Abstract
The purpose of this article was to describe the workflow from imaging, via virtual design, to manufacturing of patient-specific titanium reconstruction plates, cutting guide and mesh, and its utility in connection with surgical treatment of acquired bone defects in the mandible using additive manufacturing by electron beam melting (EBM). Based on computed tomography scans, polygon skulls were created. Following that virtual treatment plans entailing free microvascular transfer of fibula flaps using patient-specific reconstruction plates, mesh, and cutting guides were designed. The design was based on the specification of a Compact UniLOCK 2.4 Large (Synthes®, Switzerland). The obtained polygon plates were bent virtually round the reconstructed mandibles. Next, the resections of the mandibles were planned virtually. A cutting guide was outlined to facilitate resection, as well as plates and titanium mesh for insertion of bone or bone substitutes. Polygon plates and meshes were converted to stereolithography format and used in the software Magics for preparation of input files for the successive step, additive manufacturing. EBM was used to manufacture the customized implants in a biocompatible titanium grade, Ti6Al4V ELI. The implants and the cutting guide were cleaned and sterilized, then transferred to the operating theater, and applied during surgery. Commercially available software programs are sufficient in order to virtually plan for production of patient-specific implants. Furthermore, EBM-produced implants are fully usable under clinical conditions in reconstruction of acquired defects in the mandible. A good compliance between the treatment plan and the fit was demonstrated during operation. Within the constraints of this article, the authors describe a workflow for production of patient-specific implants, using EBM manufacturing. Titanium cutting guides, reconstruction plates for fixation of microvascular transfer of osteomyocutaneous bone grafts, and mesh to replace resected bone that can function as a carrier for bone or bone substitutes were designed and tested during reconstructive maxillofacial surgery. A clinically fit, well within the requirements for what is needed and obtained using traditional free hand bending of commercially available devices, or even higher precision, was demonstrated in ablative surgery in four patients.
Keywords: implant, reconstruction, electron beam melting
In craniomaxillofacial surgery, reconstruction of congenital and acquired defects of the skull and facial regions is challenging due to the complex anatomy, sensitivity of the involved systems, and with respect to appearance.1 Today, computer-based treatment planning systems are available, and have strongly facilitated diagnosis faster and surgery that is more precise and implementation of computer-assisted surgery.2 This is partly due to the increasing capabilities of medical imaging devices that have undergone a transformation, from analog to digital computed tomography (CT), volume tomography, or cone beam computed tomography (CBCT).3 These high-resolution data have to be visualized, analyzed, and interpreted which is done using computer hardware and software. The CT is unique in that image values are defined in the absolute Hounsfield scale. The radio-density of distilled water at standard pressure and temperature (STP) is defined as zero Hounsfield units (HU). From the CT or CBCT examination, it is possible to narrow down the information by changing the HU as to depict bone, and thereby creating a three-dimensional (3-D) reconstruction of the facial skeleton.
In introducing new technology in the field of reconstructive craniomaxillofacial surgery, the goal is to increase precision, as well as to decrease morbidity and operation time, thereby reducing costs, hospitalization, and improving the quality of life. This can be achieved preoperative by a virtual plan and design in an advanced 3-D environment and then transfer the plan to the operation theater.4,5 During reconstruction of the mandible, one crucial point is to optimize the reconstruction and fixate the neo mandible to the native mandible.
A reconstruction can be accomplished in minor defects in nonradiated patients using bone blocks, a mesh bent, packed with particulate bone or bone substitute, and the mesh screw fixated to the native mandible. Alternatively, the neo mandible can be reconstructed with a microvascular bone graft. Regardless of method, a cutting guide is a good and precise method to bring the virtual resection plan to real time surgery.4
Following resection and bone replacement, the reconstruction has to be fixated. This is usually done with plates and screws, that is, weight-bearing plates, which can carry the biomechanical load exerted on the reconstruction. Titanium plates of varying thickness constitute the standard today. To obtain an acceptable fit these plates have to be manually bent during the fixation procedure. There are different methods described for presurgical contouring to reduce the operating time 12. Most methods described are based on preproduction of physical guides.5 In addition, predesigned plates of different size provided by the manufacturer are available based on statistical shape models from a large number of CT scan, originating from various adult populations.6 The combination of anatomic design and manufacturing processes allows reduction of stress in the plate compared to classical intraoperative bending of a flat plate. In addition, there are different mechanical and biological requirements depending on the anatomical site to be rehabilitated, and on the functional requirements. Given this changing demand for plates, an individual approach is necessary. A first advancement would be the transfer of the information preoperatively for manufacturing of the specific devices needed for a single patient. This is already a reality using available additive manufacturing technology, for example, electron beam melting (EBM).
