Abstract
Background:
Hypoxemia is an immediate consequence of obstructive sleep apnea. Oxygen (O2) administration has been used as an alternative treatment in patients with obstructive sleep apnea (OSA) who do not adhere to continuous positive airway pressure (CPAP) in order to reduce the deleterious effects of intermittent hypoxemia during sleep. This systematic review aims to investigate the effects of O2 therapy on patients with OSA.
Method:
We conducted a systematic search of the databases Medline, Embase, Cochrane Central Register of Controlled Trials (1st Quarter 2011), Cochrane Database of Systematic Reviews (from 1950 to February 2011). Our search strategy yielded 4,793 citations. Irrelevant papers were excluded by title and abstract review, leaving 105 manuscripts. We reviewed all prospective studies that included: (1) a target population with obstructive sleep apnea, (2) O2 therapy and/or CPAP as a study intervention, (3) the effects of O2 on the apnea-hypopnea index (AHI), nocturnal hypoxemia, or apnea duration.
Results:
We identified 14 studies including a total of 359 patients. Nine studies were of single cohort design, while 5 studies were randomized control trials with 3 groups (CPAP, oxygen, and placebo/sham CPAP). When CPAP was compared to O2 therapy, all but one showed a significant improvement in AHI. Ten studies demonstrated that O2 therapy improved oxygen saturation vs. placebo. However, the average duration of apnea and hypopnea episodes were longer in patients receiving O2 therapy than those receiving placebo.
Conclusion:
This review shows that O2 therapy significantly improves oxygen saturation in patients with OSA. However, it may also increase the duration of apnea-hypopnea events.
Citation:
Mehta V; Vasu TS; Phillips B; Chung F. Obstructive sleep apnea and oxygen therapy: a systematic review of the literature and meta-analysis. J Clin Sleep Med 2013;9(3):271-279.
Keywords: OSA, CPAP, oxygen therapy
Obstructive sleep apnea (OSA) is the periodic reduction (hypopnea) or cessation (apnea) of airflow due to narrowing of the upper airway during sleep, often accompanied by hypoxemia and sleep disturbance.1 The prevalence of OSA is estimated to be between 2% and 25% in the general population. OSA is linked to hypertension, ischemic heart disease, stroke, premature death, and motor vehicle crash.2–7
Oxygen desaturation is an immediate consequence of obstructive sleep apnea. Intermittent hypoxemia increases sympathetic activity and norepinephrine levels and leads to hypertension.8,9 It has also been associated with an increased risk of diabetes.10 Indeed, most of the sequelae of obstructive sleep apnea are more strongly linked to the degree and duration of oxygen desaturation than to the numbers of apneas and hypopneas or disruptions in sleep architecture.11 The resolution of nocturnal intermittent hypoxemia associated with sleep apnea is a major goal of the treatment of patients with obstructive sleep apnea.
Many treatment approaches have been employed for the treatment of moderate to severe OSA, but CPAP is the treatment of choice and has been widely prescribed.12,13 In placebo-controlled and uncontrolled studies, CPAP has been shown to reduce apnea-hypopnea index (AHI) and to improve hypoxemia associated with respiratory events during sleep.14,15 CPAP adherence has been reported to be as low as 50%, at least in part because it is a burdensome treatment.16
Oxygen administration has been used as an alternative treatment in patients with OSA who are not somnolent or not compliant with CPAP; the purpose of supplemental oxygen in this situation is to reduce the deleterious effects of transient hypoxemia during sleep.17 Supplemental oxygen has been shown to be effective in improving the AHI, respiratory arousal index, and nocturnal desaturation during apneic episodes.18 However, oxygen therapy may lengthen apnea duration, thus accelerating CO2 retention.19
This systematic review aims to investigate the effects of CPAP and oxygen on patients with OSA. This review addresses the following questions: (1) Does evidence from controlled trials support the preferential use of CPAP over oxygen for improving OSA symptoms? (2) Can oxygen therapy be safely used in patients who are non-adherent with CPAP?
METHODS
For purposes of this analysis, the target population consisted of adult humans with a diagnosis of obstructive sleep apnea defined as an AHI > 5 events per hour. The diagnosis of OSA was made using polysomnography (PSG). The study intervention included either the CPAP and oxygen therapy or oxygen therapy compared with the placebo. Outcomes of interest included the effects on AHI, nocturnal hypoxemia, apnea duration, and arousal index.
Literature Search
The literature search was performed according to the PRISMA (Preferred Reporting Items for Systematic reviews and meta-analysis) guidelines.20 The databases Medline, Embase, Cochrane Central Register of Controlled Trials (1st Quarter 2011), Cochrane Database of Systematic Reviews (from 1950 to Feb 2011) were thoroughly searched to include all available evidence for the systematic review. We developed and executed the search strategy with the help of an expert librarian familiar with the literature search protocol of the Cochrane Collaboration. The following target population keywords were used for the literature search: “obstructive sleep apnea,” “obstructive sleep apnea syndrome,” “obstructive sleep apnea-hypopnea syndrome,” “sleep disordered breathing,” “obesity hypoventilation syndrome” and “apnea-hypopnea,” “sleep apnea syndrome and “apnea.” The target intervention keywords used were “oxygen”, “oxygen therapy,” “oxygen inhalational therapy,” “CPAP,” “positive airway pressure,” and “continuous positive airway pressure.” The results of the target population were combined with the target intervention results (using an “and”). Studies focusing on central sleep apnea were excluded by including “NOT central sleep apnea” in the search strategy. The search strategy was limited to English language abstracts and adult human population. Duplicate records, if any were removed from the final search result. We also reviewed the reference lists of relevant articles to retrieve potentially relevant articles.
Medline (Ovid SP) (1948 to Feb 2011)
EMBASE (1980 to Feb 2011)
Cochrane Database of Systematic Reviews (1st quarter 2011)
Cochrane Central Controlled Trials Registry (1st quarter 2011)
The databases of the Cochrane Library were used to confirm the completeness of the search. The time period searched was 1948 to 2011.
Study Selection
The search results were evaluated by two independent reviewers (VM, TSV). First, irrelevant papers were excluded by reviewing the title of the records. Next, the abstract and/or full text articles of the remaining papers were retrieved and carefully evaluated to determine if they met the eligibility criteria.
