Introduction
Infertility affects 10–15% of all couples, with infertility attributable to the male partner accounting for 30–40% of all infertility (Jequier et al. 1993). The most common form of male infertility involves impairment in spermatogenesis, which is reflected in an abnormal semen analysis (deKretser 1997). Most frequently, this manifests by a decreased sperm concentration, decreased sperm motility and/or abnormal sperm morphology, with each additional abnormality multiplying the likelihood of infertility (Guzick et al. 2001). Unfortunately, over 80% of men with infertility due to abnormal semen quality do not have a medically treatable cause, such as a gonadotropin deficiency. In such men, techniques such as in vitro fertilization or testicular sperm extraction coupled with intracytoplasmic sperm injection offer some hope of fertility; however, these procedures are invasive, expensive, and unsuccessful in some cases (Schlegel et al. 2009). Novel approaches to improving sperm quality that allow infertile men to father a pregnancy without assisted reproductive techniques are needed.
The necessary role of retinoic acid in spermatogenesis has been appreciated in animal models (Koubova et al. 2006; Doyle et al. 2007; Anderson et al. 2008; Chung et al. 2009). Retinoic acid functions within the nucleus of cells by activating three types of retinoic acid receptors: RARα , RARβ, and RARγ (Ghyselinck et al. 2006). Retinoic acid receptors are present in both Sertoli cells and developing germ cells (Dufour & Kin 1999), and knockouts of any of the three main retinoic acid receptors results in infertility due to a breakdown in spermatogenesis (Kastner et al. 1996; Lohnes et al. 1993; Lufkin et al. 1993). Retinoic acid plays several roles in spermatogenesis, including necessary functions in spermatogonial differentiation, spermatid adhesion to Sertoli cells and spermiation (Chung et al. 2009). Although the exact molecular mechanisms by which retinoic acid mediates these effects have yet to be completely elucidated, it is thought that retinoic acid controls spermatogonia differentiation by influencing both the somatic and the germ cell compartments of the seminiferous epithelium (Gely-Pernot et al. 2012). The central role of neonatal retinoic acid exposure on adult spermatogenesis was recently shown in a study where exposure of neonatal, but not adult mice, to retinoic acid resulted in synchronized adult spermatogenesis (Snyder et al. 2011).
Retinol (vitamin A) is the main circulating retinoid, but it is biologically inactive and requires conversion to its active metabolite retinoic acid in tissues for activity. Retinoic acid has been reported to exist as at least five isomers, including all-trans retinoic acid, 9-cis retinoic acid, 13-cis retinoic acid, 9,13 di-cis retinoic acid, and 11-cis retinoic acid (Kunchala et al. 2000). The concentration of retinoic acid in murine testes has been reported to be three times higher than in mouse serum (Vernet et al. 2006), but intratesticular retinoic acid is not derived from the serum as is the case for most tissues. Indeed, less than 1% of the retinoic acid present in the testes comes from the serum (Kurlandsky et al. 1995). Instead, testicular retinoic acid appears to be synthesized from retinol in the testes as both Sertoli cells and germ cells express the necessary alcohol and aldehyde dehydrogenases needed to convert retinol to retinoic acid (Vernet et al. 2006). Indeed, the lumen of the seminiferous tubules may be “protected” from exogenous retinoic acid due to high expression of the retinoic acid metabolizing enzyme CYP26A1 in the peritubular myoid cells (Vernet et al. 2006; Wu et al. 2008). Taken together, these observations suggest that the testes generate the retinoic acid required for spermatogenesis in situ, and that the developing germ cells are otherwise shielded from circulating retinoic acid, allowing for tight local control of intratesticular retinoic acid concentrations.
Given the crucial role of retinoic acid in spermatogenesis, it seems possible that some men with infertility have intratesticular concentrations of retinoic acid below levels necessary to initiate and/or maintain spermatogenesis, either from reduced activity of the enzymes involved in the biosynthesis of retinoic acid from retinol, or due to increased metabolism of intratesticular retinoic acid by CYP26. A relatively low concentration of intratesticular retinoic acid might result in sub-normal semen quality; however, the concentrations of intratesticular retinoic acid in man and their associations with parameters of semen quality in man have not previously been reported. We hypothesized that intratesticular concentrations of retinoic acid would be lower in men with abnormal semen as compared to men with normal semen. Therefore, we conducted a pilot study to determine the intratesticular retinoic acid concentration in man, and to examine the association between intratesticular retinoic acid and sperm concentration, motility and morphology in a convenience sample of men undergoing scrotal or penile surgery to determine if intratesticular retinoic acid differed between men with normal and abnormal semen analyses.
Methods
Subjects
Thirty men, between 18–65 years of age, who were scheduled to undergo previously planned scrotal or penile surgery (e.g. varicocelectomy, hydrocelectomy or resection of a spermatocele) were recruited from the University of Washington Medical Center Urology clinic after expressing interest in the study. After written informed consent was obtained, two pre-operative visits occurred. At the first, a physical examination, including a detailed andrological examination was performed. In addition, serum was obtained for measurement of luteinizing hormone (LH), follicle stimulating hormone (FSH), testosterone, dihydrotestosterone (DHT), all-trans-retinoic acid, 13-cis-retinoic acid, 9-cis retinoic acid and 9,13 di-cis retinoic acid and subjects provided a seminal fluid sample (after a minimum of 48 hours of abstinence) for analysis of sperm concentration, motility and morphology. Subjects had a repeat blood draw and provided another semen sample at a second pre-operative visit within 72 hours of surgery.
On the day of surgery, testicular biopsy tissue was obtained by a board certified urologist from one testis following incision of the tunica albuginea as described previously (Matthiesson et al. 2005). The biopsy was taken using curved scissors to minimize scarring. Approximately 100mg of tissue (roughly the volume of a large pea) was obtained for determination of testicular retinoid concentrations. Each biopsy was immediately frozen to prevent exposure of the sample to light and isomerization of the retinoids in the sample. All subjects were seen for a routine post-op follow-up visit within 6 weeks of surgery. Exclusion criteria include the use of anabolic steroids, illicit drugs or the ingestion of more than 4 alcoholic beverages daily, or the use of drugs known to interfere with hormones or spermatogenesis in man, such as: GnRH analogues, ketoconazole, finasteride, dutasteride, methadone or lithium. The study was approved by the University of Washington Institutional Review Board.
