced liver injury
- FIND
Foundation for Innovation of New Diagnostics
- FM
Fluroscent microscopy
- IGRA
Interferon Gamma Release Assays
- IPT
Isoniazid prophylaxis therapy
- LAM
lipoarabinomanann
- LED
Light emitting diode
- LEE
Liver Enzyme Elevation
- LJ
Lowenstein Jensen
- MGIT
Mycobacteria Growth Indicator Tube
- MODS
mycobacteria observation drug susceptibility
- MTB
mycobacteria tuberculosis
- MVA85A
Modified Vaccinia Ankara-85 subunit
- NNRTI
None nucleoside reverse transcriptase inhibitors
- PI
Protease inhibitors
- TAT
Turn-around time
- TB
Tuberculosis
- TB-IRIS
Tuberculosis Inflammatory Reconstitution Syndrome
- TST
tuberculin skin test
- UGT
uridine diphosphate glucuronosyltransferase
- UNAIDS
The Joint United Nations Programme on HIV/AIDS
- UNICEF
The United Nations Children's Fund
- WHO
World health organization
- ZN
Ziehl-Neelsen
References
- 1.Abdool Karim SS, Naidoo K, Grobler A, et al. Timing of initiation of antiretroviral drugs during tuberculosis therapy. N Engl J Med. 2010;362:697–706. doi: 10.1056/NEJMoa0905848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abdool Karim SS, Naidoo K, Grobler A, et al. Timing of initiation of antiretroviral drugs during tuberculosis therapy. N Engl J Med. 2010;362:697–706. doi: 10.1056/NEJMoa0905848. Supplementary Appendix. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abdool Karim SS, Naidoo K, Grobler A, Padayatchi N, Baxter C, Gray AL, Gengiah T, Gengiah S, Naidoo A, Jithoo N, Nair G, El-Sadr WM, Friedland G, Abdool Karim Q. Integration of antiretroviral therapy with tuberculosis treatment. (20).N Engl J Med. 2011;365(16):1492–1501. doi: 10.1056/NEJMoa1014181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abdool Karim SS, Naidoo K, Padayatchi N, et al. Optimal timing of ART during TB therapy: findings of the SAPiT trial; 18th Conference on Retroviruses and Opportunistic Infections; Boston. abstract 39LB. [Google Scholar]
- 5.Akolo C, Adetifa I, Shepperd S. Treatment of latent tuberculosis infection in HIV infected persons. Cochrane Database of Systematic Reviews. 2010;1:CD000171. doi: 10.1002/14651858.CD000171.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Badri M, Wilson D, Wood R. Effect of highly active antiretroviral therapy on incidence of tuberculosis in South Africa: a cohort study. Lancet. 2002;359:2059–2064. doi: 10.1016/S0140-6736(02)08904-3. [DOI] [PubMed] [Google Scholar]
- 7.Black GF, Weir RE, Floyd S, Bliss L, Warndorff DK, Crampin AC. BCG-induced increase in interferon-gamma response to mycobacterial antigens and efficacy of BCG vaccination in Malawi and the UK: two randomized controlled studies. Lancet. 2002;359(9315):1393–1401. doi: 10.1016/S0140-6736(02)08353-8. [DOI] [PubMed] [Google Scholar]
- 8.Boehme C, Nabeta P, Hillemann D, et al. Rapid Molecular Detection of Tuberculosis and Rifampin Resistance. N Engl J Med. 2010;363:1005–1015. doi: 10.1056/NEJMoa0907847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brainard DM, Kassahun K, Wenning LA, Petry AS, Liu C, Lunceford J, Hariparsad N, Eisenhandler R, Norcross A, DeNoia EP, Stone JA, Wagner JA, Iwamoto M. Lack of a Clinically Meaningful Pharmacokinetic Effect of Rifabutin on Raltegravir: In Vitro/In Vivo Correlation. J Clin Pharmacol. 2011;51:943–950. doi: 10.1177/0091270010375959. [DOI] [PubMed] [Google Scholar]