Additive manufacturing refers to methods where a physical object is built up, layer by layer. These methods rely on the existence of a digital 3-D model and this model is sliced up in thin layers, typically ranging from 50 to 300µm. The layers are then manufactured, one at a time, until the physical part is finished. The materials used for additive manufacturing is typically plastics, ceramics, wax, or metals. A handful of methods are able to manufacture parts in dense metals where the material properties are comparable to wrought materials, one of them being EBM. The bulk material properties obtained from the process conforms with the ASTM F136 (American Society for Testing and Material) standard and the material is therefore suitable for implants and has previously been tested in an animal study.7,8,9 The EBM method operates in vacuum and uses an electron beam to melt consecutive layers of metal powder to form the solid object. When the build is completed, the parts are left in the machine to cool down and excess powder is then removed in a blaster chamber.10
Considering the above, the first aim of the present work was to describe a workflow of a method to design and create a virtual saw guide, a mesh for harboring bone or bone substitutes, reconstruction plates, and then producing these devices in titanium using EBM. Secondly, the authors wanted to implement this in reconstructive surgery, and to apply and evaluate this in a clinical setting.
Materials and Methods
Study Design
Three different types of patient-specific devices were planned, produced, and clinically tested:
First a cutting guide to ensure the exact resection of the affected bone in accordance with a virtual plan to guarantee a precise transfer of the plan to the operating room during real-time surgery.
Secondly, load-bearing reconstruction plates for fixation of free microvascular transfer of fibula osteomyocutaneous flaps.
Thirdly, mesh with attached load-bearing plates to harbor nonvascularized bone grafts or bone substitutes as an alternative the microvascular procedure.
In this study four cases were selected to test the applicability of the approach, one patient from the Department of Oral and Maxillofacial Surgery, University of Gothenburg, Gothenburg, Sweden, and three from Uppsala University Hospital, the Department of Plastic and Maxillofacial Surgery, Uppsala, Sweden. The first patient was diagnosed with osteoradionecrosis of the mandible involving the parasymphyseal and symphyseal area on the right side. The second patient presented an extensive recurrent squamous cell carcinoma (SCC) on the left side of the mandible, and the third patient presented a very fast growing SCC also on the left side emerging from the third molar region. Finally, the fourth patient exhibited an osteoradionecrosis of the right side of the mandible from the cuspid to the angular region.
Radiological Examination
CT examinations were performed and the DICOM© files (Digital Imaging and Communication in Medicine, NEMA) obtained from the PACS station (Picture Archiving and Communication System). The postoperative CT scans from patient number three were also retrieved and reconstructed for control against the virtual plan.
Virtual Design
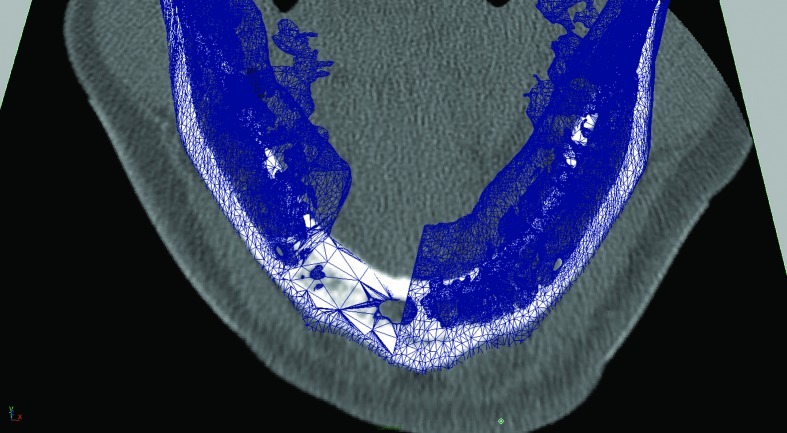
An isosurface reconstruction was carried out in Osirix© 3.2 (Wavefront Technology, Edmonton, Alberta, Canada) using a Hounsfield level of 500. The reconstruction was exported from Osirix© as a geometry definition file, *.obj. The polygon jaw was then transferred to Autodesk Maya 2009 (Autodesk®, San Rafael, CA). The DICOM images stack was exported to *.tiff (Adobe System Inc., San Jose, CA) and imported as an image sequence into Maya® 2009 (Autodesk®, San Rafael, CA). The polygon jaw was then aligned with the image sequence and virtual resection was carried out (Fig. 1), and a saw guide was designed (Fig. 2A, B). The virtual saw guide was compared to the image sequence for treatment plan accuracy. In the remaining cases, the treatment plan was carried out in Surgicase© (Materialise©, Leuven, Belgium). The *.stl-file (3D System Corp, Rock Hill, SC) from the reconstructed mandible/fibula was transferred to a Macintosh© Mac Book Pro©, 2.16 MHz, Duel Core Intel® processor with two Gigabyte RAM, (Apple© Corp., Cupertino, CA) and imported into 3-D animation program Maya 2009. The specification of a Compact UniLOCK® 2.4 large plate (Synthes®, Switzerland) was used. The geometry was scaled down by 25%. The polygon mesh was skinned onto a skeleton, according to Dérand and Hirsch5. Plates were constructed with individualized screw holes (Fig. 3) and exported as a geometry definition file, *.obj. The titanium mesh was constructed by mirroring the contralateral side and shaped with Fracture-FX (plug-in, Maya® 2009). All data were transferred over Internet to the Department of Engineering and Sustainable Development (Mid Sweden University, Östersund, Sweden) for additive manufacturing of the devices.
