Abstract
Acute lymphoblastic leukemia (ALL) is the most common cancer in children. The current treatment protocol for ALL involves an intense chemotherapy regimen yielding cure rates of nearly 80%. However, new therapies need to be designed not only to increase the survival rate but also to combat the risk of severe therapy associated toxicities including secondary malignancies, growth problems, organ damage, and infertility. The c-Myb proto-oncogene is highly expressed in immature hematopoietic cells. In this study, we demonstrate that loss of c-Myb itself decreased the viability of these leukemic cells. Additionally, the inhibition of c-Myb caused a decrease in cell proliferation, significantly increased the number of cells in G0/G1 phase of the cell cycle, increased the sensitivity of pre-B-ALL cells to cytotoxic agents in vitro, and significantly delayed disease onset in a mouse model of leukemia. Furthermore, we demonstrate that Bcl-2 is a target of c-Myb in pre-B-ALL cells. Our results identify c-Myb as a potential therapeutic target in pre-B-ALL and suggest that suppression of c-Myb levels or activity, in combination with currently used therapies and/or dose reduction, may lead to a decrease in toxicity and an increase in patient survival rates. Because c-Myb is aberrantly expressed in several other malignancies, targeting c-Myb will have broad clinical applications.
Introduction
Acute lymphoblastic leukemia (ALL) is the most common malignancy in children, with nearly 4,000 cases being diagnosed annually in the United States [1,2]. ALL is thought to originate from various genetic lesions in hematopoietic cells that are committed to differentiate in the T-cell or B-cell pathway, including mutations that impart the capacity for unlimited self renewal and those that lead to precise stage specific developmental arrest [3]. ALL leads to overproduction of white blood cells in the bone marrow, and it is classified based on the immunophenotype of the malignant lymphoblasts [4]. The majority of patients diagnosed with pediatric leukemias fall into the precursor-B subtypes, and there are fewer cases of mature B and T-cell immunophenotypes [2,4]. Improvements in treatment protocols, including an intensive reinduction course and prolonged maintenance therapy, have increased the cure rates from 15 to 75–80% [1,5]. However, close to 1 in 4 children suffer a recurrence, and their outcome is dismal [1]. Drug resistance remains a major contributor to treatment failure in ALL. Additionally, significant risks of both short- and long-term toxicities, which include infertility, secondary malignancies, auditory complications, cardiovascular dysfunction, gastrointestinal/hepatic dysfunction, neurocognitive sequelae, and growth delay, persist [6]. Furthermore, the incidence of severe late effects is ~25% in pediatric cancer survivors [6,7]. It is also challenging to treat children with relapsed ALL, and their survival rate remains poor with contemporary treatment protocols [8]. A t(1;19) chromosomal translocation is commonly found in pre-B-ALL, resulting in the fusion of two transcription factors, E2A and PBX1 [9,10]. Patients possessing the E2A-PBX1 translocation have a poor prognosis relative to other ALL biologic subsets [11]. Thus, new targets need to be developed for more effective treatment regimens for resistant and relapsed ALL patients and also to increase the efficacy and/or reduce the toxicity of current chemotherapy regimens.
c-Myb is the cellular progenitor of the v-Myb oncogene, which is carried by the avian myeloblastosis virus and E26 retroviruses [12,13]. The c-Myb transcription factor is highly expressed in immature and proliferative cellular stages, and its expression is turned off during maturation of the hematopoietic lineage [14–17]. The murine c-Myb locus is also a common site of retroviral insertion in lymphoid and myeloid leukemia [18–22]. In humans, the c-Myb gene is located at chromosomal band 6q23.3, and interestingly, chromosome 6q is frequently involved in chromosomal abnormalities in human cancer including hematologic malignancies [23]. Furthermore, duplication of c-Myb is also seen in a subset of T-ALL patients. However, despite intensive studies in a large range of human neoplasias, the role of c-Myb in pediatric B-ALL remains unknown and needs to be elucidated.
In this study, we wished to determine the role of c-Myb in pre-B-ALL cells. The cell line used for this study was 697 pre-B-ALL cells that are E2A-PBX1 positive and hence a valuable model as they may simulate patients with poor prognosis. We used a controllable lentiviral based shRNA, specific for c-Myb, as a tool to manipulate c-Myb levels and study the role of c-Myb in pre-B-ALL. Using both in vitro and in vivo models, we provide compelling evidence that c-Myb inhibition increases the sensitivity of the cells to chemotherapeutic drugs and significantly delays the onset of disease in a xenograft mouse model of leukemia. These data suggest that targeting c-Myb may be particularly powerful in the treatment of pre-B-ALL.
Materials and Methods
Cell lines and cell culture
HEK-293T cells (ATCC, CRL-11268, Manassas, VA) were grown in DMEM supplemented with 10% fetal bovine serum (Invitrogen, Carlsbad, CA) and 4 mM L-glutamine, and they were incubated at 37° C and 5% CO2. The human pre-B-ALL cell line, 697, was obtained from Dr. Noreen M. Robertson, Drexel University School of Medicine, Philadelphia, PA. These cell lines and their transduced derivatives (697 sh-c-Myb and 697 sh-NSC) were grown in RPMI 1640 culture medium supplemented with 10% fetal bovine serum and 2 mM L-glutamine, and they were incubated at 37° C and 5% CO2.
Ethics statement
All experiments involving animals conformed to the relevant regulatory standards as approved by the Louisiana State University Institutional Animal Use and Care Committee.
Xenograft mouse model of leukemia
Six-week-old female non-obese-diabetic/severe-combined-immunodeficient NOD.CB17-Prkdcscid/J mice (NOD-Scid; The Jackson Laboratory) were sublethally irradiated (335 Rads) 24 hr before injection using Gammacell® 40 Exactor (MDS Nordion, Ontario, Canada). The mice were then injected intravenously with 1 × 107 697 sh-c-Myb cells and 697 sh-NSC (control) cells. On day 7 after tumor injection, the mice were injected intraperitoneally with 10 mg/kg of doxycycline (Doxy) three times a week to maintain the knockdown of c-Myb in vivo. As controls, mice were injected with phosphate buffer saline (PBS), starting at day 7 after tumor injection and were also treated three times a week with Doxy. The mice were monitored daily and sacrificed upon physical signs of advanced leukemia (weight loss, abnormal posture, decreased activity, and/or hind limb paralysis).
Upon sacrifice, whole blood and bone marrow were harvested for flow cytometry analysis of the human cell surface protein CD19, using a CD19-FITC antibody (BD Biosciences (Franklin Lanes, NJ; Cat. #-555412). The isotype control, IgG1-FITC, was obtained from Beckman Coulter (Brea, CA). Leukemic engraftment was defined by physical presentation of symptoms as defined above and the presence of at least 5% human (CD19+) cells in the bone marrow or peripheral blood. Kaplan-Meier survival curves were constructed.
