Abstract
Noroviruses are a common cause of gastroenteritis worldwide, but outbreaks appear to be more common in industrialized countries than in developing countries, possibly reflecting differences in exposure and immunity. In this study, age-stratified sera from India and UK populations were analysed for the presence of norovirus-genogroup II specific IgG by a time resolved immunofluorescence assay and relative levels of antibodies in the two populations were compared. Antibody levels were higher among all age groups in India than in UK and increased with age in India, whereas in the UK, levels of antibody decreased in adulthood. These results indicate different patterns of exposure to noroviruses in the two countries.
Introduction
Noroviruses (NoV) are a major cause of sporadic gastroenteritis worldwide, as well as of outbreaks in hospitals and semi-closed communities such as residential and nursing homes [1]–[2]. Transmission is mainly via the fecal-oral route, through person-to-person contact or through contaminated food, water or the environment [3]. Infection elicits incomplete and short lived immunity, and previous studies with volunteers demonstrated that although pre-existing serum antibodies to NoV do not protect from re-infection, antibody levels to NoV are associated with protection after repeated exposure [4]. Seroprevalence studies have demonstrated that NoV infections are ubiquitous and infections occur early in life with prevalence reaching >95% in adults [5]–[7].
Reports of NoV prevalence in India are primarily from hospital and community based studies of sporadic acute gastroenteritis [8]–[11] and only one outbreak in a semi-closed setting has been reported [12].
This study examined the levels of human NoV-specific antibodies in age-stratified sera from India and UK populations as a measure of exposure to NoV infections in the two populations throughout life and demonstrated a difference which may, at least in part, explain the low incidence of NoV outbreaks in India in comparison to the UK.
Materials and Methods
Ethics Statement
The study was approved by the Institutional Review Boards of the Christian Medical College, Vellore, India. Prior to enrollment, written informed consent was obtained from the parents or legal guardians of eligible children.
Study Area and Population
In India, 1044 sera included sera collected in a probability proportional to size cluster survey conducted in Vellore from August 1999 to February 2000 [13] (samples from 0–40 years), and sera from the Biochemistry laboratory and the Community Health and Development Hospital, Vellore from residents in geographic areas of the serosurvey (sera from >40 years of age). The studies were approved by the Institutional Review Board of the Christian Medical College, Vellore. In the UK, 1034 sera collected in 2000 or 2005 from the HPA Seroepidemiology Programme (http://www.hpa.org.uk/webw/HPAweb&Page&HPAwebAutoListName/Page/1158313434390?p=1158313434390) were used. Sera from both cohorts were distributed in 5 age groups (Table 1).
Table 1. Summary of sera tested.
| Age Group | India Cohort | UK Cohort |
| <6 months | 83 | 47 |
| 7 m–5 y | 170 | 160 |
| 10–15 y | 237 | 207 |
| 20–30 y | 189 | 207 |
| 40–45 y | 158 | 206 |
| 50–60 y | 207 | 207 |
| Total | 1044 | 1034 |
Time Resolved Fluorescence Immunoassay (TRFIA)
Detection of human NoV-specific serum IgG was done by Time Resolved Fluorescence Immunoassay (TRFIA), which incorporates lanthanide labels that confer high sensitivity and a wider dynamic range than ELISA assays [14], and hence allows quantitation without the need for inclusion of a dilution series. Human NoV virus like particles (VLPs) were produced from an epidemic strain GII.4v2 [15], circulating in the UK during 2002 and later detected worldwide, the viral protein 1 (VP1) has 94% amino acid homology to the predominant Indian strain. The VLPs were used at a concentration of 2 µg/ml in 0.05 M carbonate/bicarbonate buffer, pH 9.6 to coat 96-well high-binding microwell plates (Costar, Corning, N.Y.), overnight at 4°C. Plates were washed 5 times with DELFIA wash buffer (PerkinElmer, UK) and 100 µl of serum diluted 1∶100 in DELFIA assay buffer (PerkinElmer, UK) was loaded per test well. Each plate included a standard curve of 8 serial two-fold dilution of a positive serum sample starting at 1∶100, a blank (no serum) and positive control and a negative control (goat serum). The plates were incubated in a humid chamber for 2 h at 37°C and then washed 5 times. A total of 100 µl of europium (Eu) labelled anti-human IgG conjugate (PerkinElmer, UK) diluted 1∶500 in DELFIA buffer was added to each well using a multi-channel pipette, followed by incubation for 1 h at 37°C and washing as before. A total of 150 µl of DELFIA enhancement solution (PerkinElmer, UK) was added to each well and plates were incubated in the dark at room temperature for 15 min with gentle rotation. Plates were read using a DELFIA 1234 reader (PerkinElmer, UK) and data analyzed using Multicalc software, version 4.0 (Wallac Oy, Finland). The distribution of the Eu counts was normalized by performing a natural log transformation. The plate-to-plate variability between the assays was eliminated by standardizing the slope and intercept across all experimental runs of the log-transformed europium counts, against a reference plate. Data were further analysed using STATA 10.0 for Windows (STATA Corp., TX, USA), using student’s t-test and ANOVA, as appropriate.
