Abstract
The mTOR pathway is aberrantly stimulated in many cancer cells, including pancreatic ductal adenocarcinoma (PDAC), and thus it is a potential target for therapy. However, the mTORC1/S6K axis also mediates negative feedback loops that attenuate signaling via insulin/IGF receptor and other tyrosine kinase receptors. Suppression of these feed-back loops unleashes over-activation of upstream pathways that potentially counterbalance the antiproliferative effects of mTOR inhibitors. Here, we demonstrate that treatment of PANC-1 or MiaPaCa-2 pancreatic cancer cells with either rapamycin or active-site mTOR inhibitors suppressed S6K and S6 phosphorylation induced by insulin and the GPCR agonist neurotensin. Rapamycin caused a striking increase in Akt phosphorylation at Ser473 while the active-site inhibitors of mTOR (KU63794 and PP242) completely abrogated Akt phosphorylation at this site. Conversely, active-site inhibitors of mTOR cause a marked increase in ERK activation whereas rapamycin did not have any stimulatory effect on ERK activation. The results imply that first and second generation of mTOR inhibitors promote over-activation of different pro-oncogenic pathways in PDAC cells, suggesting that suppression of feed-back loops should be a major consideration in the use of these inhibitors for PDAC therapy. In contrast, metformin abolished mTORC1 activation without over-stimulating Akt phosphorylation on Ser473 and prevented mitogen-stimulated ERK activation in PDAC cells. Metformin induced a more pronounced inhibition of proliferation than either KU63794 or rapamycin while, the active-site mTOR inhibitor was more effective than rapamycin. Thus, the effects of metformin on Akt and ERK activation are strikingly different from allosteric or active-site mTOR inhibitors in PDAC cells, though all these agents potently inhibited the mTORC1/S6K axis.
Introduction
The mammalian target of rapamycin (mTOR) is a highly evolutionarily conserved protein kinase that plays a key role in the integration of growth factor, nutrient and energy status of the cells [1]. mTOR functions as a catalytic subunit in two distinct multiprotein complexes, mTOR complex 1 (mTORC1) and mTORC2. mTORC1, characterized by the regulatory subunit Raptor, controls at least two regulators of protein synthesis, the 40S ribosomal protein subunit S6 kinase (S6K) and the eukaryotic translation initiation factor 4E (eIF4E)-binding protein 1, referred as 4E-BP1 [1], [2]. The heterodimer of the tumor suppressor TSC2 (tuberin) and TSC1 (hamartin) represses mTORC1 signaling by acting as the GTPase-activator protein for the small G protein Rheb (Ras homolog enriched in brain), a potent activator of mTORC1 signaling in its GTP-bound state [3], [4]. Phosphorylation of TSC2 by Akt and/or ERK/p90RSK suppresses its GTPase activating activity towards Rheb, leading to mTORC1 activation [5]. mTORC1 is acutely and allosterically inhibited by rapamycin through binding to FKBP12. mTORC2, characterized by Rictor, is not inhibited by short-term treatment with this agent and phosphorylates several AGC protein kinases, including Akt at Ser473 [6], [7]. The mTORC1 pathway plays a key role in insulin/IGF receptor signaling [8], [9] and is aberrantly activated in many cancers, including pancreatic ductal adenocarcinoma (PDAC), one of the most lethal human diseases. Accordingly, PDAC cells express insulin and IGF-1 receptors and over-express IRS-1 and IRS-2 [10]–[12] and PDAC (but not normal) tissue display activated (phosphorylated) IGF-1R [13]. Gene variations in the IGF-1 signaling system have been associated to worse survival in patients with PDAC [14]. Inactivation of p53, as seen during the progression of 50–70% of PDAC, up-regulates the insulin/IGF-1/mTORC1 pathway [15]. Crosstalk between insulin/IGF-1 receptors and G protein-coupled receptor (GPCR) signaling systems potently stimulate mTORC1, DNA synthesis and cell proliferation in a panel of PDAC cells [16]–[20]. mTORC1 signaling plays a pivotal role in the proliferation and survival of PDAC cells [21] and is activated in pancreatic cancer tissues [20], [22]–[24]. Consequently, mTORC1 has emerged as an attractive therapeutic target in PDAC and other common malignancies.
