Abstract
Background
Trypanosoma cruzi, the causative agent of Chagas disease, displays significant genetic variability revealed by six Discrete Typing Units (TcI-TcVI). In this pathology, oral transmission represents an emerging epidemiological scenario where different outbreaks associated to food/beverages consumption have been reported in Argentina, Bolivia, Brazil, Ecuador and Venezuela. In Colombia, six human oral outbreaks have been reported corroborating the importance of this transmission route. Molecular epidemiology of oral outbreaks is barely known observing the incrimination of TcI, TcII, TcIV and TcV genotypes.
Methodology and Principal Findings
High-throughput molecular characterization was conducted performing MLMT (Multilocus Microsatellite Typing) and mtMLST (mitochondrial Multilocus Sequence Typing) strategies on 50 clones from ten isolates. Results allowed observing the occurrence of TcI, TcIV and mixed infection of distinct TcI genotypes. Thus, a majority of specific mitochondrial haplotypes and allelic multilocus genotypes associated to the sylvatic cycle of transmission were detected in the dataset with the foreseen presence of mitochondrial haplotypes and allelic multilocus genotypes associated to the domestic cycle of transmission.
Conclusions
These findings suggest the incrimination of sylvatic genotypes in the oral outbreaks occurred in Colombia. We observed patterns of super-infection and/or co-infection with a tailored association with the severe forms of myocarditis in the acute phase of the disease. The transmission dynamics of this infection route based on molecular epidemiology evidence was unraveled and the clinical and biological implications are discussed.
Author Summary
Chagas disease represents a serious health problem affecting more than 10 million people in the Americas. The oral transmission route has emerged as a new epidemiological scenario that needs to be considered in prevention and control strategies. Herein was developed a high-resolution molecular characterization using mtMLST and MLMT tools in order to unravel the molecular epidemiology and transmission dynamics drivers in six well-characterized human oral outbreaks in Colombia. We observed the majority of clones typed as TcI and one clone as TcIV. The analysis of mitochondrial haplotypes allowed us to observe a high frequency of sylvatic haplotypes and a low proportion of domestic haplotypes. Likewise, a tailored allelic profile by each outbreak was observed. Our results suggest that sylvatic populations of T. cruzi are the causative agents of Chagas disease oral outbreaks and these findings should help to pursue new initiatives of control and prevention in those areas where domiciliated vectorial transmission has been interrupted.
Introduction
Chagas disease caused by Trypanosoma cruzi is considered a zoonotic and neglected disease that represents an important public health problem in the Americas. This parasite is mainly transmitted by the faeces of triatomine insects and shows tremendous genetic variability reflected in at least six Discrete Typing Units (DTU's) classified as T. cruzi I – T. cruzi VI [1], [2]. Some authors have demonstrated intraspecific genetic diversity within T. cruzi I suggesting the existence of genotypes associated to the domestic (TcIa), peridomestic (TcIb) and sylvatic (TcId) transmission cycles [3]. Other routes of disease transmission, such as blood transfusion, vertical transmission, organ transplantation and accidental laboratory contamination are considered relevant routes [4]. Trypanosoma cruzi transmission by vertical vias and by transfusion of contaminated blood products has become the main mechanisms of transmission in non-endemic countries, including the USA and Spain [5], [6]. Currently, it is estimated that 7,694,500 people are infected with this parasite in South, Central American countries and Mexico where control initiatives are being conducted to interrupt T. cruzi transmission by triatomines and the transfusion of blood products [4].
Transmission via oral route is a relevant and emerging scenario of Chagas disease. This via displays a habitual character in the primitive and endemic cycle of the parasite occurring through consumption of contaminated food and/or beverages by dejection of triatomines faeces [4]. Also by ingestion of uncooked meat, food or beverages contaminated with urine or anal secretions of infected marsupials [7]. The emergence of Chagas disease from oral routes of transmission, especially in the Amazon Region are based on the consumption of food contaminated by the failure to adopt adequate hygiene practices in food handling and human establishment in sylvatic habitats, which increases the risks associated with the proximity of sylvatic vectors and reservoirs [7]. Oral outbreaks of Chagas disease were first reported in the Brazilian Amazon basin in 1965, followed by reports in Argentina, Bolivia, Colombia, Ecuador and in Venezuela where specific outbreaks have occurred in urban areas [8]. The previously mentioned cases of T. cruzi oral transmission resulted from consumption of sugarcane (Saccharum spp.) juice, açai (Euterpe oleracea) juice, Guava (Psidium guajava) juice and meat from hunting animals. The majority source of contamination has been attributed to T. cruzi infected insects that are macerated with the sugarcane or the fruits used for juice preparation [8]–[13]. All of these acute Chagas disease outbreaks associated with food/beverage consumption display severe clinical features in comparison with those of patients that have been infected with T. cruzi by other transmission routes [4], [11], [14].
