Abstract
Study Objectives:
Assess the association between REM predominant obstructive sleep apnea (OSA), sleepiness, and quality of life in a community-based cohort of men ≥ 65 years-old.
Design, Intervention and Measurements:
A cross-sectional analysis of 2,765 subjects from the Outcomes of Sleep Disorders in Older Men (MrOS Sleep) Study was performed to identify subjects with an apnea hypopnea index (AHI) < 15 (n = 2,044). Subjects were divided into groups based on the AHI in REM sleep (< 5 [referent group], 5 to < 15, 15 to < 30, and ≥ 30). Daytime somnolence, sleep-related quality of life, sleep disturbance, general quality of life, depressive symptoms, and health status were quantified using Epworth Sleepiness Scale (ESS), Functional Outcomes of Sleep Questionnaire (FOSQ), Pittsburgh Sleep Quality Index (PSQI), Short Form-12 (SF-12), Geriatric Depression Scale-15 (GDS), and self-perceived health status, respectively.
Results:
Prevalence of REM-predominant OSA (AHI-REM ≥ 5) was 42.8% if OSA was defined as AHI ≥ 15 and 14.4% if OSA was defined as AHI ≥ 5. Higher AHI-REM was associated with polysomnographic indices of poorer sleep architecture (reduced total sleep time, sleep efficiency, REM sleep duration and proportion). Adjusting for age, BMI, and study site, higher AHI-REM was not associated with subjective sleep measures (ESS, FOSQ, PSQI), lower quality of life (SF-12), or greater depressive symptoms (GDS).
Conclusions:
In a community-based sample of older adult men ≥ 65 years-old, REM-predominant OSA was highly prevalent and was associated with objective indices of poorer sleep quality on polysomnography but not with subjective measures of daytime sleepiness or quality of life.
Citation:
Khan A; Harrison SL; Kezirian EJ; Ancoli-Israel S; O'Hearn D; Orwoll E; Redline S; Ensrud K; Stone KL. Obstructive sleep apnea during rapid eye movement sleep, daytime sleepiness, and quality of life in older men in osteoporotic fractures in men (MrOS) sleep study. J Clin Sleep Med 2013;9(3):191-198.
Keywords: Sleep apnea syndromes, sleep apnea, obstructive, sleep, rapid eye movement, disorders of excessive somnolence, epidemiology, older adults, quality of life
Obstructive sleep apnea (OSA), as defined by an apnea hypopnea index (AHI) ≥ 5, is present in 24% to 62% of community dwelling elderly (age ≥ 65 years), and moderate to severe OSA with an AHI ≥ 15 has been noted in 19% to 44%.1–3 OSA has been associated with excessive daytime sleepiness, decreased quality of life, impaired cognition, hypertension, stroke, diastolic dysfunction, left atrial enlargement, and insulin resistance.4–13 Little is known about OSA confined only to REM sleep (REM-predominant OSA).14–17 This is of particular interest, given that disordered breathing events in REM sleep are often of longer duration and associated with a greater degree of oxygen desaturation than NREM sleep.18,19
REM-predominant OSA also poses a difficult dilemma for the practicing clinician. There is lack of data regarding the association between REM-predominant OSA and quality of life as well as cardiovascular and metabolic outcomes. It is not clear if isolated REM-only OSA needs to be treated or if during continuous positive airway pressure (CPAP) titration, pressure should be increased for REM events if the overall AHI is in the normal range. The prevalence and impact of REM-predominant OSA is not clear due to differences in sample size and patient populations of various studies and the adjustments used in various studies to compensate for the effect of AHI in NREM (AHI-NREM) on AHI in REM (AHI-REM) sleep.14–17,20 This is further confounded by REM-predominant OSA being more prominent in women and relatively younger population and less common in individuals with severe OSA.14–17,20,21
BRIEF SUMMARY
Current Knowledge/Study Rationale: It is not known if REM-predominant obstructive sleep apnea (OSA) affects quality of life and sleepiness and if isolated REM-only OSA needs to be treated.
Study Impact: In a community-based sample of older adult men ≥ 65 years-old, REM-predominant OSA was highly prevalent and was associated with objective indices of poorer sleep quality on polysomnography but not with subjective measures of daytime sleepiness or quality of life.
The prevalence and impact of REM-predominant OSA in individuals older than 65 years is not known. The average age of the subjects in most studies of OSA has been between 49-65 years, and individuals older than 65 have been excluded in most studies.17,22–24 In some studies REM related OSA was more common in the younger population,15,16 while in other studies such as the Sleep Heart Health Study, subjects with more severe REM-predominant OSA were older.22 Our primary objective was to define the prevalence of REM-predominant OSA in the Osteoporotic Fractures in Men Sleep study (MrOS Sleep Study) cohort and determine whether REM-predominant OSA was associated with sleepiness and quality of life in older male adults. Our secondary objective was to describe the demographic and polysomnography (PSG) characteristics of older men with REM-predominant OSA.
METHODS
Population
From March 2000 through April 2002, 5,994 men aged 65 years and older were recruited for participation in the prospective Osteoporotic Fractures in Men (MrOS) Study from population-based listings in 6 areas of the United States: Birmingham, Alabama; Minneapolis, Minnesota; Palo Alto, California; Pittsburgh, Philadelphia; Portland, Oregon and San Diego, California.25,26 To be eligible for the study, men had to be able to walk without assistance, not have had bilateral hip replacement or a medical condition that (in the judgment of the investigator) would result in imminent death at the time of enrollment, and were willing to give informed consent and undergo the study procedures. There were no other exclusion criteria.25
In an ancillary study, from December 2003 through March 2005, subjects were invited to have in-home polysomnography (PSG) and actigraphy as part of Outcomes of Sleep Disorders in Older Men (MrOS Sleep) Study.2 Of the 5,994 men enrolled in the MrOS Study, 3,135 (52.3%) were enrolled in the MrOS Sleep Study. All participants provided written informed consent, and the study protocols were approved by the institutional review boards at each respective study site and at the data coordinating center in San Francisco, California. The MrOS sleep visit was completed between December 2003 and March 2005. A total of 3,135 participants attended the sleep visit. Men were screened for use of mechanical devices during sleep including continuous positive airway pressure (CPAP) or bilevel positive airway pressure (BPAP), mouthpiece for snoring or sleep apnea, or oxygen therapy. In general, those who reported nightly use of any of these devices were excluded from the MrOS Sleep Study; however, the study sample does include 11 men who reported use of one of these devices < 2 times per week. In the present analysis, data were analyzed from 2,765 subjects who took part in the MrOS Sleep Study and had adequate PSG signal quality available for ≥ 4 h, as well as adequate REM staging data (Figure 1).2
Figure 1. Distribution of subjects in MrOS Sleep Study with AHI < 15.