- 10.Burman WJ, Goldberg S, Johnson JL, Muzanye G, Engle M, Mosher AW, Choudhri S, Daley CL, Munsiff SS, Zhao Z, Vernon A, Chaisson RE. Moxifloxacin versus ethambutol in the first 2 months of treatment for pulmonary tuberculosis. Am J Respir Crit Care Med. 2006;174:331–338. doi: 10.1164/rccm.200603-360OC. [DOI] [PubMed] [Google Scholar]
- 11.Burman WJ, Gallicano K, Peloquin C. Therapeutic implications in the treatment of human immunodeficiency virus-related tuberculosis. Clin Infect Dis. 1999;28:419–430. doi: 10.1086/515174. [DOI] [PubMed] [Google Scholar]
- 12.Cattamanchi A, Dowdy DW, Davis JL, Worodria W, Yoo S, Joloba M, Matovu J, Hopewell PC, Huang L. Sensitivity of direct versus concentrated sputum smear microscopy in HIV-infected patients suspected of having pulmonary tuberculosis. BMC Infect Dis. 2009;9:53. doi: 10.1186/1471-2334-9-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Metcalfe JZ, Everett CK, Steingart KR, Cattamanchi A, Huang L, Hopewell PC, Pai M. Interferon-gamma release assays for the diagnosis of latent tuberculosis infection in HIV-infected individuals - A systematic review and meta-analysis. JAIDS. 2011 doi: 10.1097/QAI.0b013e31820b07ab. Publish Ahead of Print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colditz G, Brewer F, Berkey C, Wilson M, Burdick E, Fineberg H. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA. 1994;271(9):698–702. [PubMed] [Google Scholar]
- 15.Conde MB, Efron A, Loredo C, De Souza GR, Graça NP, Cezar MC, Ram M, Chaudhary MA, Bishai WR, Kritski AL, Chaisson RE. Moxifloxacin versus ethambutol in the initial treatment of tuberculosis: a double-blind, randomised, controlled phase II trial. Lancet. 2009;373:1183–1189. doi: 10.1016/S0140-6736(09)60333-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dean GL, Edwards SG, Ives NJ, Matthews G, Fox EF, Navaratne L, Fisher M, Taylor GP, Miller R, Taylor CB, de Ruiter A, Pozniak AL. Treatment of tuberculosis in HIV-infected persons in the era of highly active antiretroviral therapy. AIDS. 2002;16:75–83. doi: 10.1097/00002030-200201040-00010. [DOI] [PubMed] [Google Scholar]
- 17.Dietrich J, Andersen C, Rappuoli R, Doherty TM, Jensen CG, Andersen P. Mucosal administration of Ag85B-ESAT-6 protects against infection with Mycobacterium tuberculosis and boosts prior bacillus Calmette-Guerin immunity. J Immunol. 2006;177(9):6353–6360. doi: 10.4049/jimmunol.177.9.6353. [DOI] [PubMed] [Google Scholar]
- 18.Diacon AH, Patientia RF, Venter A, van Helden PD, Smith PJ, McIlleron H, Maritz JS, Donald PR. Early bactericidal activity of high-dose rifampicin in patients with pulmonary tuberculosis evidenced by positive sputum smears. Antimicrob Agents Chemother. 2007;51:2994–2996. doi: 10.1128/AAC.01474-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dodd PJ, Garnett GP, Hallet TB. Examining the promise of ‘Test and Treat’ in hyper endemic settings. AIDS. 2010;24(5):729–735. doi: 10.1097/QAD.0b013e32833433fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fox W, Ellard GA, Mitchison DA. Studies on the treatment of tuberculosis undertaken by the British Medical Research Council Tuberculosis Units, 1946–1986. Int J Tuberc Lung Dis. 1999;3:S231–S279. [PubMed] [Google Scholar]
- 21.Getahun H, Kittikraisak W, Heilig CM, Corbett EL, Ayles H, et al. Development of a Standardized Screening Rule for Tuberculosis in People Living with HIV in Resource-Constrained Settings: Individual Participant Data Meta-analysis of Observational Studies. PLoS Med. 2011;8(1):e1000391. doi: 10.1371/journal.pmed.1000391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gil-Setas A, Torroba L, Fernandez JL, Martinez-Artola V, Olite J. Evaluation of the MB/BacT system compared with Middlebrook 7H11 and Lowenstein-Jensen media for detection and recovery of mycobacteria from clinical specimens. Clin Microbiol Infect. 2004;10:224–228. doi: 10.1111/j.1198-743x.2004.00733.x. [DOI] [PubMed] [Google Scholar]