In addition to growth-promoting signaling, mTORC1/S6K also mediates negative feedback loops that restrain signaling through insulin/IGF receptor and other tyrosine kinase receptors via phosphorylation and transcriptional repression of IRS-1 [25]–[30] and phosphorylation of Grb10 [31], [32]. Consequently, suppression of mTORC1 activity by rapamycin prevents inhibitory IRS-1 phosphorylations and degradation, thereby augmenting PI3K/Akt activation in several cancer cell types [30], [33]–[35]. These studies imply that the potential anti-cancer activity of rapamycin (or analogs) can be counterbalanced by release of feedback inhibition of PI3K/Akt activation [25], [30], [33]–[35]. Furthermore, rapamycin incompletely inhibits 4E-BP-1 phosphorylation [36]–[40]. Accordingly, the clinical antitumor activity of rapamycin and its analogs (rapalogs) has been rather limited in many types of cancer [41], [42], including PDAC [43], [44]. In an effort to target the mTOR pathway more effectively, novel inhibitors of mTOR that act at the catalytic active site (active-site mTOR inhibitors) have been identified, including PP242 [37], Torin [45], KU63794 [38] and its analogue AZD8055 [46]. These compounds inhibit 4E-BP-1 phosphorylation at rapamycin-resistant sites (e.g. Thr37/46) and block Akt phosphorylation at Ser473 through inhibition of mTORC2. However, active-site mTOR inhibitors also eliminate feedback loops that restrain PI3K activation [25] and consequently, their therapeutic effectiveness can also be diminished by activation of upstream pathways that oppose their anti-proliferative effects.
mTORC1 is also negatively regulated by metformin, the most widely used drug in the treatment of type 2 diabetes mellitus (T2DM). Metformin is emerging as a potential novel agent in cancer chemoprevention. Recent epidemiological studies linked administration of metformin to reduced incidence, recurrence and mortality of a variety of cancers in T2DM patients [20], [47]–[56], including PDAC [54], [56]. At the cellular level, metformin indirectly stimulates AMP–activated protein kinase (AMPK) activation [57], though other mechanisms of action have been proposed at very high concentrations of this biguanide. AMPK inhibits mTORC1 activation through stimulation of TSC2 function [58]–[60], leading to accumulation of Rheb-GDP (the inactive form) and by direct phosphorylation of Raptor, which disrupts its association with mTOR, leading to dissociation of the mTORC1 complex [61]. The precise consequence of suppression of negative feedback loops mediated by the mTORC1/S6K axis in response to metformin remains poorly defined and, in particular, it is not known whether rapamycin, active-site mTOR inhibitors and metformin lead to over-activation of similar upstream pathways in PDAC cells.
Here, we demonstrate that treatment of PANC-1 or MiaPaCa-2 pancreatic cancer cells with either rapamycin or active-site mTOR inhibitors suppressed S6K and S6 phosphorylation induced by insulin, a combination of insulin and the GPCR agonist neurotensin or serum. Rapamycin caused a striking augmentation of Akt phosphorylation at Ser473 while the active-site mTOR inhibitors KU63794 and PP242 completely abrogated Akt phosphorylation at this site. A salient feature of the results presented here is that active-site inhibitors of mTOR, in contrast to rapamycin, cause a marked increase in ERK activation in PDAC cells. The results imply that first and second generation mTOR inhibitors promote over-activation of different pro-oncogenic pathways in PDAC cells, namely Akt and ERK. Metformin also abolished mTORC1 activation but without over-stimulating Akt phosphorylation on Ser473. Furthermore, metformin prevented ERK activation in response to cross-talking agonists in PDAC cells. Our results demonstrate that the effects of metformin on Akt and ERK activation are strikingly different from those elicited by allosteric or active-site mTOR inhibitors, though all these agents potently inhibited the mTORC1/S6K axis.
Materials and Methods
Cell culture
The human pancreatic cancer cell lines PANC-1 and MiaPaCa-2 were obtained from the American Type Culture Collection (ATCC, Manassas, VA). These cell lines harbor activating mutations in the KRAS oncogene. Cells were grown in Dulbecco's modified Eagle Medium (DMEM) with 2 mM glutamine, 1 mM Na-pyruvate, 100 units/mL penicillin, and 100 µg/mL streptomycin and 10% fetal bovine serum (FBS) at 37°C in a humidified atmosphere containing 10% CO2.
Western blot analysis
Confluent cultures of PANC-1 or MIA PaCa-2 cells grown on 3 cm dishes were washed and then incubated for 24 hr with DMEM containing 5 mM glucose and 1% FBS. The cells were washed twice with DMEM containing 5 mM glucose and incubated in serum-free medium for 4 h and then treated as described in individual experiments. The cultures were then directly lysed in 2× SDS-PAGE sample buffer [200 mM Tris-HCl (pH 6.8), 2 mM EDTA, 0.1 M Na3VO4, 6% SDS, 10% glycerol, and 4% 2-mercaptoethanol], followed by SDS-PAGE on 10% gels and transfer to Immobilon-P membranes (Millipore, Billerica, MA). Western blots were then performed on membranes incubated overnight with the specified antibodies in phosphate-buffered saline (PBS) containing 0.1% Tween-20. The immunoreactive bands were detected with ECL (enhanced chemiluminescence) reagents (GE Healthcare Bio-Sciences Corp, Piscataway, NJ). The antibodies used detected the phosphorylated state of S6K at Thr389, S6 at Ser235/236, 4E-BP1 at Thr37/46 and Thr70, Akt at Ser473 and Thr308 and ERK1/2 at Thr202 and Tyr204 or the total levels of these proteins.