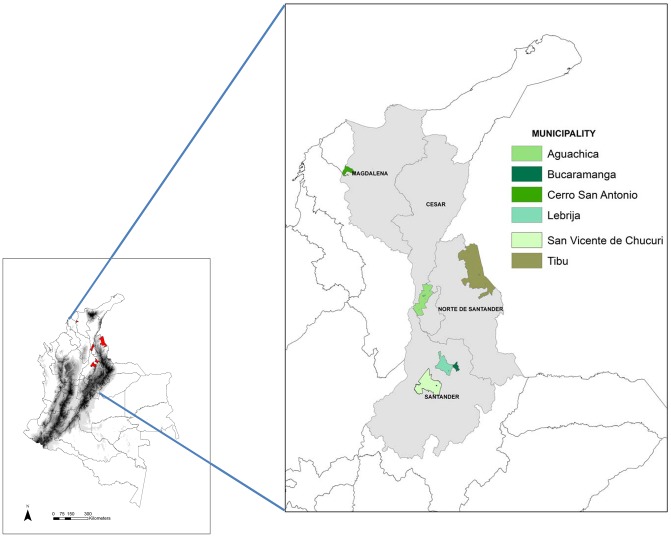
In Colombia, six cases of acute Chagas disease outbreaks attributed to food/beverages consumption and catalogued as cases of oral transmission according to the National Health Institute (NHI) harboring four municipalities have been reported. The first outbreak occurred in a group of soldiers in the municipality of Tibú (Norte de Santander) in 1992. Six cases of acute Chagas disease myocarditis were confirmed. The NHI studied a group of 144 soldiers, of which 24 (17%) were confirmed as serologically positive by Immunofluorescent Assay (IFAT); Fifty-two percent of the seropositive patients presented electrocardiographic abnormalities [15]. The second outbreak was reported in the municipality of Guamal (Magdalena) in 1999. The surveys provided information from 607 patients; of these, 9 (3.2%) presented fevers. Sera samples were taken from 102 patients and 13% (13/102) of them were IFAT positive with cardiac alterations [16]. The third outbreak took place in Lebrija (Santander) in 2008, two patients died. Diagnosis was confirmed in 10 cases, which were 100% reactive to ELISA and IFAT. In addition, three cases presenting severe myocarditis were confirmed by histopathology [17]. The fourth outbreak took place in the city of Bucaramanga in 2009, when one child died due to severe myocarditis attributed to T. cruzi ethiology and a total of 5 cases were confirmed in the same family. The NHI found that the most frequent symptoms were fever (100%), abdominal pain (60%), and cardiomegaly (40%). Pericardial effusion was detected in 80% of the cases in the 2009 outbreak in Bucaramanga. The diagnosis was confirmed in 80% of the cases using serological tests (ELISA and IFAT). The two last outbreaks were reported in San Vicente de Chucurí (Santander) and Aguachica (Cesar) in 2010, when no deaths were reported but serological diagnosis confirmed that the symptoms were a result of Chagas disease. In the outbreak of Aguachica, 12 cases were confirmed by serological diagnosis using ELISA and IFAT; two cases presented pericardial effusion (Figure 1).
Figure 1. Geographical distribution and location of the municipalities in Colombia where oral transmission outbreaks have been reported.
There is scarce available information regarding T. cruzi DTU's detected in the oral cases of Chagas disease. In studies developed by Marcili (2009), T. cruzi genotypes associated with food consumption were characterized from wild primates, triatomines and humans in the Brazilian Amazon; all isolates were genotyped as TcI and TcIV [18]. Andrade (2011) examined T. cruzi strains isolated from oral Chagas disease patients in Santa Catarina, Brazil and reported the presence of DTU's mixtures (TcI, TcII, TcV) [19]. Despite these efforts, there is not an evidence-based link between the severe clinical features of food-borne Chagas disease and T. cruzi genotypes [20]–[22]. The objective of this work was to develop a high-throughput analysis using polymorphic microsatellite markers based on a Multilocus Microsatellite Typing strategy (MLMT) and gene sequencing of the spliced leader intergenic region of mini-exon gene (SL-IR) and a mitochondrial Multilocus Sequence Typing (mtMLST) strategy to understand the molecular epidemiology of the clones isolated from six oral Chagas disease outbreaks in Colombia.
Methods
Study samples, isolates and clones
Eight T. cruzi isolates from humans (Tibú, Lebrija, Bucaramanga and San Vicente de Chucurí outbreaks) and two from triatomines (Guamal and Aguachica outbreaks) were cloned by a poisson-distributed limiting dilution assay, obtaining five clones from each isolate (a total of 50 clones analyzed) [23] (Table 1). Blood in guanidine buffer (GEB) from six patients (Lebrija outbreak) were collected and submitted to molecular characterization analysis by five genomic regions in order to discriminate T. cruzi DTU's [22], parasitic load quantification using a SYBR Green quantitative real-time PCR assay (qPCR), using methods previously reported [24] and Nested PCR of seven polymorphic microsatellite markers previously reported by following the amplification conditions described by Duque (2011) to determine the T. cruzi populations circulating in the patients [25], [26]. These microsatellite markers were only applied on this set of samples in terms of the sensitivity obtained since working with blood samples the number of parasites is scarce. An automated capillary sequencer (AB3730, Applied Biosystems, UK) was employed to obtain the allelic products using a fluorescent-tagged size standard. The metadata was checked manually for errors and all samples were typed in blind to avoid user bias.