Measurements
Semen analyses were performed following guidelines of the WHO protocol (WHO lab manual 5th edition 2010), in a certified clinical andrology laboratory. Sperm concentration was determined by hemacytometer, and manual and computer-assisted motility analyses were performed. For the computer assisted sperm analysis, six microliters of well-mixed, liquefied semen was loaded into one chamber of a Leja (Leja Products B.V., Nieuw Vennep, Netherlands) standard count 2-chamber, 20 micron deep permanently coverslipped slide. Leja slides were kept at 37°C during loading and analysis. If required, semen with a high sperm concentration was diluted up to 1:3 with HTF-HEPES culture medium. Loaded Leja slides were analyzed in a Hamilton-Thorne Biosciences (Beverly, MA) HTM-IVOS computerized sperm motility analyzer running Version 12 software with Edit Tracks and Sort options. An Olympus 10x (A10NH) negative phase contrast objective was used with a phase contrast condenser. Setup values included: 30 frames acquired at 60 frames per second; 2 pixels minimum cell size; minimum contrast 80; 80% straightness threshold and15 microns per second progressive minimum average path velocity (VAP) identified sperm with progressive motility; slow cells (<5.0 microns per second VAP and <11.0 microns per second straight line velocity (VSL) were counted as motile; illumination was adjusted to provide maximum contrast. Rapid and linear motility was defined using the Sort option as sperm tracks with at least 25 points and VSL ≥ 25.0. Sufficient fields were collected to analyze at least 200 motile sperm. The percent motility (progressive and non-progressive) obtained from manual observation was used for "% Motile". "Percent Progressively Motile" was calculated by the following formula: (IVOS percent progressive / IVOS percent motile) × % Motile. "Percent Rapid and Linear Motility" was calculated by multiplying IVOS rapid and linear motility by "% Motile"/100. Average progressive velocity (VSL, or straight-line velocity) was obtained directly from the IVOS analysis. The following kinetic parameters were included in our analysis: Straight-Line Velocity (VSL), Linearity, which is the best kinematic measures of sperm in semen, % progressive and % rapid and linear motility (Table). Normal semen was defined as having a sperm concentration of greater than 15 million sperm/mL, greater than 40% motility, and greater than 4% normal (strict) morphology. Abnormal semen analyses were defined as failing to meet one or more of these criteria. For the testicular histology, appoximately 25 mg of testicular tissue was placed into Bouin’s fixative for 2–5 hours, rinsed in 70% ethoanol and dehydrated, embedding in Paraplast and sectioned at 4 um sections were made. Sections were stained with Harris hematoxylin and periodic acid-Schiff reagent, and photographed using a 20x bright field objective on a Leitz photomicroscope.
Table.
Sperm parameters, serum reproductive hormone and serum retinoids in study subjects (n=24). Values are expressed as medians and interquartile ranges, stratified by normal (n=17) or abnormal (n=7) semen analyses.
| Normal Semen Analyses (n=17) |
Abnormal Semen Analyses (n=7) |
p- value* |
|
|---|---|---|---|
| Serum Hormones | |||
| Testosterone (ng/ml) |
4.3 (3.1, 6.1) | 4.7 (4.2, 7.7) | 0.37 |
| Dihydrotestosterone (ng/ml) |
0.61 (0.46, 0.97) | 0.63 (0.44, 0.88) | 0.91 |
| Luteinizing hormone (IU/L) |
4.0 (2.9, 4.8) | 5.1 (3.1, 6.5) | 0.27 |
| Follicle stimulating hormone (IU/L) |
2.5 (1.8, 3.4) | 2.6 (2.1, 4.4) | 0.56 |
| Sperm Parameters | |||
| Sperm concentration (millions/ml) |
85 (36, 124) | 22 (0.4, 26) | 0.01 |
| Total sperm count (millions/ejaculate) |
181 (117, 365) | 96 (0.5, 135) | 0.01 |
| Sperm morphology (% strict normal) |
19 (16, 25) | 6 (3, 12) | 0.01 |
| Sperm motility (% forward movement) |
60 (48, 63) | 28 (13, 58) | 0.02 |
| Straight-line velocity (µm/second) |
52 (49, 58) | 36 (0, 51) | 0.03 |
| Progressive motility (%) | 35 (25, 45) | 26 (0, 39) | 0.10 |
| Rapid and linear motility (%) |
19 (13, 28) | 6 (0, 23) | 0.12 |
| Serum Retinoids | |||
| All-trans retinoic acid (nmol/L) |
2.6 (2.1, 3.0) | 3.2 (2.9, 3.3) | 0.06 |
| 13-cis retinoic acid (nmol/L) |
3.3 (3.1, 4.0) | 3.8 (3.0, 4.2) | 0.36 |
By Wilcoxon rank-sum test
Luteinizing hormone, follicle stimulating hormone were measured by immunofluorometric assays (Delfia, Wallac Oy, Turku, Finland). The sensitivity of the assay for FSH and LH are 0.016 IU/L and 0.019 IU/L, respectively with intraassay and interassay coefficients of variation of 3.0% and 5% for FSH and 5.6% and 13% for LH for mid-normal range values. Serum testosterone and DHT were measured using high-performance liquid chromatography-mass spectroscopy on an Aquity UPLC coupled with a Micromass Premiere-XE tandem quadrupole mass spectrometer (Waters Corp., Milford, MA, USA) as described previously (Kalhorn et al. 2007). The lower limit of quantification for all both testosterone and DHT was 0.04 pg/ml. The intra- and inter-assay coefficient of variation for testosterone was 3.5% and 7.7% respectively, and for DHT was 3.5% and 6.3% for mid-normal range values.