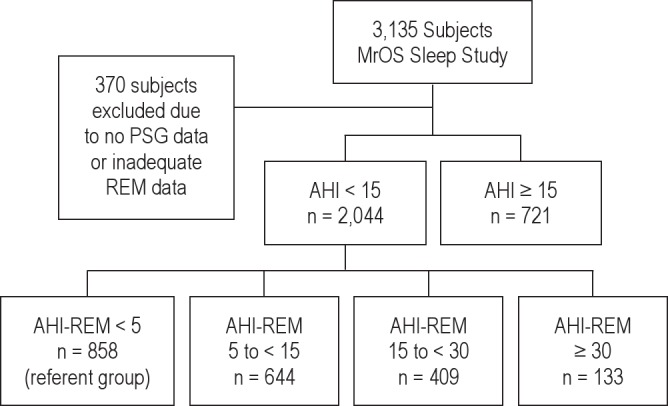
AHI, apnea hypopnea index; AHI-REM, apnea hypopnea index-rapid eye movement sleep; PSG, polysomnography. REM-predominant OSA was defined as AHI < 15 but AHI-REM ≥ 5.
Polysomnography
In-home sleep studies were performed using unattended PSG (Safiro, Compumedics, Inc., Melbourne, Australia). The recording montage consisted of 2 central (C3/A2 and C4/A1) electroencephalographic leads, bilateral electroculograms, a bipolar submental electromyogram, thoracic and abdominal respiratory inductance plethysmography, airflow (using nasal-oral thermocouple and nasal pressure cannula), finger pulse oximetry, electrocardiogram, body position (mercury switch sensor), and bilateral leg movements (piezoelectric sensors). Trained certified staff members performed home visits for setup of the sleep study units. After sensors were placed and calibrated, signal quality and impedance were checked, and sensors were repositioned as needed to improve signal quality, replacing electrodes if impedances were greater than 5 kΩ, using approaches similar to those used in the Sleep Heart Health Study.27–29 Staff returned the next morning to collect the equipment and download the data to the Central Sleep Reading Center (Cleveland, OH) for centralized scoring by trained technicians using standard criteria.27,29
Subjects included in the present analysis included MrOS Sleep Study participants who had PSG with adequate scoring of REM sleep based on availability of reliable staging data. Less than 1% of the subjects had ≤ 10 min of REM sleep; all subjects with any REM sleep were included in the analysis. Sleep stages (REM, stages 1-4 NREM) were scored using standard criteria.27,29 The reliability of indices of sleep architecture, determined by rescoring studies over time, indicates that the inter-scorer reliability (ICC) for the percent of sleep time spent in sleep stages 1, 2, slow wave sleep, and REM were 0.60, 0.91, 0.96, and 0.94, respectively.2,30 AHI was calculated as the total number of apneas and hypopneas per hour of sleep. The inter-scorer reliability for AHI was high (ICC = 0.99).30
Apneas were identified if the amplitude of the airflow signal was flat or nearly flat for > 10 seconds. Obstructive apneas were scored if persistence of effort on abdominal or thoracic inductance plethysmography was noted, and central apneas were scored if there was no evident effort on either the abdominal and thoracic plethysmography bands. Hypopneas were scored using Sleep Heart Health Study criteria (requiring a “discernible” [> 30%] reduction in amplitude of respiratory effort or airflow) and, in secondary analyses, using the American Academy of Sleep Medicine (AASM) criteria (> 50% reduction in amplitude of signals), considered according to the following hierarchy: summed inductance plethysmography channel, abdominal or thoracic inductance plethysmography, nasal pressure, or thermistor.28 For the current study, apneas and hypopneas associated with ≥ 4% oxygen desaturation were included, and the Sleep Heart Health Study definition was used for primary analyses.30 The total AHI, as well as AHI-REM and AHI-NREM were computed as the number of apneas plus hypopneas per hour of total, REM, and NREM sleep, respectively.
Selection and Classification of Subjects
OSA was defined as AHI ≥ 15 during the entire sleep period. REM-predominant OSA was defined as AHI < 15, but AHIREM ≥ 5 (Figure 1). From the entire population of MrOS study participants, men were first classified on the basis of OSA: AHI < 15 (absence of moderate to severe OSA group) and AHI ≥ 15 (OSA group). The 2,015 men with AHI < 15 (analytical cohort) were further subdivided based upon the AHI-REM sleep: AHIREM < 5 (referent group) and AHI-REM 5 to < 15, AHI-REM 15 to < 30, and AHI-REM ≥ 30 (Figure 1). Baseline characteristics of men in the analytical cohort were compared across category of AHI-REM sleep (Figure 1, Table 1). The analysis was repeated defining OSA as AHI ≥ 5/h during the entire sleep period and REM-only OSA as AHI < 5/h and AHI-REM ≥ 5/h (supplemental material Figure S1, Table S1).