Figure 1.
The DICOM files were converted to *.tiff and then imported into Autodesk Maya 2009 (Autodesk). The image was aligned with the polygon mandible to check the resection.
Figure 2.
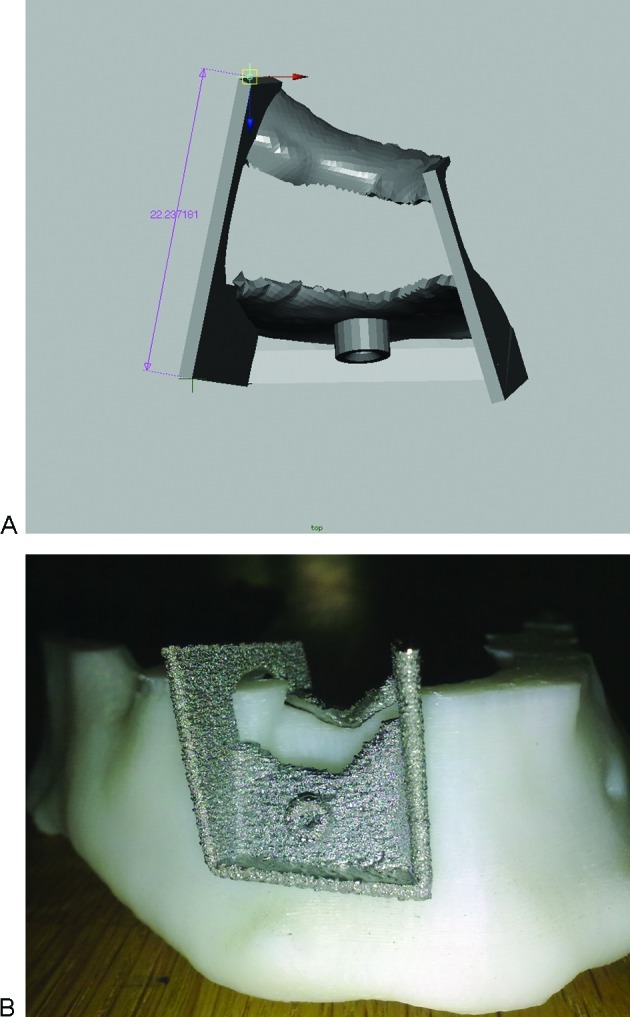
(A) A saw guide was designed. (B) The EBM-produced titanium saw guide shown on a plastic model of the mandible.
Figure 3.
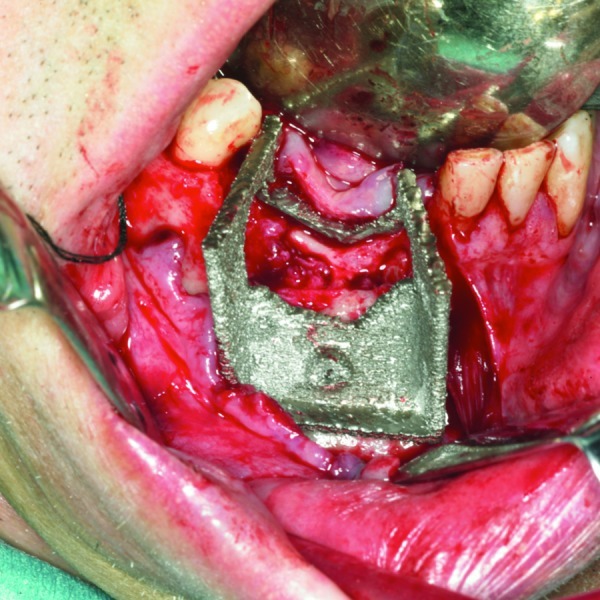
The saw guide was checked against the patient mandible.