Lentivirus production, titering, and transduction of target cell lines
Lentiviral particles were produced, and the titering was performed, as described previously [24]. Five constitutive c-Myb shRNAmir lentiviral constructs (oligo ID numbers, V2LHS_36795, V2LHS_36796, V2LHS_36797, V2LHS_36798, V2LHS_36799; Open Biosystems, Huntsville, AL) were tested for the ability to knock down c-Myb levels. Our efforts to constitutively knock down the expression of c-Myb in 697 pre-B-ALL cells gave positive results for five different c-Myb shRNAmir constructs at short times after transduction, but this was lost with extended times of cell culture (data not shown). Therefore, we decided to use a doxycycline (Doxy) controllable system to knock down the expression of c-Myb. We transferred two of these shRNA mirs (oligo ID numbers, V2LHS_36796 and V2LHS_36798) into the Expression Arrest™ TRIPZ lentiviral shRNAmir system (Open Biosystems, Huntsville, AL) to produce lentiviral particles containing Doxy-controllable c-Myb-specific shRNA constructs, according to the manufacturer’s protocol, via HEK-293T transfection. We also obtained another c-Myb shRNAmir inducible construct (oligo ID number, V2LHS_36794) from the same company. Although all three of these exhibited the controllable knockdown of c-Myb (data not shown), the V2LHS_36794-containing construct had the best knockdown, and so it was chosen for the remainder of the experiments. 697 parental cell lines were transduced with a multiplicity of infection of 0.3, and they were subsequently selected via puromycin treatment (shRNA-containing provirus contains a puromycin resistance gene) to produce two new cell lines, 697 sh-c-Myb and 697 sh-NSC. A puromycin killing curve was completed on the parental 697 cell line to determine the correct puromycin selection dosage, which was 1.0 μg/ml. Clonal populations of each new cell line were obtained by flow cytometry cell sorting using the BD FACSAria instrument (BD Biosciences, San Jose, CA). A stable cell line, 697 sh-c-Myb, was produced by transducing parental 697 cells with lentiviral particles containing a c-Myb specific, Doxy controllable shRNAmir. Also, another cell line was produced that contained an integrated provirus with a controllable shRNAmir sequence that was not specific for any human gene—the nonsilencing control (NSC) cell line, 697 sh-NSC.
Real time quantitative reverse transcription polymerase chain reaction (QRT-PCR)
The PCR DNA primer and Taqman® probe sequences specific for c-Myb were previously described [25,26]. For analysis of Bcl-2, the following probe and primers were used: Taqman Probe, 6-FAM-5′-CCTGGTGGACAACATCGCCCTGT-3′-TAMRA; forward primer, 5′-CATGTGTGTGGAGAGCGTCAA-3′, and reverse primer 5′-CCGG TTCAGGTACTCAGTCA-3′. For c-Myc, primers with the sequence 5′-GGACGACGAGACCTTCATCAA-3′ and 5′-CCAGCTTCTCTGAGAC GAGCTT-3′ were used together with the Taqman Probe, 6-FAM-5′-AGAAGCCGCTCCACATACAGTCCTGG-3′-TAMRA. QRT-PCR reactions were normalized to the concentration of their internal 18S rRNA concentration. Real time analysis was completed with the iScript™ One-Step RT-PCR Kit for Probes on the Bio-Rad (Hercules, CA) CFX96 system following the manufacturer’s instructions. Total RNA used in QRT-PCR reactions was isolated from cell samples using the Qiagen RNeasy Mini RNA isolation kit. All RNA samples were treated with DNase I according to the manufacturer’s (Qiagen, Valencia, CA) on-column digestion instructions.
Real-time PCR was also performed to confirm the reduction of c-Myb expression in CD19+ ALL cells harvested from the bone marrow of mice which were injected with 697 sh-c-Myb cells treated with Doxy as described above. Similarly, the presence of c-Myb expression was confirmed in CD19+ ALL cells collected from mice injected with 697 sh-c-Myb cells treated with PBS as well as 697 sh-NSC cells treated with PBS as well as Doxy.
Western blotting
Cells were collected, washed with PBS, and lysed with 1× Laemmli sample buffer containing 1/100 volume of protease inhibitor cocktail (Sigma, St. Louis, MO) and 1 mM PMSF. Proteins were resolved by SDS-PAGE and transferred to 0.45-μm nitrocellulose membranes. The membranes were blocked with 5% nonfat milk and blotted with protein-specific antibodies. The antibodies, anti-c-Myb (sc-7874), and anti-GAPDH (sc-25778) were from Santa Cruz Biotechnologies (Santa Cruz, CA). The anti-TurboRFP (AB321) antibody was from Evrogen (Moscow, Russia). The Poly ADP Ribose Polymerase (PARP) antibody (9542) was from Cell Signaling Technology (Danvers, MA). The monoclonal Bcl-2 antibody (Cat # 610538) was obtained from BD Biosciences (San Diego, CA).
Measurement of [3H] thymidine incorporation
The cells were cultured in 96-well plates and treated with or without Doxy for 96 hr. [3H] thymidine (1 μCi per well; PerkinElmer, San Jose, CA) was added for the last 24 hr of the 96-hr assay. The cells were collected onto glass-fiber filters (Unifilter GF/B; Packard Instrument, Meriden, CT), and radioactivity was counted in a beta counter (Microplate Scintillation and Luminescence Counter, TopCount; Packard) as described previously [27]. Data are expressed as the average counts per minute (CPM) (mean ± SD) of thymidine incorporated for at least three separate experiments.
Cell viability assays
The Vybrant® Apoptosis Assay Kit #4 (Invitrogen, Carlsbad, CA) was used to determine cell viability. The cells were collected by centrifugation and washed two times with PBS. After washing, the cells were resuspended in 1 ml PBS, and the reagents were added and incubated according to manufacturer’s instructions. Cell viability was measured by flow cytometry (FACSCalibur, BD Biosciences, Franklin Lakes, NJ).
Cell cycle analysis
The cells were treated with Doxy for 66 hr. Cells were collected, washed twice with ice-cold PBS, and fixed with 70% ethanol overnight at −20° C. The cells were then stained with propidium iodide and analyzed by flow cytometry (FACSCalibur, BD Biosciences, Franklin Lakes, NJ).
Isolation of human CD19+ cells
Bone marrow was harvested from the mice for the isolation of human CD19+ cells to confirm the knockdown of c-Myb in vivo at the time of sacrifice. The cells were labeled with anti-human CD19-FITC antibody (BD Pharmigen -555412), followed by the isolation of FITC-labeled cells using anti-FITC-magnetic beads separation kit (Miltenyi Biotech, Auburn, CA). Human pre-B-ALL CD19+ cell purity ranged between 95 and 99% as tested by flow cytometry. Fluorescence acquisition and analysis were done using a Coulter-Epics-XL flow cytometer (Beckman-Coulter) with a 488-nm argon laser.
Statistical analysis
Standard statistical tests including the Student’s t test were performed to determine the level of significance. A P value of less than 0.05 was considered to indicate statistical significance.