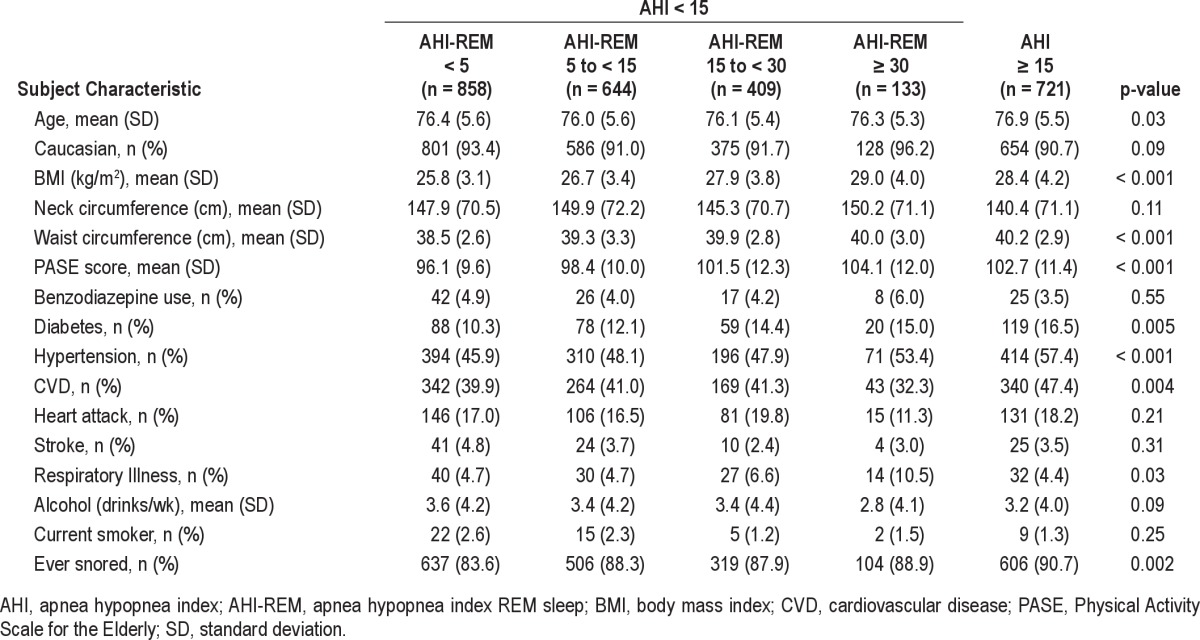
Table 1.
Baseline subject characteristics based on AHI and AHI-REM sleep of subjects in MrOS Sleep Study
Sleepiness and Functional Outcome Measures
Subjective sleepiness was measured using the Epworth Sleepiness Score (ESS), a self-administered questionnaire which provides a measurement of the subject's general level of daytime sleepiness.12 Individuals rate the chances that they would doze off or fall asleep when in 8 different situations commonly encountered in daily life on a 0- to 3-point scale. Possible scores range from 0 to 24. An ESS score > 10 is considered sleepy and correlates with sleep latency measured during the multiple sleep latency test and during overnight PSG.12
Sleep-related quality of life was measured using the Functional Outcomes of Sleep Questionnaire (FOSQ).31,32 FOSQ is a 30-item disease specific self-reported measure designed to assess the impact of disorders of excessive sleepiness on multiple activities of everyday living.31,32 Mean-weighted item scores are used to generate 5 subscales (activity level, vigilance, intimacy and sexual relationships, general productivity, and social outcome) that together produce a composite score.31 The total score ranges from 5 to 20, and lower scores indicate greater dysfunction. FOSQ can successfully discriminate between normal subjects and those who should seek medical attention for a sleep problem.
Subjective sleep symptoms, disturbance, and patterns were assessed using the Pittsburgh Sleep Quality Index (PSQI).33–35 PSQI is a self-reported questionnaire that assesses sleep quality and disturbances over a 1-month time interval.33 Nineteen individual items generate 7 component scores (subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medication, and daytime dysfunction), the sum of which yields a global score. The total global score ranges from 0 to 21; greater scores indicate higher levels of sleep symptoms. A global PSQI score > 5 yielded a diagnostic sensitivity of 89.6% and specificity of 86.5% in distinguishing good and poor sleepers.33
General quality of life was measured using Short Form 12 (SF-12), a generic 12-question survey which is weighted and summed to provide interpretable scales for physical and mental health.36 The physical and mental health composite summary scores are computed using the scores of the 12 questions and range from 0 to 100, where a 0 score indicates the lowest level of health measured by the scales and 100 indicates the highest level of health. SF-12 does not target a specific age or disease group and the physical and mental summary scores tend to vary over the life span and for different age groups.
Depressive symptoms were assessed using the Geriatric Depression Scale (GDS).37–39 GDS is a brief questionnaire in which participants are asked to respond to either 15 or 30 questions by answering yes or no in reference to how they felt on the day of administration.37,38 GDS-15 was used in this study, and scores ≥ 6 on this scale indicates depression.37,39
Self-reported health status was scaled as excellent, good, fair, poor, or very poor; the responses were collapsed into 2 categories excellent/good health and fair/poor/very poor health.40
Other Measurements
Study participants completed an anthropometric evaluation and questionnaire assessment of medical history including self-reported provider–diagnosed disease which included hypertension, diabetes mellitus, cardiovascular disease (CVD), heart attack, respiratory illness, and stroke. All prescription and over-the-counter medications taken 30 days prior to the last visit were recorded by the clinics and stored in an electronic medications inventory database (San Francisco Coordinating Center, San Francisco, CA). Each medication was matched to its ingredient(s) based on the Iowa Drug Information Service (IDIS) Drug Vocabulary (College of Pharmacy, University of Iowa, Iowa City, IA).41 Clinic visit examination and questionnaire measurements included age (years), body mass index (BMI = kg/m2), race, neck and waist circumference (cm), tobacco smoking, and alcohol use status. Physical activity was assessed using Physical Activity Scale for the Elderly (PASE), a research instrument that measures the level of physical activity in individuals aged 65 years or older.42
Statistical Analysis
Participant characteristics were examined across AHI-REM categories. For categorical data, χ2 test was used. Analysis of variance (ANOVA) was used for normally distributed continuous data and Kruskal-Wallis test for continuous data that were skewed. Similarly, sleep related variables were also explored across AHIREM categories where p-value for trend was calculated.