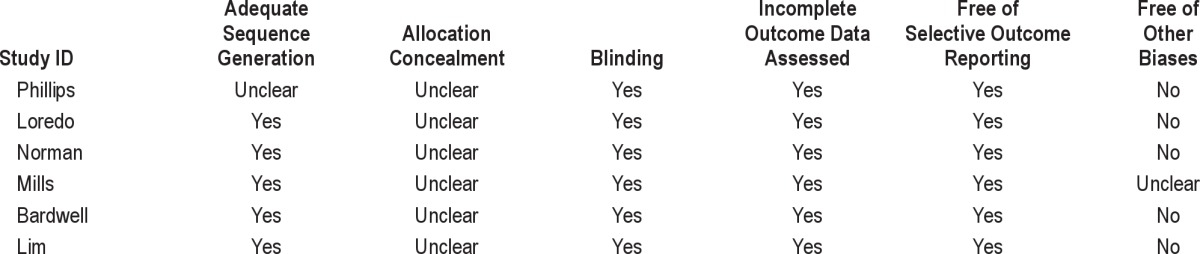
All prospective studies, including randomized and non-randomized placebo controlled trials were included if they reported the effects of CPAP treatment or oxygen therapy on AHI, oxygen saturation, apnea duration, and arousal index in patients with OSA. Studies not reporting at least one of these outcomes were excluded. All observational studies were graded for strength of evidence according to the Oxford level of evidence.21 We used the Cochrane risk of bias tool to assess the risk of bias for 6 randomized controlled trials (Table 1).22
Table 1.
Cochrane risk of bias in included studies
Data Extraction
Data extraction was completed by two reviewers (VM, TSV) and validated by the senior author (FC). Various data extracted from these studies included the type of study, level of evidence, number of patients receiving the study intervention, type of study intervention, duration and effects of intervention on AHI, SpO2, arousal index, and apnea duration. We divided the studies into 2 groups: the first group included studies which used CPAP and O2 treatment; the second group included studies which used only O2 therapy as an intervention. The methodological qualities of the included studies were independently evaluated by the first author (VM), if any doubt the senior author was consulted (FC). Individual authors were contacted via emails for the details of the results
Statistical Analysis
We performed the meta-analyses by using fixed-effects model if no heterogeneity was present. In order to assess the heterogeneity between studies, we used χ2 tests and estimated the I2 statistic. We considered the heterogeneity to be present if the p value on the χ2 test was < 0.05. In the presence of heterogeneity, we pooled the results by using random-effects (DarSemonian and Laird method) model. The standardized mean difference was used to pool continuous variables that used different scales. We performed separate random-effects meta-analyses among randomized controlled studies comparing CPAP, placebo CPAP, and oxygen. We did not correct for multiple comparisons.
RESULTS
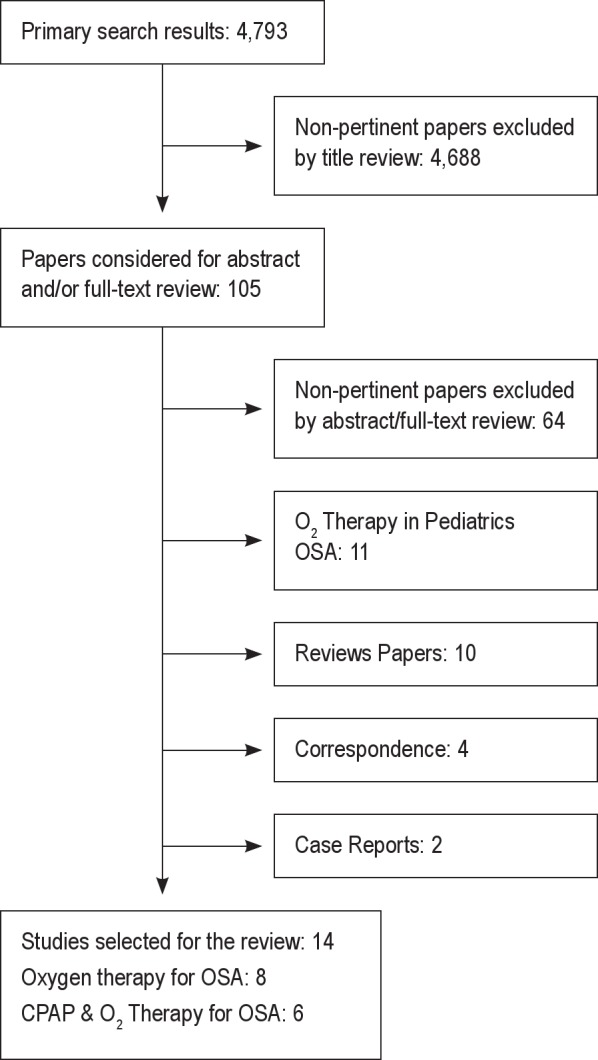
The Preferred Reporting Items for Systematic reviews and Meta-Analysis (PRISMA) guidelines were followed for the description of the search strategy. Our search strategy yielded 4,793 citations (Figure 1). In the first session of screening, most studies were eliminated based on the predetermined eligibility criteria, leaving 105 articles. In the second session, 105 articles were evaluated and 14 articles were identified as meeting the inclusion criteria, with subsequent exclusion of 91 articles. Articles were excluded for the following reasons: Non-pertinent papers—excluded by abstract/full-text review (n = 64), O2 therapy in pediatric OSA (n = 11), reviews papers (n = 10), correspondence (n = 4), and case reports (n = 2).
Figure 1. Flowchart of the literature search indicating number of papers in each condition.
Study Characteristics
Tables 2 and 3 summarize study characteristics included in the systematic review. There were 6 studies23–28 that used a randomized control design with 3 groups, each group being assigned to CPAP, placebo CPAP, or O2 to evaluate the effects of CPAP and O2 on AHI, O2 saturation, and arousal indices. Eight studies29–36 used a single cohort in which the outcome was measured in the same study population before and after the study intervention. All of these observational studies compared the effects of room air with O2 on mean oxyhemoglobin saturation, sleep disordered breathing (SDB) events, and SDB event duration. These 8 studies were graded according to Oxford level of evidence and had a 2b level of evidence. We could not pool the results from the study by Block et al.33 because the authors did not provide the standard deviation for the outcome of interests.
Table 2.