Results
Doxycycline controllable c-Myb knockdown in 697 pre-B-ALL cells
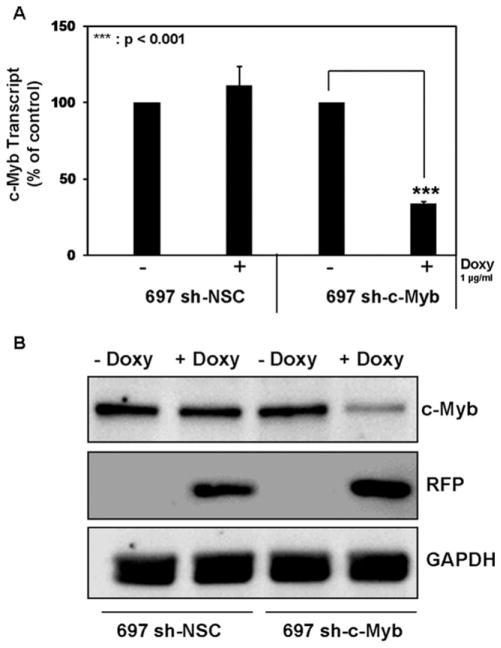
A stable cell line, 697 sh-c-Myb, was produced by transducing parental 697 cells with lentiviral particles containing a c-Myb specific, Doxy controllable shRNAmir. Also, another cell line was produced that contained an integrated provirus with a controllable shRNAmir sequence that was not specific for any human gene—the nonsilencing control (NSC) cell line, 697 sh-NSC. c-Myb RNA transcript levels were knocked down when 697 sh-c-Myb cells were treated with Doxy for 48 hr, which was not the case in the 697 sh-NSC cell line (Fig. 1A). c-Myb protein levels (Fig. 1B) were also knocked down in the 697 sh-c-Myb cell line after the addition of Doxy, whereas Doxy did not change the c-Myb protein levels in the 697 sh-NSC cell line. The expression of TurboRFP is a marker for Doxy induction and was used to validate that the Doxy inducible system was functioning correctly in the cells. These data demonstrate that the knockdown of c-Myb is controllable via Doxy treatment in the 697 sh-c-Myb cell line.
Figure 1.
Controllable knockdown of c-Myb in 697 pre-B-ALL cells A: c-Myb mRNA transcript measurements were performed via QRT-PCR with a probe and primer set specific for c-Myb transcripts on 697 cells stably transduced with a c-Myb specific shRNAmir lentivirus (697 sh-c-Myb) or with a nonsilencing control vector (697 sh-NSC). The data are normalized to endogenous 18S RNA levels. Total RNA was isolated following 48 hr of Doxy treatment. Three separate experiments were performed, and the values are the average ± SEM. ***, P < 0.001 for Doxy-treated samples vs. untreated samples. B: 697 cells stably transduced with a c-Myb specific shRNAmir lentivirus and with nonsilencing control vector were treated with 1 μg/ml of Doxy for 48 hr. Expression levels of c-Myb, TurboRFP, and GAPDH proteins were analyzed by immunoblotting. Blots representative of three independent experiments are shown.
The knockdown of c-Myb reduces thymidine incorporation in 697 pre-B-ALL cells
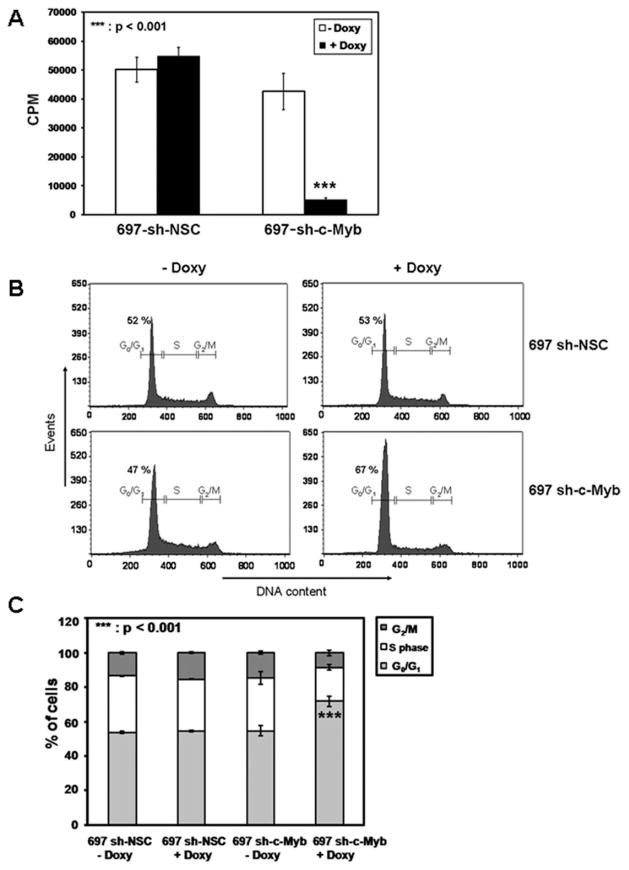
In the cell-line with a controllable knockdown of c-Myb, we noted that upon addition of Doxy to 697 sh-c-Myb cells, the cells were less confluent compared to the control 697 sh-NSC cells treated with Doxy, suggesting a decrease in cell proliferation. Although not a formal measure of cell proliferation, [3H] thymidine incorporation is indicative of DNA synthesis that is required for cell proliferation. Thus, the effect of c-Myb knockdown on [3H] thymidine incorporation was performed. The cells were treated with or without Doxy for 96 hr. [3H] thymidine was added for the last 24 hr of the 96-hr assay and [3H] thymidine uptake was measured. The [3H] uptake in case of 697 sh c-Myb cells was significantly reduced after the addition of Doxy which was not the case in 697 sh-NSC cells after the addition of Doxy (Fig. 2A). This suggests that the loss of c-Myb inhibits the proliferation of 697 pre-B-ALL cells.
Figure 2.
The knockdown of c-Myb decreases [3H] thymidine incorporation and increases the number of cells in the G0/G1 phase of the cell cycle. A: 697 sh-c-Myb cells and 697 sh-NSC cells were treated with or without 1 μg/ml Doxy for 96 hr. Doxy in the media was replenished every 48 hr. 1 μCi [3H] thymidine per well was added for the last 24 hr of the 96-h assay and [3H] thymidine uptake was measured as described in Materials and Methods. Data are expressed as counts per minute (CPM) (mean ± SD) for at least three experiments. ***, P < 0.001 for Doxy-treated samples vs. untreated samples. B and C: 697 sh-c-Myb cells and 697 sh-NSC cells were treated with or without 1 μg/ml Doxy for 66 hr. Cells were stained with propidium iodide, and cell cycle analysis was performed. Error bars represent the standard error of the mean of three separate experiments. ***, P < 0.001 for Doxy-treated samples vs. untreated samples for the % of cells in G0/G1 phase of the cell cycle.
The knockdown of c-Myb causes an arrest of the cell cycle at the G0/G1 phase
To determine if there was an earlier demonstrable biological effect of c-Myb knockdown, cell cycle analysis was performed using flow cytometry. 697 sh-c-Myb cells and 697 sh-NSC cells were treated with 1 μg/ml Doxy for 66 hr, and cell cycle analysis was performed. The 697 sh-c-Myb cells treated with Doxy exhibited a clear tendency to accumulate in the G1 phase (67.4% of the entire cell population), and the percentage of cells in the S and G2/M phases was decreased. Also, we observed no change in the number of cells in the G0/G1, S, or G2/M phases of the cell cycle after the addition of Doxy in control 697 sh-NSC cell-line (Fig. 2B,C). This shows that the knockdown of c-Myb causes an arrest of the cells in G0/G1 phase of the cell cycle, thus leading to a decrease rate of cell proliferation.