Least square means linear regression was used to examine the relationship between continuous outcomes, such as sleepiness and quality of life, and AHI-REM categories. Logistic regression was used to assess the relationship between AHI-REM categories and similar dichotomized outcomes. All models were initially adjusted for age and study site. Since OSA has been associated with obesity43 and weight loss has been associated with improvement in BMI, anthropometric measures, and OSA,44 further adjustment was done for either BMI or waist and neck circumference. All analyses were performed using SAS software, version 9.1 (SAS Institute, Cary, NC).
RESULTS
Polysomnography data quality were excellent, with a failure rate < 4% and more than 70% of studies graded as being of excellent or outstanding quality using standardized criteria. Of the 2,765 men with technically adequate PSG data (Figure 1), 2,044 subjects had AHI < 15 (analytical cohort), while 721 subjects had AHI ≥ 15. Three hundred seventy subjects were excluded due to inadequate unreliable REM/NREM data or no PSG data. There were 858 subjects with AHI-REM < 5 who served as the referent group; there were 644 subjects with AHIREM 5 to < 15, 409 subjects with AHI-REM 15 to < 30, 133 subjects with AHI-REM ≥ 30, and 721 subjects with AHI > 15. The average time spent in REM sleep time was 70.3 ± 28.2 min and in NREM 286.3 ± 55.5 min. The prevalence of REM-predominant OSA was 42.8% if OSA was defined as AHI ≥ 15 and 14.4% if OSA was defined as AHI ≥ 5.
Among our cohort of 2,041 older men without moderate or severe OSA (AHI < 15), there were no significant differences by age or race across AHI-REM categories. A higher AHI-REM was associated with higher BMI, neck circumference, and waist circumference (p value < 0.001) (Table 1). Subject-reported histories of respiratory illness and snoring were also associated with a higher AHI-REM (p < 0.05). There were no significant associations between PASE score, benzodiazepine use, smoking status, alcohol use, physician diagnosed and self-reported diabetes, stroke, cardiovascular disease, and heart attack across categories of REM-predominant OSA (Table 1). Results were similar when AHI ≥ 5 was used to define the presence of OSA (supplemental material Table S1).
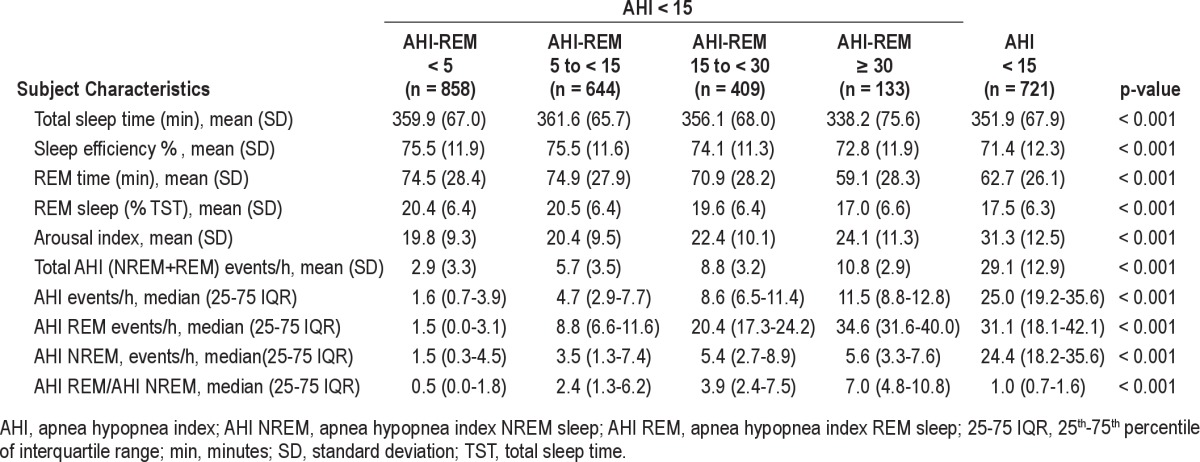
There was a significant trend in decreasing total sleep time, sleep efficiency, and minutes and percentage of time in REM sleep across AHI-REM categories (Table 2). A higher AHIREM was associated with a higher total and NREM AHI as well as a higher AHI-REM-to-AHI-NREM ratio and arousal index (Table 2). The trend was similar when AHI ≥ 5 was used as definition of OSA (supplemental material Table S2).
Table 2.
Polysomnography characteristics of subjects in MrOS Sleep Study
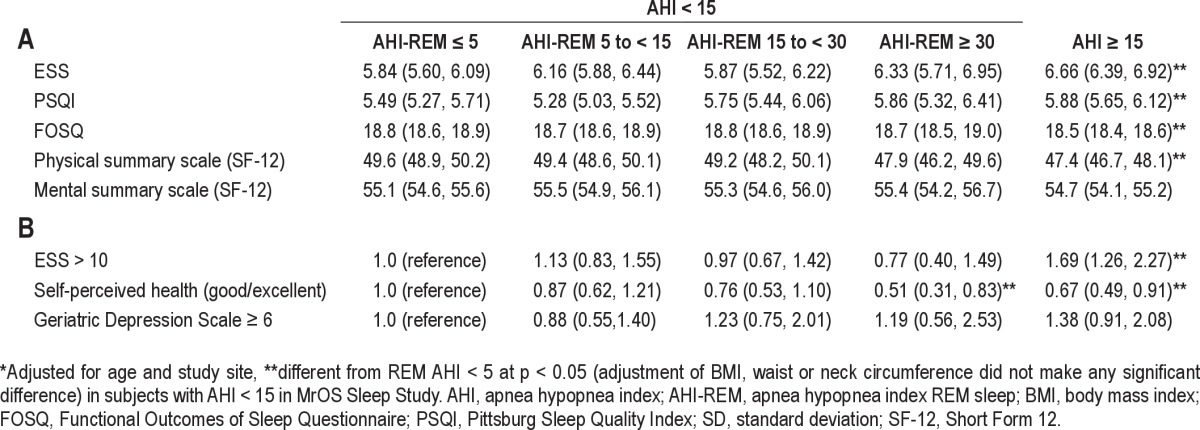
Adjusted for age, study site, and anthropometric measures, AHI-REM ≥ 30 was associated with worse self-perceived health status than AHI-REM ≤ 5 (Tables 3 and 4). After adjustment for age and site, there was no evidence that level of sleepiness or vitality (as measured by ESS, PSQI, and FOSQ) or quality of life (as assessed by SF-12 physical, mental summary scale, and GDS) varied across categories of REM-predominant OSA (Tables 3 and 4). Adjusting for BMI or neck or waist circumference did not change the results (data not shown). Results were not altered in analyses when OSA was defined as AHI ≥ 5 during the entire sleep period and REM-predominant OSA was defined as AHI < 5 with AHI-REM ≥ 5 (supplemental material Table S3 and S4).