Additive EBM Manufacturing
To complete the fabrication of the patient-specific implants the earlier described method was used. All data transferred were imported to Magics (Materialise, Leuven, Belgium). To compensate for shrinkage in the manufacturing process, the plates were scaled with 1.0086, 1.0086, and 1.0093 in x, y, and z direction, respectively, were z is the build direction. The plates were positioned 3 mm above the start plate and solid supports between the start plate and the reconstruction plates were designed. The files were then prepared and sliced into 0.1 mm layer thickness in the software Build Assembler 3.0 (ARCAM AB, Gothenburg, Sweden). The titanium parts were manufactured by an ARCAM EBM A2 machine loaded with gas-atomized Ti6Al64V ELI powder with a particle size ranging from 45 to 100 µm. The build temperature range was kept in the range +600 to +750°C and the vacuum pressure held constant at 2 × 10−3 mbar. The parts were manufactured in three different batches approximately 1 to 2 weeks before each surgery. The build time varied between the builds, due to different build heights, and the average build speed was 50 s/layer. For example, the plates and the meshes for the last case had a build height of 36 mm, yielding a build time of 5 hours. The build was then cooled down inside the machine in a helium atmosphere of 400 mbar to approximately 70°C. The build was then taken out of the machine and excess powder was removed in a blaster chamber using the same powder as for the manufacturing. As a last step, holes with a diameter for nonlocking screws were drilled in the plates according to the outline in the virtual plan. The solid supports were removed using pliers and the parts were put into plastic bags and send to the Department of Plastic and Maxillofacial Surgery, Uppsala University Hospital, Uppsala, Sweden.
Cleaning and Sterilization
All implants were ultrasonically cleaned in sonicator (Whaledent AG, Biosmic, Switzerland) with a standard cleaning solution at about 50–65 °C for 10 minutes to remove any loose particles, followed by the same procedure in absolute alcohol. After the ultrasonic cleaning, all components were rinsed under sterile water then packed and standard steam-sterilized.
Clinical Application
In addition to the patient-specific implants the virtual reconstruction plans were reproduced in additive-manufactured plastic mandible models. These models were used to orientate and check the fit of the customized implants before sterilization.
The saw guide was used in the operating theater, checked against the mandible (Fig. 4), and then used to perform the planned osteotomies.
Figure 4.
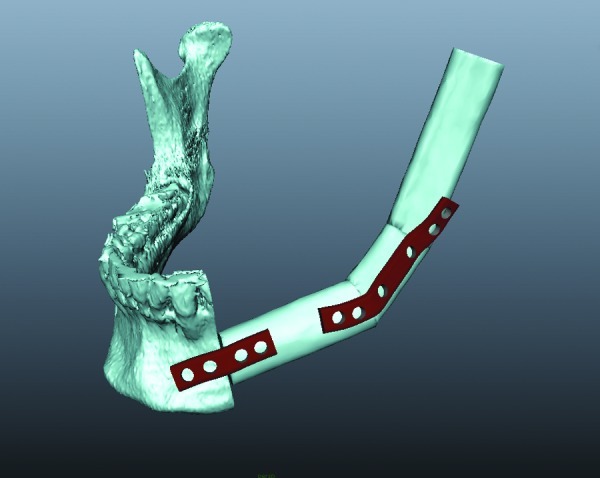
A four-hole and a six-hole polygon plate was constructed against the treatment plan conducted in SurgiCase (Materialise, Leuven, Belgium).
During the operations, plates were applied on the segmented fibula flaps and screw fixated with standard titanium nonlocking screws (Synthes®, Switzerland). The titanium mesh was tried for fit after the planned osteotomies were performed.
Results
This project has shown that commercially available software programs are sufficient to virtually plan and transform the information to production of patient-specific titanium implants.
The additive manufacturing method EBM produces custom-designed implants fully usable under clinical conditions in reconstruction of acquired defects in the mandible with good agreement between the treatment plan and the fit as demonstrated during operation (Figs. 4 and 5) and the subsequent short-term clinical as well as the superimposed preoperative treatment plan with the radiological outcome (Fig. 6). Clinically, the custom-made cutting guide, mesh, and the load-bearing reconstruction plates were appreciated during surgery as the implants were easy to handle, fixate, and reduced operating time significantly.
Figure 5.