Results and Discussion
Using a cut-off of +3SD above the mean of negative controls, the prevalence of human NoV-specific antibody in both cohorts was high, 99.5% and 99.4% in the India and the UK cohort, respectively. With a higher arbitrary Eu count cut-off of 7000, 4% of samples from UK and 1.6% samples from India were below this cut-off, mainly in the 0–5 year age group, with younger children within the group having fewer antibodies. The high prevalence found in this study, particularly for the UK cohort, when compared to previous studies may be due to the use of a more sensitive assay and also the choice of antigen, which used the recently widely circulating GII.4 strain, although a subset of sera were evaluated with a GII.4 VLP made with a pre-2002 strain also showed complete cross-reactivity (data not shown). Other studies have shown that the antibodies detected in human NoV-specific EIA are largely cross-reactive across different NoV genotypes [16]–[19]. Studies examining seroprevalence to GII NoVs reported overall seropositivity of 91.2%, 74.1% 87% and 96.5% in Italy, France, Japan and South Africa, respectively [20]–[23] using conventional ELISAs. A study from China showed that the seropositivity increased from 70% among one year olds to 98% among 8–9 year old children for human norovirus GII and from 65% in the one year olds to 100% among the 8–9 year old children for human norovirus GI [24].
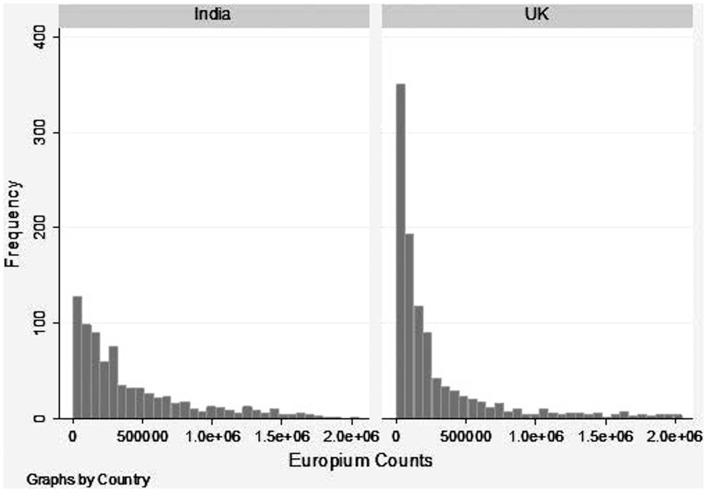
Although the proportion of seropositives to human NoV antibody was comparable in the two populations, the levels of antibodies as represented by the distribution of Eu counts were markedly different (Figure 1). Due to the lack of a standard serum for quatification of human NoV-specific antibodies, the results are presented as relative quantitation of antibody levels between the two cohorts rather than absolute quantification.
Figure 1. Distribution of Europium counts obtained with sera from the India and UK sera cohorts.
The europium counts were log transformed for a normal distribution. The first plate from India was the reference category and a slope and intercept was calculated for all other plates from both India and the UK. The slope and the intercept values were used to standardize the log-transformed europium counts across experimental runs. Standardized log-transformed values were then used for all analyses.