The knockdown of c-Myb reduces the cell viability and induces apoptosis in pre-B-ALL 697 cells
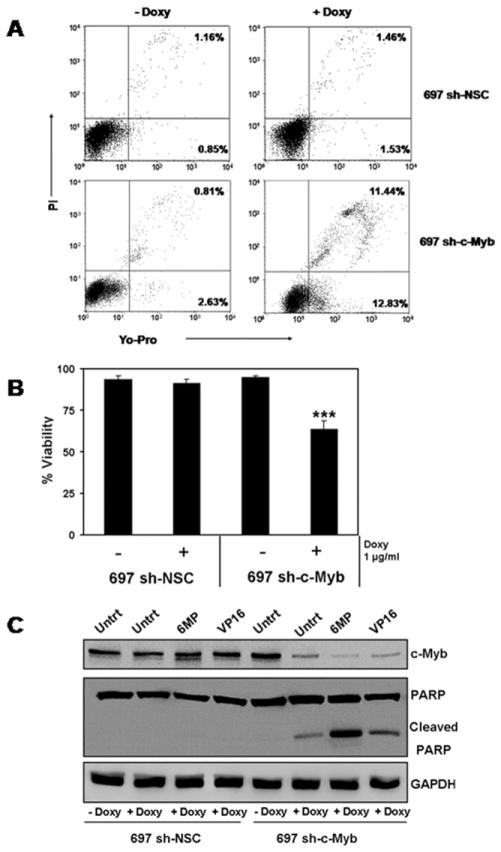
Our inability to constitutively knock down the expression of c-Myb in 697 pre-B-ALL cells was perplexing. Also, in the cell-line with a controllable knockdown of c-Myb, we noted that upon addition of Doxy to 697 sh-c-Myb cells, the cells were less confluent compared to the control 697 sh-NSC cells treated with Doxy. Also, there was a significant decrease in cell proliferation when 697 sh-c-Myb cells were treated with Doxy. One possible explanation was that the knockdown of c-Myb, by itself, reduces cellular viability. To test the effect of the loss of c-Myb on cell viability, 697 sh-c-Myb cells were treated with or without Doxy for 84 hr and cell viability was then measured. The cells were stained with YO-PRO1 and propidium iodide to permit flow cytometry quantification of cell viability by taking into account the number of apoptotic and dead cells. Representative flow cytometry profiles are shown in Fig. 3A. Cells undergoing apoptosis are stained with YO-PRO1 but are impermeable to propidium iodide (bottom, right quadrant). Dead cells and cells in late apoptosis are permeable to both dyes (upper, right quadrant). Viable cells are not stained by either dye (lower, left quadrant). We observed a significant increase in the number of apoptotic cells and the number of dead cells when 697 sh-c-Myb cells were treated with Doxy to induce the knockdown of c-Myb (Fig. 3A). That is, we observed a significant decrease in the viability of 697 sh-c-Myb cells when c-Myb was knocked down, whereas no change was observed in the control 697 sh-NSC cell line, even after the addition of Doxy (Fig. 3A,B). This demonstrates that the knockdown of c-Myb induces apoptosis, suggesting that c-Myb is essential for the viability of pre-BALL 697 cells.
Figure 3.
The knockdown of c-Myb reduces the viability and induces apoptosis in 697 pre-B-ALL cells. A and B: 697 sh-c-Myb cells and 697 sh-NSC cells were treated with or without 1 μg/ml Doxy for 84 hr. Cell viability was then measured using the Vybrant #4 apoptosis assay. Apoptotic and dead cells were identified by flow cytometry analysis of cells stained with YO-PRO1 and propidium iodide. A: Representative flow cytometry profiles are shown. Cells undergoing apoptosis are stained with YO-PRO1 but are impermeable to propidium iodide. Dead cells and cells in late apoptosis are permeable to both dyes. Viable cells are not stained by either dye. Apoptotic cells are shown in the bottom right quadrants and dead cells are shown in the upper right quandrants. B: Cumulative data demonstrate a significant decrease in cell viability when c-Myb is knocked down. Error bars represent the standard error of the mean of three separate experiments. **, P < 0.001 for Doxy-treated samples vs. untreated samples C: 697 sh-c-Myb cells and 697 sh-NSC cells were treated with or without 1 μg/ml Doxy for 48 hr. The doxy-treated cells were then exposed to 5 μM 6-MP and 150 nM VP-16 for 24 hr. Whole cell lysates were prepared, and the expression of c-Myb, PARP, and GAPDH were determined by immunoblotting. Blots representative of three independent experiments are shown.
Detection of PARP cleavage using western blotting has been used extensively as an indicator of apoptosis [28–31]. Thus, another approach to verify if the cells were undergoing apoptosis was to evaluate if PARP was getting cleaved when c-Myb was knocked down. 697 sh-c-Myb cells, and 697 sh-NSC cells were pretreated with or without 1 μg/ml Doxy for 48 hr. The Doxy treated cells were then exposed to 5 μM 6-mercaptopurine (6-MP) or 150 nM VP-16 (etoposide) for 24 hr. Whole cell lysates were prepared, and the expression of c-Myb, PARP, and GAPDH (loading control) were determined by immunoblotting. As expected, we observed a decrease in c-Myb protein levels when Doxy was added to the 697 sh-c-Myb cells but not in the 697 sh-NSC cell line after the addition of Doxy (Fig. 3C). Furthermore, PARP cleavage was observed when 697 sh-c-Myb cells were treated with Doxy, and an increase in PARP cleavage occurred when 697 sh-Myb cells treated with Doxy were also treated with the chemotherapeutic drugs 6-MP and VP-16. However, there was no cleavage of PARP in the control 697 sh-NSC cells (Fig. 3C) even, under these conditions, when the chemotherapeutic drugs were used to treat the cells. The cleavage of PARP further demonstrates that the knockdown of c-Myb is leading to apoptosis.
The knockdown of c-Myb downregulates Bcl-2 expression in pre-B-ALL
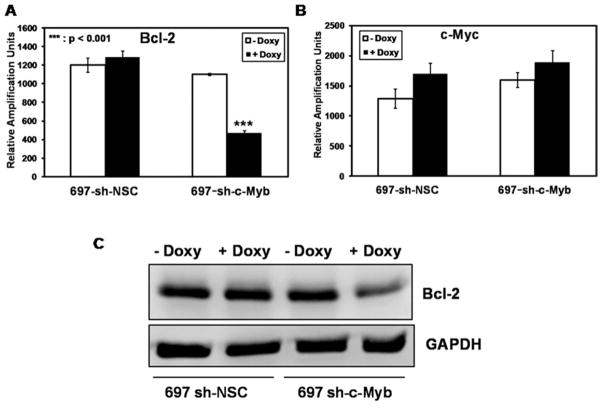
The antiapoptotic protein Bcl-2 and the proto-oncogene c-Myc have been shown to be targets of c-Myb in other cell types [32–34]. Given the observed increase in apoptosis after c-Myb inhibition, we examined whether c-Myb targets Bcl-2 and c-Myc in 697 pre-B-ALL cells. 697 sh-c-Myb cells and 697 sh-NSC cells were treated with 1 μg/ml Doxy for 48 hr. Bcl-2 mRNA transcript levels were significantly reduced in 697 sh-c-Myb cells treated with Doxy (Fig 4A), whereas c-Myc transcript levels were unaffected after the knockdown of c-Myb (Fig. 4B). Bcl-2 protein levels (Fig. 4C) were also decreased in the 697 sh-c-Myb cell line after the addition of Doxy, whereas Doxy did not change the c-Myc protein levels in the 697 sh-c-Myb cell line (data not shown). Furthermore, the knockdown of c-Myb did not affect the levels of total Akt, p-Akt, total mTOR, and p-mTOR (data not shown) proteins that affect apoptosis in other cells lines. These data demonstrate that the loss of c-Myb causes a specific downregulation of the antiapoptotic protein Bcl-2.