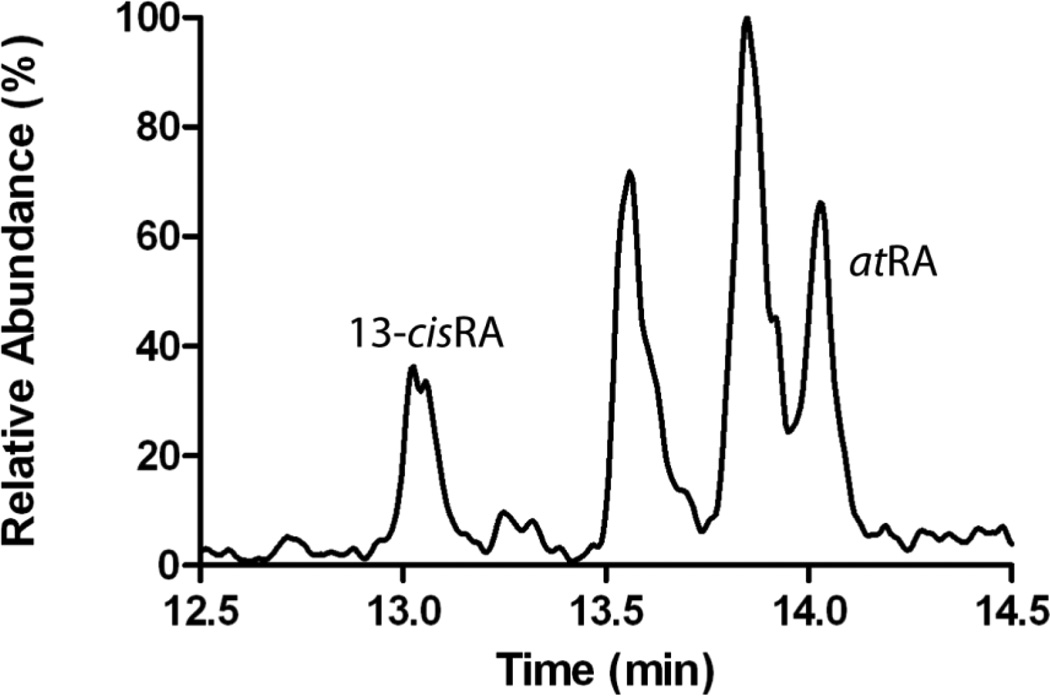
Serum retinoic acid concentrations, including all-trans retinoic acid, 13-cis retinoic acid, 9-cis retinoic acid and 9,13 di-cis retinoic acid were measured by LC-MS/MS as described previously (21). All sample processing, preparation and extraction was conducted on ice under red light. For the measurement of testicular tissue retinoic acid concentrations, 55–114 mg testicular tissue was homogenized using a Precellys 24 bead homogenizer and kept cold with liquid nitrogen using an advanced temperature controller Cryolys (Bertin Technologies, Villeurbanne France). Each tissue sample was homogenized in 500 µL 0.1% NaCl using 2 mL ceramic bead tubes (Omni International, Kennesaw, GA). To each sample, 20 µL of 13-cisRA-d5 (1 µM in 6:4 ACN:H2O mixture) was added as an internal standard. In brief, 60 µl 4 N HCl was added to each sample followed by 500 µL ACN. The samples were vortexed and retinoids were extracted twice with 5 mL of Hexanes. The organic phase was separated by centrifugation at 1000 rpm for 3 minutes and evaporated to dryness under nitrogen stream at 32°C. The dry residue was reconstituted in 60 µL of 6:4 ACN:H2O and transferred to an amber autosampler vial. The retinoic acid isomers were separated using an Ascentis Express RP Amide column (2.7 µm, 150mm × 2.1 mm) (Sigma, St. Louis, MO, USA). Gradient elution with a flow rate of 0.5 mL/min using water (A) and acetonitrile (B) with 40% methanol and 0.1% formic acid in A and B was used. The gradient was from initial 60% (A) for 2 minutes to 45% (A) over 8 minutes and then to 10% (A) over 7 minutes. The column was then washed with 95% (B) for 3 minutes and returned to initial conditions. The column heater was set to 40°C. Samples were kept in the autosampler at 4°C in amber vials with the light turned off. 20 µL of sample was injected for analysis. Analytes were detected using an AB Sciex 5500 qTrap Q-LIT mass spectrometer (Foster City, CA) operated in positive ion APCI mode. Concentrations of all analytes were calculated using a five-point standard curve with concentrations of 0, 0.5, 1, 5, 10, and 15 nM of 9-cisRA, 13-cis RA, and atRA, spiked into 500 µL of blank charcoal stripped human serum. From the standard curve, the retinoid to internal standard peak area ratio for each analyte was plotted as a function of the amount of each retinoid injected on the column. The amount of the retinoids present in samples was calculated from the amount detected in each injection according to the standard curve multiplied by the total sample amount. To determine the concentration in the tissue sample, the total amount of the retinoid in the sample was divided by the tissue amount used from each biopsy in sample preparation. Intraday assay variability and retinoid recovery were determined using dog testicular homogenate spiked with retinoids. The intraday coefficient of variation was below 15% for atRA and 13-cisRA. The recovery of retinoids from testicular homogenate was 49% for atRA and 47% for 13-cisRA. Blood counts, serum chemistries, and liver function tests were measured by the University of Washington clinical laboratory.
Statistical Analysis
Due to non-normal distributions, hormone and retinoic acid concentrations and sperm parameters were expressed as medians, with 25th and 75th percentiles included in parentheses. Values from each subject’s two semen samples were averaged prior to analysis. For serum retinoic acid and reproductive hormones, the sample obtained nearest surgery was used for analysis. Differences in sperm parameters, serum reproductive hormone concentrations and retinoid concentrations between men with normal and abnormal semen analyses were compared with a Wilcoxon rank-sum test. Correlations between intratesticular retinoids and sperm parameters were performed using Spearman’s technique. For all comparisons, a two-sided alpha of <0.05 was considered significant. Statistical analyses were performed using STATA version 10.0 (College Park, TX).