- 23.Ginsberg AM, Spigelman M. Challenges in tuberculosis drug research and development. Nat Med. 2007;13:290–294. doi: 10.1038/nm0307-290. [DOI] [PubMed] [Google Scholar]
- 24.Grode L, Seiler P, Baumann S, et al. Increased vaccine efficacy against tuberculosis of recombinant Mycobacterium bovis bacilli Calmette-Guérin mutants that secrete listeriolysin. J Clin Invest. 2005;115(9):2472–2479. doi: 10.1172/JCI24617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Havlir DV, Kendall MA, Ive P, Kumwenda J, Swindells S, Qasba SS, Luetkemeyer AF, Hogg E, Rooney JF, Wu X, Hosseinipour MC, Lalloo U, Veloso VG, Some FF, Kumarasamy N, Padayatchi N, Santos BR, Reid S, Hakim J, Mohapi L, Mugyenyi P, Sanchez J, Lama JR, Pape JW, Sanchez A, Asmelash A, Moko E, Sawe F, Andersen J, Sanne I. AIDS Clinical Trials Group Study A5221. Timing of antiretroviral therapy for HIV-1 infection and tuberculosis. (20).N Engl J Med. 2011;365(16):1482–91. doi: 10.1056/NEJMoa1013607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meintjes G, Lawn SD, Scano F, Maartens G, French MA, Worodria W, Elliott JH, Murdoch D, Wilkinson RJ, Seyler C, John L, van der Loeff MS, Reiss P, Lynen L, Janoff EN, Gilks C, Colebunders R. International Network for the Study of HIV-associated IRIS Tuberculosis-associated immune reconstitution inflammatory syndrome: case definitions for use in resource-limited settings. Lancet Infect Dis. 2008;8(8):516–523. doi: 10.1016/S1473-3099(08)70184-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Granich RM, Gilks CF, Dye C, De Cock KM, Williams BG. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet. 2009;373:48–57. doi: 10.1016/S0140-6736(08)61697-9. [DOI] [PubMed] [Google Scholar]
- 28.Kayanja HK, Debanne S, King C, Whalen CC. Tuberculosis infection among health care workers in Kampala, Uganda. Int J Tuberc Lung Dis. 2005;9(6):686–688. [PubMed] [Google Scholar]
- 29.Khan FA, Minion Jessica, Pai1 Madhukar, Royce Sarah, William Burman, Anthony D Harries, Dick Menzies. Treatment of Active Tuberculosis in HIV-Coinfected Patients: A Systematic Review and Meta-Analysis. Clin Infect Dis. 2010;50(9):1288–1299. doi: 10.1086/651686. (2010) [DOI] [PubMed] [Google Scholar]
- 30.Cain Kevin P, McCarthy Kimberly D, Heilig Charles M, et al. An Algorithm for Tuberculosis Screening and Diagnosis in People with HIV. N Engl J Med. 2010;362:707–804. doi: 10.1056/NEJMoa0907488. [DOI] [PubMed] [Google Scholar]
- 31.Lalvani A, Pareek M. Infereon gamma release assays: Principles and Practice. Enfemadades Infecciosas Y Microbiologia Clinica. 2009 doi: 10.1016/j.eimc.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 32.Lawn SD, Wood R. Incidence of tuberculosis during highly active antiretroviral therapy in high-income and low-income countries. Clin Infect Dis. 2005;15:1783–1786. doi: 10.1086/498308. [DOI] [PubMed] [Google Scholar]
- 33.Leith J, Piliero P, Storfer S, Mayers D, Hinzmann R. Appropriate use of nevirapine for long-term therapy. J Infect Dis. 2005 Aug 1;192(3):545–546. doi: 10.1086/431606. [DOI] [PubMed] [Google Scholar]