Figure 4.
c-Myb targets the antiapoptotic protein Bcl-2 in 697 pre-B-ALL cells A: Bcl-2 and c-Myc mRNA transcript measurements were performed via QRT-PCR on 697 cells stably transduced with a c-Myb specific shRNAmir lentivirus (697 sh-c-Myb) or with a nonsilencing control vector (697 sh-NSC). The data are normalized to endogenous 18S RNA levels. Total RNA was isolated following 48 hr of Doxy treatment. Three separate experiments were performed, and the values are the average ± SEM. ***, P <0.001 for Doxy-treated samples vs. untreated samples. B: 697 sh-c-Myb and 697 sh-NSC cells were treated with 1 μg/ml of Doxy for 48 hr. Expression levels of the Bcl-2 and GAPDH proteins were analyzed by immunoblotting. A blot that is representative of three independent experiments are shown.
Inhibition of c-Myb increases the chemosensitivity of leukemia cells
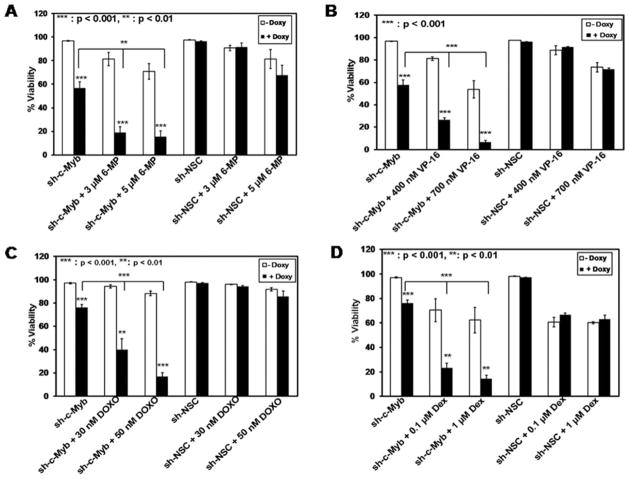
Given the observed increase in apoptosis after c-Myb inhibition and the increase in PARP cleavage when cells with lowered c-Myb levels were treated with 6-MP and VP-16, we investigated whether inhibition of c-Myb would enhance the sensitivity of leukemia cells to various cytotoxic agents. 697 sh-NSC and 697 sh-c-Myb cells were pretreated with or without Doxy for 48 hr followed by treatment with the chemotherapeutic agents 6-MP or VP-16 for 48 hr, or dexamethasone (Dex) or doxorubicin (DOXO) for 24 hr, all at clinically relevant concentrations [35–39]. Two concentrations of each drug were used, based on dose response curves that were performed earlier (data not shown). Cell viability was determined by flow cytometry analysis of cells stained with YO-PRO1 and propidium iodide. Cumulative analyses of three independent experiments revealed a dramatic decrease in cell viability when 697 sh-c-Myb cells treated with Doxy were also treated with the chemotherapeutic agents (6-MP, VP-16, Dex, or DOXO), compared to 697 sh-c-Myb cells not treated with Doxy, as well as when compared to control 697 sh-NSC cells treated with or without Doxy (Fig. 5). Thus, the decrease in viability of the cells after the knockdown of c-Myb and the related increase in chemosensitivity suggest that targeting c-Myb along with standard leukemia chemotherapy may lead to a better therapeutic response in pre-B-ALL.
Figure 5.
Inhibition of c-Myb expression results in increased chemosensitivity in 697 pre-B-ALL cells. 697 sh-c-Myb cells and 697 sh-NSC cells were treated with or without 1 μg/ml Doxy for 48 hr. The cells were then treated with the indicated concentrations of 6-mercaptopurine (6-MP) or etoposide (VP-16) for 48 hr, or with Doxorubicin (DOXO) or Dexamethasone (Dex) for 24 hr. Cell viability was then measured using the Vybrant #4 apoptosis assay. The knockdown of c-Myb caused a significant increase in chemosensitivity to all the four drugs in 697 cells. Error bars represent the standard error of the mean of three separate experiments. **, P < 0.01, ***, P < 0.001 for Doxy-treated samples vs. untreated sample.
Inhibition of c-Myb delays the progression of leukemia in a mouse xenograft model
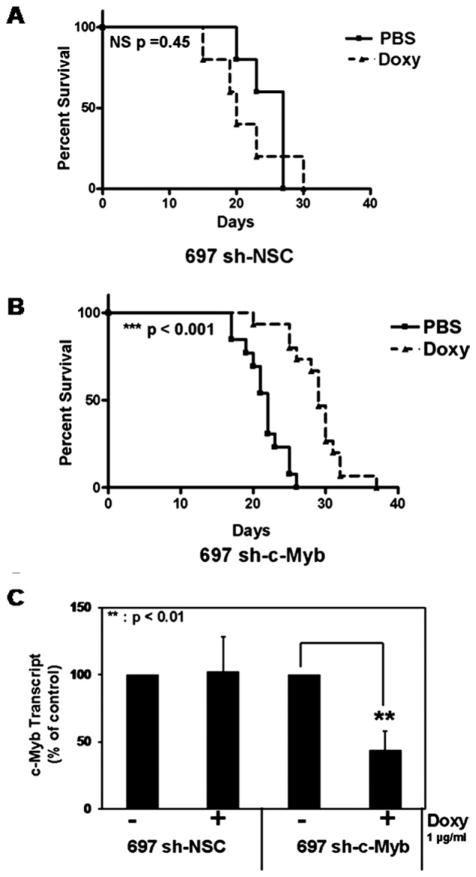
In an attempt to validate c-Myb as a therapeutic target in an in vivo setting, we evaluated the oncogenicity of control and c-Myb knockdown 697 cells in a mouse xenograft model of leukemia. Sublethally irradiated NOD-SCID mice were injected with the indicated cell lines. The mice were treated with Doxy 3 days a week to knock down the expression of c-Myb in vivo or were treated with PBS (control). The mice were monitored daily and killed upon signs of advanced leukemia. Kaplan-Meier survival curves demonstrated that c-Myb knockdown significantly delayed the progression of the disease when 697 sh-c-Myb cells were treated with Doxy (Fig. 6B; P < 0.0001), but not in the control cell line treated with Doxy (Fig. 6A; P = 0.45). CD19+ cells were isolated from the bone marrow of these mice, and QRT-PCR was performed to determine if c-Myb was indeed knocked down in vivo. We observed a significant decrease in the amount of c-Myb mRNA in mice injected with 697 sh-c-Myb cells and treated with Doxy, compared to mice injected with 697 sh-c-Myb cells treated with PBS (control) and mice injected with 697 sh-NSC cells treated with or without Doxy (Fig. 6C). We also performed flow cytometric analysis of cells isolated from the bone marrow of the mice. We observed that mice treated with Doxy had RFP positive cells, whereas the mice treated with PBS did not have RFP positive cells, demonstrating that the pTRIPZ controllable knockdown system was functioning well in vivo (data not shown). These data indicate that c-Myb is critical for leukemogenesis and that inhibition of c-Myb slows the progression of disease in vivo.
Figure 6.
