Abstract
Uncoupling protein 3 (UCP3) is implicated in mild uncoupling and the regulation of mitochondrial ROS production. We previously showed that UCP3 turns over rapidly in C2C12 myoblasts, with a half-life of 0.5–4 h, and that turnover can be reconstituted in vitro. We show here that rapid degradation of UCP3 in vitro in isolated brown adipose tissue mitochondria required the 26S proteasome, ubiquitin, ATP, succinate to generate a high membrane potential, and a pH of 7.4 or less. Ubiquitin containing lysine-48 was both necessary and sufficient to support UCP3 degradation, implying a requirement for polyubiquitylation through this residue. The 20S proteasome did not support degradation. UCP3 degradation was prevented by simultaneously blocking matrix ATP generation and import, showing that ATP in the mitochondrial matrix was required. Degradation did not appear to require a transmembrane pH gradient, but was very sensitive to membrane potential: degradation was halved when membrane potential decreased 10–20 mV from its resting value, and was not significant below about 120 mV. We propose that matrix ATP and a high membrane potential are needed for UCP3 to be polyubiquitylated through lysine-48 of ubiquitin and exported to the cytosolic 26S proteasome, where it is de-ubiquitylated and degraded.
Keywords: uncoupling protein, UCP3, mitochondria, in vitro, ubiquitin, 26S proteasome
1. INTRODUCTION
The uncoupling proteins, UCP1, UCP2 and UCP3, are closely-related members of the mitochondrial anion carrier family, integral membrane proteins that transport metabolic substrates and ions across the mitochondrial inner membrane [1]. The crystal structure is known for one member, the adenine nucleotide translocase (ANT); it has six transmembrane helices and its N- and C-termini extend into the intermembrane space [2]. The primary sequences of other members of the family, including the UCPs, fit comfortably into this ANT structure [3].
UCP1 mediates adaptive thermogenesis in brown adipose tissue. By regulated protonophoric action it lowers the protonmotive force that drives oxidative phosphorylation and so stimulates respiration and heat production [4]. UCP2 protein is found in kidney, pancreas, lung, immune cells, and brain [5, 6], while UCP3 is mainly restricted to skeletal muscle and brown adipose tissue [5]. Though multiple functions for UCP2 and UCP3 have been proposed, there is good evidence that they attenuate production of reactive oxygen species by partially dissipating protonmotive force [6].
UCP2 and UCP3 protein are degraded rapidly in cells, with half-lives of <4 h, and this degradation can be prevented by proteasome inhibitors [7–9]. In contrast, the closely-related UCP1 and ANT display turnover rates of 1–5 days [10, 11], consistent with general mitophagy [10]. Rapid degradation combined with tight transcriptional and translational control allows dynamic regulation of UCP2 protein levels in response to physiological changes [12], implying that protein level is an important control over the activity of these proteins.
Rapid degradation of both UCP2 [8] and UCP3 [9] can be reconstituted in vitro by incubation of isolated, energized mitochondria with purified 26S proteasome and components of the ubiquitylation machinery. Degradation of UCP2 requires the 26S proteasome, ATP, succinate, and protonmotive force [8], leading to a model in which energized mitochondria facilitate the translocation and 26S-targeted degradation of UCP2. Degradation of UCP3 is less well-characterized, but is known to require the 26S proteasome [9] and is presumed to share the same degradation pathway as UCP2.
In the present paper we characterize the molecular components and biochemical conditions required for degradation of UCP3 in vitro. We show that it requires K48-linked polyubiquitylation and matrix ATP, and is very sensitive to small decreases in mitochondrial membrane potential.
2. MATERIALS AND METHODS
2.1 Animals
Female Wistar rats (Harlan Laboratories) were housed at 22 ± 2°C, 45 ± 15% humidity, 12/12 h light/dark cycle, with standard chow and water ad libitum, and tissue was collected at 4–6 weeks of age following sacrifice by CO2 asphyxia and cervical dislocation. All animal experiments were carried out following standards and guidelines of the American Veterinary Medical Association and the Association for the Assessment and Accreditation of Laboratory Animal Care and approved by the Buck Institute Institutional Animal Care and Use Committee (protocol number 10080).
2.2 Mitochondrial isolation
Brown adipose tissue mitochondria were isolated as previously described [9]. Briefly, brown adipose tissue from 1–4 animals was collected into buffer A (250 mM sucrose, 10 mM TES, 1 mM EDTA and 1% w/v defatted BSA) on ice and tissue was chopped then disaggregated using a Dounce homogenizer. The homogenate was filtered through two layers of gauze and centrifuged at 8500 g for 10 min. The pellet was gently resuspended in buffer B (250 mM sucrose, 20 mM TES, 1 mM EGTA and 0.4% w/v BSA) and centrifuged at 700 g for 10 min. The supernatant was centrifuged at 8500g for 10 min, and the final pellet was resuspended in buffer B. Protein concentrations were determined using the biuret method.