Study characteristics and effects of CPAP vs. oxygen therapy in OSA patients
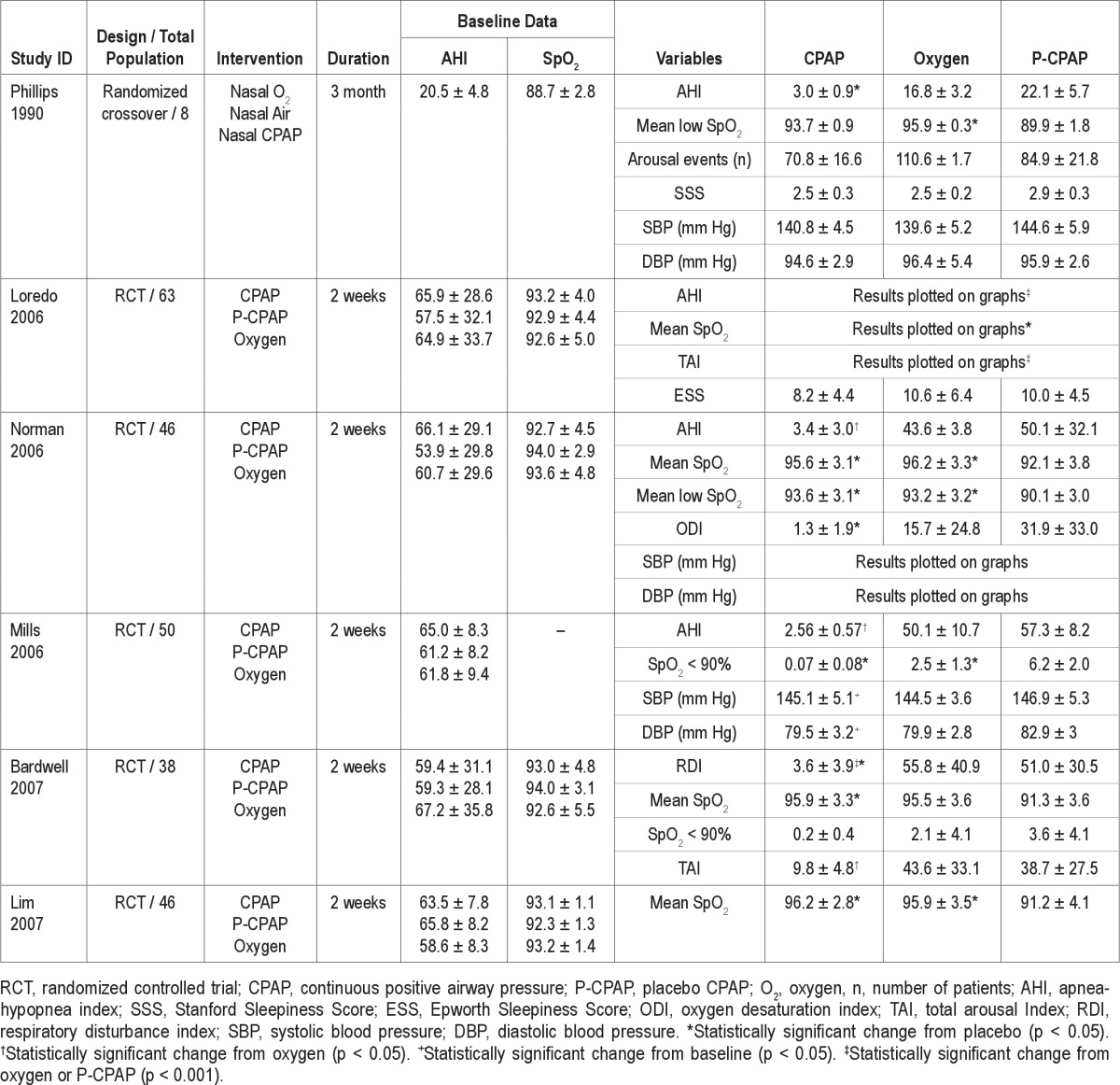
Table 3.
Study characteristics and effects of oxygen therapy in OSA patients
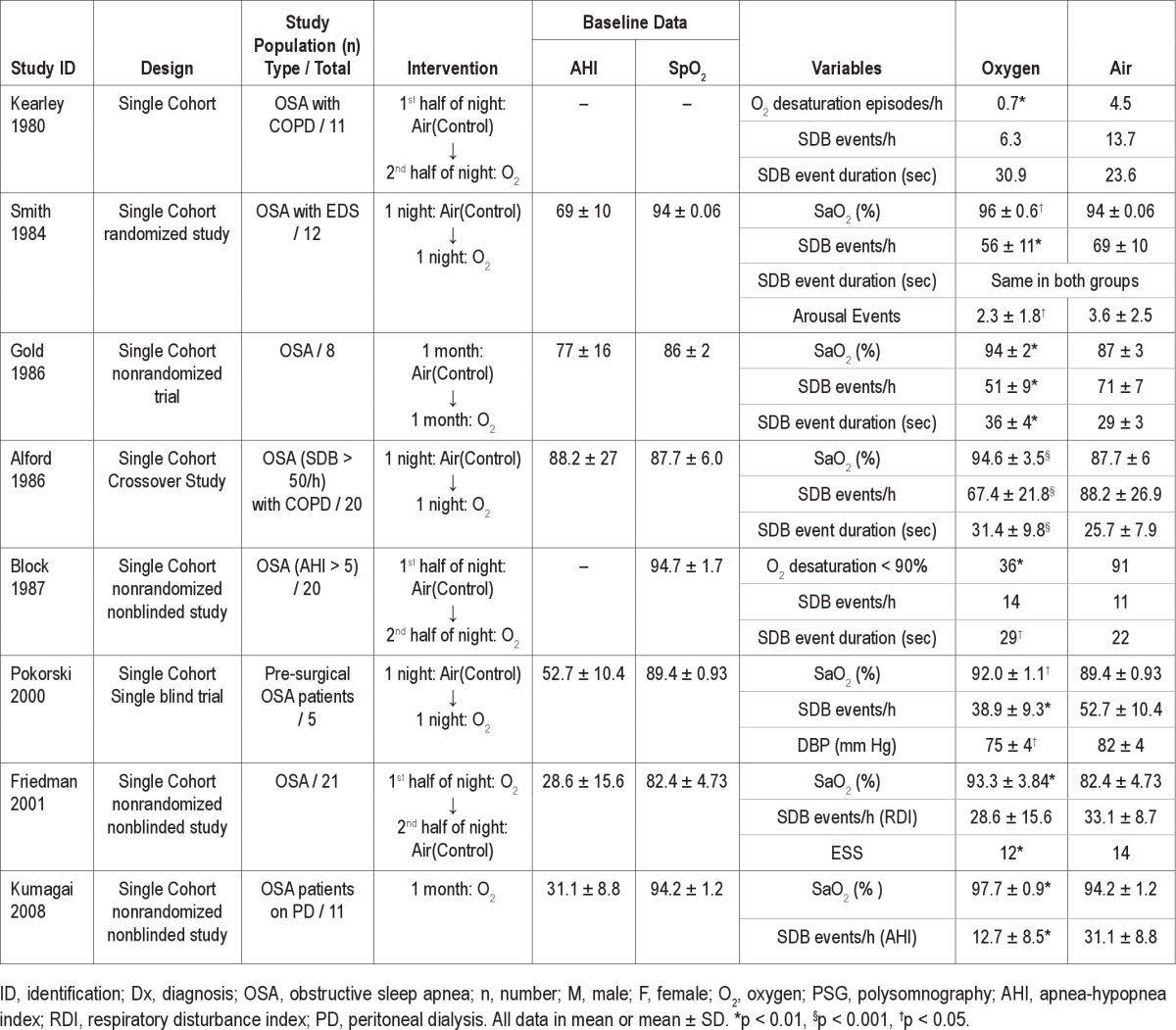
Patient Characteristics
Table 2 represents a group of patients who received CPAP and O2 intervention versus placebo CPAP. Table 3 represents a group of patients who received O2 intervention compared with control (air). A total of 359 patients were included in the 14 studies. All the patients had a diagnosis of OSA confirmed by in-laboratory polysomnography. The inclusion criteria of the patients differed among the studies selected for the review. Two studies23,33 used AHI > 5; 5 studies24–28 used AHI > 15; one study32 used SDB event > 50/h, and one study35 used RDI > 20 for OSA patient selection. Four studies30,31,34,36 selected patients with a confirmed diagnosis of OSA following overnight PSG with no description of any specific AHI criteria. Most of the patients were male, accounting for 89% of the study population. All the patients in the reported studies had moderate to severe OSA with AHI ranging from 20.5 ± 5 to 88.2 ± 27. The duration of the study intervention across the different studies was in the range of 1 night to 3 months.