Results
Subjects
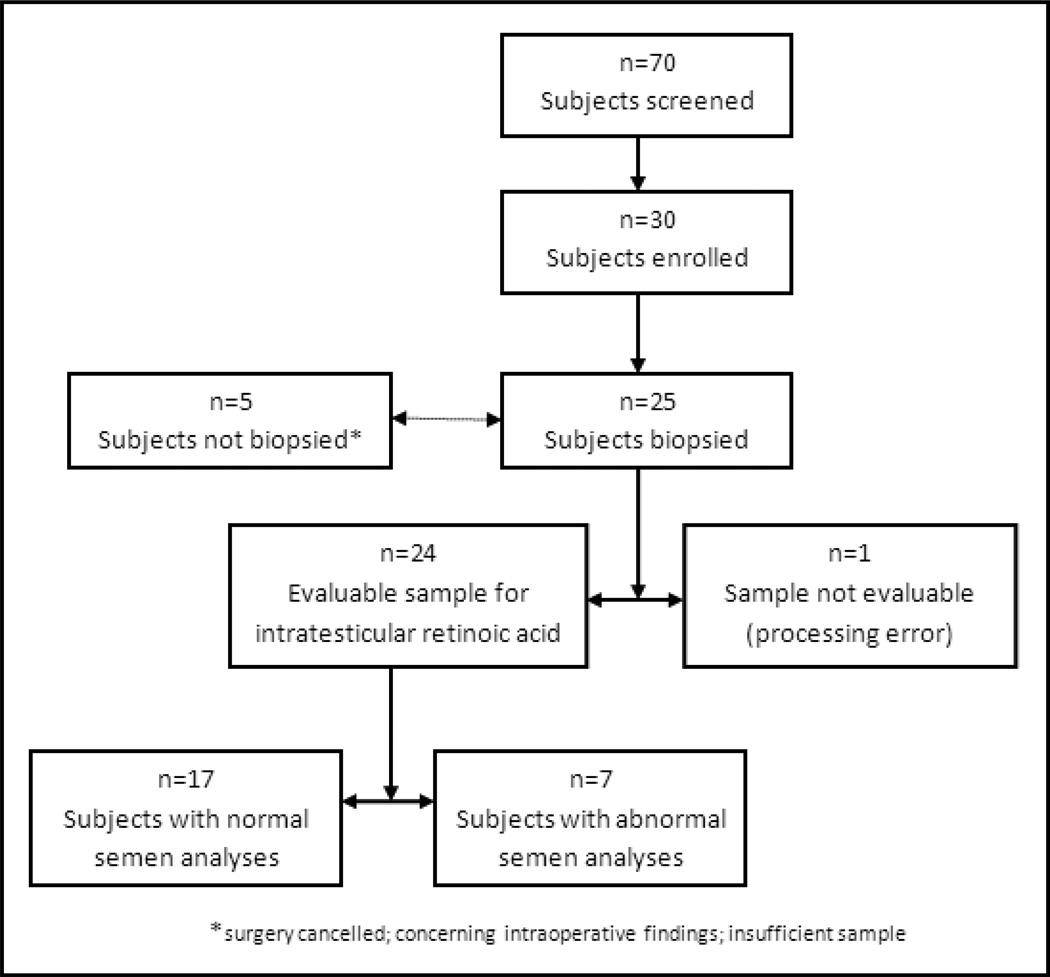
Seventy men undergoing scrotal or penile surgery for a variety of benign indication not directly related to male infertility were screened for participation in the study. Thirty were eligible and enrolled in the study (Figure 2). Reasons for non-participation included: the presence of exclusion criteria, difficulty scheduling the preoperative study visits, inability to provide the semen samples in clinic, and unwillingness to undergo a testicular biopsy during their planned surgical procedure. Twenty-five of the thirty enrolled subjects underwent a successful testicular biopsy at the time of surgery. Four enrolled subjects did not undergo testicular biopsy due to cancellation of the procedure (3 subjects) or intra-operative findings concerning for cancer (1 subject), and a fifth subject had insufficient testicular tissue. Due to a processing error, tissue from one subject remained at room temperature for 30 minutes before it was frozen in liquid nitrogen. The intratesticular retinoic acid concentration from this subject was several orders of magnitude higher than any other sample. As a result, values from this subject were not included in the final analysis. Therefore, analyses focused on the remaining twenty-four subjects. Of these twenty-four men, eleven had a varicocelectomy, six had a hydrocelectomy, three had a spermatocelectomy, three had adult circumcisions, and one had a vasectomy. All procedures were performed with the patient under general anesthesia. No subject was lost to follow-up. No adverse events related to the testicular biopsy occurred and routine lab testing revealed no abnormalities in study subjects.
Semen Analyses and Testicular Histology
Seven men had abnormal semen analyses as defined by the presence of one of the following: sperm concentration of less than 15 million sperm/ml, sperm motility of less than 50% or strict sperm morphology of less than 4%. Seventeen men were normal by all three sperm criteria. When stratified as normal or abnormal, these groups were significantly different in terms of sperm concentration, morphology, and motility, including straight-line velocity, % progressive motility and % rapid and linear motility (Table). Five of the seven men (72%) with abnormal semen were undergoing varicocelectomy and two were undergoing hydrocelectomy. Of the seven men with abnormal semen analyses, four had only one of the three criteria that were abnormal (two with abnormal motility of 13% and 40% and two with abnormal morphology of 3% and 1%) and one had two abnormal criteria (concentration of 1.1 million sperm/ml of ejaculate and motility of 28%). Two men were abnormal in all three criteria, one of whom was azoospermic, and one of whom had severe oligoteratoasthenozoospermia with an average sperm concentration of 400,000 sperm/ml of ejaculate, an average sperm motility of 28% and an average normal morphology of 1% when both semen samples were combined. Interestingly, in the operating room, the subject with azoospermia was found to have very large (>300 cc) bilateral hydroceles. Histological analysis of his testicular tissue from this subject revealed normal spermatogenesis, implying that his azoospermia was obstructive in nature. Testicular histology from the other twenty-three subjects also demonstrated complete spermatogenesis in every case (data not shown).
Serum Retinoic Acid and Reproductive Hormones
There were no significant differences in serum reproductive hormones between men with normal and abnormal semen analyses (Table). All subjects had serum gonadotropin values within the normal range, and serum hormones were normal in all men except one with a slightly elevated testosterone and one with a slightly reduced testosterone. Both of these men had normal semen analyses. There was a trend towards a lower serum all-trans retinoic acid concentration in men with normal semen analyses. Serum all-trans retinoic acid was 2.6 (2.1, 3.0) nmol/L in men with normal semen analyses compared with 3.2 (2.9, 3.3) nmol/L in men with abnormal semen analyses (p=0.06). Otherwise, the serum concentrations of 13-cis retinoic acid, 9-cis retinoic acid and 9,13 di-cis retinoic acid were similar between groups (Table). There were no significant correlations between serum retinoids and sperm concentration, count motility or morphology in the semen samples.