2.3 In vitro degradation assay
Isolated mitochondria were incubated with 26S proteasome (Enzo Life Sciences, #PW9310), ubiquitin (Boston Biochem #U-100) and partially purified E1–E3 ligase fractions (Boston Biochem, catalogue number K-960, or Calbiochem, catalogue number 662096), succinate, and an ATP-regenerating system as previously described [9]. The initial reconstitution system (250 µL total volume) contained 1 mg/mL brown adipose tissue mitochondria, 1 mM ATP, 10 mM phosphocreatine, 20 U/ml creatine kinase, ubiquitylation fractions (1.6 µg Fraction 1 and 1.6 µg Fraction 2), 120 µM purified ubiquitin, 12 µg/mL 26S proteasome, and 20 mM succinate. This medium was used for Figs 1 and 2, but the ligase fractions were omitted in Figs 3–7 as Fig. 2 demonstrates their dispensability. Experiments were carried out at 37°C in a modified hypotonic potassium-HEPES medium (50 mM KCl, 5 mM HEPES, 1 mM EGTA, 4 mM KH2PO4, 5 mM MgCl2, pH 7.2 except where noted), or, where indicated in Fig. 1, in a high-sucrose medium [9] comprising 250 mM sucrose, 5 mM Tris-HCl, 2 mM EGTA, pH 7.4. As a negative control, mitochondria were incubated with all components except 26S proteasome. After different incubation times, reaction was stopped by pelleting mitochondria and resuspending in 1X Laemmli protein loading buffer (50 mM Tris-HCl, 10% v/v glycerol, 2% SDS, 1 mM EDTA, 1% v/v β-mercaptoethanol, 0.01% w/v bromophenol blue, pH 6.8) supplemented with 1X complete protease inhibitor (Roche) and 25 mM N-ethylmaleimide. UCP3 levels were subsequently assayed by Western blot.
Figure 1. In vitro reconstitution of UCP3 degradation in mitochondria isolated from brown adipose tissue.
(A) Brown adipose tissue mitochondria were incubated in high-sucrose (STE) (circles) or hypotonic potassium-HEPES (KHE) medium (diamonds) containing all reconstitution components (+, filled symbols) or lacking only the 26S proteasome (−, open symbols) (section 2.3). Reaction was stopped at the times shown and the samples were assayed by Western blot. All data are normalized first to UCP1 in the same lane and then to the appropriate zero timepoint, and represent means ± SEM (n = 6 independent experiments). (B) Representative immunoblot of mean intensity. Proteins (5 µg of protein/lane) were separated by SDS/PAGE and immunoblotted for UCP3 and reblotted for UCP1. (C) Statistical significance was determined by calculating the area under the curve and performing one-way ANOVA on the resulting values. ctl, control lacking 26S proteasome. Data represent individual values with means ± SEM. (D) Intensity of signals obtained by Western blot for UCP3 and UCP1 for different loadings of total mitochondrial protein. Data represent means ± SEM (n = 3–4). (E) quantification of the signal intensity of UCP1 (circles) and ANT (squares) during incubation under degradation conditions (KHE, reconstitution components, 26S proteasome). Raw band intensity was normalized to total protein loaded (n = 11)
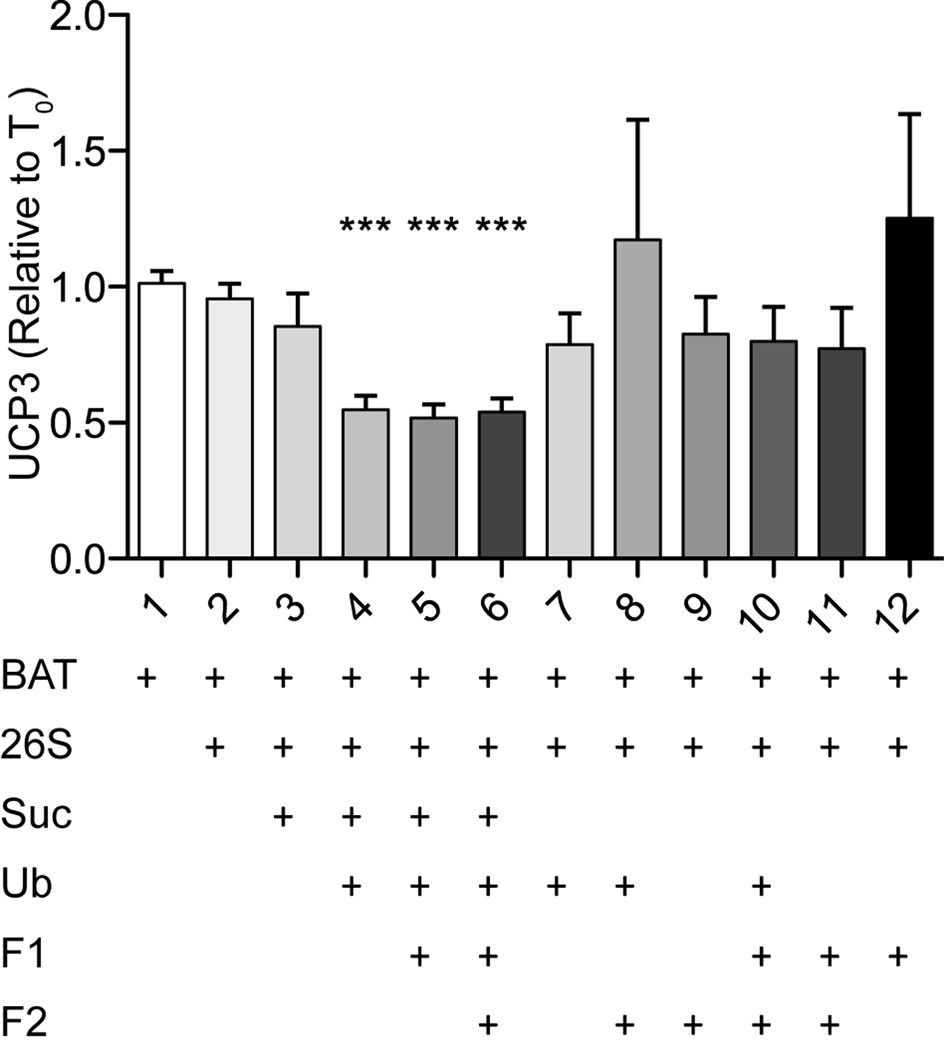
Figure 2. Components required for UCP3 degradation in vitro.