Effects on Oxygenation, Respiratory Events, and Sleepiness
Table 2 summarizes the effects of the different treatment modalities on AHI, SpO2 and arousal events studied by 6 RCTs. The respiratory disturbances occurring during the nighttime in OSA patients were measured using AHI, respiratory disturbance index (RDI), or SDB events. When CPAP was compared with O2, CPAP was significantly more effective in reducing AHI, while O2 was shown to be more effective in elevating the mean SpO2 and mean nadir SpO2 during hypoxemic events. Both CPAP and O2 improved the oxygenation as compared to placebo (sham) CPAP; this effect was statistically significant (p < 0.05). Four studies showed that CPAP versus O2 therapy was more effective in improving the arousal events/total arousal index, but we could not pool the arousal events for the meta-analysis because of insufficient data.
The effects of CPAP and oxygen supplementation on the daytime somnolence was evaluated by 2 studies.23,24 In one study, nasal CPAP was more effective in improving objectively measured daytime sleepiness than oxygen. This effect was apparent due to the significant efficacy of CPAP in lengthening the multiple sleep latency test (MSLT) time compared to baseline.23 Similarly, another study showed the effectiveness of CPAP in reducing Ep-worth Sleepiness Scale score; however, it was not statistically different from placebo-CPAP or supplemental oxygen.24
Effects on Systemic Blood Pressure
Three studies showed the treatment outcome on systemic blood pressure in patients treated with CPAP, oxygen, and placebo-CPAP.23,25,26 Two studies showed that CPAP effectively reduced both the systolic as well as the diastolic blood pressure as compared to oxygen (p < 0.05).25,26 In one study, CPAP and oxygen both had the effects in lowering the systolic blood pressure as compared to diastolic blood pressure; however, the changes were not statistically significant.23
The effects of O2 therapy on the oxygen saturation and SDB events are summarized in Table 3. Seven studies showed that oxygen therapy was effective in improving the oxygenation as compared to air (control) in OSA patients. The SDB events showed a decreasing trend in the number of events when the patients received O2 therapy after breathing room air. One study demonstrated an improved cardiovascular status in OSA patients following oxygen enrichment night.34 Similarly, another study showed an improvement in daytime somnolence in patients receiving oxygen therapy.35
Meta-Analysis of Randomized Controlled Trials
Effects on Apnea Hypopnea Index (Figure 2)
Figure 2. Effect of CPAP versus oxygen on apnea hypopnea index (AHI).
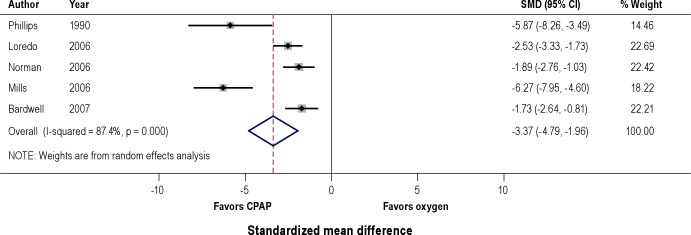
A pooled analysis of 5 randomized controlled trials demonstrated that the use of therapeutic CPAP lead to a statistically significant reduction in the AHI versus nocturnal administration of oxygen (SMD -3.37, 95% CI -4.79 to -1.96). There was also a statistically significant reduction in AHI in CPAP group versus placebo (SMD -3.65, 95% CI -5.31 to -1.98). Nocturnal oxygen did not show significant reduction in AHI compared to placebo CPAP (SMD -0.32, 95% CI -0.74 to 0.08).
Effects on Mean Nocturnal Oxyhemoglobin Saturation (Figure 3)
Figure 3. Effect of CPAP versus oxygen on nocturnal mean oxyhemoglobin saturation (SpO2).
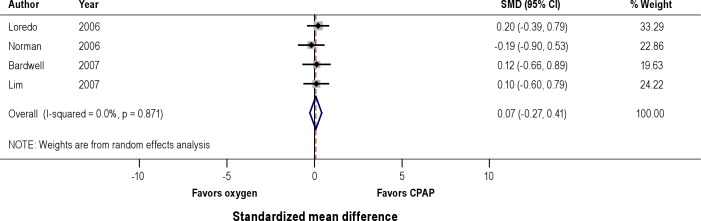
A pooled analysis of 4 studies that reported mean oxyhemoglobin saturation showed that both therapeutic CPAP and nocturnal administration of oxygen lead to significant improvement in oxyhemoglobin saturation compared to placebo CPAP. Comparison of CPAP to nocturnal oxygen did not demonstrate a significant difference in the degree of improvement in oxygenation (SMD 0.07, 95% CI -0.27 to 0.41).