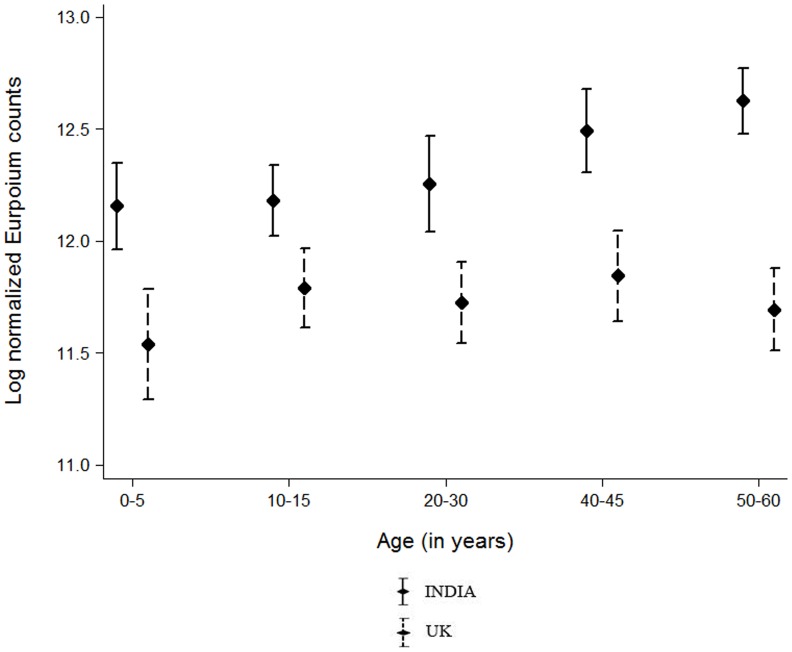
For the comparative analysis between the two cohorts, exclusion of the negative samples did not alter the results, and data shown includes the complete cohorts. Sera from India showed significantly higher levels of norovirus-specific IgG than the sera from UK across all 5 age groups (Fig. 2). The Indian panel showed a steady rise in human NoV-specific antibody levels with age, reaching the highest levels in the age group of 50–60 years of age (Fig. 2). In the UK panel, the antibody levels was lowest among the 0–5 year old children with a mean (SD) log normalized Eu count of 11.5 (1.8), increasing to 11.8 (1.3) in 10–15 years age group; the differences were not statistically significant (P = 0.264). Among the Indian samples, antibody levels were significantly different across age groups, with higher levels among the 50–60 year age group [Mean (SD) 12.6 (1.1)] in comparison to the younger age groups 0–5 [Mean (SD) 12.2(1.5), P = 0.003] and 10–15 year age groups [Mean (SD) 12.2(1.5), P<0.001]. No significant difference was observed (at p = 0.003 level, after Bonferroni correction) for any of the other age groups (Fig. 2). The antibody levels were comparable between males and females for all age groups except the 20–30 year olds. In this age group the antibody levels were higher in females (12.1, 1.4) than in males (11.8, 1.4, P = 0.016). Although not statistically significant, this relationship held true for both India [12.4 (1.5) vs. 12.0 (1.5), P = 0.102] and UK [11.8 (1.3) vs. 11.6 (1.3), P = 0.188] panels (data not shown).
Figure 2. Distribution of log normalized Europium counts among the different age strata in India and the UK.
The dots in the center represent the mean Europium counts for that age group whereas the horizontal error bars represent +2 standard deviations. Europium counts were measured by time resolved immunofluresecence assay.
The results show higher levels of human NoV-specific antibodies in the population in India compared to the UK, with the widest difference between the two populations in the youngest and the oldest age groups analysed. These findings suggest more constant exposure to noroviruses across all age groups in India when compared to the UK. A number of factors are known to play a role in the increased prevalence of some infectious diseases in developing countries. These include poor hygiene and sanitation, less access to healthcare, overcrowding as well as socio-cultural differences wherein people in India live in larger family units when compared to developed countries [25]–[28]. Norovirus infections, similar to rotavirus infections, are endemic in infants and children. Recently it has been demonstrated that in India, first rotavirus infections occur earlier than in the middle and high-income countries, and that number of reinfections is higher [29] and that seroprevalence of rotavirus group A antibodies is 100% in the same age-stratified population as evaluated here [30]. The same pattern could be expected for human norovirus infections, which would be consistent with the data presented here and with other studies from developing countries [23]–[24], [31]. In the UK, the peak of human norovirus-specific antibody levels in the 5–10 year olds is likely to reflect increased opportunities for infection through contact with other children. Indeed, in Europe, social contact rates are highest in school age children [32]. The lower human norovirus–specific antibody levels in the >50 years old could also correlate with the increased susceptibility in this age group that translates into the high frequency of norovirus outbreaks that are seen each winter in the UK, particularly among elderly patients in hospitals and residential care institutions. In addition to the social contact across all ages, it is likely that in India there are also greater opportunities for food, water and environmental transmission of noroviruses, contributing to greater and steady exposure and boosting of immunity. This may result in re-infections being mostly subclinical, and outbreaks being rare in this population in contrast to the high incidence of outbreaks involving adults and the elderly in the UK and other high income countries.