Degradation of UCP3 in brown adipose tissue mitochondria in 12 variants of the in vitro assay in the presence (+) or absence (blank) of the components indicated. Bars represent Western blot intensities at 240 min incubation relative to time zero. Values are means ± SEM (n = 8–9 independent experiments). BAT, mitochondria isolated from brown adipose tissue; 26S, purified 26S proteasome; Suc, succinate; Ub, purified ubiquitin; F1, partially purified cellular fraction containing E1-E2 ligases; F2, partially purified cellular fraction containing E3 ligases and deubiquitylases.
Figure 3. Ubiquitin mutants alter UCP3 degradation in vitro.
(A) Degradation of UCP3 in brown adipose tissue mitochondria in vitro in the presence of different purified mutant ubiquitins (Boston Biochem UM-K48R, UMK480, U-501). Symbols: -26S (open circles): all required in vitro components, including wild-type ubiquitin, minus the 26S proteasome. Ub (closed circles): wildtype ubiquitin. Ub-K48R (open diamonds) contains a Lys-Arg substitution at position 48, disrupting polymerization. Ub-K48O (closed diamonds) retains Lys48, while the six remaining Lys residues are substituted with Arg. Ub-Ch4 (closed inverted triangles) is methylated at the terminal amines of all Lys residues, blocking polymerization. No Ub (open inverted triangles) no added ubiquitin. (B) Area under the curve values of the individual experiments in (A). Data in (A) and (B) depict means ± SEM (n = 4–7).
Figure 7. Requirement for the 26S proteasome in UCP3 degradation in vitro.
(A) Chymotrypsin-like peptidase cleavage of suc-LLVY-amc by purified 20S and 26S proteasomes under degradation assay conditions. (B) Degradation of UCP3 in brown adipose tissue mitochondria in vitro using either 20S or 26S proteasomes. Symbols: -26S (open circles): all required in vitro components minus the 26S proteasome. 26S (closed circles): all required in vitro components, 20S (closed triangles): 20S core proteasome. Data represent means ± SEM (n = 4).
2.4 Immunoblots
Samples (5 µg mitochondrial protein) were resolved by 10% SDS-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes (Amersham Biosciences). Western blot analysis was performed with the indicated primary antibodies (UCP3: PA1-044, Thermo; UCP1: U6382, Sigma) overnight at 4°C and appropriate secondary antibodies (31463, Pierce) for 1 h at ~20°C. Protein was visualized with enhanced chemiluminescence (Amersham) and X-ray film. For stripping and re-probing, blots were incubated with Restore Stripping Buffer (Pierce) and washed in 1X TBST (20 mM Tris-HCl, 137 mM NaCl, 0.1% Tween 20, pH 7.6) prior to re-probing with additional primary antibody.
2.5 Proteasome activity
Chymotrypsin-like peptidase activities of the 20S (Boston Biochem) and 26S (Enzo) proteasomes were measured using the fluorogenic substrate Suc-LLVY-amc (S-280, Boston Biochem). Proteasome was incubated with 50 µM substrate in degradation assay buffer (above) and samples were read on a Molecular Devices microplate reader at em-ex 380–460 using SoftMax Pro software. Fluorescence was calibrated to free aminomethylcoumarin to give activity in pmol substrate/min/µg protein.
2.6 Mitochondrial membrane potential
Mitochondrial membrane potential was measured using a triphenylmethyl phosphonium (TPMP+)-sensitive electrode in an oxygen electrode chamber (Rank Bros, UK) monitored by a Powerlab 4/20 (AD Instruments) and analyzed with Powerlab Chart software [13]. Brown adipose tissue mitochondria (0.35 mg protein/mL) were incubated in hypotonic potassium-HEPES medium as above, with the addition of 5 µM rotenone, 0.1 µM nigericin, 1 µg/mL oligomycin, and 2 µM TPMP. To mimic the in vitro degradation assay, ubiquitin, succinate, and the ATP regenerating system components were added. To titrate membrane potential in Fig. 5A–C, different ratios of succinate and malonate were added to a combined final concentration of 20 mM. For Fig. 5D, 20 mM succinate was added to an incubation containing nigericin, oligomycin and TPMP as above, and aliquots were placed in the TPMP electrode chamber at the times shown and the signal was recorded. After depolarization by addition of 30 µM FCCP, four further additions of 0.5 µM TPMP were made, allowing back-calibration of the TPMP signal. For consistency, the experiment in Fig. 5A was calibrated in the same way. This departure from the standard calibration [13] caused an underestimation of membrane potential by 15–20 mV.
Figure 5. Effect of mitochondrial membrane potential on UCP3 degradation in vitro.