Meta-Analysis of Observational Studies
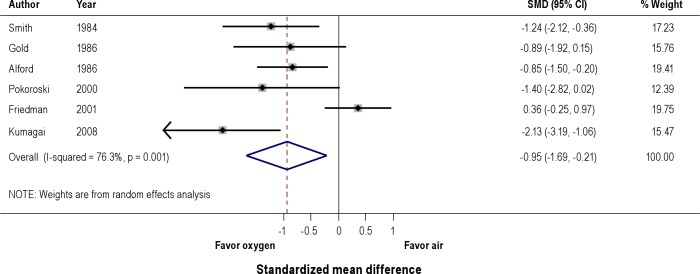
Effects on SDB events (Figure 4)
Figure 4. Effect of oxygen versus air on sleep disordered breathing (SDB) events.
A pooled analysis of 6 observational studies showed significant reduction in SDB events with oxygen compared to air (SMD -0.95, 95% CI -1.69 to -0.21).
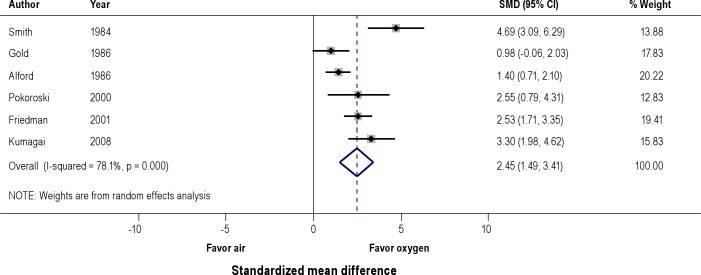
Effects on Mean Nocturnal Oxyhemoglobin Saturation (Figure 5)
Figure 5. Effect of oxygen versus air on nocturnal mean oxyhemoglobin saturation (SpO2).
A pooled analysis of 6 observational studies showed signifi-cant improvement in mean oxyhemoglobin saturation with oxygen compared to air (SMD 2.45, 95% CI 1.49 to 3.4).
Effects on Sleep Disordered Breathing (SDB) Event Duration
We identified 5 observational studies reporting the SDB event duration as an outcome.30–33 We could not pool the results of these studies for statistical analysis due to the lack of sufficient data. However, 3 of these studies reported that administration of oxygen lead to the prolongation of SDB event duration.31–33
DISCUSSION
In this systematic review, we identified and reviewed 14 studies evaluating the effects of oxygen supplementation for the treatment of intermittent nocturnal hypoxemia in patients with OSA. We performed a meta-analysis of the six randomized controlled trials that evaluated the effect of CPAP, placebo CPAP, versus oxygen on AHI and SpO2. In this analysis, patients with obstructive sleep apnea who used CPAP had signifi-cant reduction in AHI compared to those who used nocturnal oxygen. However, both nocturnal oxygen and CPAP improved oxyhemoglobin saturation equally.
Obstructive sleep apnea is a prevalent disorder with its serious health related consequences.37 Many patients with sleep apnea have intermittent episodes of hypoxemia at night secondary to the periods of the upper airway obstruction. These episodes have been shown to be associated with harmful sequelae including insulin resistance, cognitive deficit, and the development of other cardiovascular morbidity.38–40 Both nasal CPAP and nocturnal administration of oxygen improve oxy-hemoglobin saturation, but nocturnal oxygen has little effect on the blood pressure surge following apneas in patients with sleep apnea.41–43 On the other hand, CPAP has been shown to lower the blood pressure variability in patients with sleep apnea.25,26 This suggests that there might be some other factors such as hypercapnia, arousals, respiratory efforts, intrathoracic pressure changes, or fragmented sleep contributing to the increase in the blood pressure seen in sleep apnea.44–47 In a study in human adults, the arousals from NREM sleep was shown to increase the sympathetic discharge with increase in the systolic blood pressure.48
Patients with OSA frequently have cognitive dysfunction and excessive daytime sleepiness (EDS), possible secondary to the combination of hypoxemia and fragmented sleep. These symptoms worsen with increasing severity of hypoxemia and increasing frequency of arousals. Nasal CPAP improves both the arousals and hypoxemia and thereby has been shown to improve the sleepiness in contrast to the nocturnal administration of oxygen.23,49–51 On the other hand, both CPAP and oxygen supplementation have been shown to improve psychological symptoms, including depression.27
CPAP is clearly the treatment of choice in patients with OSA due to its immediate efficacy. It has been shown to improve AHI, hypoxemia, and arousals, thereby improving sleepiness and hypertension in contrast to the nocturnal administration of oxygen. However, patient adherence to CPAP is less than optimum.52 In one study, adherence to CPAP was reported to be higher in patients who had consultation with the sleep physician prior to undergoing the sleep study,53 but adherence to CPAP is between 50% and 70%, even with excellent management.54
Hypoxemia is a major problem for patients with OSA in the postoperative period and hypoxemic episodes have been reported to occur mostly between the postoperative nights two to five.55 Up to 40% of patients undergoing abdominal or thoracic surgery may experience postoperative hypoxemia.56 In particular, surgical patients with OSA are at high risk of having postoperative complications.57,58 A recent cohort study showed that oxygen desaturation with SpO2 < 90% was the most common postoperative complication in patients with OSA.59 These hypoxemic episodes have been shown to have serious consequences, including poor wound healing, cardiac arrhythmias, and delirium.60,61 The use of supplemental oxygen in the perioperative period has been shown to reduce nausea and vomiting and hospital length of stay, and to improve wound healing.62–64
Long-term oxygen therapy (LTOT) has been shown to improve survival and quality of life in patients with COPD.65–67 However, its role in obstructive sleep apnea treatment is more controversial. The administration of nocturnal oxygen leads to the improvement of intermittent hypoxemia in patients with OSA. It may be considered in hypoxemic patients with OSA who are intolerant to the other treatment modalities for sleep apnea. However, the long-term consequences of chronic nocturnal administration of oxygen are unknown in patients with OSA. Further nocturnal oxygen has been shown to prolong apnea duration in patients with OSA, perhaps as a result of the suppression of the hypoxic respiratory drive.31–33 In an observational study, the rise in blood pressure following each apneic episode was primarily linked to apnea duration and was not linked to hypoxemia.42 Prolonged apnea duration may also increase the severity of hypercarbia and acidosis in patients with OSA.19,31,32 This potential risk mandates careful monitoring for arrhythmias and other consequences of hypercarbia, especially in those with comorbid lung disease.