Table 1. List of Trypanosoma cruzi stocks isolated from oral Chagas disease outbreaks in Colombia.
| Isolate | Gender | Age (Years) | Outbreak | Year | Host | IFAT/ELISA | Cardiac alterations |
| MHOM/CO/92/FCH | Male | 22 | Tibú (Norte de Santander) | 1992 | Homo sapiens | NA | NA |
| IRHO/CO/99/SAN | - | - | Guamal (Magdalena) | 1999 | Rhodnius pallescens | + | + |
| MHOM/CO/09/LER | Male | 21 | Lebrija (Santander) | 2008 | Homo sapiens | + | + |
| MHOM/CO/09/EH | Male | 22 | Lebrija (Santander) | 2008 | Homo sapiens | + | + |
| MHOM/CO/09/XCH | Female | 25 | Bucaramanga (Santander) | 2009 | Homo sapiens | + | + |
| MHOM/CO/09/NCH | Male | 48 | Bucaramanga (Santander) | 2009 | Homo sapiens | + | + |
| MHOM/CO/09/LJVP | Female | 22 | Bucaramanga (Santander) | 2009 | Homo sapiens | + | + |
| MHOM/CO/10/SMA | Female | 24 | San Vicente de Chucurí (Santander) | 2010 | Homo sapiens | + | + |
| MHOM/CO/10/GC | Female | 52 | San Vicente de Chucurí (Santander) | 2010 | Homo sapiens | NA | NA |
| IRHO/CO/10/RPALL | - | - | Aguachica (Cesar) | 2010 | R.pallescens | + | + |
Available sera samples from patients collected in the different outbreaks (four from Bucaramanga, two from San Vicente de Chucurí and four from Lebrija) were tested by TESA-blot. TESA-blot analysis was performed on sera previously positive by IFAT and ELISA. Briefly, TESAs from T. cruzi II strain Y were obtained from the supernatant of infected LLC-MK2 cells and used for immunoblotting. Membrane strips (5 mm) were later incubated with serum diluted 1∶200 in Tris-buffered saline (TBS)–1% milk for 2 h with mechanical agitation. After four 5-min washes in TBS, the bound antibodies were detected by using peroxidase-conjugated anti-human immunoglobulin G (Sigma) diluted 1∶2,500 in TBS–1% milk for 2 h. After a new cycle of washes, the immune complexes were revealed by the addition of H2O2 and 4-chloro-1-naphthol. The reaction was stopped with deionized water [27].
Ethics statement
All of the appropriate ethical clearance was considered and approval of the ethics committee from Universidad de Los Andes under the form number 066/2006. Written consent was obtained in all human patients included as part of the epidemiological surveillance developed by NIH and Universidad de Los Andes under the same form number 066/2006.
T. cruzi molecular characterization, microsatellite amplification and gene sequencing
Fifty clones corresponding to ten stocks were harvested until they reached logarithmic phase and 200 µL aliquots were taken for DNA extraction using the mini prep Qiagen kit (Table 1). We always used paired samples in order to compare DNA sequences and microsatellite allele profiles. The molecular characterization of the clones obtained was performed by amplifying the SL-IR and domain (D7) of the 24Sα region [28]. In order to detect genotypes within TcI, direct sequencing of SL-IR region and ten mitochondrion maxicircle DNA fragments was performed [28], [29]. Primers flanking variable regions of the pre-edited (12S rRNA and 9S rRNA), 5′ edited (Cytochrome b (Cytb), MUF1 (MurfA), NADH Dehydrogenase 1 (ND1), MURF2 (MurfB)), internal editing (Cytochrome oxidase II (COII)) and the non-edited regions, (NADH dehydrogenase 4 (ND4) and NADH dehydrogenase 5 (ND5a and ND5b), were amplified [29]. The amplification of the SL-IR and the ten maxicircle gene fragments was performed in a final volume of 20 µL using 1× Buffer (Corpogen, COL), 50 mM MgCl2, 10 µM of each primer, 5 U/µL of Taq Tucan (Corpogen, COL) and 10 ng of DNA. The mix was submitted to 29 cycles of amplification and the amplicons were visualized in 2% agarose gels stained with ethidium bromide. The PCR products were cleaned up by isopropanol precipitation and sequenced by the dideoxy-terminal method in an automated capillary sequencer (AB3730, Applied Biosystems, UK). The resulting sequences were edited in MEGA 5.0 [30]. All edited sequences were deposited in GenBank and assigned accession numbers KC282902–KC282951 (Table S1). The final sequences were concatenated in SeaView 4.0 according to the orientation of the kDNA maxicircle [31], [32]. The sequences of the maxicircle molecule were aligned and a matrix was constructed in Nexus format to develop a haplotype network analysis including reference strains from domestic and sylvatic mitochondrial haplotypes (EM – Homo sapiens-TcIa and YDm1M – Didelphis marsupialis-TcId), using the median-joining method and the default parameters on Network 2.0 to observe the polymorphism differences among the sequences. Twenty-four microsatellite loci were amplified as previously described [33]. These markers are spread over eight different chromosomes. An automated capillary sequencer (AB3730, Applied Biosystems, UK) was used to obtain the allelic products using a size standard with a fluorescent tag. The metadata was checked manually for errors and all samples were typed in blind to avoid user bias. Finally, individual level pair-wise distances were calculated using the DAS algorithm (1-proportion of shared alleles at all loci/n) and estimated using MICROSAT to construct a genetic distances Neighbor-joining tree (NJ).