Isolated brown adipose tissue mitochondria incubated under degradation assay conditions (all required components minus 26S proteasome) with the addition of nigericin and oligomycin, which do not alter UCP3 degradation kinetics (Fig 4). (A) Membrane potential of isolated brown adipose tissue mitochondria approximately 3 minutes after substrate addition, incubated under degradation assay conditions at different malonate concentrations (combined succinate plus malonate kept at 20 mM). Data represent means ± SEM (n = 3). (B) Degradation of UCP3 in brown adipose tissue mitochondria in vitro in the presence of identical malonate/succinate conditions as in (A). Symbols: -26S (open circles): all required in vitro components minus the 26S proteasome. 26S (closed circles): all required in vitro components. Data represent area under the curve individual values and means ±SEM. (C) Dependence of UCP3 degradation on membrane potential. Area under the curve values from (B) plotted against the membrane potentials from (D) Membrane potential over time under in vitro degradation assay conditions with the addition of TPMP. Samples were removed at times indicated for membrane potential measurement (section 2.6). Dotted line at 140 marks a 20 mV drop from resting membrane potential. Data represent means ± SEM (n = 3). Inset: cytochrome c release from isolated mitochondria during in vitro incubation, plotted as nmol cyt c per mg total mitochondrial protein against time. Datapoints at 0 and 120 min represent n = 5 technical replicates; error bars are within data symbols. (E) Membrane potential measurements 60 minutes after substrate addition under degradation assay conditions, plus and minus 26S proteasome.
2,7 Cytochrome c release
Isolated mitochondria (1 mg protein) were incubated under the degradation assay conditions in 1 mL of hypotonic potassium-HEPES buffer as described in Section 2.3. At each time point, samples were centrifuged at 10,000 × g for 10 min. The resulting supernatant was removed and frozen for subsequent analysis. Cytochrome c levels were determined by measuring the 414 nm peak of non-reduced samples using a dual-beam spectrophotometer zeroed with incubation buffer. Total cytochrome c levels were determined by addition of 0.5% w/v sodium deoxycholate to the incubated samples, permeabilizing mitochondria prior to centrifugation.
2.8 Data analysis
UCP3 degradation was monitored by quantitative Western blot. Blots were scanned to generate digital images and analyzed using NIH ImageJ software. All UCP3 band intensities were normalized to UCP1 before further analysis. The resulting degradation profiles were used to calculate area under the curve values using trapezoidal curve fitting with a cutoff at 0.5. Statistical analysis was performed using repeated-measures ANOVA against negative control conditions followed by Dunnet’s post-hoc testing. *p<0.05; **p<0.01; ***p<0.005. Where appropriate, one-phase decay curves were fitted to degradation profiles as trend lines and to allow crude estimation of half-lives. Such curve fits likely overestimate the true half-life, as decreases in membrane potential during the incubation (Fig. 5) slow degradation, invalidating an assumption of one-phase decay for the total incubation period. However, one-phase decay curves clarify the plotted data, and so are included here. To determine statistical differences between treatments, values of area under the curve were preferred, to minimize assumptions about the type of curve. Where shown, representative blot images were chosen for best visual fit to the mean values plotted after normalization to UCP1 as a protein loading control.
3. RESULTS
3.1 UCP3 degradation in vitro
UCP3 degradation in vitro was previously investigated in a high-sucrose medium. It is possible that the resulting condensation of mitochondrial cristae alters UCP3 degradation [9]. For the present studies, we used a hypo-osmotic potassium-based medium designed to optimize the respiratory activity of brown adipose tissue mitochondria by expanding the cristae [14]. UCP3 degradation was similar in the two buffers (Fig. 1A), showing that the UCP3 degradation mechanism is largely unaffected by osmotic and other differences between the two media. Figs 1A and 1B show that UCP3 was degraded only in the presence of the 26S proteasome, while Figs 1B and 1E demonstrate that UCP1 and ANT were not similarly degraded, confirming previous results [9]. Fig. 1C shows the statistical analysis of the data in Fig. 1A. In the total protein range loaded per well, (5 µg protein), the band intensities for UCP3, UCP1, and ANT were dependent on total protein loaded (Fig. 1D), validating the use of signal intensity to quantify relative protein levels. UCP1 and ANT levels remained constant as a function of time during the degradation assay (Fig. 1E)
3.2 Requirements for UCP3 degradation in vitro
Rapid degradation of UCP2 in intact cells requires the membrane potential component of the protonmotive force, and is prevented by proteasome inhibitors [8]. Degradation of UCP2 in vitro requires addition of succinate and the 26S proteasome, and the presence of protonmotive force [8]. We have previously shown that UCP3 degradation in cells is also rapid and prevented by proteasome inhibition [9], and that degradation of UCP3 in vitro in the presence of a cocktail of ingredients that supports degradation of UCP2 requires the 26S proteasome ([8], Fig. 1B), but otherwise nothing is known about the requirements for turnover of UCP3.
To determine the components required for UCP3 degradation in isolated mitochondria in vitro, we added the minimal components of an ATP regenerating system (needed to support proteasome activity), and the 26S proteasome. We then tested different additions of succinate, purified ubiquitin, and partially characterized ubiquitin E1/E2 (Fraction 1) and E3/de-ubiquitylase (Fraction 2) ligase fractions. Only assays containing both succinate and ubiquitin supported degradation of UCP3 (Fig. 2). The requirement for succinate presumably reflects the requirement for protonmotive force (see below). Further addition of the ubiquitin ligase fractions did not enhance degradation, showing that these components are dispensable in vitro and suggesting that the ubiquitin ligase machinery that is presumably needed for UCP3 degradation is already present in the isolated brown adipose tissue mitochondrial preparation.