Loss of c-Myb delays the progression of leukemia in a mouse xeno-graft model of human leukemia. A: Sublethally irradiated NOD-SCID mice were injected with 1 × 107 697 sh-NSC cells. Seven days after injecting the cells, the mice were treated with Doxy or PBS three times a week. Kaplan-Meier survival curve revealed no significant difference in leukemia free survival in these mice. B: Sublethally irradiated NOD-SCID mice were injected with 1 × 107 697 sh-c-Myb cells. Seven days after injecting the cells, the mice were treated with Doxy or PBS three times a week. Kaplan-Meier survival curve revealed a significant increase in leukemia free survival of mice injected with 697 sh c-Myb cells treated with Doxy. C: Real-time PCR was performed to confirm the reduction of c-Myb expression in CD19+ ALL cells harvested from the bone marrow of mice which were injected with 697 sh-c-Myb cells treated with Doxy. Similarly, the unchanged levels of c-Myb expression were confirmed in CD19+ ALL cells collected from mice injected with 697 sh-c-Myb cells treated with PBS as well as 697 sh-NSC cells treated with PBS as well as Doxy. Error bars represent the standard error of the mean of three separate experiments. **, P <0.01 for Doxy-treated samples vs. untreated sample.
Discussion
ALL is the most common cancer in children and a leading cause of cancer-related deaths in children [2]. Mutations affecting genes that code transcriptional regulators are frequently associated with pre-B-ALL [40]. The proto-oncogene c-Myb encodes a transcription factor that is expressed in the cells of the hematopoietic lineage. Studies using antisense oligonucleotides and dominant negative forms of c-Myb have shown that c-Myb is essential for the continued proliferation of acute myeloid leukemia and chronic myeloid leukemia [41,42]. More recently, c-Myb gene duplications or translocations of the c-Myb gene to the TCRβ locus have been described in a significant proportion of childhood T-ALLs [43,44]. Although c-Myb has been shown to play a role in the B-cell differentiation pathway, the role of c-Myb in pre-BALL has not been previously studied. We used a controllable shRNA to specifically knock down the expression of c-Myb in pre-B-ALL cells. Our study demonstrates the potential importance of targeting c-Myb in pre-B-ALL.
At early treatment times, the knockdown of c-Myb caused a cell cycle arrest in 697-pre-B-ALL cells, as evidenced by a significant increase in the number of cells in G0/G1 phase of the cell cycle. This is consistent with previous studies showing that c-Myb plays an important role in regulating cell cycle transition in hematopoietic cells [45,46]. With the prolonged knockdown of c-Myb, there was a significant decrease in cell proliferation and cell viability in the 697 pre-B-ALL cells. This explains why we were unsuccessful in getting stable long-term c-Myb knockdown with the constitutive c-Myb-shRNA-mir constructs, as those cells in the pool that had a strong knockdown probably died, thereby selecting for cells with no, or only a weak, knockdown of c-Myb. This result is consistent with studies done in normal B-lymphoid cell differentiation, which have shown that c-Myb plays an important role in B-cell development [47]. Thus, it appears that c-Myb may be a B-cell viability factor, at least at some, probably early, stages of B-cell development. Also, we demonstrate that Bcl-2 is a target of c-Myb in pre-B-ALL cells. Therefore, the loss of c-Myb causes a downregulation of the antiapoptotic protein Bcl-2, which may be one of the reasons why the cells undergo apoptosis.
The functional significance of this study is highlighted by our finding that loss of c-Myb expression increases the sensitivity of pre-B-ALL cells to multiple chemotherapeutic agents. Importantly, 6-MP, Doxo, and Dex are used in standard pediatric chemotherapy regimens, and VP-16 is a major component of relapse therapy. The increased sensitivity of 697 sh-c-Myb cells to chemotherapeutic agents was mediated at least in part by a decrease in viability and increased induction of apoptosis by the knockdown of c-Myb. The most important finding of this study is that the knockdown of c-Myb in vivo was sufficient to delay onset and/or progression of the disease in a mouse model of leukemia, indicating an important role of c-Myb in leukemogenesis in vivo. This result is consistent with our in vitro result that the knockdown of c-Myb in itself decreases the viability of the leukemic cells. Thus, an intense apoptotic stimulus, such as chemotherapy, is not required to observe the effects of c-Myb inhibition on cancer cell survival. Our results suggest the possibility of targeting c-Myb as a therapeutic strategy in pre-B-ALL, as that would inhibit leukemogenesis and increase the efficacy of standard chemotherapy. Interestingly, there are already examples of some solid tumors in which c-Myb dysregulation is neoplastic. About a third of BRCA1 mutated hereditary breast cancers show amplification of c-Myb, leading to an overexpression of c-Myb mRNA and protein [48]. Also, amplification of c-Myb has been observed in ~10% of pancreatic cancers, as well as in two colorectal cancer cell lines and two glioblastoma cell lines [49–51]. The human c-Myb gene is located on chromosome 6q22-24 [52], and abnormalities in this locus have been observed in acute myelogenous leukemia, T-cell leukemia, colon carcinoma, and melanomas [50,53,54]. Moreover, a recent study published during the preparation of this manuscript suggests that c-Myb regulates the process of p190 BCR/ABL – dependent B-cell leuke-mogenesis through its effect on the levels of Bmi1 [55]. Thus, the results that we have obtained for a cell line containing a t(1;19), which results in a fusion of E2A and PBX1 and very poor prognosis, are not restricted to this particular B-cell line but appear to extend to other types of B-cell hematopoietic malignancies as well.
In conclusion, this study provides evidence for a role of c-Myb in pre-B-ALL. The current challenge for the management of ALL is to treat relapsed ALL patients and to minimize the significant risk of side effects of high dose chemotherapy. Our results suggest that new agents that target c-Myb or its expression may directly inhibit leukemogenesis. Also, a combination of Myb inhibition with standard chemotherapeutic agents may permit dose reduction and decreased toxicity, thus minimizing the side-effects of chemotherapeutic agents. Furthermore, c-Myb inhibition may be most beneficial for patients who currently have a poor prognosis, namely those with chemoresistant or recurrent disease. Although the focus of our study was pre-B-ALL, the widespread aberrant expression of c-Myb in human cancers suggests that inhibitors that target c-Myb activity and expression may also prove to be useful for the treatment of other hematological malignancies and solid tumors.
Acknowledgments
The authors thank Dr. Beatriz F. Finkel-Jimenez for flow cytometry assistance. The authors thank Dr. Jovanny Zabaleta, (LSUHSC, New Orleans) for help with the [3H] thymidine incorporation assay.
Contract grant sponsors: NCI; Contract grant number: CA116042. Contract grant sponsor: NIH; Contract grant number: 2P20RR021970-06
Footnotes
Conflict of interest: Nothing to report.