In conclusion, the evidence from the controlled trials does support the preferential use of CPAP over oxygen in patients with OSA since CPAP significantly improves the oxyhemoglobin saturation and reduces AHI and systemic blood pressure with improvement in daytime sleepiness. On the other hand, oxygen therapy is a double-edged sword, which not only lengthens the apnea duration but potentially increases the risk of hypercarbia with minimal to no effect on blood pressure and daytime sleepiness. Hence, at present it is difficult to recommend oxygen therapy for patients who are non-adherent with CPAP until the results of a multicenter clinical trial, Heart Biomarker Evaluation in Apnea Treatment, are available.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors would like to thank Marina Englesakis, BA (Hons), MLIS, Information Specialist, Health Sciences Library, University Health Network, Toronto, Ontario, Canada for her assistance with the literature search.
Author Roles:
Vanita Mehta: Conception and study design, data extraction, data analysis, and manuscript preparation
Tajender Vasu: Data extraction, meta-analysis, and manuscript preparation
Barabara Phillips: Manuscript preparation
Frances Chung: Conception and study design, acquisition and interpretion of data, manuscript preparation
This study was funded by the Physicians' Services Incorporated Foundation, University Health Network Foundation and Department of Anesthesia, University Health Network, University of Toronto.
References
- 1.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 2.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: A population health perspective. Am J Respir Crit Care Med. 2002;165:1217–39. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 3.Ohayon MM, Guilleminault C, Priest RG, Zulley J, Smirne S. Is sleep-disordered breathing an independent risk factor for hypertension in the general population (13,057 subjects)? J Psychosom Res. 2000;48:593–601. doi: 10.1016/s0022-3999(00)00142-2. [DOI] [PubMed] [Google Scholar]
- 4.Kuniyoshi FH, Garcia-Touchard A, Gami AS, et al. Day-night variation of acute myocardial infarction in obstructive sleep apnea. J Am Coll Cardiol. 2008;52:343–6. doi: 10.1016/j.jacc.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan W, Coutts SB, Hanly P. Sleep apnea in patients with transient ischemic attack and minor stroke: opportunity for risk reduction of recurrent stroke? Stroke. 2010;41:2973–75. doi: 10.1161/STROKEAHA.110.596759. [DOI] [PubMed] [Google Scholar]
- 6.Gami AS, Howard DE, Olson EJ, Somers VK. Day-night pattern of sudden death in obstructive sleep apnea. N Engl J Med. 2005;352:1206–14. doi: 10.1056/NEJMoa041832. [DOI] [PubMed] [Google Scholar]
- 7.Shiomi T, Arita AT, Sasanabe R, et al. Falling asleep while driving and automobile accidents among patients with obstructive sleep apnea-hypopnea syndrome. Psychiatry Clin Neurosci. 2002;56:333–4. doi: 10.1046/j.1440-1819.2002.01004.x. [DOI] [PubMed] [Google Scholar]
- 8.Gilmartin GS, Tamisier R, Curley M, Weiss JW. Ventilatory, hemodynamic, sympathetic nervous system, and vascular reactivity changes after recurrent nocturnal sustained hypoxia in humans. Am J Physiol Heart Circ Physiol. 2008;295:H778–85. doi: 10.1152/ajpheart.00653.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fletcher EC. Hypertension in patients with sleep apnoea: a combined effect? Thorax. 2000;55:726–8. doi: 10.1136/thorax.55.9.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muraki I, Tanigawa T, Yamagishi K, et al. Nocturnal intermittent hypoxia and the development of type 2 diabetes: the Circulatory Risk in Communities Study (CIRCS) Diabetologia. 2010;53:481–8. doi: 10.1007/s00125-009-1616-0. [DOI] [PubMed] [Google Scholar]
- 11.Gami AS, Pressman G, Caples SM, et al. Association of atrial fibrillation and obstructive sleep apnea. Circulation. 2004;110:364–7. doi: 10.1161/01.CIR.0000136587.68725.8E. [DOI] [PubMed] [Google Scholar]
- 12.Giles TL, Lasserson TJ, Smith B, White J, Wright JJ, Cates CJ. Continuous positive airways pressure for obstructive sleep apnea in adults. Cochrane Database Syst Rev. 2006:CD001106. doi: 10.1002/14651858.CD001106.pub2. [DOI] [PubMed] [Google Scholar]
- 13.Gay P, Weaver T, Loube D, Iber C. Evaluation of positive airway pressure treatment for sleep related breathing disorders in adults. Sleep. 2006;29:381–401. doi: 10.1093/sleep/29.3.381. [DOI] [PubMed] [Google Scholar]
- 14.Engleman H, Kingshott RN, Wraith PK. Randomized placebo-controlled crossover trial of continuous positive airway pressure for mild sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med. 1999;159:461–7. doi: 10.1164/ajrccm.159.2.9803121. [DOI] [PubMed] [Google Scholar]
- 15.Montserrat JM, Montserrat F, Hernandez L, et al. Effectiveness of CPAP treatment in daytime function in sleep apnea syndrome: a randomized controlled study with an optimized placebo. Am J Respir Crit Care Med. 2001;164:608–13. doi: 10.1164/ajrccm.164.4.2006034. [DOI] [PubMed] [Google Scholar]
- 16.Shapiro GK, Shapiro CM. Factors that influence CPAP adherence: an overview. Sleep Breath. 2010;14:323–35. doi: 10.1007/s11325-010-0391-y. [DOI] [PubMed] [Google Scholar]
- 17.Farney RJ, Walker JM, Elmer JC, et al. Transtracheal oxygen, nasal CPAP and nasal oxygen in five patients with obstructive sleep apnea. Chest. 1992;101:1228–35. doi: 10.1378/chest.101.5.1228. [DOI] [PubMed] [Google Scholar]
- 18.Chauncey JB, Aldrich MS. Preliminary findings in the treatment of obstructive sleep apnea with transtracheal oxygen. Sleep. 1990;13:167–74. [PubMed] [Google Scholar]
- 19.Motta J, Guilleminault C. Sleep apnea syndromes. New York: 1978. Effects of oxygen administration in sleep-induced apneas; pp. 137–44. [Google Scholar]
- 20.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oxford Centre for Evidence-Based Medicine. Levels of evidence. [Accessed December 15, 2011]. http://www.cebm.net/index.aspx?o=1025.