- 34.Lienhardta C, Vernonb A, Raviglione M. New drugs and new regimens for the treatment of tuberculosis: review of the drug development pipeline and implications for national programmes. Current Opinion in Pulmonary Medicine. 2010;16:186–193. doi: 10.1097/MCP.0b013e328337580c. [DOI] [PubMed] [Google Scholar]
- 35.Manosuthi W, Chottanapand S, Thongyen S, Chaovavanich A, Sungkanuparph S. Survival rate and risk factors of mortality among HIV/tuberculosis co-infected patients with and without antiretroviral therapy. J Acquir Immune Defic Syndr. 2006;43:42–46. doi: 10.1097/01.qai.0000230521.86964.86. [DOI] [PubMed] [Google Scholar]
- 36.Meintjes G, Wilkinson RJ, Morroni C, Pepper DJ, Rebe K, Rangaka MX, Oni T, Maartens G. Randomized placebo-controlled trial of prednisone for paradoxical tuberculosis-associated immune reconstitution inflammatory syndrome. AIDS. 2010;24(15):2381–2390. doi: 10.1097/QAD.0b013e32833dfc68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meintjes G, Lawn SD, Scano F, Maartens G, French MA, Worodria W, Elliott JH, Murdoch D, Wilkinson RJ, Seyler C, John L, van der Loeff MS, Reiss P, Lynen L, Janoff EN, Gilks C, Colebunders R. International Network for the Study of HIV-associated IRIS Tuberculosis-associated immune reconstitution inflammatory syndrome: case definitions for use in resource-limited settings. Lancet Infect Dis. 2008;8(8):516–523. doi: 10.1016/S1473-3099(08)70184-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McShane H, Pathan AA, Sander CR, et al. Recombinant modified vaccinia virus Ankara expressing antigen 85A boosts BCG-primed and naturally acquired antimycobacterial immunity in humans. Nat Med. 2004;10(11):1240–1244. doi: 10.1038/nm1128. [DOI] [PubMed] [Google Scholar]
- 39.Moore DA, Benepal T, Portsmouth S, et al. Etiology and natural history of neutropenia in Human immunodeficiency virus disease: a prospective study. Clinical therapeutics. 2004;26:92–97. doi: 10.1086/318495. [DOI] [PubMed] [Google Scholar]
- 40.Moore D, Evans A, Gilman R, et al. Microscopic-Observation Drug-Susceptibility Assay for the Diagnosis of TB. N Engl J Med. 2006;355(15):1539–1550. doi: 10.1056/NEJMoa055524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moore D, Liechty C, Ekwaru P, et al. Prevalence, incidence and mortality associated with tuberculosis in HIV-infected patients initiating antiretroviral therapy in rural Uganda. AIDS. 2007;21:713–719. doi: 10.1097/QAD.0b013e328013f632. [DOI] [PubMed] [Google Scholar]
- 42.Mutetwa R, Boehme C, Dimairo M, et al. Diagnostic accuracy of commercial urinary lipoarabinomannan detection in African tuberculosis suspects and patients. Int J Tuberc Lung Dis. 2009;13:1253–1259. [PMC free article] [PubMed] [Google Scholar]
- 43.Muyoyeta M, Schaap JA, De Haas P, et al. Comparison of four culture systems for Mycobacterium tuberculosis in the Zambian National Reference Laboratory. Int J TB & Lung Dis. 2009;13(4):460–465. (6) [PubMed] [Google Scholar]
- 44.Ngadaya ES, Mfinanga GS, Wandwalo E, et al. Detection of pulmonary tuberculosis among patients with cough attending outpatient departments in Dar Es Salaam, Tanzania: does duration of cough matter? BMC Health Services Research. 2009;9:112. doi: 10.1186/1472-6963-9-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Samandari T, et al. Preliminary results of the Botswana Isoniazid Preventive Therapy (IPT) Clinical Trial (6 months vs 36 months); 40th Union World Lung Conference; 2009; Cancun. [Google Scholar]