Results
High-resolution molecular characterization of T. cruzi clones
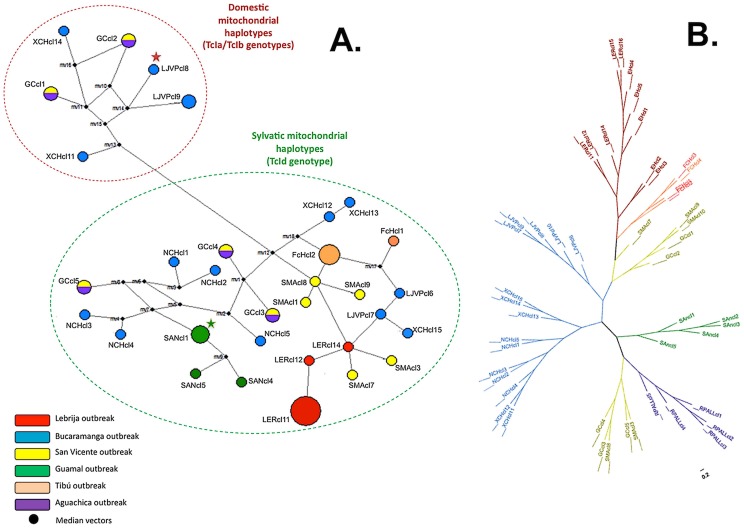
Molecular typing was performed in clones from the stocks, which resulted in the presence of T. cruzi I in 49 (98%) clones and T. cruzi IV in 1 (2%) clone (FCHcl5). When the sequencing of SL-IR was performed, occurrence of 11 (22%) clones as TcIa, 7 (14%) clones as TcIb and 31 (64%) clones as TcId was observed. According to the sequences of the ten gene fragments from the maxicircle molecule, a network was developed based on a median-joining algorithm, which allowed us to observe 32 different haplotypes among the dataset. From the network, we could establish six mitochondrial haplotypes associated to the domestic cycle and 24 mitochondrial haplotypes associated to the sylvatic cycle of transmission based on reference strains (TcIa and TcId), demonstrating the features displayed by each clone (Figure 2A). Based on the microsatellite patterns, a Neighbor-Joining tree was constructed using the genetic distances of shared alleles. We determined the genetic relationships between clones from the same oral outbreak, observing specific alleles groups in each oral outbreak (evidenced by the different number of multilocus genotypes), displaying that independent populations are the causative agents of each outbreak (Figure 2B; Table S1).
Figure 2. Network haplotype and un-rooted DAS tree of T. cruzi biological closes isolated from the six cases of oral Chagas disease transmission.
A. Median-Joining haplotype network based on MLSTmt of 49 TcI clones isolated from oral outbreaks in Colombia shows the presence of 32 different haplotypes and the discrimination of domestic and sylvatic mitochondrial haplotypes according to the median vectors. Red star indicate the reference sequence of TcI domestic mithocondrial haplotype (EM) and previously typed as TcIa using SL-IR region. Green star indicate the reference sequence of TcI sylvatic mitochondrial haplotype and previously typed as TcId using SL-IR region. There is congruence between MLSTmt and SL-IR genotypes that convey in domestic haplotypes for TcIa/TcIb and sylvatic haplotypes for TcId. The black spots are considered ‘mv’ that can be biologically interpreted as possibly extant unsampled sequences or extinct ancestral sequences. B. Neighbor-Joining genetic distance tree based on MLMT of 49 TcI clones isolated from the oral outbreaks in Colombia shows the clustering of the allelic profiles within each outbreak. A significant number of allelic multilocus genotypes can be considered within each cluster. The topology of the un-rooted tree demonstrates the allelic relatedness among the clones isolated within the same oral outbreak with the presence of some outliers.
Parasitic load and molecular characterization of GEB samples and TESA-blot results
The molecular characterization of GEB samples resulted in the detection of different genotypes within the same patient and absolute parasitic loads ranging from 110 to 250 parasites/mL (Table 2). In the cases of AM and EH, a co-infection with genotypes TcIa and TcId (SL-IR) was observed and also presenting the same allelic profile based on microsatellite data. Hence, patients JR and EB were found infected with the TcIa genotype and the same T. cruzi population. In the cases of patients FM and WM, they were infected with only the TcId genotype and they had the same allele profile based on microsatellite data. All of the samples analyzed by TESA-blot showed bands between 130–200 kDa, which corresponds to the SAPA antigen only detected in the acute phase of Chagas disease.
Table 2. DTU discrimination, TcI genotypes and polymorphic microsatellites (Allele sizes in base pair) results from GEB samples.