Intratesticular Retinoic Acid
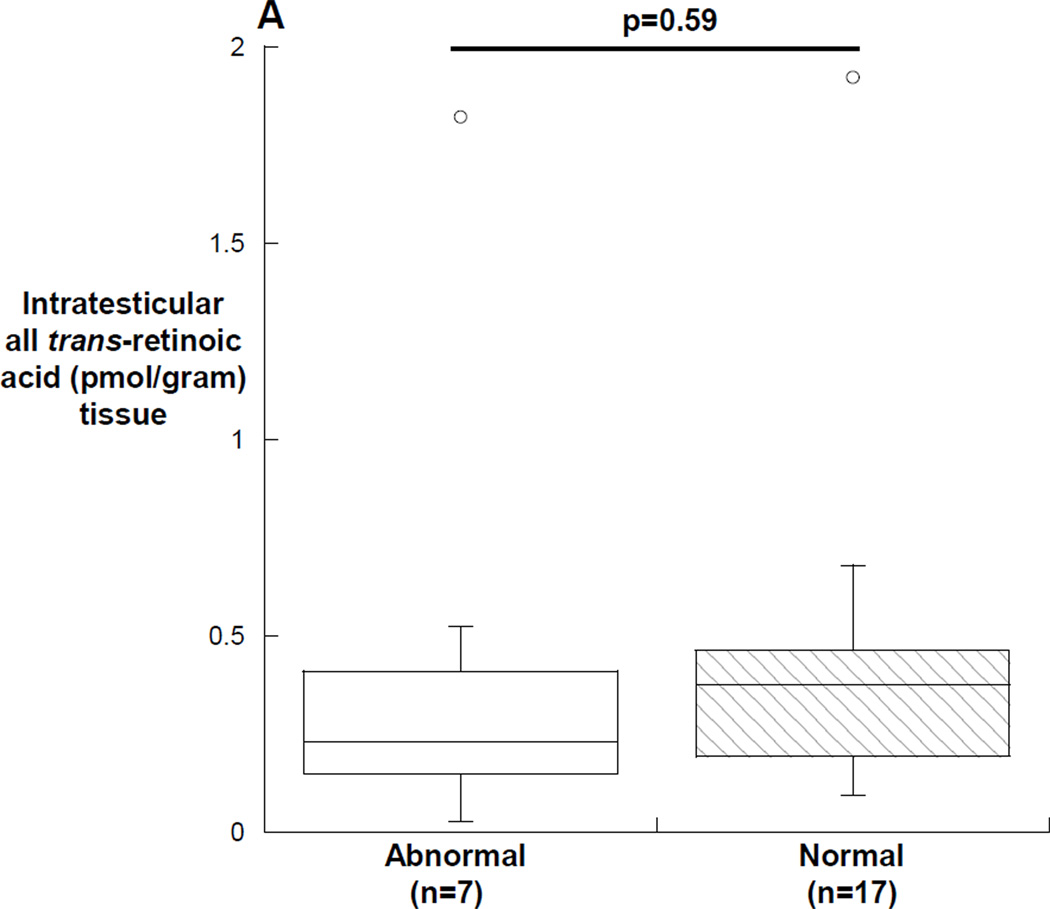
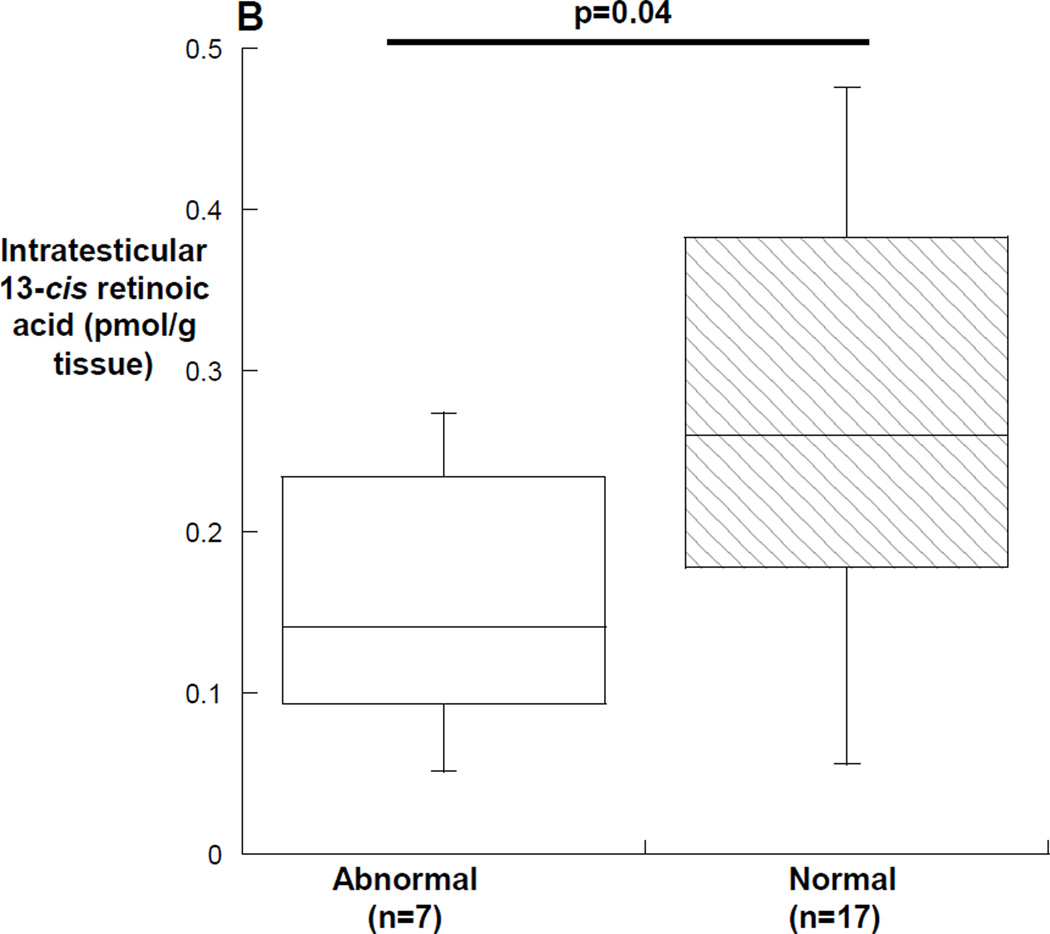
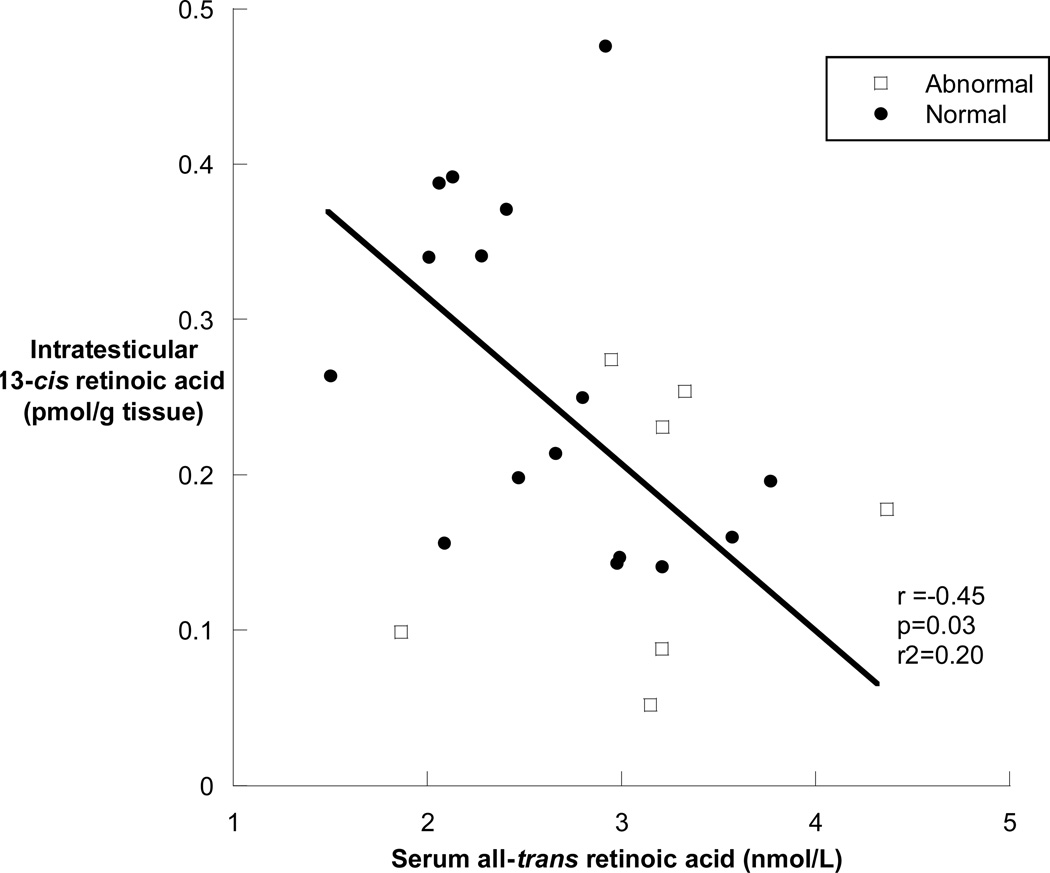
In men with abnormal semen analyses, the median (25th, 75th percentile) all-trans retinoic acid was 0.23 (0.12, 0.42) pmol/gram tissue in men with abnormal semen analyses compared with 0.37 (0.19, 0.46) pmol/gram tissue in men with normal semen analyses (p=0.59) (Figure 3A). The median (25th, 75th percentile) intratesticular 13-cis retinoic acid was 0.14 (0.08, 0.25) pmol/gram tissue in men with abnormal semen analyses compared with 0.26 (0.18, 0.38) pmol/gram tissue in men with normal semen analyses (p=0.04) (Figure 3B). Of the subjects with abnormal semen analyses, the subjects with concentrations of intratesticular 13-cis retinoic acid below 0.1 pmol/gram of tissue all had impaired sperm motility (18%, 28% and 33%), and the subject with the lowest intratesticular 13-cis retinoic acid concentration had both impaired motility (18%) and severe oligozoospermia with an average sperm concentration of 1.1 million sperm/ml of ejaculate. In contrast, the subject with azoospermia and the subject with severe oligoteratoasthenozoospermia had intratesticular 13-cis and all-trans retinoic acid concentrations that were near the medians of the group of men with normal spermatogenesis. Men having surgery for varicocele repair had significantly lower intratesticular 13-cis retinoic acid concentrations compared to men undergoing surgery for other reasons (p=0.01), but there was no similar difference in intratesticular concentrations of all-trans retinoic acid by indication for surgery. Lastly, there were no significant correlations between intratesticular retinoic acid and sperm concentration, count motility or morphology in the semen samples; however, there was a significant inverse correlation between the intratesticular 13-cis retinoic acid concentration and the concentration of all-trans retinoic acid in the serum of subjects (r= −0.45, p=0.03) (figure 4).
Discussion
Despite its central role in spermatogenesis, the concentrations of intratesticular retinoic acid in man have never previously been reported. We measured intratesticular retinoic acid concentrations in a sample of men undergoing scrotal or penile surgery in whom the risk of testicular biopsy was minimal. We hypothesized that intratesticular concentrations of retinoic acid would be lower in men with abnormal semen as compared to men with normal semen. We observed that intratesticular 13-cis retinoic acid concentration were significantly lower in men with abnormal semen compared to men with normal semen, but intratesticular all-trans retinoic acid