3.3 Ubiquitin Lys-48 is necessary and sufficient for UCP3 degradation
Since ubiquitylation of proteins occurs by addition of either monomers or polymers of ubiquitin, with linkages through one or more of ubiquitin's seven lysine residues [15], we tested which properties of ubiquitin were required for successful UCP3 degradation (Fig. 3A). Use of purified mutant ubiquitins in the in vitro assay revealed that Ub-CH4 (which supports only mono-ubiquitylation) was unable to support UCP3 degradation, suggesting that polyubiquitylation is required. Ub-K48R (lacking lys-48) was also unable to support UCP3 degradation, suggesting that K48-linked polyubiquitin is necessary to support proteasomal degradation of UCP3. Further, a ubiquitin mutant with Lys-Arg substitutions at every residue except K48 (K48O, K48-only) did support UCP3 degradation, confirming the sufficiency of K48 for proper degradation of UCP3 by the 26S proteasome. Only wild-type ubiquitin and the K48O mutant could significantly decrease UCP3 levels (Fig. 3B). Thus, K-48 linkage of ubiquitin is both necessary and sufficient to support UCP3 degradation in vitro.
3.4 UCP3 degradation requires matrix ATP
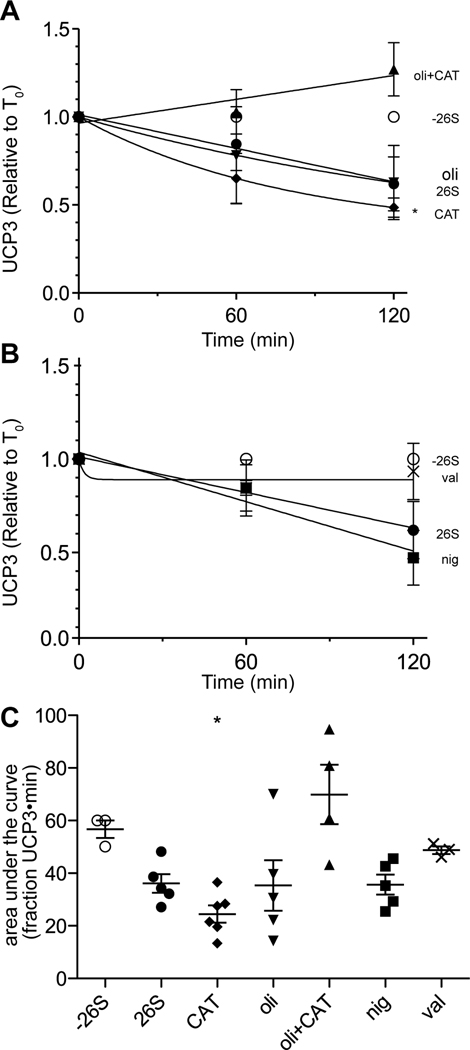
Since the assembly and activity of the 26S proteasome is ATP-dependent [16], extramitochondrial ATP is required for proteasomal degradation of UCP2 and UCP3 in vitro. We investigated whether ATP is also required for intramitochondrial steps of UCP3 degradation. We assayed degradation in the presence of carboxyatractylate (CAT), which blocks the transport of ATP into the matrix on the adenine nucleotide translocase, and oligomycin, which inhibits the FoF1 ATP synthase and prevents ATP synthesis in the mitochondrial matrix. Treatment with CAT or oligomycin alone had no effect on UCP3 degradation. Together, however, they blocked UCP3 degradation (Fig. 4A,C). With oligomycin inhibiting ATP synthesis, matrix ATP can still be maintained through import by the ANT. Conversely, CAT blocks the ANT, but matrix ATP can be generated by the ATP synthase. Thus, only with both inhibitors present is matrix ATP not maintained. The inhibition of UCP3 degradation under these conditions demonstrates the requirement for matrix ATP to support UCP3 degradation by the proteasome.
Figure 4. Effects of bioenergetic inhibitors and ionophores on UCP3 degradation in vitro.
Degradation of UCP3 in brown adipose tissue mitochondria in vitro in the presence of (A) 1 µg/mL oligomycin (oli), 2.5 µM carboxyatractylate (CAT), or both, or (B) 0.1 µM nigericin (nig) or 0.5 µM valinomycin (val). Symbols: -26S (open circles): all required in vitro components minus the 26S proteasome. 26S (closed circles): all required in vitro components, including 26S proteasome. CAT: closed diamonds. Oli closed inverted triangles. Oli+CAT: triangles. Nig: closed squares. Val: “x” symbols. (C) Area under the curve values of the individual experiments in (A) and (B). Data represent means ± SEM (n = 4–6).
3.5 UCP3 degradation in vitro requires membrane potential but not a pH gradient
In high-potassium medium, nigericin (a K+/H+ exchanger) collapses the pH gradient across the mitochondrial membrane and increases the membrane potential [17]. UCP3 degradation was not obviously affected by addition of nigericin (Fig. 4B,C), suggesting that a transmembrane pH gradient is not required. Addition of valinomycin, which collapses the electrical potential (and, by causing swelling, also fully dissipates the protonmotive force), prevented UCP3 degradation. Thus, degradation of UCP3, like degradation of UCP2 [8], appears to be independent of the pH gradient, but requires the membrane potential component of protonmotive force.