Funding Statement
This study was funded by British Council, with grant number SA08-040. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Blanton LH, Adams SM, Beard RS, Wei G, Bulens SN, et al. (2006) Molecular and epidemiologic trends of caliciviruses associated with outbreaks of acute gastroenteritis in the United States, 2000–2004. J Infect Dis 193: 413–421. [DOI] [PubMed] [Google Scholar]
- 2. Vipond IB, Caul EO, Hirst D, Carmen B, Curry A, et al. (2004) National epidemic of Lordsdale Norovirus in the UK. J Clin Virol 30: 243–247. [DOI] [PubMed] [Google Scholar]
- 3. Atmar RL (2010) Noroviruses - State of the Art. Food Environ Virol 2: 117–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Johnson PC, Mathewson JJ, DuPont HL, Greenberg HB (1990) Multiple-challenge study of host susceptibility to Norwalk gastroenteritis in US adults. J Infect Dis 161: 18–21. [DOI] [PubMed] [Google Scholar]
- 5. Gray JJ, Jiang X, Morgan-Capner P, Desselberger U, Estes MK (1993) Prevalence of antibodies to Norwalk virus in England: detection by enzyme-linked immunosorbent assay using baculovirus-expressed Norwalk virus capsid antigen. J Clin Microbiol 31: 1022–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. O’Ryan ML, Vial PA, Mamani N, Jiang X, Estes MK, et al. (1998) Seroprevalence of Norwalk virus and Mexico virus in Chilean individuals: assessment of independent risk factors for antibody acquisition. Clin Infect Dis 27: 789–795. [DOI] [PubMed] [Google Scholar]
- 7. Cubitt WD, Green KY, Payment P (1998) Prevalence of antibodies to the Hawaii strain of human calicivirus as measured by a recombinant protein based immunoassay. J Med Virol 54: 135–139. [PubMed] [Google Scholar]
- 8. Chhabra P, Chitambar SD (2008) Norovirus genotype IIb associated acute gastroenteritis in India. J Clin Virol 42: 429–432. [DOI] [PubMed] [Google Scholar]
- 9. Chhabra P, Dhongade RK, Kalrao VR, Bavdekar AR, Chitambar SD (2009) Epidemiological, clinical, and molecular features of norovirus infections in western India. J Med Virol 81: 922–932. [DOI] [PubMed] [Google Scholar]
- 10. Nayak MK, Chatterjee D, Nataraju SM, Pativada M, Mitra U, et al. (2009) A new variant of Norovirus GII.4/2007 and inter-genotype recombinant strains of NVGII causing acute watery diarrhoea among children in Kolkata, India. J Clin Virol 45: 223–229. [DOI] [PubMed] [Google Scholar]
- 11. Monica B, Ramani S, Banerjee I, Primrose B, Iturriza-Gomara M, et al. (2007) Human caliciviruses in symptomatic and asymptomatic infections in children in Vellore, South India. J Med Virol 79: 544–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Girish R, Broor S, Dar L, Ghosh D (2002) Foodborne outbreak caused by a Norwalk-like virus in India. J Med Virol 67: 603–607. [DOI] [PubMed] [Google Scholar]
- 13. Kang G, Samuel R, Vijayakumar TS, Selvi S, Sridharan G, et al. (2005) Community prevalence of antibodies to human immunodeficiency virus in rural and urban Vellore, Tamil Nadu. Natl Med J India 18: 15–17. [PubMed] [Google Scholar]
- 14. Smith DR, Rossi CA, Kijek TM, Henchal EA, Ludwig GV (2001) Comparison of dissociation-enhanced lanthanide fluorescent immunoassays to enzyme-linked immunosorbent assays for detection of staphylococcal enterotoxin B, Yersinia pestis-specific F1 antigen, and Venezuelan equine encephalitis virus. Clin Diagn Lab Immunol 8: 1070–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Allen DJ, Noad R, Samuel D, Gray JJ, Roy P, et al. (2009) Characterisation of a GII-4 norovirus variant-specific surface-exposed site involved in antibody binding. Virol J 6: 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cannon JL, Lindesmith LC, Donaldson EF, Saxe L, Baric RS, et al. (2009) Herd immunity to GII.4 noroviruses is supported by outbreak patient sera. J Virol 83: 