did not differ between these groups. The fact that the intratesticular all-trans retinoic acid concentrations were similar between groups implies that the lower concentrations of intratesticular 13-cis retinoic acid observed in men with poor sperm quality were not likely due to differences in the average number of spermatozoa between groups. Instead, this finding suggests that the relatively lower intratesticular 13-cis retinoic acid concentrations observed in the men with abnormal semen analysis may play a role in their poor sperm quality. Interestingly, the two subjects with the poorest sperm quality had relatively normal intratesticular retinoic acid concentrations. Therefore, these men appear to have a defect in spermatogenesis this is independent of the retinoid pathway. Indeed, the subject with azoospermia and very large bilateral hydroceles had normal spermatogenesis on histological examination of his testicular tissue, demonstrating that his azoospermia was obstructive in nature, and probably independent of his intratesticular retinoic acid concentration.
Whether the reduced intratesticular 13-cis retinoic acid in men with abnormal semen analyses is due to decreased biosynthesis or increased metabolism is unknown, but will be the focus of future study. In addition, future studies focusing on the intratesticular concentration of retinoic acid in men presenting with complaints of infertility and more severe impairments of spermatogenesis such as non-obstructive azoospermia will be necessary to determine if these differences are more significant in such men than those observed here. Lastly, our results suggest that certain types of infertility, such as defects in sperm motility, may be more strongly associated with reduced concentrations of intratesticular retinoic acid.
Interestingly, the concentrations of retinoic acid we observed in human testicular tissues were ten times lower than those previously reported in murine testes (Kane et al. 2008). It is unknown whether this is due to variation between species or differences in assay methodology. Interestingly, we did note that a delay in freezing the testicular tissue, as occurred in one sample in our study, resulted in a markedly increased tissue concentrations of retinoic acid. Possibly, this is due to impaired tissue metabolism of retinoic acid when samples are exposed to light and room temperature for more than a brief period of time. Future research will be conducted to better understand the intratesticular retinoic acid concentrations in a variety of species to determine whether intra-species differences are really this large, or whether the observed difference is due to other factors.