- 46.Sarner L, Fakoya A. Acute onset lactic acidosis and pancreatitis in the third trimester of pregnancy in HIV-1 positive women taking antiretroviral medication. Sex Transm Infect. 2002 Feb;78(1):58–59. doi: 10.1136/sti.78.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Showalter HD, Denny WA. A roadmap for drug discovery and its translation to small molecule agents in clinical development for tuberculosis treatment. Tuberculosis. 2008;(Edinb) 88(Suppl 1):S3–S17. doi: 10.1016/S1472-9792(08)70032-5. [DOI] [PubMed] [Google Scholar]
- 48.Steingart KR, Henry M, Ng V, et al. Fluorescence versus conventional sputum smear microscopy for tuberculosis: a systematic review. Lancet Infect Dis. 2006;6:570–581. doi: 10.1016/S1473-3099(06)70578-3. [DOI] [PubMed] [Google Scholar]
- 49.Vilaplana C, Montané E, Pinto S, et al. Double- blind, randomized, placebo-controlled Phase I Clinical Trial of the therapeutical antituberculous vaccine RUTI. Vaccine. 2010;28(4):1106–1116. doi: 10.1016/j.vaccine.2009.09.134. [DOI] [PubMed] [Google Scholar]
- 50.Von Reyn CF, Mtei L, Arbeit RD, et al. Prevention of tuberculosis in Bacille Calmette- Guérin-primed, HIV-infected adults boosted with an inactivated whole-cell mycobacterial vaccine. AIDS. 2010;24(5):675–685. doi: 10.1097/QAD.0b013e3283350f1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.WHO, author. antiretroviral therapy for HIV infection in adults and adolescents: recommendations for a public health approach. Geneva: World Health Organization; 2010. [PubMed] [Google Scholar]
- 52.WHO, author. Policy statement. Geneva: World Health Organization; 2010. Fluorescent light emitting diode (led) microscopy for diagnosis of tuberculosis. [PubMed] [Google Scholar]
- 53.Towards universal access, scaling up priority HIV/AIDS interventions in the health sector. 2010. WHO, UNAIDS, UNICEF progress report. [Google Scholar]
- 54.WHO, author. Global tuberculosis control: surveillance, planning, financing. Geneva: World Health Organization; 2010. [Google Scholar]
- 55.WHO, author. Intensified case-finding (ICF), isoniazid preventive therapy (IPT) and TB infection control (IC) for people living with HIV. Geneva: World Health Organization; 2008. Three I's for HIV/TB Meeting Report. [Google Scholar]
- 56.WHO, author. WHO policy on TB infection control in healthcare facilities, congregate settings and households. Geneva: World Health Organization; 2009. [PubMed] [Google Scholar]
- 57.WHO, author. Treatment of Tuberculosis: Guidelines. 4th edition. Geneva: World Health Organization; 2010. [PubMed] [Google Scholar]
- 58.Williams BG, Dye C. Antiretroviral drugs for tuberculosis control in the era of HIV/AIDS. Science. 2003;301:1535–1537. doi: 10.1126/science.1086845. [DOI] [PubMed] [Google Scholar]
- 59.Williams-Bouyer N, Yorke R, Lee HI, Woods GL. Comparison of the BACTEC MGIT 960 and ESP culture system II for growth and detection of mycobacteria. J Clin Microbiol. 2000;38:4167–4170. doi: 10.1128/jcm.38.11.4167-4170.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Worodria W, Massinga-Loembe M, Mayanja-Kizza H, et al. Antiretroviral Treatment-Associated Tuberculosis in a Prospective Cohort of HIV-Infected Patients Starting ART. Clinical & developmental immunology. 2011:758350. doi: 10.1155/2011/758350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Worodria W, Okot-Nwang M, Yoo S D, Aisu T. Causes of lower respiratory infection in HIV-infected Ugandan adults who are sputum AFB smear-negative. INT J TUBERC LUNG DIS. 2003;7(2):117–123. [PubMed] [Google Scholar]