References
- 1.Pui CH. Recent research advances in childhood acute lymphoblastic leukemia. J Formos Med Assoc. 2010;109:777–787. doi: 10.1016/S0929-6646(10)60123-4. [DOI] [PubMed] [Google Scholar]
- 2.Pui C-H, Robison LL, Look AT. Acute lymphoblastic leukaemia. Lancet. 2008;371:1030–1043. doi: 10.1016/S0140-6736(08)60457-2. [DOI] [PubMed] [Google Scholar]
- 3.Gurney JG, Severson RK, Davis S, Robison LL. Incidence of cancer in children in the United States. Sex-, race-, and 1-year age-specific rates by histologic type. Cancer. 1995;75:2186–2195. doi: 10.1002/1097-0142(19950415)75:8<2186::aid-cncr2820750825>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 4.Pui CH. Childhood Leukemias. Cambridge: Cambridge University Press; 2006. pp. 21–47. [Google Scholar]
- 5.Raimondi SC, Behm FG, Roberson PK, et al. Cytogenetics of pre-B-cell acute lymphoblastic leukemia with emphasis on prognostic implications of the t(1;19) J Clin Oncol. 1990;8:1380–1388. doi: 10.1200/JCO.1990.8.8.1380. [DOI] [PubMed] [Google Scholar]
- 6.Oeffinger KC, Eshelman DA, Tomlinson GE, et al. Grading of late effects in young adult survivors of childhood cancer followed in an ambulatory adult setting. Cancer. 2000;88:1687–1695. [PubMed] [Google Scholar]
- 7.Winick NJ, Carroll WL, Hunger SP. Childhood leukemia—New advances and challenges. N Engl J Med. 2004;351:601–603. doi: 10.1056/NEJMe048154. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen K, Devidas M, Cheng SC, et al. Factors influencing survival after relapse from acute lymphoblastic leukemia: A Children’s Oncology Group study. Leukemia. 2008;22:2142–2150. doi: 10.1038/leu.2008.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carroll AJ, Crist WM, Parmley RT, et al. Pre-B cell leukemia associated with chromosome translocation 1;19. Blood. 1984;63:721–724. [PubMed] [Google Scholar]
- 10.Hunger SP, Galili N, Carroll AJ, et al. The t(1;19)(q23;p13) results in consistent fusion of E2A and PBX1 coding sequences in acute lymphoblastic leukemias. Blood. 1991;77:687–693. [PubMed] [Google Scholar]
- 11.Crist WM, Carroll AJ, Shuster JJ, et al. Poor prognosis of children with pre-B acute lymphoblastic leukemia is associated with the t(1;19)(q23;p13): A Pediatric Oncology Group study. Blood. 1990;76:117–122. [PubMed] [Google Scholar]
- 12.Klempnauer KH, Gonda TJ, Bishop JM. Nucleotide sequence of the retroviral leukemia gene v-myb and its cellular progenitor c-myb: The architecture of a transduced oncogene. Cell. 1982;31:453–463. doi: 10.1016/0092-8674(82)90138-6. [DOI] [PubMed] [Google Scholar]
- 13.Leprince D, Gegonne A, Coll J, et al. A putative second cell-derived oncogene of the avian leukaemia retrovirus E26. Nature. 1983;306:395–397. doi: 10.1038/306395a0. [DOI] [PubMed] [Google Scholar]
- 14.Mucenski ML, McLain K, Kier AB, et al. A functional c-myb gene is required for normal murine fetal hepatic hematopoiesis. Cell. 1991;65:677–689. doi: 10.1016/0092-8674(91)90099-k. [DOI] [PubMed] [Google Scholar]
- 15.Emambokus N, Vegiopoulos A, Harman B, et al. Progression through key stages of haemopoiesis is dependent on distinct threshold levels of c-Myb. EMBO J. 2003;22:4478–4488. doi: 10.1093/emboj/cdg434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sandberg ML, Sutton SE, Pletcher MT, et al. c-Myb and p300 regulate hematopoietic stem cell proliferation and differentiation. Dev Cell. 2005;8:153–166. doi: 10.1016/j.devcel.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 17.Sakamoto H, Dai G, Tsujino K, et al. Proper levels of c-Myb are discretely defined at distinct steps of hematopoietic cell development. Blood. 2006;108:896–903. doi: 10.1182/blood-2005-09-3846. [DOI] [PubMed] [Google Scholar]
- 18.Shen-Ong GL, Potter M, Mushinski JF, et al. Activation of the c-myb locus by viral insertional mutagenesis in plasmacytoid lymphosarcomas. Science. 1984;226:1077–1080. doi: 10.1126/science.6093260. [DOI] [PubMed] [Google Scholar]
- 19.Hwang HC, Martins CP, Bronkhorst Y, et al. Identification of oncogenes collaborating with p27Kip1 loss by insertional mutagenesis and high-throughput insertion site analysis. Proc Natl Acad Sci USA. 2002;99:11293–11298. doi: 10.1073/pnas.162356099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanter MR, Smith RE, Hayward WS. Rapid induction of B-cell lymphomas: Insertional activation of c-myb by avian leukosis virus. J Virol. 1988;62:1423–1432. doi: 10.1128/jvi.62.4.1423-1432.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim R, Trubetskoy A, Suzuki T, et al. Genome-based identification of cancer genes by proviral tagging in mouse retrovirus-induced T-cell lymphomas. J Virol. 2003;77:2056–2062. doi: 10.1128/JVI.77.3.2056-2062.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wolff L. Myb-induced transformation. Crit Rev Oncog. 1996;7:245–260. doi: 10.1615/critrevoncog.v7.i3-4.60. [DOI] [PubMed] [Google Scholar]
- 23.Harper ME, Franchini G, Love J, et al. Chromosomal sublocalization of human c-myb and c-fes cellular onc genes. Nature. 1983;304:169–171. doi: 10.1038/304169a0. [DOI] [PubMed] [Google Scholar]
- 24.Schwartz JR, Sarvaiya PJ, Vedeckis WV. Glucocorticoid receptor knock down reveals a similar apoptotic threshold but differing gene regulation patterns in T-cell and pre-B-cell acute lymphoblastic leukemia. Mol Cell Endocrinol. 2010;320:76–86. doi: 10.1016/j.mce.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pedersen KB, Vedeckis WV. Quantification and glucocorticoid regulation of glucocorticoid receptor transcripts in two human leukemic cell lines. Biochemistry. 2003;42:10978–10990. doi: 10.1021/bi034651u. [DOI] [PubMed] [Google Scholar]
- 26.Geng CD, Vedeckis WV. A new, lineage specific, autoup-regulation mechanism for human glucocorticoid receptor gene expression in 697 pre-B-acute lymphoblastic leukemia cells. Mol Endocrinol. 2011;25:44–57. doi: 10.1210/me.2010-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zabaleta J, McGee DJ, Zea AH, et al. Helicobacter pylori arginase inhibits T cell proliferation and reduces the expression of the TCR zeta-chain (CD3zeta) J Immunol. 2004;173:586–593. doi: 10.4049/jimmunol.173.1.586. [DOI] [PubMed] [Google Scholar]
- 28.Duriez PJ, Shah GM. Cleavage of poly(ADP-ribose) polymerase: A sensitive parameter to study cell death. Biochem Cell Biol. 1997;75:337–349. [PubMed] [Google Scholar]
- 29.Joashi UC, Greenwood K, Taylor DL, et al. Poly(ADP ribose) polymerase cleavage precedes neuronal death in the hippocampus and cerebellum following injury to the developing rat forebrain. Eur J Neurosci. 1999;11:91–100. doi: 10.1046/j.1460-9568.1999.00409.x. [DOI] [PubMed] [Google Scholar]