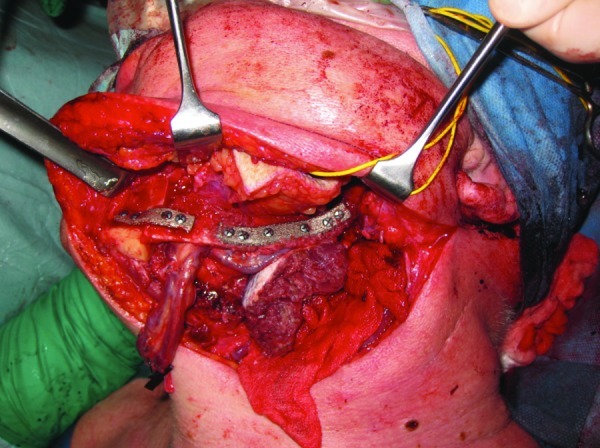
The EBM-produced titanium reconstruction plates in place.
Figure 6.
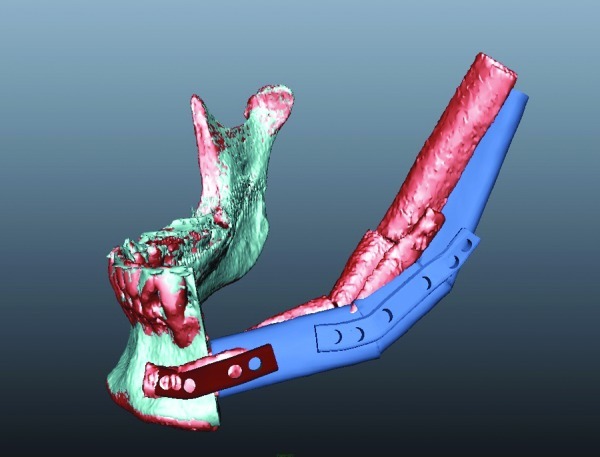
Difference between the preoperative treatment plan and treatment outcome. Dark blue is the preoperative treatment plan. Red being the postoperative treatment outcome.
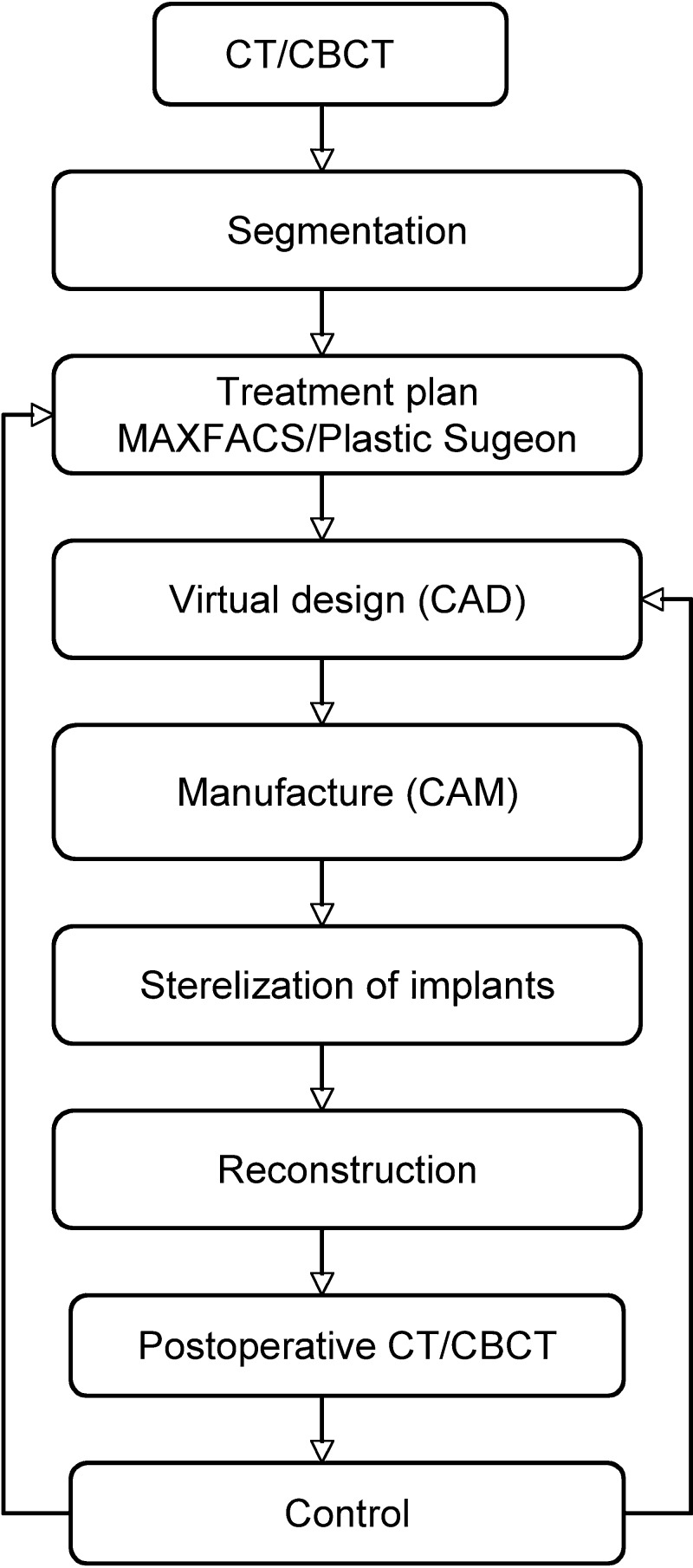
Predesigning plates in advance of the surgery when performing time-consuming procedures, as in this case, is an advantage. The calculated reduction of operation time is approximated to 30 minutes. Including all personnel present in the operation theatre (anesthetists, nurses, involved surgeons), a total reduction of time for staff is estimated to more than 3.5 hours. The workflow, as shown in Fig. 7 has been applied.