- 22.Higgins JPT, Altman D. Higgins JPT, Green S, editors. Assessing risk of bias in included studies. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.0.0. The Cochrane Collaboration; updated February 2008. [Google Scholar]
- 23.Phillips BA, Schmitt FA, Berry DT, Lamb DG, Amin M, Cook YR. Treatment of obstructive sleep apnea. A preliminary report comparing nasal CPAP to nasal oxygen in patients with mild OSA. Chest. 1990;98:325–30. doi: 10.1378/chest.98.2.325. [DOI] [PubMed] [Google Scholar]
- 24.Loredo JS, Ancoli-Israel S, Kim EJ, Lim WJ, Dimsdale JE. Effect of continuous positive airway pressure versus supplemental oxygen on sleep quality in obstructive sleep apnea: A placebo-CPAP- controlled study. Sleep. 2006;29:564–71. doi: 10.1093/sleep/29.4.564. [DOI] [PubMed] [Google Scholar]
- 25.Norman D, Loredo JS, Nelesen RA, et al. Effects of continuous positive airway pressure versus supplemental oxygen on 24-hour ambulatory blood pressure. Hypertension. 2006;47:840–5. doi: 10.1161/01.HYP.0000217128.41284.78. [DOI] [PubMed] [Google Scholar]
- 26.Mills PJ, Kennedy BP, Loredo JS, Dimsdale JE, Ziegler MG. Effects of nasal continuous positive airway pressure and oxygen supplementation on norepinephrine kinetics and cardiovascular responses in obstructive sleep apnea. J Appl Physiol. 2006;100:343–8. doi: 10.1152/japplphysiol.00494.2005. [DOI] [PubMed] [Google Scholar]
- 27.Bardwell WA, Norman D, Ancoli-Israel S, et al. Effects of 2-week nocturnal oxygen supplementation and continuous positive airway pressure treatment on psychological symptoms in patients with obstructive sleep apnea: a randomized placebo-controlled study. Behav Sleep Med. 2007;5:21–38. doi: 10.1207/s15402010bsm0501_2. [DOI] [PubMed] [Google Scholar]
- 28.Lim WJ, Barwell WA, Loredo JS, et al. Neuropsychological effects of 2-week continuous positive airway pressure treatment and supplemental oxygen in patients with obstructive sleep apnea: a randomized placebo-controlled study. J Clin Sleep Med. 2007;3:380–6. [PMC free article] [PubMed] [Google Scholar]
- 29.Kearley R, Wynne JW, Block AJ, Boysen PG, Lindsey S, Martin C. The effect of low flow oxygen on sleep-disordered breathing and oxygen desaturation: A study of patients with chronic obstructive lung disease. Chest. 1980;78:682–5. doi: 10.1378/chest.78.5.682. [DOI] [PubMed] [Google Scholar]
- 30.Smith PL, Haponik EF, Bleecker ER, et al. The effects of oxygen in patients with sleep apnea. Am Rev Respir Dis. 1984;132:220–3. doi: 10.1164/arrd.1984.130.6.958. [DOI] [PubMed] [Google Scholar]
- 31.Gold AR, Schwartz AR, Bleecker ER, et al. The effect of chronic nocturnal oxygen administration upon sleep apnea. Am Rev Respir Dis. 1987;134:925–9. doi: 10.1164/arrd.1986.134.5.925. [DOI] [PubMed] [Google Scholar]
- 32.Alford NJ, Fletcher EC, Nickeson D. Acute oxygen in patients with sleep apnea and COPD. Chest. 1986;89:30–8. doi: 10.1378/chest.89.1.30. [DOI] [PubMed] [Google Scholar]
- 33.Block AJ, Hellard DW, Cicale MJ. Snoring, nocturnal hypoxemia, and the effect of oxygen inhalation. Chest. 1987;92:411–7. doi: 10.1378/chest.92.3.411. [DOI] [PubMed] [Google Scholar]
- 34.Pokorski M, Jernajczyk U. Nocturnal oxygen enrichment in sleep apneoa. J Int Med Res. 2000;28:1–8. doi: 10.1177/147323000002800101. [DOI] [PubMed] [Google Scholar]
- 35.Friedman M, Landsberg R, Ascher-Landsberg J. Treatment of hypoxemia in obstructive sleep apnea. Am J Rhinol. 2001;15:311–3. [PubMed] [Google Scholar]
- 36.Kumagai T, Ishibashi Y, Kawarazaki H, et al. Effects of nocturnal oxygen therapy on sleep apnea syndrome in peritoneal dialysis patients. Clin Nephrol. 2008;70:332–9. doi: 10.5414/cnp70332. [DOI] [PubMed] [Google Scholar]
- 37.Lindberg E, Gislason T. Epidemiology of sleep related obstructive breathing. Sleep Med Rev. 2000;4:411–33. doi: 10.1053/smrv.2000.0118. [DOI] [PubMed] [Google Scholar]
- 38.Punjabi NM, Shahar E, Redline S, Gottlieb DJ, Givelber R, Resnick HE Sleep Heart Health Study Investigators. Sleep-disordered breathing, glucose intolerance, and insulin resistance: the Sleep Heart Health Study. Am J Epidemiol. 2004;160:521–30. doi: 10.1093/aje/kwh261. [DOI] [PubMed] [Google Scholar]
- 39.Sharafkhaneh A, Giray N, Richardson P, Young T, Hirshkowitz M. Association of psychiatric disorders and sleep apnea in a large cohort. Sleep. 2005;28:1405–11. doi: 10.1093/sleep/28.11.1405. [DOI] [PubMed] [Google Scholar]
- 40.Gilmartin GS, Lynch M, Tamisier R, Weiss JW. Chronic intermittent hypoxia in humans during 28 nights results in blood pressure elevation and increased muscle sympathetic nerve activity. Am J Physiol Heart Circ Physiol. 2010;299:H925–31. doi: 10.1152/ajpheart.00253.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schnider HJ, Schaub CD, Chen CA, et al. Neural and local effects of hypoxia on cardiovascular responses to obstructive apnea. Appl Physiol. 2000;88:1093–102. doi: 10.1152/jappl.2000.88.3.1093. [DOI] [PubMed] [Google Scholar]
- 42.Ali NJ, Davies RJ, Fleetham JA, Stradling JR. The acute effects of continuous positive airway pressure and oxygen administration on blood pressure during obstructive sleep apnea. Chest. 1992;101:1526–32. doi: 10.1378/chest.101.6.1526. [DOI] [PubMed] [Google Scholar]
- 43.Ringler JJ, Basner RC, Shannon R, et al. Hypoxemia alone does not explain blood pressure elevations after obstructive apneas. Appl Physiol. 1990;69:2143–8. doi: 10.1152/jappl.1990.69.6.2143. [DOI] [PubMed] [Google Scholar]