| PATIENT | qPCR (parasites/mL) | DTU | TcI SL-IR genotype | TcAAAT6 | TcAAT8 | TcGAG10 | TcCAA10 | TcATT14 | TcTAC15 | TcTAT20 |
| AM | 125 | TcI | TcIa-TcId | 251/251 | 226/226 | 144/144 | 125/125 | 250/253 | 93/93 | 181/181 |
| EH | 255 | TcI | TcIa-TcId | 251/251 | 226/226 | 144/144 | 125/125 | 250/253 | 93/93 | 181/181 |
| JR | 130 | TcI | TcIa | 251/251 | 226/226 | 144/144 | 125/125 | 250/253 | 93/93 | 181/181 |
| EB | 110 | TcI | TcIa | 255/255 | 226/229 | 144/144 | 122/122 | 253/253 | 93/93 | 181/181 |
| WM | 250 | TcI | TcId | 251/255 | 226/229 | 144/144 | 122/125 | 250/253 | 93/93 | 181/181 |
| FM | 133 | TcI | TcId | 251/255 | 226/229 | 144/144 | 122/125 | 250/253 | 93/93 | 181/181 |
Discussion
To our knowledge, this is the first report of a set of high-resolution molecular markers being applied to a group of clones isolated from cases of oral Chagas disease transmission. There are few reports where molecular discrimination of DTU's has been performed. In Brazil, there are reports of 14 isolates typed as TcI and TcIV in the Amazonian region [18]. In Santa Catarina, Brazil, a full study using several markers allowed the authors to find TcI, TcII, TcV and mixed infections of the isolates obtained from an oral outbreak in 2005 [19]. In Bolivia, a recent outbreak allowed the authors to find TcI and TcIV [34]. This suggests that our results showing a high prevalence of TcI and one clone typed as TcIV, are in accordance with the genotypes found in stocks isolated from other oral transmission cases. These findings are also in accordance with the biological and ecological distributions of TcI and TcIV, where these genotypes are related to the sylvatic transmission cycles and also display an interesting intersection feature being isolated from domestic and sylvatic hosts in a same niche [2], [33], [35], [36].
Information regarding the epidemiological surveillance was obtained in the cases of Lebrija, Bucaramanga and Aguachica. In Lebrija, the community collected two triatomines (Panstrongylus geniculatus and Rhodnius pallescens) from the sylvatic cycle where T. cruzi infection was detected in P. geniculatus. Regarding the reservoirs, one D. marsupialis was collected and not infected with trypanosomatids. As a result, we could determine those cases as oral transmission outbreaks due to the absence of domiciliated vectors. In Bucaramanga, the outbreak (nine cases) occurred in the same house during a citrus harvesting, in which the source of infection could be attributed to mandarine and/or orange juice. There was no evidence of domiciliated triatomines; P. geniculatus and R. pallescens were collected and P. geniculatus was found infected with T. cruzi. There are fruits that are sold in the area in little shops on the streets, where P. geniculatus vectors could infect the fruits by dejection of faeces, however further studies are required to validate this premise. The molecular characterization using SL-IR in the isolates and in the GEB samples showed that all clones from the Lebrija patients were typed as TcI. In addition, isolates from P. geniculatus have been found infected with TcI in the sylvatic foci, which may support that it could be the responsible vector in the outbreaks, including the similar multilocus genotypes evidenced by MLMT [23], [36]–[38]. These results are in accordance with the reported cases of acute oral Chagas disease in Venezuela, where P. geniculatus was the vector incriminated and TcI infection also confirmed [13], [39].
A significant frequency of TcI among the clones studied was found as previously reported in Colombia [28], [37]. In the samples collected, TcIa, TcIb and TcId were detected in the clones analyzed as mixed infections within stocks. Based on the analysis of SL-IR genotypes, a pattern of super-infection and/or co-infection within the same patient was observed. It is interesting to highlight the definition of super-infection in the acute phase of Chagas disease; in this context this might be suggesting that a composite of T. cruzi populations (from different triatomines and/or infected mammals) are causing the acute infection in the patients sampled. Hence, in terms of multiclonality the acute phase shows the lowest percentage of population diversity but this case is not ruled out in the oral transmission where at least two distinct genotypes were detected within the same patient. Therefore, the infection of two distinct genotypes was corroborated in the stocks LER and EH by microsatellites, demonstrating that certain alleles are associated to the domestic cycle of infection and some alleles associated with the sylvatic cycle residing within the same patient. This suggests that triatomines infected with a composite of T. cruzi populations are a source of infection or that the presence of more than one triatomine is involved in each outbreak. The TcIa genotype, which is associated with the domestic cycle of Chagas disease in Colombia, was detected but was not the most frequent. The TcIb genotype is associated with the peridomestic cycle, while TcId is associated with the sylvatic cycle and the most frequent in our dataset, which supports that the origin of infection was possible triatomines from sylvatic foci. The hypothesis about the origin of infection from sylvatic triatomines is supported by the presence of P. geniculatus and R. pallescens in the dwellings and because these triatomines were found to be mostly infected with genotypes TcIb and TcId [39]–[41].