Table 3.
Self-reported perceived health status, quality of life measures and sleepiness in subjects in MrOS Sleep Study
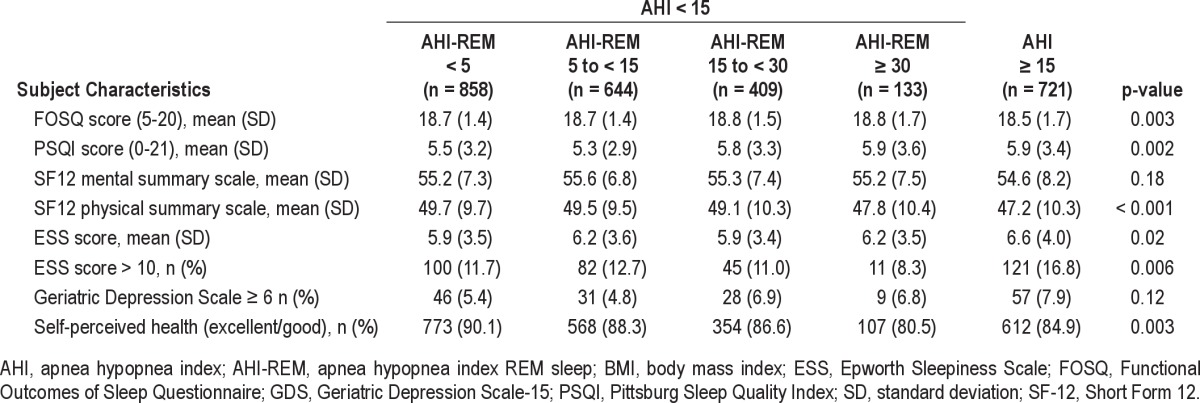
Table 4.
Adjusted* means (A) and odds ratios (B) (95%CI) of functional outcome measures by AHI categories
DISCUSSION
In a large, community-based sample of adults > 65 years old, we assessed the association between REM-predominant OSA, sleepiness and quality of life in subjects without OSA (using either AHI ≥ 15 or AHI ≥ 5 to define OSA). Our findings show that in this population of older men, an elevated AHI-REM sleep was highly prevalent even when the AHI was either low (< 5) or modest (< 15). Similar to what is known regarding total AHI levels, a higher AHI-REM was associated with a higher BMI, neck circumference, and waist circumference. Of note, even among those without an elevated AHI (overall AHI < 5 or < 15), a higher AHI-REM was associated with reduced total sleep time, sleep efficiency, and REM time and percentage. This suggests that even when the predominant number of respiratory disturbances occur in REM sleep, sleep architecture is disturbed. However, increasing REM-specific AHI levels were not linearly associated with increased sleepiness as determined by the ESS. In this elderly population, only adjusted AHI-REM ≥ 30 was associated with a decrease in self-perceived health status. There were no other associations between REM-predominant OSA and the behavioral measures studied.
Prior literature has provided conflicting reports regarding whether REM-predominant OSA is associated with excessive daytime sleepiness. Excessive daytime sleepiness can affect 10% to 33% of the elderly and has been associated with significant consequences, including an increased incidence of functional impairment, falls, cognitive deficits, and mortality.13,45–47 The presence of OSA has been shown to be an important risk factor for mortality from excessive daytime sleepiness in older adults.13,24 In our study, REM-predominant OSA was not associated with increased daytime sleepiness using the ESS. This is similar to two large studies which showed that OSA in NREM sleep was associated with excessive daytime sleepiness while patients with REM-predominant OSA did not have evidence of excessive daytime sleepiness.17,22
Similar to data from the Sleep Heart Health Study,22 our results show that after adjusting for age, BMI, and study site, in subjects with either AHI < 15 or < 5, REM-predominant OSA did not have a significant association with quality of life measures as measured by FOSQ, PSQI, SF-12 physical and mental summary scale, or GDS-15. Worse self-perceived health, however, was associated with REM-AHI ≥ 30 (Table 3 and 4). Similar to our results, the Sleep Heart Health Study showed that REM-predominant SDB was not independently associated with daytime sleepiness, impaired health-related quality of life, or self-reported sleep disruption.22 Self-perceived health status was not reported in the Sleep Heart Health Study.
We choose AHI ≥ 15 as the primary definition of OSA in our analysis because of the high prevalence of modestly elevated AHI levels in older adults that has no significant clinical consequences.1–3,48,49 Morbidity and mortality in the elderly with lower AHI levels have been shown to be similar to those without OSA.50,51 We further analyzed our data defining OSA as AHI ≥ 5 during the entire sleep period and defining REM-predominant OSA as AHI < 5 with AHI-REM ≥ 5 and the results were similar (supplemental material Tables S3 and S4).