Our study has some limitations. Firstly, observational studies can only report associations, and cannot demonstrate causality. The finding that there are relatively lower concentrations of 13-cis retinoic acid in men with abnormal semen analyses could be secondary to other changes in the testes of men with abnormal semen. In addition, our sample size is limited due to the difficulty of obtaining human testicular tissue from men. Due to the preliminary nature of this observational study, care must be taken to not draw a definite correlation between lower intratesticular 13-cis retinoic acid and abnormal semen analyses. Nevertheless, this study provides novel information on the expected ranges and variances of intratesticular retinoic acid in men, something not previously reported. In addition, it should be acknowledged that semen analysis is not the perfect indicator of a man’s fertility status, since its measures are limited to features of sperm number, motility and morphology. Further, there are no strictly defined or verified minimum values for these measures in relation to fertility. Lastly, some sperm functions required for fertilization such as acrosome reactivity or fertilizing capacity are not measured in the semen analysis.
Previous investigators have sought to determine if known testicular products such as inhibin-B, which is a protein product of Sertoli cells, have diagnostic significance in infertile men. Indeed, a positive correlation between serum inhibin-B and sperm concentration has been noted (Jensen et al. 1997); however, there is no strong evidence that a single serum inhibin-B concentration can be used as a predictive marker of persistent spermatogenesis in patients with non-obstructive azoospermia. In contrast, one group of investigators found that a seminal plasma inhibin-B concentration was a useful predictor of the presence of testicular sperm in men with non-obstructive azoospermia (Nagata et al. 2005). Our future study of intratesticular retinoids in infertile men will examine these associations. In addition, there has been great interest in determining if differences in seminal proteins are present between fertile and infertile men. One study found that 45 proteins were increased and 56 proteins decreased in the semen of 38 asthenozoospermic men as compared to 20 normal controls (Wang et al., 2009). None of these reported proteins, however, appear to be related to retinoic acid synthesis, metabolism or function.
Ultimately, this work is directed at understanding whether a relative deficiencies intratesticular retinoic acid might contribute to male infertility. Given the crucial role of retinoic acid in spermatogenesis, it seems possible that some men with infertility have intratesticular concentrations of retinoic acid below those necessary for normal spermatogenesis. Low intratesticular concentrations retinoic acid could either be due to dietary deficiency or problems in biosynthesis or transport, or could be due to increased metabolism within the testes. Intriguingly, during the development of 13-cis retinoic acid for the treatment of acne, three small human studies examining the effect of long-term oral administration of 13-cis retinoic acid on sperm production in men were performed. Men in these studies exhibited improved sperm quality during treatment suggesting that 13-cis retinoic acid administration enhanced spermatogenesis in these men (Vogt et al. 1985; Torok et al. 1987; Hoting et al. 1992). If the administration of retinoic acid was shown to improve spermatogenesis in infertile men, it would have tremendous significance for our current approach to the treatment of male infertility. Although this is purely speculative, retinoic acid therapy could be incorporated into male infertility treatment algorithms as it is already approved for use in humans, potentially improving a man’s chances of successfully fathering a pregnancy without the need assisted reproductive technologies that are invasive, expensive and may increase the risk of birth defects (Davies et al. 2012).
In conclusion, we have observed that intratesticular 13-cis retinoic acid is significantly lower in men with abnormal semen analyses compared to men with normal semen analyses, but there is no significant difference in intratesticular all-trans retinoic acid between these groups. Additional study of mechanisms underlying these differences and study of intratesticular retinoic acid in larger populations of infertile men may provide insight into whether relative deficiencies of intratesticular retinoic acid may play a role in male infertility.
Acknowledgements
The authors would like to acknowledge Ms. Dorothy McGuiness for performing the hormone assays, and Ms. Erin R. Pagel and Ms. Heather Christianson of the Male Fertility Laboratory for performing the semen analyses. The authors would like to thank William J. Bremner MD, PhD and David W. Amory Sr. MD, PhD for critical review of the manuscript.
Research Support: This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, a division of the National Institute of Health through cooperative agreements #U01 HD060408, U54 HD 04245 and, in part, by NIH grant R01GM081569-02S1.
Footnotes
Disclosure Statement: No conflicts of interest to disclose
References
- Anderson EL, Baltus AE, Roepers-Gajadein HL, Hassold TJ, de Rooij DG, van Pelt AM, et al. Stra8 and its inducer, retinoic acid, regulate meiotic initiation in both spermatogenesis and oogenesis in mice. Proc Natl Acad Sci USA. 2008;105:14976–14980. doi: 10.1073/pnas.0807297105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold SLM, Amory JK, Walsh TJ, Isoherranen N. A sensitive and specific method for measurement of multiple retinoids in human serum with UHPLC-MS/MS. J Lipid Res. 2012;53:587–598. doi: 10.1194/jlr.D019745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung SSW, Wang W, Wolgemuth DJ. Expression of retinoic acid receptor alpha in the germline is essential for proper cellular association and spermiogenesis during spermatogenesis. Development. 2009;136:2091–2100. doi: 10.1242/dev.020040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies MJ, Moore VM, Wilson KJ, Van Essen P, Priest K, Scott H, et al. Reproductive technologies and the risk of birth defects. N Engl J Med. 2012;366:1803–1813. doi: 10.1056/NEJMoa1008095. [DOI] [PubMed] [Google Scholar]
- deKretser DM. Male Infertility. Lancet. 1997;349:787–790. doi: 10.1016/s0140-6736(96)08341-9. [DOI] [PubMed] [Google Scholar]