A strong association across different haplotypes and the outbreaks was observed as well as the high genetic diversity among these clones represented in 32 different haplotypes, observing the occurrence of domestic and sylvatic haplotypes based on the reference strains sequences employed (Figure 2A) that correlates with the TcIa/TcIb (domestic genotypes) and TcId (sylvatic genotype) genotypes. The topology of the network suggests that the population affecting the two patients in Lebrija is similar and that the infection is harbored by a composite of T. cruzi populations, including a unique clone that has a hybrid maxicircle mosaic [37]. The network allows to observe the majority occurrence of sylvatic mitochondrial haplotypes and the low frequency of domestic mitochondrial haplotypes corroborating the premise of the likely incrimination of T. cruzi sylvatic DTU's in the oral infection outbreaks. P. geniculatus has been found naturally infected in Colombia with TcI, TcII and TcIII [23], [25], [42]. The presence of these DTU's in P. geniculatus is of paramount importance to understand the dynamics of T. cruzi DTUs in oral transmission outbreaks. The topology of the NJ tree based on polymorphic microsatellite data showed a strong association between each outbreak and the allelic profile of the clones obtained from each stock (Figure 2B). We did find defined clusters in most of the outbreaks with some outliers as seen in the San Vicente de Chucurí outbreak showing that the composite of T. cruzi populations causing the oral infection are quite alike and display a marked genetic variability pattern. There were two fatal cases in the Bucaramanga outbreak, which may suggest that the composition of multiclonal infections in oral outbreaks is closely related to the clinical manifestations of cardiomyopathy in acute cases of Chagas disease. Microsatellite markers were able to show the high multiclonality events observed in the stocks analyzed (a significant number of distinct allelic multilocus genotypes within hosts), which brings up the question whether the source of infection is the result of a single group of triatomines infected with the same T. cruzi population, or a composite of T. cruzi populations from the sylvatic cycle of transmission of Chagas disease in the region [35], [43]. The molecular characterization in GEB samples showed co-infections in two patients (AM and EH) with TcIa and TcId which supports the findings observed in the high-resolution molecular characterization performed in the clones from these patients (Table 2). In addition, the co-infection patterns could not be confirmed with the microsatellite markers employed. This premise emerges in the light of the absence of triple peaks among the loci studies or heterozygosis status; only locus TcATT14 showed heterozygosis and the rest of loci employed were homozygous, this could be explained by a low resolution power displayed by the microsatellite markers employed since parasite DNA yield was high (Table 2). Additionally, the multi-copy arrangement that SL-IR displays and the presence of null alleles and/or aneuploidy could explain these premises but further testing is required [44].
In conclusion, we developed a high-resolution molecular typing of the stocks isolated from cases of Chagas disease transmitted by an oral infection route in Colombia. We determined that the prevalent DTU in the stocks was TcI, with one clone typed as TcIV. Based on our results, the T. cruzi populations causing acute infections are those associated with the sylvatic foci and vectored by P. geniculatus and R. pallescens. The DTU discrimination in the oral infection outbreaks allowed us to highlight the significant impact of the DTU's from the sylvatic cycle, TcI and TcIV, in the outbreaks studied, which implies that oral transmission is a relevant epidemiological scenario that has emerged in the natural life cycle of T. cruzi. This suggests that, in areas where vectorial transmission has been interrupted, new acute cases of Chagas disease may emerge as a potential problem in public health.
Supporting Information
Microsatellite alleles, SL-IR genotypes and accession numbers of the clones analyzed.
(DOC)
Acknowledgments
We thank E.S. Umezawa from Universidad de Sao Paulo for kindly providing the TESA-blot strips, C. Gonzalez from Universidad de los Andes for the construction of the map.
Funding Statement
Financial support was provided by the Project Chagas EpiNet from The European Union Seventh Framework Programme, contract no. 223034. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Zingales B, Andrade S, Briones M, Campbell DA, Chiari E, et al. (2009) A new consensus for Trypanosoma cruzi intraspecific nomenclature: second revision meeting recommends TcI to TcVI. Mem Inst Oswaldo Cruz 104: 1051–1054. [DOI] [PubMed] [Google Scholar]
- 2. Zingales B, Miles MA, Campbell DA, Tibayrenc M, Macedo AM, et al. (2012) The revised Trypanosoma cruzi subspecific nomenclature: Rationale, epidemiological relevance and research applications. Infect Genet Evol 12: 240–253. [DOI] [PubMed] [Google Scholar]
- 3. Guhl F, Ramírez JD (2011) Trypanosoma cruzi I diversity: Towards the need of genetic subdivision? Acta Trop 119: 1–4. [DOI] [PubMed] [Google Scholar]
- 4.WHO (2007) World Health Organization Global health atlas. Available: http://www.who.int/globalatlas/. Accessed 2008 Mar 2.
- 5. Gascon J, Bern C, Pinazo Ma-Js (2010) Chagas disease in Spain, the United States and other non-endemic countries. Acta Trop 115: 22–27. [DOI] [PubMed] [Google Scholar]
- 6. Schmunis GA, Yadon ZE (2010) Chagas disease: A Latin American health problem becoming a world health problem. Acta Trop 115: 14–21. [DOI] [PubMed] [Google Scholar]
- 7.PAHO (2009) ENFERMEDAD DE CHAGAS: Guia para la vigilancia, prevención, control y manejo clínico de la enfermedad de Chagas aguda transmitida por alimentos. Available: www.paho.org.