In our cohort, the average time spent in REM sleep time was 70.3 ± 28.2 min and in NREM 286.3 ± 55.5 min. It is possible that some of the effects of REM-predominant OSA were mitigated by a decrease in time spent in REM sleep with increasing AHI-REM (Table 2). This could be due to increased events in REM sleep leading to arousals and decreasing the time in REM sleep. The total AHI in all subgroups of AHI-REM was low, with the highest being 10.8 ± 2.9 in the subgroup with AHIREM > 30. The total AHI in subjects with AHI ≥ 15 was 29.1 ± 12.9. This significant difference may have also been responsible for the lack of any effect of AHI-REM on sleepiness and quality of life measures.
AHI levels increase with age1,48; however, it has not been clear if REM-predominant OSA increases with age. The prevalence of REM-predominant OSA in our population was 42.8%, which is higher than the 14% in men ≥ 70 years old in another published trial with a similar definition of REM-predominant OSA.16 More severe REM-predominant OSA was associated with a higher BMI, neck and waist circumference, and an increased prevalence of snoring. It has been suggested that narrowing of the upper airway in REM sleep due to REM-associated atonia may lead to changes in airway closing pressure in REM sleep.52 It is possible that an increase in BMI or neck and waist circumference lead to an increased AHI-REM before any changes were observed in AHI-NREM which may explain some of our findings. The strength of our study is that we were able to evaluate the association of REM-predominant OSA with daytime sleepiness in a large, community-based sample of older adults, a population group that has not been evaluated before for the effects of REM-predominant OSA.
Our study has several limitations. This is an observational study and with associated limitations on inferences regarding causality. Our study population consists of community dwelling, ambulatory men aged 65 years or older with ability to walk without the assistance, who did not have either bilateral hip replacements or a medical condition that would result in imminent death at the time of enrollment.25 There were no other exclusion criteria. Another limitation is that more than 90% of our subjects were Caucasian. Our data should be taken with caution while generalizing it to other populations and racial groups. We only studied male subjects and although we used validated instruments to measure subjective indices of sleepiness, these instruments may have lower levels of discrimination in older subjects, who may also have other competing causes of sleepiness.53,54
CONCLUSION
In a cohort of community dwelling males, 67 years or older, REM-predominant OSA was associated with small differences in objectively measured sleep quality: decreased total sleep time, sleep efficiency, minutes and percentage of time in REM sleep. However, increasing AHI-REM levels were not linearly associated with subjective measures of excessive daytime sleepiness or quality of life. Severe REM-predominant OSA was associated with worse self-perceived health status.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Khan has received grant support from United Therapeutics. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The Osteoporotic Fractures in Men (MrOS) Study is supported by National Institutes of Health funding. The following institutes provide support: the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Institute on Aging (NIA), the National Center for Research Resources (NCRR), and NIH Roadmap for Medical Research under the following grant numbers: U01 AR45580, U01 AR45614, U01 AR45632, U01 AR45647, U01 AR45654, U01 AR45583, U01 AG18197, U01-AG027810, and UL1 RR024140. The National Heart, Lung, and Blood Institute (NHLBI) provided funding for the MrOS Sleep ancillary study “Outcomes of Sleep Disorders in Older Men” under the following grant numbers: R01 HL071194, R01 HL070848, R01 HL070847, R01 HL070842, R01 HL070841, R01 HL070837, R01 HL070838, and R01 HL070839. NIA R01 AG08415 for Sonia Ancoli-Israel. This publication was made possible with support from the Oregon Clinical and Translational Research Institute (OCTRI), grant number UL1 RR024140 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research.
Author Contributions: Akram Khan and Katie Stone were involved in the conception, hypotheses delineation, and design and interpretation of the analysis. Stephanie L Harrison was involved in the analysis and interpretation of data. All authors were involved in the writing the article or substantial involvement in its revision prior to submission.
SUPPLEMENTAL MATERIAL
AHI, apnea hypopnea index; AHI-REM, apnea hypopnea index REM sleep; PSG, polysomnography.
Subject characteristics based on AHI and AHI-REM sleep in subjects with AHI < 5/h in MrOS Sleep Study
Polysomnography Characteristics of subjects with AHI < 5/h in MrOS Sleep Study
Self-reported perceived health status, quality of life measures and sleepiness in subjects with the REM-only OSA vs. those with no OSA in patients with AHI < 5/h in MrOS Sleep Study
Adjusted# means (A) and odds ratios (B) (95%CI) of functional outcome measures by AHI categories
References
- 1.Ancoli-Israel S, Kripke DF, Klauber MR, Mason WJ, Fell R, Kaplan O. Sleep-disordered breathing in community-dwelling elderly. Sleep. 1991;14:486–95. doi: 10.1093/sleep/14.6.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mehra R, Stone KL, Blackwell T, et al. Prevalence and correlates of sleep-disordered breathing in older men: osteoporotic fractures in men sleep study. J Am Geriatr Soc. 2007;55:1356–64. doi: 10.1111/j.1532-5415.2007.01290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Young T, Shahar E, Nieto FJ, et al. Predictors of sleep-disordered breathing in community-dwelling adults: the Sleep Heart Health Study. Arch Intern Med. 2002;162:893–900. doi: 10.1001/archinte.162.8.893. [DOI] [PubMed] [Google Scholar]