Figure 7.
Schematic workflow used when designing and producing patient-specific implants.
Discussion
The authors consider virtual planning of reconstructive procedures as a mean to significantly increase precision in surgical outcome. Fitting implants virtually and producing these devices on a patient-specific basis in biocompatible material as demonstrated here is a further step towards creating value for the patient and for the health care, which can be measured, in health economic terms. This opens for further advancement in implant technology.11
There is no necessity to develop new software as the feasibility of the approach was demonstrated here using only commercially available softwares. Two immediate problems arise, that is, to have competence to manage the total flow of the process and to implement the process in routine work. This report makes suggestions how to handle the workflow which is the initial problem when a number of specialties are involved. These problems are usually of a transient nature.
There are quite a few reports on using a stereolithography model as a template as described by Kernan and Wimsatt,12 or other templates or guides to preoperatively bend a reconstruction plate that is functional and results in acceptable precision.5 However, combining different software for virtual planning and production of guides is less common. Virtual planning systems have been presented for skeletal movements with special reference to mandible distraction osteogenesis13,14,15,16 and for soft tissue prediction.17,18 In minimal invasive implant surgery, the virtually placed implants are transferred to the patient via a drilling guide manufactured to transfer the virtual plan to real-time surgery.19 Using such an approach, Zinser et al published an article on preoperative manufacturing surgical wafers before orthognathic surgery.20 The orthognathic surgery was carried out virtually and when the bones had been moved, wafers were manufactured and used during real-time surgery. Furthermore, Leiggener et al presented a method for speeding up the assembly of the bone transplant based on virtual planning of the cutting planes for a free fibula graft.4 The osteotomies were translated into a rapid prototyping guide, which was applied during surgery. The circulation could thus be kept intact during this part thereby reducing the ischemic time significantly. The above-described technique can easily be implemented in all aspects of reconstructive bone surgery where plates are used. By supplying patient-specific plates, the authors have brought the process of simplifying, increasing precision, and reducing operation time one step further. So far, however, there is limited information in the literature regarding manufacturing of patient-specific plates, screws, and mesh in advance of surgery using CAD (computer aided design) in the field of craniomaxillofacial surgery.
From a surgical point of view, the customized guide facilitates the identification of the most appropriate site with regard to bone volume, blood supply, and precisely assists in resection of the desired bony segment in a correct angle both at the donor site as well as at the recipient site. In addition, a guide is useful in sectioning a bone graft in predetermined length and angles.4 The customized plates also function as a guide to assemble the segments according to the virtual plan to create the neo mandible and to fixate the bony fragments in the correct position at the recipient site. In a microvascular reconstruction situation the whole procedure can be performed with intact circulation, which shortens the ischemic time considerably. Thus, bending and fitting of a reconstruction plate in the symphysic area of the mandible where the plate has to be bent in three dimensions is time-consuming compared to dorsally in the mandible where it is less difficult. Altogether, the work normally requires approximately 25 minutes. To do this preoperatively is time saving, particularly as the patient-specific plates, during surgery, only takes a few minutes to fit. This is expected to have an impact on the reconstruction outcome of procedures where a microvascular osseous flap is used. In a situation where for any reason there is a change of the operation plan, traditional methods are always available as reported previously.5
The virtual planning allows for predetermining plate screw length eliminating all perioperative length measuring and vital anatomical structures can be avoided. The bony flap, the plates, and screws could be designed in such a way that osseointegrated dental implants can be installed with plates and screws in situ, in connection with the reconstruction, or at a later stage and still reaching optimal function and aesthetics. This is best planned by combining commercially available systems for guided-dental implant surgery (Nobel guide©, Nobel Biocare© Sweden, Simplant©, Materialise©, Leuven, Belgium) with the algorithms presented here.