- 44.Trinder MR, Rosenberg JI, Fitzgerald F, Kleiman J, Douglas Bradley T. Patho-physiological interactions of ventilation, arousals, and blood pressure oscillations during Cheyne-Stokes respiration in patients with heart failure. Am J Respir Crit Care Med. 2000;162:808–13. doi: 10.1164/ajrccm.162.3.9806080. [DOI] [PubMed] [Google Scholar]
- 45.O'Donnell CP, Ayuse T, King ED, Schwartz AR, Smith PL, Robotham JL. Airway obstruction during sleep increases blood pressure without arousal. J Appl Physiol. 1996;80:773–81. doi: 10.1152/jappl.1996.80.3.773. [DOI] [PubMed] [Google Scholar]
- 46.Stradling JR, Barbour C, Glennon J, Langford BA, Crosby JH. Which aspects of breathing during sleep influence the overnight fall of blood pressure in a community population? Thorax. 2000;55:393–8. doi: 10.1136/thorax.55.5.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Redline S, Min NI, Shahar E, Rapoport D, O'Connor G. Polysomnographic predictors of blood pressure and hypertension: is one index best? Sleep. 2005;28:1122–30. doi: 10.1093/sleep/28.9.1122. [DOI] [PubMed] [Google Scholar]
- 48.Morgan BJ, Crabtree DC, Puleo DS, Badr MS, Toiber F, Skatrud JB. Neurocirculatory consequences of abrupt change in sleep state in humans. J Appl Physiol. 1996;80:1627–36. doi: 10.1152/jappl.1996.80.5.1627. [DOI] [PubMed] [Google Scholar]
- 49.Montserrat JM, Ferrer M, Hernandez L, et al. Effectiveness of CPAP treatment in daytime function in sleep apnea syndrome: a randomized controlled study with an optimized placebo. Am J Respir Crit Care Med. 2001;164:608–13. doi: 10.1164/ajrccm.164.4.2006034. [DOI] [PubMed] [Google Scholar]
- 50.Jenkinson C, Davies RJ, Mullins R, Stradling JR. Comparison of therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised prospective parallel trial. Lancet. 1999;353:2100–05. doi: 10.1016/S0140-6736(98)10532-9. [DOI] [PubMed] [Google Scholar]
- 51.McArdle N, Douglas NJ. Effect of continuous positive airway pressure on sleep architecture in the sleep apnea-hypopnea syndrome: a randomized controlled trial. Am J Respir Crit Care Med. 2001;164:1459–63. doi: 10.1164/ajrccm.164.8.2008146. [DOI] [PubMed] [Google Scholar]
- 52.Collen J, Lettieri C, Kelly W, Roop S. Clinical and polysomnographic predictors of short-term CPAP compliance. Chest. 2009;135:704–9. doi: 10.1378/chest.08-2182. [DOI] [PubMed] [Google Scholar]
- 53.Pamidi S, Knutson KL, Ghods F, Mokhlesi B. The impact of sleep consultation prior to a diagnostic polysomnogram on continuous positive airway pressure adherence. Chest. 2012;141:51–7. doi: 10.1378/chest.11-0709. [DOI] [PubMed] [Google Scholar]
- 54.Kohler M, Smith D, Tippett V, Stradling JR. Predictors of long-term compliance with continuous positive airway pressure. Thorax. 2010;65:829–32. doi: 10.1136/thx.2010.135848. [DOI] [PubMed] [Google Scholar]
- 55.Rosenberg J, Ullstad T, Rasmussen J, Hjørne FP, Poulsen NJ, Goldman MD. Time course of postoperative hypoxemia. Eur J Surg. 1994;160:137–43. [PubMed] [Google Scholar]
- 56.Squadrone V, Coha M, Cerutti E, et al. Continuous positive airway pressure for treatment of postoperative hypoxemia: a randomized controlled trial. JAMA. 2005;293:589–95. doi: 10.1001/jama.293.5.589. [DOI] [PubMed] [Google Scholar]
- 57.Hwang D, Shakir N, Limann B, et al. Association of sleep-disordered breathing with postoperative complications. Chest. 2008;133:1128–34. doi: 10.1378/chest.07-1488. [DOI] [PubMed] [Google Scholar]
- 58.Gupta RM, Parvizi J, Hanssen AD, Gay PC. Postoperative complications in patients with obstructive sleep apnea syndrome undergoing hip or knee replacement: a case-control study. Mayo Clin Proc. 2001;76:897–905. doi: 10.4065/76.9.897. [DOI] [PubMed] [Google Scholar]
- 59.Kaw R, Pasupulti V, Walker E, Ramaswamy A, Schafer NF. Postoperative complications in patients with obstructive sleep apnea. Chest. 2012;141:436–41. doi: 10.1378/chest.11-0283. [DOI] [PubMed] [Google Scholar]
- 60.Galatius-Jensen S, Hansen J, Rasmussen V, Bildsøe J, Therboe M, Rosenberg J. Nocturnal hypoxaemia after myocardial infarction: association with nocturnal myocardial ischaemia and arrhythmias. Br Heart J. 1994;72:23–30. doi: 10.1136/hrt.72.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kehlet H, Rosenberg J. Late post-operative hypoxaemia and organ dysfunction. Eur J Anaesthesiol Suppl. 1995;10:31–4. [PubMed] [Google Scholar]
- 62.Chura JC, Boyd A, Argenta PA. Surgical site infections and supplemental peri-operative oxygen in colorectal surgery patients: a systematic review. Surg Infect (Larchmt) 2007;8:455–61. doi: 10.1089/sur.2006.034. [DOI] [PubMed] [Google Scholar]
- 63.Grief R, Laciny S, Rapf B, Hickle RS, Sessler DI. Supplemental oxygen reduces the incidence of postoperative nausea and vomiting. Anesthesiology. 1999;91:1246–52. doi: 10.1097/00000542-199911000-00014. [DOI] [PubMed] [Google Scholar]
- 64.Greif R, Akça O, Horn EP, Kurz A, Sessler DI. Supplemental perioperative oxygen to reduce the incidence of surgical-wound infection. N Engl J Med. 2000;342:161–7. doi: 10.1056/NEJM200001203420303. [DOI] [PubMed] [Google Scholar]
- 65.Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: a clinical trial. Nocturnal Oxygen Therapy Trial Group. Ann Intern Med. 1980;93:391–8. doi: 10.7326/0003-4819-93-3-391. [DOI] [PubMed] [Google Scholar]
- 66.Report of the Medical Research Council Working Party. Long-term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema. Lancet. 1981;1:681–6. [PubMed] [Google Scholar]
- 67.Cranston JM, Crockett AJ, Moss JR, Alpers JH. Domiciliary oxygen for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2005:CD001744. doi: 10.1002/14651858.CD001744.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]