- 4.Baldwin CM, Griffith KA, Nieto FJ, O'Connor GT, Walsleben JA, Redline S. The association of sleep-disordered breathing and sleep symptoms with quality of life in the Sleep Heart Health Study. Sleep. 2001;24:96–105. doi: 10.1093/sleep/24.1.96. [DOI] [PubMed] [Google Scholar]
- 5.Khan A, Latif F, Hawkins B, Tawk M, Sivaram CA, Kinasewitz G. Effects of obstructive sleep apnea treatment on left atrial volume and left atrial volume index. Sleep Breath. 2008;12:141–7. doi: 10.1007/s11325-007-0142-x. [DOI] [PubMed] [Google Scholar]
- 6.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–53. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 7.Punjabi NM, Shahar E, Redline S, Gottlieb DJ, Givelber R, Resnick HE. Sleep-disordered breathing, glucose intolerance, and insulin resistance: the Sleep Heart Health Study. Am J Epidemiol. 2004;160:521–30. doi: 10.1093/aje/kwh261. [DOI] [PubMed] [Google Scholar]
- 8.Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease. Cross-sectional Results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 9.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034–41. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 10.Becker HF, Jerrentrup A, Ploch T, et al. Effect of nasal continuous positive airway pressure treatment on blood pressure in patients with obstructive sleep apnea. Circulation. 2003;107:68–73. doi: 10.1161/01.cir.0000042706.47107.7a. [DOI] [PubMed] [Google Scholar]
- 11.Gottlieb DJ, Whitney CW, Bonekat WH, et al. Relation of sleepiness to respiratory disturbance index: the Sleep Heart Health Study. Am J Respir Crit Care Med. 1999;159:502–7. doi: 10.1164/ajrccm.159.2.9804051. [DOI] [PubMed] [Google Scholar]
- 12.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 13.Gooneratne NS, Richards KC, Joffe M, et al. Sleep disordered breathing with excessive daytime sleepiness is a risk factor for mortality in older adults. Sleep. 2011;34:435–42. doi: 10.1093/sleep/34.4.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kass JE, Akers SM, Bartter TC, Pratter MR. Rapid-eye-movement-specific sleep-disordered breathing: a possible cause of excessive daytime sleepiness. Am J Respir Crit Care Med. 1996;154:167–9. doi: 10.1164/ajrccm.154.1.8680674. [DOI] [PubMed] [Google Scholar]
- 15.Haba-Rubio J, Janssens J-P, Rochat T, Sforza E. Rapid eye movement-related disordered breathing: clinical and polysomnographic features. Chest. 2005;128:3350–7. doi: 10.1378/chest.128.5.3350. [DOI] [PubMed] [Google Scholar]
- 16.Koo BB, Patel SR, Strohl K, Hoffstein V. Rapid eye movement-related sleep-disordered breathing: influence of age and gender. Chest. 2008;134:1156–61. doi: 10.1378/chest.08-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Punjabi NM, Bandeen-Roche K, Marx JJ, Neubauer DN, Smith PL, Schwartz AR. The association between daytime sleepiness and sleep-disordered breathing in NREM and REM sleep. Sleep. 2002;25:307–14. [PubMed] [Google Scholar]
- 18.Findley LJ, Wilhoit SC, Suratt PM. Apnea duration and hypoxemia during REM sleep in patients with obstructive sleep apnea. Chest. 1985;87:432–6. doi: 10.1378/chest.87.4.432. [DOI] [PubMed] [Google Scholar]
- 19.Series F, Cormier Y, La Forge J. Influence of apnea type and sleep stage on nocturnal postapneic desaturation. Am Rev Respir Dis. 1990;141:1522–6. doi: 10.1164/ajrccm/141.6.1522. [DOI] [PubMed] [Google Scholar]
- 20.Conwell W, Patel B, Doeing D, et al. Prevalence, clinical features, and CPAP adherence in REM-related sleep-disordered breathing: a cross-sectional analysis of a large clinical population. Sleep Breath. 2012;16:519–26. doi: 10.1007/s11325-011-0537-6. [DOI] [PubMed] [Google Scholar]
- 21.O'Connor C, Thornley KS, Hanly PJ. Gender differences in the polysomnographic features of obstructive sleep apnea. Am J Respir Crit Care Med. 2000;161:1465–72. doi: 10.1164/ajrccm.161.5.9904121. [DOI] [PubMed] [Google Scholar]
- 22.Chami HA, Baldwin CM, Silverman A, et al. Sleepiness, quality of life, and sleep maintenance in REM versus non-REM sleep-disordered breathing. Am J Respir Crit Care Med. 2010;181:997–1002. doi: 10.1164/rccm.200908-1304OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chervin RD, Aldrich MS. The relation between multiple sleep latency test findings and the frequency of apneic events in REM and non-REM sleep. Chest. 1998;113:980–4. doi: 10.1378/chest.113.4.980. [DOI] [PubMed] [Google Scholar]
- 24.Mokhlesi B, Pamidi S, Yaggi HK. Sleep disordered breathing and subjective sleepiness in the elderly: a deadly combination? Sleep. 2011;34:413–5. doi: 10.1093/sleep/34.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Orwoll E, Blank JB, Barrett-Connor E, et al. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study-a large observational study of the determinants of fracture in older men. Contemp Clin Trials. 2005;26:569–85. doi: 10.1016/j.cct.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 26.Blank JB, Cawthon PM, Carrion-Petersen ML, et al. Overview of recruitment for the osteoporotic fractures in men study (MrOS) Contemp Clin Trials. 2005;26:557–68. doi: 10.1016/j.cct.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 27.Rechtschaffen A, Kales A. Maryland: US Dept of Health, Education, and Welfare; National Institutes of Health; 1968. A Manual of Standardized Terminology, Techniques and Scoring System For Sleep Stages of Human Subjects. [Google Scholar]
- 28.Redline S, Sanders MH, Lind BK, et al. Methods for obtaining and analyzing unattended polysomnography data for a multicenter study. Sleep Heart Health Research Group. Sleep. 1998;21:759–67. [PubMed] [Google Scholar]
- 29.EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15:173–84. [PubMed] [Google Scholar]
- 30.Whitney CW, Gottlieb DJ, Redline S, et al. Reliability of scoring respiratory disturbance indices and sleep staging. Sleep. 1998;21:749–57. doi: 10.1093/sleep/21.7.749. [DOI] [PubMed] [Google Scholar]