3.6 Sensitivity of UCP3 degradation to mitochondrial membrane potential
We investigated the dependence of UCP3 degradation on membrane potential. Fig. 5A shows a titration of membrane potential with malonate (a competitive inhibitor of succinate oxidation) performed under standard degradation assay conditions. The combined concentration of succinate plus malonate was kept at 20 mM. UCP3 degradation was measured under identical conditions (Fig. 5B); degradation was not significantly greater than the negative control (minus 26S) when malonate made up 3% or more of the substrate mixture. Plotting UCP3 degradation (Fig. 5B) against membrane potential (Fig. 5A) in Fig. 5C revealed that UCP3 degradation was very sensitive to membrane potential: it decreased sharply as membrane potential dropped by 20 mV from the fully energized state. At lower potentials UCP3 degradation appeared to decrease further, but the degradation was not significantly different from zero (Fig. 5B), showing that significant rapid UCP3 degradation requires a high potential, above about 120 mV.
This acute sensitivity to membrane potential may explain the lack of complete degradation in vitro of UCP3 ([9] and the present paper) (and UCP2 [8]), since mitochondria may not be able to maintain a high membrane potential when incubated at 37°C for more than 2 h (e.g., Fig. 1). To examine this possibility, we incubated mitochondria under in vitro degradation conditions minus proteasome and removed samples at various times for measurement of membrane potential (Fig. 5D). By 60–90 min, the membrane potential had fallen by 10–20 mV. This likely explains why degradation of UCP3 in vitro slows or even stops prior to completion, and suggests that the kinetics of decay would be faster and more complete if potential was maintained than they are in these in vitro degradation assays where the mitochondria are not fully functional after an hour or so at 37°C. To examine the possibility that the 26S proteasome affects membrane potential over time, we measured membrane potential at 60 min in the presence of 12 µg/mL purified 26S proteasome and found no differences between samples minus and plus proteasome (Fig. 5E).
Though UCP3 degradation kinetics are the same in cells and in vitro, it is possible that the in vitro degradation mechanism is affected by prolonged exposure to a hypotonic medium, allowing swelling and perhaps breakage of the outer membrane. To test this, we assayed mitochondrial outer membrane permeability by monitoring cytochrome c release into the incubation medium under in vitro degradation conditions. We found that by 60 min, the apparent time threshold of a functioning UCP3 degradation mechanism, approximately 11% of the total cytochrome c was detectable in the supernatant of samples minus and plus 26S proteasome (Fig. 5D, inset). Under identical conditions, 40–50% of UCP3 is degraded when 26S proteasome is present. Addition of 10 µM FCCP, which abolishes both membrane potential and UCP3 degradation (experiments not shown), increased cytochrome c release, further separating UCP3 degradation from outer membrane instability. This demonstrates that breakage of the outer membrane is not the underlying cause driving UCP3 degradation by exogenous 26S proteasome.
3.7 pH dependence UCP3 degradation in vitro
We tested whether changing the pH within the optimal range of proteasome activity (pH 7–8) affected UCP3 degradation (Fig. 6A,B). Varying the extramitochondrial pH in steps of 0.2 units revealed a dramatic shift in the ability to degrade UCP3. Degradation was optimal between pH 7.0 and 7.4, peaking at 7.2, and absent at 7.6 and above. Since the ubiquitin hydrolase activity of the 19S regulatory particle of the 26S proteasome has a pH optimum slightly more alkaline than does the 20S core, this may implicate 19S regulatory particle activity in the pathway of degradation of UCP3.
Figure 6. Effect of pH on UCP3 degradation in vitro.
(A) Degradation of UCP3 in brown adipose tissue mitochondria in vitro at different pH values. Symbols: -26S (open circles): all required in vitro components minus the 26S proteasome. Closed symbols: all required in vitro components, varying pH. (B) Area under the curve values of the individual experiments in (A). Data represent means ± SEM (n = 3–6).
3.8 Involvement of the assembled 26S proteasome in UCP3 degradation
To differentiate between a requirement for the 20S core particle and a fully assembled 26S proteasome (including the19S regulatory particle), we compared the abilities of the 26S proteasome and the 20S proteasome core particle to degrade UCP3. Fig. 7A confirms that both the 20S and 26S proteasomes were active under the in vitro degradation assay conditions. Fig. 7B shows that only the 26S proteasome supported UCP3 degradation. These data suggest that UCP3 degradation depends on an intact 26S complex, including its de-ubiquitylation and protein unfolding activities.
4. DISCUSSION
In the work presented here we identify the molecular components (K48 ubiquitin, assembled 26S proteasome) and mitochondrial status (matrix ATP present, mitochondrial membrane potential within 20 mV of fully energized) required for rapid UCP3 degradation in isolated mitochondria. This in vitro approach has important advantages: it allows positive confirmation of the necessary components, and permits extensive manipulation to refine our understanding of mechanisms. The results reported here suggest several potential regulatory steps for UCP3 degradation.