In addition to the patient-specific load-bearing plates titanium mesh was virtually designed with plates attached. These patient-specific meshes were produced as an exact anatomical duplicate of the preexisting anatomy. If the anatomy were to be destroyed due to bone decease, the intact contralateral side can be mirrored and used as a template.
Three different designs were tested with varying mesh size. They all had a perfect fit when tried during operation. The idea is to use these meshes as an alternative to a nonvascularized block bone graft. A mesh can be packed with particulate bone or any proven bone substitute.21
One other benefit with EBM manufacturing is the ability to investigate and build a variety porous of titanium scaffolds (Fig. 8) that combined with a load-bearing plates can still be a load-bearing structure.
Figure 8.
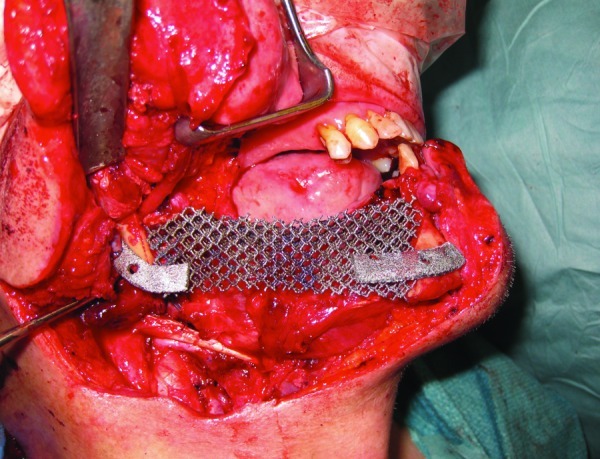
Porous titanium scaffolds in place with load-bearing plates.
During the procedure, the clinically verified difference between the virtual model and the EBM-produced reconstruction plate was insignificant. In reality this difference in precision may, however, be significant because it is impossible during real-time surgery to inspect the plate from all angles to verify the difference in exact terms.
The economic feasibility of using an additive approach for the manufacturing of implants instead of using conventional methods such as milling and turning has been discussed by Cronskär et al.22 Seven customized hip stem implants were manufactured using both EBM and conventional methods, and the EBM-based manufacturing cost reported to reduce 65% of the conventional cost. The reported build time was 36 hours for seven implants, but also that the build time would be extended only by 2 hours for a build with 14 implants. A batch of four reconstruction plates will most probably yield a high cost for each plate, but by running the EBM process at full capacity, that is, by maximizing the number of parts on the start plate area, the price for each manufactured part will decrease rapidly.
Acknowledgement
This study was supported by grants from Uppsala County Council, Sweden, and from the Swedish Agency for Economic and Regional Growth – Tillväxtverket, European Regional Development Fund.
References
- 1.Juergens P, Krol Z, Zeilhofer H F. et al. Computer simulation and rapid prototyping for the reconstruction of the mandible. J Oral Maxillofac Surg. 2009;67(10):2167–2170. doi: 10.1016/j.joms.2009.04.104. [DOI] [PubMed] [Google Scholar]
- 2.Hirsch D L, Garfein E S, Christensen A M, Weimer K A, Saddeh P B, Levine J P. Use of computer-aided design and computer-aided manufacturing to produce orthognathically ideal surgical outcomes: a paradigm shift in head and neck reconstruction. J Oral Maxillofac Surg. 2009;67(10):2115–2122. doi: 10.1016/j.joms.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 3.Eggers G, Mühling J, Hofele C. Clinical use of navigation based on cone-beam computer tomography in maxillofacial surgery. Br J Oral Maxillofac Surg. 2009;47(6):450–454. doi: 10.1016/j.bjoms.2009.04.034. [DOI] [PubMed] [Google Scholar]
- 4.Leiggener C, Messo E, Thor A, Zeilhofer H F, Hirsch J M. A selective laser sintering guide for transferring a virtual plan to real time surgery in composite mandibular reconstruction with free fibula osseous flaps. Int J Oral Maxillofac Surg. 2009;38(2):187–192. doi: 10.1016/j.ijom.2008.11.026. [DOI] [PubMed] [Google Scholar]
- 5.Dérand P, Hirsch J M. Virtual bending of mandibular reconstruction plates using a computer-aided design. J Oral Maxillofac Surg. 2009;67(8):1640–1643. doi: 10.1016/j.joms.2009.03.039. [DOI] [PubMed] [Google Scholar]
- 6.Wang D, Shi L, Chu W C, Cheng J C, Heng P A. Segmentation of human skull in MRI using statistical shape information from CT data. J Magn Reson Imaging. 2009;30(3):490–498. doi: 10.1002/jmri.21864. [DOI] [PubMed] [Google Scholar]