5363–5374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Green KY, Lew JF, Jiang X, Kapikian AZ, Estes MK (1993) Comparison of the reactivities of baculovirus-expressed recombinant Norwalk virus capsid antigen with those of the native Norwalk virus antigen in serologic assays and some epidemiologic observations. J Clin Microbiol 31: 2185–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Parker S, Cubitt D, Jiang JX, Estes M (1993) Efficacy of a recombinant Norwalk virus protein enzyme immunoassay for the diagnosis of infections with Norwalk virus and other human “candidate” caliciviruses. J Med Virol 41: 179–184. [DOI] [PubMed] [Google Scholar]
- 19. Widdowson MA, Rockx B, Schepp R, van der Poel WH, Vinje J, et al. (2005) Detection of serum antibodies to bovine norovirus in veterinarians and the general population in the Netherlands. J Med Virol 76: 119–128. [DOI] [PubMed] [Google Scholar]
- 20. Pelosi E, Lambden PR, Caul EO, Liu B, Dingle K, et al. (1999) The seroepidemiology of genogroup 1 and genogroup 2 Norwalk-like viruses in Italy. J Med Virol 58: 93–99. [PubMed] [Google Scholar]
- 21. Nicollier-Jamot B, Pico V, Pothier P, Kohli E (2003) Molecular cloning, expression, self-assembly, antigenicity, and seroepidemiology of a genogroup II norovirus isolated in France. J Clin Microbiol 41: 3901–3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Honma S, Nakata S, Numata K, Kogawa K, Yamashita T, et al. (1998) Epidemiological study of prevalence of genogroup II human calicivirus (Mexico virus) infections in Japan and Southeast Asia as determined by enzyme-linked immunosorbent assays. J Clin Microbiol 36: 2481–2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Smit TK, Steele AD, Peenze I, Jiang X, Estes MK (1997) Study of Norwalk virus and Mexico virus infections at Ga-Rankuwa Hospital, Ga-Rankuwa, South Africa. J Clin Microbiol 35: 2381–2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jing Y, Qian Y, Huo Y, Wang LP, Jiang X (2000) Seroprevalence against Norwalk-like human caliciviruses in beijing, China. J Med Virol 60: 97–101. [DOI] [PubMed] [Google Scholar]
- 25. Dai YC, Nie J, Zhang XF, Li ZF, Bai Y, et al. (2004) Seroprevalence of antibodies against noroviruses among students in a Chinese military medical university. J Clin Microbiol 42: 4615–4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Parashar UD, Li JF, Cama R, DeZalia M, Monroe SS, et al. (2004) Human caliciviruses as a cause of severe gastroenteritis in Peruvian children. J Infect Dis 190: 1088–1092. [DOI] [PubMed] [Google Scholar]
- 27. Koopmans M (2008) Progress in understanding norovirus epidemiology. Curr Opin Infect Dis 21: 544–552. [DOI] [PubMed] [Google Scholar]
- 28. Garcia C, DuPont HL, Long KZ, Santos JI, Ko G (2006) Asymptomatic norovirus infection in Mexican children. J Clin Microbiol 44: 2997–3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gladstone BP, Ramani S, Mukhopadhya I, Muliyil J, Sarkar R, et al. (2011) Protective effect of natural rotavirus infection in an Indian birth cohort. N Engl J Med 365: 337–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mukhopadhya I, Anbu D, Iturriza-Gomara M, Gray JJ, Brown DW, et al. (2010) Anti-VP6 IgG antibodies against group A and group C rotaviruses in South India. Epidemiology and Infection 138: 442–447. [DOI] [PubMed] [Google Scholar]
- 31. Smit TK, Bos P, Peenze I, Jiang X, Estes MK, et al. (1999) Seroepidemiological study of genogroup I and II calicivirus infections in South and southern Africa. J Med Virol 59: 227–231. [DOI] [PubMed] [Google Scholar]
- 32. Mossong J, Hens N, Jit M, Beutels P, Auranen K, et al. (2008) Social contacts and mixing patterns relevant to the spread of infectious diseases. PLoS Med 5: e74. [DOI] [PMC free article] [PubMed] [Google Scholar]