- 8. Sánchez LV, Ramírez JD (2012) Congenital and oral transmission of American trypanosomiasis: an overview of physiopathogenic aspects. Parasitol 139: 1–13. [DOI] [PubMed] [Google Scholar]
- 9. Dias JCP (2006) Notas sobre o Trypanosoma cruzi e suas características bio-ecológicas, como agente de enfermidades transmitidas por alimentos. Rev Soc Bras Med Trop 39: 370–375. [DOI] [PubMed] [Google Scholar]
- 10. Moncayo Å, Silveira AC (2009) Current epidemiological trends for Chagas disease in Latin America and future challenges in epidemiology, surveillance and health policy. Mem Inst Oswaldo Cruz 104: 17–30. [DOI] [PubMed] [Google Scholar]
- 11.Pereira KS, Schmidt FvL, Barbosa RL, Gusraldo AM, Franco RM, et al. (2010) Chapter 3 - Transmission of Chagas Disease (American Trypanosomiasis) by Food. In: Steve LT, ed. Adv Food Nutr Res 59: : 63–85. [DOI] [PubMed] [Google Scholar]
- 12. Nóbrega AA, Garcia MH, Tatto E, Obara MT, Costa E, et al. (2009) Oral transmission of Chagas disease by consumption of açaí palm fruit, Brazil. Emerg Infect Dis 15: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Alarcón de Noya B, Díaz-Bello Z, Colmenares C, Ruiz-Guevara R, Mauriello L, et al. (2010) Large Urban Outbreak of Orally Acquired Acute Chagas Disease at a School in Caracas, Venezuela. J Infect Dis 201: 1308–1315. [DOI] [PubMed] [Google Scholar]
- 14. Coura JR (2007) Chagas disease: what is known and what is needed - A background article. Mem Inst Oswaldo Cruz 102: 113–122. [DOI] [PubMed] [Google Scholar]
- 15. Bohórquez RMB, Blanco M, Nicholls S, Hernández C, Gualdrón L (1992) Estudio de una epidemia de Carditis Aguda en Población Adulta. Act Med Col 17: 4. [Google Scholar]
- 16. Cáceres D, Nicholls RS, Corredor A, Gualdrón L, Slait E, et al. (1999) Investigación de un brote de síndrome febril con miocarditis aguda en Guamal,Magdalena, 7 a 11 de junio de 1999. Inf Quinc Epidemiol Nac 4: 170–178. [Google Scholar]
- 17. Hernández LM, Ramírez A, Cucunubá Z, Zambrano P (2009) Brote de Chagas agudo en Lebrija, Santander, 2008. Rev Obs S Pub San 4: 28–36. [Google Scholar]
- 18. Marcili A, Valente VC, Valente SA, Junqueira AC, da Silva FM, et al. (2009) Trypanosoma cruzi in Brazilian Amazonia: Lineages TCI and TCIIa in wild primates, Rhodnius spp. and in humans with Chagas disease associated with oral transmission. Int J Parasitol 39: 615–623. [DOI] [PubMed] [Google Scholar]
- 19. Andrade SG, Campos RlF, Steindel M, Guerreiro ML, Magalhaes JB, et al. (2011) Biological, biochemical and molecular features of Trypanosoma cruzi strains isolated from patients infected through oral transmission during a 2005 outbreak in the state of Santa Catarina, Brazil: its correspondence with the new T. cruzi Taxonomy Consensus. Mem Inst Oswaldo Cruz 106: 948–956. [DOI] [PubMed] [Google Scholar]
- 20. Burgos JM, Diez M, Vigliano C, Bisio M, Risso M, et al. (2010) Molecular Identification of Trypanosoma cruzi Discrete Typing Units in End-Stage Chronic Chagas Heart Disease and Reactivation after Heart Transplantation. Clin Infect Dis 51: 485–495. [DOI] [PubMed] [Google Scholar]
- 21. Macedo AM, Machado CR, Oliveira RP, Pena SDJ (2004) Trypanosoma cruzi: genetic structure of populations and relevance of genetic variability to the pathogenesis of chagas disease. Mem Inst Oswaldo Cruz 99: 1–12. [DOI] [PubMed] [Google Scholar]
- 22. Ramírez JD, Guhl F, Rendón LMa, Rosas F, Marin-Neto JA, et al. (2010) Chagas Cardiomyopathy Manifestations and Trypanosoma cruzi Genotypes Circulating in Chronic Chagasic Patients. PLoS Negl Trop Dis 4: e899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ramírez JD, Duque MC, Guhl F (2011) Phylogenetic reconstruction based on Cytochrome b (Cytb) gene sequences reveals distinct genotypes within Colombian Trypanosoma cruzi I populations. Acta Trop 119: 61–65. [DOI] [PubMed] [Google Scholar]
- 24. Moreira OC, Ramírez JD, Velázquez E, Melo M, Lima-Ferreira C, et al. Towards the establishment of a consensus Real-Time qPCR to monitor Trypanosoma cruzi parasitemia in patients with chronic Chagas disease cardiomyopathy: a substudy from the BENEFIT trial. Acta Trop In press. [DOI] [PubMed] [Google Scholar]
- 25. Duque M, Ramírez JD, Rendón L, Guhl F (2011) Evaluación de la variabilidad genética de aislamientos colombianos de Trypanosoma cruzi I mediante marcadores microsatélites. Infectio 15: 4. [Google Scholar]
- 26. Valadares HMS, Pimenta JR, de Freitas JM, Duffy T, Bartholomeu DC, et al. (2008) Genetic profiling of Trypanosoma cruzi directly in infected tissues using nested PCR of polymorphic microsatellites. Int J Parasitol 38: 839–850. [DOI] [PubMed] [Google Scholar]