- 7.Christensen A Lippincott A Kircher R Qualification of Electron Beam Melted (EBM) Ti6Al4V-ELI for Orthopaedic Implant Application Available at; www.medicalmodeling.com/ebm/ 2010–02–25
- 8.Murr L E, Quinones S A, Gaytan S M. et al. Microstructure and mechanical behavior of Ti-6Al-4V produced by rapid-layer manufacturing, for biomedical applications. J Mech Behav Biomed Mater. 2009;2(1):20–32. doi: 10.1016/j.jmbbm.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 9.Palmquist A, Lindberg F, Emanuelsson L, Brånemark R, Engqvist H, Thomsen P. Morphological studies on machined implants of commercially pure titanium and titanium alloy (Ti6Al4V) in the rabbit. J Biomed Mater Res B Appl Biomater. 2009;91(1):309–319. doi: 10.1002/jbm.b.31404. [DOI] [PubMed] [Google Scholar]
- 10.Rännar L-E, Glad A, Gustafson C-G. Efficient cooling with tool inserts manufactured by electron beam melting. Rapid Prototyping J. 2007;13:128–135. [Google Scholar]
- 11.Xia W, Lindahl C, Lausmaa J. et al. Biomineralized strontium-substituted apatite/titanium dioxide coating on titanium surfaces. Acta Biomater. 2010;6(4):1591–1600. doi: 10.1016/j.actbio.2009.10.030. [DOI] [PubMed] [Google Scholar]
- 12.Kernan B T, Wimsatt J A. Use of a stereolithography model for accurate, preoperative adaptation of a reconstruction plate. J Oral Maxillofac Surg. 2000;58(3):349–351. doi: 10.1016/s0278-2391(00)90071-5. [DOI] [PubMed] [Google Scholar]
- 13.Schmidt J G Berti G Fingberg J Cao J Wollny G A Finita Element Based Tool Chain for the Planning and Simulation of Maxillo-Facial Surgery European Congress on Computational Methods in Applied Sciences and Engineering, 2004
- 14.Yeshwant K, Seldin E B, Gateno J. et al. Analysis of skeletal movements in mandibular distraction osteogenesis. J Oral Maxillofac Surg. 2005;63(3):335–340. doi: 10.1016/j.joms.2004.06.057. [DOI] [PubMed] [Google Scholar]
- 15.Robiony M, Salvo I, Costa F. et al. Virtual reality surgical planning for maxillofacial distraction osteogenesisn: the role of reverse engineering rapid prototyping and cooperative work. J Oral Maxillofac Surg. 2007;65:1198–1208. doi: 10.1016/j.joms.2005.12.080. [DOI] [PubMed] [Google Scholar]
- 16.Rodt T, Bartling S O, Zajaczek J E. et al. Evaluation of surface and volume rendering in 3D-CT of facial fractures. Dentomaxillofac Radiol. 2006;35(4):227–231. doi: 10.1259/dmfr/22989395. [DOI] [PubMed] [Google Scholar]
- 17.Zachow S, Hierl T, Erdmann B. On the predictability of tissue changes for osteotomy planning in maxillofacial surgery: a comparison with postoperative results. Int Congr Ser. 2004;1268:648–653. [Google Scholar]
- 18.Westermark A, Zachow S, Eppley B L. Three-dimensional osteotomy planning in maxillofacial surgery including soft tissue prediction. J Craniofac Surg. 2005;16(1):100–104. doi: 10.1097/00001665-200501000-00019. [DOI] [PubMed] [Google Scholar]
- 19.Azari A, Nikzad S. Flapless implant surgery: review of the literature and report of 2 cases with computer-guided surgical approach. J Oral Maxillofac Surg. 2008;66(5):1015–1021. doi: 10.1016/j.joms.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 20.Zinser M J, Mischkowski R A, Durond M, Zoeller J E. Computer-assisted orthognathic surgery based on 3D cephalometry: a new approach with 3D surgical wavers. J Oral Maxillofac Surg. 2007;65 01:42000–420000. [Google Scholar]
- 21.Kokemueller H, Spalthoff S, Nolff M. et al. Prefabrication of vascularized bioartificial bone grafts in vivo for segmental mandibular reconstruction: experimental pilot study in sheep and first clinical application. Int J Oral Maxillofac Surg. 2010;39(4):379–387. doi: 10.1016/j.ijom.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 22.Cronskär M Rännar L-E Koptioug A Bäckström M Application of Electron Beam Melting to Titanium Hip Stem Implants 2nd International Congress on Additive Technologies, Ptuj, Slovenia, September 17–18; 2008