- 31.Weaver TE, Laizner AM, Evans LK, et al. An instrument to measure functional status outcomes for disorders of excessive sleepiness. Sleep. 1997;20:835–43. [PubMed] [Google Scholar]
- 32.Gooneratne NS, Weaver TE, Cater JR, et al. Functional outcomes of excessive daytime sleepiness in older adults. J Am Geriatr Soc. 2003;51:642–9. doi: 10.1034/j.1600-0579.2003.00208.x. [DOI] [PubMed] [Google Scholar]
- 33.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 34.Cole JC, Motivala SJ, Buysse DJ, Oxman MN, Levin MJ, Irwin MR. Validation of a 3-factor scoring model for the Pittsburgh sleep quality index in older adults. Sleep. 2006;29:112–6. doi: 10.1093/sleep/29.1.112. [DOI] [PubMed] [Google Scholar]
- 35.Buysse DJ, Reynolds CF, 3rd, Monk TH, Hoch CC, Yeager AL, Kupfer DJ. Quantification of subjective sleep quality in healthy elderly men and women using the Pittsburgh Sleep Quality Index (PSQI) Sleep. 1991;14:331–8. [PubMed] [Google Scholar]
- 36.Ware J, Jr., Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–33. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 37.Sheikh JI, Yesavage J. New York: The Haworth Press; 1986. Geriatric depression scale (GDS): recent evidence and development of a shorter version. [Google Scholar]
- 38.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 39.Almeida OP, Almeida SA. Short versions of the geriatric depression scale: a study of their validity for the diagnosis of a major depressive episode according to ICD-10 and DSM-IV. Int J Geriatr Psychiatry. 1999;14:858–65. doi: 10.1002/(sici)1099-1166(199910)14:10<858::aid-gps35>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 40.Kezirian EJ, Harrison SL, Ancoli-Israel S, et al. Behavioral correlates of sleep-disordered breathing in older men. Sleep. 2009;32:253–61. doi: 10.1093/sleep/32.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pahor M, Chrischilles EA, Guralnik JM, Brown SL, Wallace RB, Carbonin P. Drug data coding and analysis in epidemiologic studies. Eur J Epidemiol. 1994;10:405–11. doi: 10.1007/BF01719664. [DOI] [PubMed] [Google Scholar]
- 42.Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46:153–62. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 43.Grunstein R, Wilcox I, Yang TS, Gould Y, Hedner J. Snoring and sleep apnoea in men: association with central obesity and hypertension. Int J Obes Relat Metab Disord. 1993;17:533–40. [PubMed] [Google Scholar]
- 44.Foster GD, Borradaile KE, Sanders MH, et al. A randomized study on the effect of weight loss on obstructive sleep apnea among obese patients with type 2 diabetes: the Sleep AHEAD study. Arch Intern Med. 2009;169:1619–26. doi: 10.1001/archinternmed.2009.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Empana JP, Dauvilliers Y, Dartigues JF, et al. Excessive daytime sleepiness is an independent risk indicator for cardiovascular mortality in community-dwelling elderly: the three city study. Stroke. 2009;40:1219–24. doi: 10.1161/STROKEAHA.108.530824. [DOI] [PubMed] [Google Scholar]
- 46.Foley DJ, Vitiello MV, Bliwise DL, Ancoli-Israel S, Monjan AA, Walsh JK. Frequent napping is associated with excessive daytime sleepiness, depression, pain, and nocturia in older adults: findings from the National Sleep Foundation ‘2003 Sleep in America’ Poll. Am J Geriatr Psychiatry. 2007;15:344–50. doi: 10.1097/01.JGP.0000249385.50101.67. [DOI] [PubMed] [Google Scholar]
- 47.Hays JC, Blazer DG, Foley DJ. Risk of napping: excessive daytime sleepiness and mortality in an older community population. J Am Geriatr Soc. 1996;44:693–8. doi: 10.1111/j.1532-5415.1996.tb01834.x. [DOI] [PubMed] [Google Scholar]
- 48.Ancoli-Israel S, Coy T. Are breathing disturbances in elderly equivalent to sleep apnea syndrome? Sleep. 1994;17:77–83. doi: 10.1093/sleep/17.1.77. [DOI] [PubMed] [Google Scholar]
- 49.Dickel MJ, Mosko SS. Morbidity cut-offs for sleep apnea and periodic leg movements in predicting subjective complaints in seniors. Sleep. 1990;13:155–66. doi: 10.1093/sleep/13.2.155. [DOI] [PubMed] [Google Scholar]
- 50.Ancoli-Israel S, Kripke DF, Klauber MR, et al. Morbidity, mortality and sleep-disordered breathing in community dwelling elderly. Sleep. 1996;19:277–82. doi: 10.1093/sleep/19.4.277. [DOI] [PubMed] [Google Scholar]
- 51.Phillips BA, Berry DT, Lipke-Molby TC. Sleep-disordered breathing in healthy, aged persons. Fifth and final year follow-up. Chest. 1996;110:654–8. doi: 10.1378/chest.110.3.654. [DOI] [PubMed] [Google Scholar]
- 52.Schwartz AR, Smith PL, Wise RA, Gold AR, Permutt S. Induction of upper airway occlusion in sleeping individuals with subatmospheric nasal pressure. J Appl Physiol. 1988;64:535–42. doi: 10.1152/jappl.1988.64.2.535. [DOI] [PubMed] [Google Scholar]
- 53.Beaudreau SA, Spira AP, Stewart A, et al. Validation of the Pittsburgh Sleep Quality Index and the Epworth Sleepiness Scale in older black and white women. Sleep Med. 2012;13:36–42. doi: 10.1016/j.sleep.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Spira AP, Beaudreau SA, Stone KL, et al. Reliability and Validity of the Pittsburgh Sleep Quality Index and the Epworth Sleepiness Scale in Older Men. J Gerontol A Biol Sci Med Sci. 2012;67:433–9. doi: 10.1093/gerona/glr172. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
AHI, apnea hypopnea index; AHI-REM, apnea hypopnea index REM sleep; PSG, polysomnography.
Subject characteristics based on AHI and AHI-REM sleep in subjects with AHI < 5/h in MrOS Sleep Study
Polysomnography Characteristics of subjects with AHI < 5/h in MrOS Sleep Study
Self-reported perceived health status, quality of life measures and sleepiness in subjects with the REM-only OSA vs. those with no OSA in patients with AHI < 5/h in MrOS Sleep Study
Adjusted# means (A) and odds ratios (B) (95%CI) of functional outcome measures by AHI categories