- 27. Umezawa ES, Nascimento MS, Kesper N, Coura JR, Borges-Pereira J, et al. (1996) Immunoblot assay using excreted-secreted antigens of Trypanosoma cruzi in serodiagnosis of congenital, acute and chronic Chagas' disease. J Clin Microbiol 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Herrera C, Bargues MD, Fajardo A, Montilla M, Triana O, et al. (2007) Identifying four Trypanosoma cruzi I isolate haplotypes from different geographic regions in Colombia. Infect Genet Evol 7: 535–539. [DOI] [PubMed] [Google Scholar]
- 29. Messenger LA, Llewellyn MS, Bhattacharyya T, Franzén O, Lewis MD, et al. (2012) Multiple Mitochondrial Introgression Events and Heteroplasmy in Trypanosoma cruzi Revealed by Maxicircle MLST and Next Generation Sequencing. PLoS Negl Trop Dis 6: e1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tamura K, Peterson D, Peterson N, Stecher G, Nei M, et al. (2011) MEGA5: Molecular Evolutionary Genetics Analysis Using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Mol Biol Evol 28: 2731–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gouy M, Guindon Sp, Gascuel O (2010) SeaView Version 4: A Multiplatform Graphical User Interface for Sequence Alignment and Phylogenetic Tree Building. Mol Biol Evol 27: 221–224. [DOI] [PubMed] [Google Scholar]
- 32. Westenberger S, Cerqueira G, El-Sayed N, Zingales B, Campbell D, et al. (2006) Trypanosoma cruzi mitochondrial maxicircles display species- and strain-specific variation and a conserved element in the non-coding region. BMC Genomics 7: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Llewellyn MS, Miles MA, Carrasco HJ, Lewis MD, Yeo M, et al. (2009) Genome-Scale Multilocus Microsatellite Typing of Trypanosoma cruzi Discrete Typing Unit I Reveals Phylogeographic Structure and Specific Genotypes Linked to Human Infection. PLoS Pathog 5: e1000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Santalla J, Carrasco P, Espinoza E, Rios T, Brutus L (2011) First reported outbreak of Chagas disease in the Bolivian amazonean zone: a report of 14 cases of oral transmission of acute Trypanosoma cruzi in Guayamerín, Beni-Bolivia. Biofar 19: 52–58. [Google Scholar]
- 35. Llewellyn MS, Lewis MD, Acosta N, Yeo M, Carrasco HJ, et al. (2009) Trypanosoma cruzi IIc: Phylogenetic and Phylogeographic Insights from Sequence and Microsatellite Analysis and Potential Impact on Emergent Chagas Disease. PLoS Negl Trop Dis 3: e510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Marcili A, Lima L, Valente VC, Valente SA, Batista JS, et al. (2009) Comparative phylogeography of Trypanosoma cruzi TCIIc: New hosts, association with terrestrial ecotopes, and spatial clustering. Infect Genet Evol 9: 1265–1274. [DOI] [PubMed] [Google Scholar]
- 37. Ramírez JD, Guhl F, Messenger L, Lewis M, Montilla M, et al. Contemporary cryptic sexuality in Trypanosoma cruzi . Mol Ecol In press. [DOI] [PubMed] [Google Scholar]
- 38. Mejía-Jaramillo AM, Peña VH, Triana-Chavez O (2009) Trypanosoma cruzi: Biological characterization of lineages I and II supports the predominance of lineage I in Colombia. Exp Parasito 121: 83–91. [DOI] [PubMed] [Google Scholar]
- 39. Carrasco HJ, Torrellas A, García C, Segovia M, Feliciangeli MD (2005) Risk of Trypanosoma cruzi I (Kinetoplastida: Trypanosomatidae) transmission by Panstrongylus geniculatus (Hemiptera: Reduviidae) in Caracas (Metropolitan District) and neighboring States, Venezuela. Int J Parasitol 35: 1379–1384. [DOI] [PubMed] [Google Scholar]
- 40. Cura CI, Mejía-Jaramillo AM, Duffy T, Burgos JM, Rodriguero M, et al. (2010) Trypanosoma cruzi I genotypes in different geographical regions and transmission cycles based on a microsatellite motif of the intergenic spacer of spliced-leader genes. Int J Parasitol 40: 1599–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Falla A, Herrera C, Fajardo A, Montilla M, Vallejo GA, et al. (2009) Haplotype identification within Trypanosoma cruzi I in Colombian isolates from several reservoirs, vectors and humans. Acta Trop 110: 15–21. [DOI] [PubMed] [Google Scholar]
- 42. Triana O, Ortiz S, Dujardin JC, Solari A (2006) Trypanosoma cruzi: Variability of stocks from Colombia determined by molecular karyotype and minicircle Southern blot analysis. Exp Parasitol 113: 62–66. [DOI] [PubMed] [Google Scholar]
- 43. Llewellyn MS, Rivett-Carnac JB, Fitzpatrick S, Lewis MD, Yeo M, et al. (2011) Extraordinary Trypanosoma cruzi diversity within single mammalian reservoir hosts implies a mechanism of diversifying selection. Int J Parasitol 41: 609–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Valadares HMS, Ramos Pimenta J, Segatto M, Veloso VM, Gomes ML, et al. (2012) Unequivocal Identification of Subpopulations in Putative Multiclonal Trypanosoma cruzi Strains by FACs Single Cell Sorting and Genotyping. PLoS Negl Trop Dis 6 7: e1722 doi:10.1371/journal.pntd.0001722. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Microsatellite alleles, SL-IR genotypes and accession numbers of the clones analyzed.
(DOC)