We show that the presence of ubiquitin, or more specifically, K48-linked polyubiquitin, is required for UCP3 degradation. These data support the hypothesis that UCP3 degradation occurs through ubiquitin-dependent targeting of UCP3 to the 26S proteasome by a K48-linked polyubiquitin moiety. Moreover, the dispensability of additional ubiquitylation components suggests that the necessary machinery is already present in the isolated mitochondrial fractions used in these experiments. These data suggest that ubiquitylation of UCP3 (and UCP2 [8]) represents a major regulatory step in its degradation. The inability of the 20S proteasome to support UCP3 degradation in vitro is also consistent with ubiquitin-dependent targeting, which generally requires the fully assembled 26S proteasome [18]. This feature potentially allows UCP3 degradation to be controlled by the concentration of 26S complexes, which in turn is regulated by factors including oxidative stress [19, 20].
Our demonstration of the high sensitivity of UCP3 degradation to mitochondrial membrane potential is particularly interesting and invites speculation. A high membrane potential is required to promote hydroxynonenal activation of mild uncoupling by UCP3 [21, 22]. Together, these observations suggest that UCP3 adopts an 'active' conformation at high membrane potential, in which its uncoupling function can be activated by hydroxynonenal, and it can also be tagged for degradation by ubiquitylation. Since high protonmotive force can also cause high mitochondrial production of reactive oxygen species [23], this suggests a scenario in which transient high protonmotive force increases superoxide production, primes UCP3 for acute activation to cause mild uncoupling (attenuating protonmotive force and superoxide production), and simultaneously primes UCP3 for degradation so that its activated state is rapidly destroyed by protein turnover and the system can quickly return to its fully coupled state when the transient stress has passed. In this way, UCP3 could provide, over tens of minutes, both a short-term memory of peaks of high membrane potential, and a transient defense against the resulting and future high oxidative stress. A complementary function of UCP3 could be to act as an adaptor in the mitochondrial membrane between protonmotive force and cellular machinery. The decreased degradation of UCP3 at slightly low membrane potential will lead to its accumulation in the membrane, and, through interaction with other proteins, this accumulation could report the depolarization to extramitochondrial pathways, such as transcriptional regulation (e.g., to stimulate mitochondrial biogenesis) or mitophagy (e.g., to degrade dysfunctional mitochondria).
The high sensitivity of UCP3 degradation to membrane potential may also explain a feature of the in vitro assay. The membrane potential falls enough within the first 60 min of incubation at 37°C (Fig. 5D) to significantly slow UCP3 degradation, perhaps explaining why degradation is incomplete. If so, decay from 0–60 min may most accurately model fully active UCP3 degradation in vitro, giving a t1/2 of 30 min or less. In some cases (e.g., Fig. 4) there is further UCP3 loss after 60 min, which may indicate residual activity, or a “primed” degradation mechanism in which initiation is potential-dependent but subsequent steps are not.
Alternative explanations for UCP3 degradation in vitro involve potential artifacts of the assay conditions and UCP3 detection methods. For example, the extended mitochondrial incubation period could alter mitochondrial integrity or activity (e.g., by swelling) in a way that facilitates UCP3 clearance or removal of the epitope-containing C-terminus. However, the rapid degradation of UCP3 in vitro matches its turnover in cell culture [9] and the turnover of UCP2 (detected by an internal eiptope) in cells and in vitro [8], making a physiological, rather than artifactual mechanism more likely. In addition, an artifact would be more likely to affect other, similar mitochondrial carriers. We do not observe rapid turnover of UCP1 or ANT, suggesting a selective mechanism.
5. CONCLUSION AND PERSPECTIVE
These data expand our current understanding of the mechanism by which UCP3 (and probably UCP2) are degraded. Based on evidence presented here, we propose that high mitochondrial membrane potential allows initiation of degradation through a conformational change, allowing UCP3 to be polyubiquitylated. Following its modification, either UCP3 or its polyubiquitin tag is exposed to the outer mitochondrial surface. The 19S regulatory cap of the 26S proteasome then recognizes and binds UCP3, and mediates its de-ubiquitylation and proteasomal degradation. At least one step in this process requires matrix ATP.
Highlights.
-
-
The rapid turnover of UCP3 was characterized in vitro.
-
-
UCP3 degradation requires matrix ATP and a high membrane potential.
-
-
K48-linked polyubiquitylation is necessary and sufficient to promote UCP3 degradation.
-
-
UCP3 degradation is highly sensitive to small changes in membrane potential.
ACKNOWLEDGEMENTS
This work was supported by the National Institutes of Health [grant numbers P01 AG025901, PL1 AG032118, P30 AG025708] and California Institute for Regenerative Medicine (CIRM) [S.A.M., grant number TG2-00152]
Abbreviations used
- UCP
uncoupling protein
- ANT
adenine nucleotide translocase
- TPMP
triphenylmethylphosphonium
- CAT
carboxyatractylate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
Shona A. Mookerjee and Martin D. Brand designed the experiments and wrote the manuscript. Shona A. Mookerjee performed all experiments and data analysis.