- Doyle TJ, Braun KW, McLean DJ, Wright RW, Griswold MD, Kim KH. Potential functions of retinoic acid receptor A in Sertoli cells and germ cells during spermatogenesis. Ann N Y Acad Sci. 2007;1120:114–130. doi: 10.1196/annals.1411.008. [DOI] [PubMed] [Google Scholar]
- Dufour JM, Kim KH. Cellular and subcellular localization of six retinoid receptors in rat testis during postnatal development: identification of potential heterodimeric receptors. Biol Reprod. 1999;61:1300–1308. doi: 10.1095/biolreprod61.5.1300. [DOI] [PubMed] [Google Scholar]
- Gely-Pernot A, Raverdeau M, Celebi C, Dennefeld C, Feret B, Klopfenstein M, et al. Spermatogonia Differentiation Requires Retinoic Acid Receptor γ. Endocrinology. 2012;153:438–449. doi: 10.1210/en.2011-1102. [DOI] [PubMed] [Google Scholar]
- Ghyselinck NB, Vernet N, Dennefeld C, Giese N, Nau H, Chambon P, et al. Retinoids and spermatogenesis: lessons from mutant mice lacking the plasma retinol binding protein. Dev Dyn. 2006;235:1608–1622. doi: 10.1002/dvdy.20795. [DOI] [PubMed] [Google Scholar]
- Guzick DS, Overstreet JW, Factor-Litvak P, Brazil CK, Nakajima ST, Coutifaris C, et al. Sperm morphology, motility, and concentration in fertile and infertile men. N Engl J Med. 2001;345:1388–1393. doi: 10.1056/NEJMoa003005. [DOI] [PubMed] [Google Scholar]
- Hoting VE, Schutte B, Schirren C. Isotretinoin and acne conglobata: andrological evaluations. Fortschur Med (German) 1992;23:427–430. [PubMed] [Google Scholar]
- Jensen TK, Andersson AM, Hjollund NH. Inhibin B as a serum marker of spermatogenesis: Correlation to differences in sperm concentration and follicle-stimulating hormone levels. A study of 349 Danish men. J Clin Endocrinol Metab. 1997;82:4059–4063. doi: 10.1210/jcem.82.12.4456. [DOI] [PubMed] [Google Scholar]
- Jequier AM, Holmes SC. Primary testicular disease presenting as azoospermia or oligozoospermia in an infertility clinic. Br J Urol. 1993;71:731–735. doi: 10.1111/j.1464-410x.1993.tb16075.x. [DOI] [PubMed] [Google Scholar]
- Kane MA, Folias AE, Wang C, Napoli J. Quantitative profiling of endogenous retinoic acid in vivo and in vitro by tandem mass spectroscopy. Anal Chem. 2008;80:1702–1708. doi: 10.1021/ac702030f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalhorn TF, Page ST, Howald WN, Mostaghel EA, Nelson PS. Analysis of testosterone and dihydrotestosterone from biological fluids as the oxime derivatives using high-performance liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom. 2007;21:3200–3206. doi: 10.1002/rcm.3205. [DOI] [PubMed] [Google Scholar]
- Kastner P, Mark M, Leid M, Gansmuller A, Chin W, Grondona JM, et al. Abnormal spermatogenesis in RAR beta mutant mice. Genes Dev. 1996;10:80–92. doi: 10.1101/gad.10.1.80. [DOI] [PubMed] [Google Scholar]
- Koubova J, Menke D, Zhou Q, Capel B, Griswold MD, Page DC. Retinoic acid regulates sex-specific timing of meiotic initiation in mice. Proc Natl Acad Sci USA. 2006;103:2472–2479. doi: 10.1073/pnas.0510813103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunchala SR, Suzuki T, Murayama A. Photoisomerization of retinoic acid and its influence on regulation of human keratinocyte growth and differentiation. Indian J Biochem Biophys. 2000;37:71–76. [PubMed] [Google Scholar]
- Kurlandsky SB, Gamble MV, Ramakrishnan R, Blaner WS. Plasma delivery of retinoic acid to tissues in the rat. J Biol Chem. 1995;270:17850–17857. doi: 10.1074/jbc.270.30.17850. [DOI] [PubMed] [Google Scholar]
- Lohnes D, Kastner P, Dierich A, Mark M, LeMeur M, Chambon P. Function of retinoic acid receptor gamma in the mouse. Cell. 1993;73:643–658. doi: 10.1016/0092-8674(93)90246-m. [DOI] [PubMed] [Google Scholar]
- Lufkin T, Lohnes D, Mark M, Dierich A, Gorry P, Gaub MP, et al. High postnatal lethality and testis degeneration in retinoic acid receptor alpha mutant mice. Proc Natl Acad Sci USA. 1993;90:7225–7299. doi: 10.1073/pnas.90.15.7225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthiesson KL, Stanton PG, O'Donnell L, Meachem SJ, Amory JK, Berger R, et al. Effects of testosterone and levonorgestrel combined with a 5alpha-reductase inhibitor or gonadotropin-releasing hormone antagonist on spermatogenesis and intratesticular steroid levels in normal men. J Clin Endocrinol Metab. 2005;90:5647–5655. doi: 10.1210/jc.2005-0639. [DOI] [PubMed] [Google Scholar]
- Nagata Y, Fujita K, Banzai J, Kojima Y, Kasima K, Suzuki M, et al. Seminal plasma inhibin-B level is a useful predictor of the success of conventional testicular sperm extraction in patients with non-obstructive azoospermia. J Obstet Gynaecol Res. 2005;31:384–388. doi: 10.1111/j.1447-0756.2005.00306.x. [DOI] [PubMed] [Google Scholar]
- Schlegel PN. Nonobstructive azoospermia: a revolutionary surgical approach and results. Semin Reprod Med. 2009;27:165–170. doi: 10.1055/s-0029-1202305. [DOI] [PubMed] [Google Scholar]
- Snyder EM, Davis JC, Zhou Q, Evanoff R, Griswold MD. Exposure to retinoic acid in the neonatal but not adult mouse results in synchronous spermatogenesis. Biol Reprod. 2011;84:886–893. doi: 10.1095/biolreprod.110.089755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torok L, Kadar L, Kasa M. Spermatological investigations in patients treated with etretinate and isotretinoin. Andrologia. 1987;19:629–633. doi: 10.1111/j.1439-0272.1987.tb01915.x. [DOI] [PubMed] [Google Scholar]
- Vernet N, Dennefeld C, Rochette-Egly C, Oulad-Abdelghani M, Chambon P, Ghyselinck NB, et al. Retinoic acid metabolism and signaling pathways in the adult and developing mouse testes. Endocrinology. 2006;147:96–110. doi: 10.1210/en.2005-0953. [DOI] [PubMed] [Google Scholar]
- Vogt HJ, Ewers R. 13-cis-retinoic acid and spermatogenesis. Der Hautarzt (German) 1995;36:281–286. [PubMed] [Google Scholar]
- Wang J, Wang J, Zhang HR, Shi HJ, Ma D, Zhao HX, et al. Proteomic analysis of seminal plasma from asthenozoospermia patients reveals proteins that affect oxidative stress responses and semen quality. Asian J Androl. 2009;11:484–491. doi: 10.1038/aja.2009.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization Department of Reproductive Health and Research. WHO Laboratory Manual for the Examination and Processing of Human Semen. 5th Edition. Geneva, Switzerland: WHO Press; 2010. [Google Scholar]
- Wu JW, Wang RY, Guo QS, Xu C. Expression of the retinoic acid metabolizing enzymes RALDH2 and CYP26 during mouse postnatal testis development. Asian J Androl. 2008;10:569–577. doi: 10.1111/j.1745-7262.2008.00408.x. [DOI] [PubMed] [Google Scholar]