- 30.Shah GM, Shah RG, Poirier GG. Different cleavage pattern for poly(ADP-ribose) polymerase during necrosis and apoptosis in HL-60 cells. Biochem Biophys Res Commun. 1996;229:838–844. doi: 10.1006/bbrc.1996.1889. [DOI] [PubMed] [Google Scholar]
- 31.Simbulan-Rosenthal CM, Rosenthal DS, Iyer S, et al. Transient poly(ADP-ribosyl)ation of nuclear proteins and role of poly(ADP-ribose) polymerase in the early stages of apoptosis. J Biol Chem. 1998;273:13703–13712. doi: 10.1074/jbc.273.22.13703. [DOI] [PubMed] [Google Scholar]
- 32.Nakagoshi H, Kanei-Ishii C, Sawazaki T, et al. Transcriptional activation of the c-myc gene by the c-myb and B-myb gene products. Oncogene. 1992;7:1233–1240. [PubMed] [Google Scholar]
- 33.Frampton J, Ramqvist T, Graf T. v-Myb of E26 leukemia virus up-regulates bcl-2 and suppresses apoptosis in myeloid cells. Genes Dev. 1996;10:2720–2731. doi: 10.1101/gad.10.21.2720. [DOI] [PubMed] [Google Scholar]
- 34.Thompson MA, Rosenthal MA, Ellis SL, et al. c-Myb down-regulation is associated with human colon cell differentiation, apoptosis, and decreased Bcl-2 expression. Cancer Res. 1998;58:5168–5175. [PubMed] [Google Scholar]
- 35.Corona G, Casetta B, Sandron S, et al. Rapid and sensitive analysis of vincristine in human plasma using on-line extraction combined with liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom. 2008;22:519–525. doi: 10.1002/rcm.3390. [DOI] [PubMed] [Google Scholar]
- 36.DiFrancesco R, Griggs JJ, Donnelly J, DiCenzo R. Simultaneous analysis of cyclophosphamide, doxorubicin and doxorubicinol by liquid chromatography coupled to tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;852:545–553. doi: 10.1016/j.jchromb.2007.02.033. [DOI] [PubMed] [Google Scholar]
- 37.Estlin EJ. Continuing therapy for childhood acute lymphoblastic leukaemia: Clinical and cellular pharmacology of methotrexate, 6-mercaptopurine and 6-thioguanine. Cancer Treat Rev. 2001;27:351–363. doi: 10.1053/ctrv.2002.0245. [DOI] [PubMed] [Google Scholar]
- 38.Pein F, Pinkerton R, Berthaud P, et al. Dose finding study of oral PSC 833 combined with weekly intravenous etoposide in children with relapsed or refractory solid tumours. Eur J Cancer. 2007;43:2074–2081. doi: 10.1016/j.ejca.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 39.Linger RM, DeRyckere D, Brandao L, et al. Mer receptor tyrosine kinase is a novel therapeutic target in pediatric B-cell acute lymphoblastic leukemia. Blood. 2009;114:2678–2687. doi: 10.1182/blood-2009-03-209247. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 40.Cobaleda C, Sanchez-Garcia I. B-cell acute lymphoblastic leukaemia: Towards understanding its cellular origin. Bioessays. 2009;31:600–609. doi: 10.1002/bies.200800234. [DOI] [PubMed] [Google Scholar]
- 41.Anfossi G, Gewirtz AM, Calabretta B. An oligomer complementary to c-myb-encoded mRNA inhibits proliferation of human myeloid leukemia cell lines. Proc Natl Acad Sci USA. 1989;86:3379–3383. doi: 10.1073/pnas.86.9.3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Calabretta B, Sims RB, Valtieri M, et al. Normal and leukemic hematopoietic cells manifest differential sensitivity to inhibitory effects of c-myb antisense oligodeoxynucleotides: An in vitro study relevant to bone marrow purging. Proc Natl Acad Sci USA. 1991;88:2351–2355. doi: 10.1073/pnas.88.6.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clappier E, Cuccuini W, Kalota A, et al. The C-MYB locus is involved in chromosomal translocation and genomic duplications in human T-cell acute leukemia (T-ALL), the translocation defining a new T-ALL subtype in very young children. Blood. 2007;110:1251–1261. doi: 10.1182/blood-2006-12-064683. [DOI] [PubMed] [Google Scholar]
- 44.Lahortiga I, De Keersmaecker K, Van Vlierberghe P, et al. Duplication of the MYB oncogene in T cell acute lymphoblastic leukemia. Nat Genet. 2007;39:593–595. doi: 10.1038/ng2025. [DOI] [PubMed] [Google Scholar]
- 45.Gewirtz AM, Anfossi G, Venturelli D, et al. G1/S transition in normal human T-lymphocytes requires the nuclear protein encoded by c-myb. Science. 1989;245:180–183. doi: 10.1126/science.2665077. [DOI] [PubMed] [Google Scholar]
- 46.Nakata Y, Shetzline S, Sakashita C, et al. c-Myb contributes to G2/M cell cycle transition in human hematopoietic cells by direct regulation of cyclin B1 expression. Mol Cell Biol. 2007;27:2048–2058. doi: 10.1128/MCB.01100-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thomas MD, Kremer CS, Ravichandran KS, et al. c-Myb is critical for B cell development and maintenance of follicular B cells. Immunity. 2005;23:275–286. doi: 10.1016/j.immuni.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 48.Kauraniemi P, Hedenfalk I, Persson K, et al. MYB oncogene amplification in hereditary BRCA1 breast cancer. Cancer Res. 2000;60:5323–5328. [PubMed] [Google Scholar]
- 49.Wallrapp C, Muller-Pillasch F, Solinas-Toldo S, et al. Characterization of a high copy number amplification at 6q24 in pancreatic cancer identifies c-myb as a candidate oncogene. Cancer Res. 1997;57:3135–3139. [PubMed] [Google Scholar]
- 50.Alitalo K, Winqvist R, Lin CC, et al. Aberrant expression of an amplified c-myb oncogene in two cell lines from a colon carcinoma. Proc Natl Acad Sci USA. 1984;81:4534–4538. doi: 10.1073/pnas.81.14.4534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Welter C, Henn W, Theisinger B, et al. The cellular myb oncogene is amplified, rearranged and activated in human glioblastoma cell lines. Cancer Lett. 1990;52:57–62. doi: 10.1016/0304-3835(90)90077-b. [DOI] [PubMed] [Google Scholar]
- 52.Zabel BU, Naylor SL, Grzeschik KH, Sakaguchi AY. Regional assignment of human protooncogene c-myb to 6q21----qter. Somat Cell Mol Genet. 1984;10:105–108. doi: 10.1007/BF01534477. [DOI] [PubMed] [Google Scholar]
- 53.Barletta C, Pelicci PG, Kenyon LC, et al. Relationship between the c-myb locus and the 6q-chromosomal aberration in leukemias and lymphomas. Science. 1987;235:1064–1067. doi: 10.1126/science.3469751. [DOI] [PubMed] [Google Scholar]
- 54.Dasgupta P, Reddy EP. Identification of alternatively spliced transcripts for human c-myb: Molecular cloning and sequence analysis of human c-myb exon 9A sequences. Oncogene. 1989;4:1419–1423. [PubMed] [Google Scholar]
- 55.Waldron T, De Dominici M, Soliera AR, et al. c-Myb and its target Bmi1 are required for p190BCR/ABL leukemogenesis in mouse and human cells. Leukemia. 2011;26:644–653. doi: 10.1038/leu.2011.264. [DOI] [PMC free article] [PubMed] [Google Scholar]