References
- 1.Kunji ERS. The role and structure of mitochondrial carriers. FEBS Lett. 2004;564:239–244. doi: 10.1016/S0014-5793(04)00242-X. [DOI] [PubMed] [Google Scholar]
- 2.Pebay-Peyroula E, Dahout-Gonzalez C, Kahn R, Trézéguet V, Lauquin GJ, Brandolin G. Structure of mitochondrial ADP/ATP carrier in complex with carboxyatractyloside. Nature. 2003;426:39–44. doi: 10.1038/nature02056. [DOI] [PubMed] [Google Scholar]
- 3.Robinson AJ, Kunji ERS. Mitochondrial carriers in the cytoplasmic state have a common substrate binding site. Proc Natl Acad Sci USA. 2006;103:2617–2622. doi: 10.1073/pnas.0509994103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nicholls DG. A history of UCP1. Biochem Soc Trans. 2001;29:751–755. doi: 10.1042/bst0290751. [DOI] [PubMed] [Google Scholar]
- 5.Vidal-Puig AJ, Solanes G, Grujic D, Flier JS, Lowell BB. UCP3: an uncoupling protein homologue expressed preferentially and abundantly in skeletal muscle and brown adipose tissue. Biochem. Biophys. Res. Commun. 1997;235:79–82. doi: 10.1006/bbrc.1997.6740. [DOI] [PubMed] [Google Scholar]
- 6.Brand MD, Esteves TC. Physiological functions of the mitochondrial uncoupling proteins UCP2 and UCP3. Cell Metab. 2005;2:85–93. doi: 10.1016/j.cmet.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 7.Rousset S, Mozo J, Dujardin G, Emre Y, Masscheleyn S, Ricquier D, Cassarddoulcier A. UCP2 is a mitochondrial transporter with an unusual very short half-life. FEBS Lett. 2007;581:479–482. doi: 10.1016/j.febslet.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 8.Azzu V, Brand MD. Degradation of an intramitochondrial protein by the cytosolic proteasome. J Cell Sci. 2010;123:578–585. doi: 10.1242/jcs.060004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Azzu V, Mookerjee SA, Brand MD. Rapid turnover of mitochondrial uncoupling protein 3. Biochem J. 2010;426:13–17. doi: 10.1042/BJ20091321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moazed B, Desaultes M. Differentiation-dependent expression of cathepsin D and importance of lysosomal proteolysis in the degradation of UCP1 in brown adipocytes. Canadian Journal of Physiological Pharmacology. 2002;80:515–525. doi: 10.1139/y02-067. [DOI] [PubMed] [Google Scholar]
- 11.Puigserver P, Herron D, Gianotti M, Palou A, Cannon B, Nedergaard J. Induction and degradation of the uncoupling protein thermogenin in brown adipocytes in vitro and in vivo. Evidence for a rapidly degradable pool. Biochem J. 1992;284:393–398. doi: 10.1042/bj2840393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Azzu V, Affourtit C, Breen E, Parker N, Brand MD. Dynamic regulation of uncoupling protein 2 content in INS-1E insulinoma cells. Biochim Biophys Acta. 2008;1777:1378–1383. doi: 10.1016/j.bbabio.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parker N, Crichton PG, Vidal-Puig AJ, Brand MD. Uncoupling protein-1 (UCP1) contributes to the basal proton conductance of brown adipose tissue mitochondria. J Bioenerg Biomembr. 2009;41:335–342. doi: 10.1007/s10863-009-9232-8. [DOI] [PubMed] [Google Scholar]
- 14.Cannon B, Nedergaard J. Studies of thermogenesis and mitochondrial function in adipose tissues. Methods Mol Biol. 2008;456:109–121. doi: 10.1007/978-1-59745-245-8_8. [DOI] [PubMed] [Google Scholar]
- 15.Kerscher O, Felberbaum R, Hochstrasser M. Modification of proteins by ubiquitin and ubiquitin-like proteins. Annu Rev Cell Dev Biol. 2006;22:159–180. doi: 10.1146/annurev.cellbio.22.010605.093503. [DOI] [PubMed] [Google Scholar]
- 16.Kleijnen MF, Roelofs J, Park S, Hathaway NA, Glickman M, King RW, Finley D. Stability of the proteasome can be regulated allosterically through engagement of its proteolytic active sites. Nat Struct Mol Biol. 2007;14:1180–1188. doi: 10.1038/nsmb1335. [DOI] [PubMed] [Google Scholar]
- 17.Nicholls DG, Ferguson SJ. Bioenergetics. 3rd ed. San Diego: Academic Press; 2002. [Google Scholar]
- 18.Glickman MH, Ciechanover A. The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev. 2002;82:373–428. doi: 10.1152/physrev.00027.2001. [DOI] [PubMed] [Google Scholar]
- 19.Wang X, Yen J, Kaiser P, Huang L. Regulation of the 26S proteasome complex during oxidative stress. Sci Signal. 2010;3:ra88. doi: 10.1126/scisignal.2001232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shringarpure R, Grune T, Mehlhase J, Davies KJA. Ubiquitin conjugation is not required for the degradation of oxidized proteins by proteasome. J Biol Chem. 2003;278:311–318. doi: 10.1074/jbc.M206279200. [DOI] [PubMed] [Google Scholar]
- 21.Parker N, Vidal-Puig AJ, Brand MD. Stimulation of mitochondrial proton conductance by hydroxynonenal requires a high membrane potential. Biosci Rep. 2008;28:83. doi: 10.1042/BSR20080002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parker N, Affourtit C, Vidal-Puig A, Brand MD. Energization-dependent endogenous activation of proton conductance in skeletal muscle mitochondria. Biochem. J. 2008;412:131. doi: 10.1042/BJ20080006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brand MD, Affourtit C, Esteves TC, Green K, Lambert AJ, Miwa S, Pakay JL, Parker N. Mitochondrial superoxide: production, biological effects, and activation of uncoupling proteins. Free Radic Biol Med. 2004;37:755–767. doi: 10.1016/j.freeradbiomed.2004.05.034. [DOI] [PubMed] [Google Scholar]