Anchorage-dependent cell proliferation
PANC-1 cells (105) were plated on 35 mm tissue culture dishes in DMEM containing 10% FBS. After 24 h of incubation at 37°C, groups of cultures were incubated with neurotensin and insulin with or without metformin in DMEM containing 0.25% FBS or Rapamycin, KU63794 or Metformin in DMEM containing 2.5% FBS. The cultures were then incubated for 4 d, and the total cell count was determined from a minimum of six wells per condition using a Coulter counter, after cell clumps were disaggregated by passing the cell suspension 10 times through a 19-gauge, and subsequently, a 21-gauge needle.
Materials
DMEM was obtained from Invitrogen (Carlsbad, CA). Neurotensin and insulin were obtained from Sigma Chemical (St. Louis, MO). Rapamycin, KU63794 and PP242 were from R&D Systems, Inc. Minneapolis. All antibodies were purchased from Cell Signaling Technology (Danvers, MA). Horseradish peroxidase–conjugated anti-rabbit IgG and anti-mouse IgG were from GE Healthcare Bio-Sciences Corp (Piscataway, NJ). All other reagents were of the highest grade available.
Results
Stimulation of p70S6K and S6 phosphorylation in response to insulin and neurotensin in PDAC cells is completely abolished by rapamycin or KU63794
Initially, we determined the influence of rapamycin and KU63794 on mTORC1-mediated phosphorylation of S6K in PDAC cells. Rapamycin is an allosteric inhibitor of mTORC1 that acts via FKBP-12 whereas KU63794 is a highly specific ATP-competitive inhibitor of mTOR that inhibits both mTORC1 and mTORC2. Cultures of PANC-1 ( Fig. 1 ) or MiaPaCa-2 ( Fig. 2 ) cells were incubated for 2 h in the absence or presence of rapamycin (at 10 or 100 nM) or KU63794 (at 1 or 5 µM). Then, the cultures were stimulated with a combination of insulin (10 ng/ml) and the GPCR agonist neurotensin (5 nM) for 2 h to elicit positive crosstalk [18], [20]. Phosphorylation of S6K on Thr389, a direct target of mTORC1, and phosphorylation of S6 (Ser235/236), a substrate of S6K, was monitored using specific antibodies that detect the phosphorylated state of those residues. Stimulation of either PANC-1 or MiaPaCa-2 cells with insulin and neurotensin induced robust phosphorylation of S6K on Thr389 and S6 ( Figs. 1 and 2 ). Exposure to either rapamycin or KU63794 completely prevented the increase in the phosphorylation of these proteins in response to stimulation by insulin and neurotensin in either PANC-1 ( Fig. 1 ) or MiaPaCa-2 cells ( Fig. 2 ). We verified that the total levels of S6K and S6 did not change in response to the treatments. The results indicate that allosteric or active-site inhibitors of mTOR potently blocked the mTORC1/S6K axis at the concentrations used in PDAC cells.
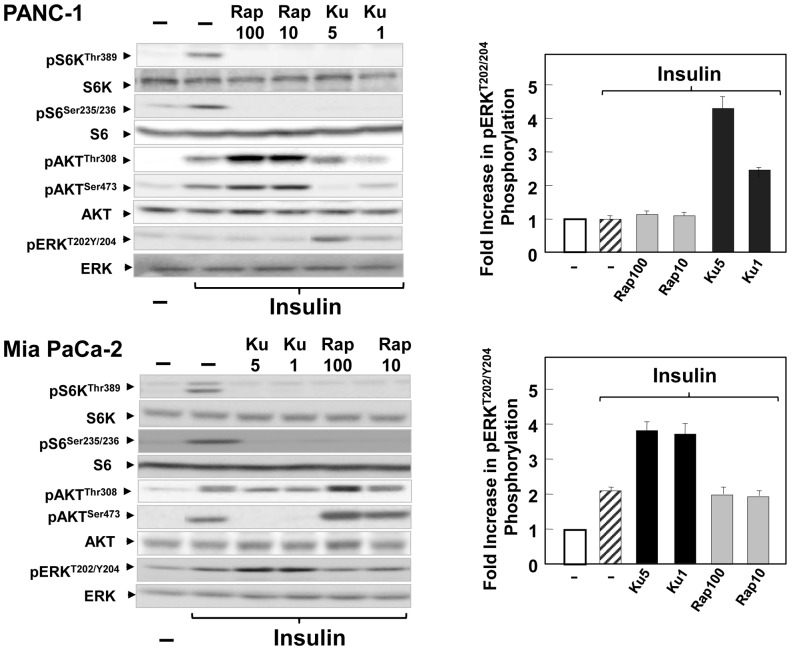
Figure 1. Differential feedback activation of Akt and ERK phosphorylation by rapamycin and KU63794 in PANC-1 cells.
Cultures of PANC-1 cells were incubated in the absence (−) or in the presence of KU63794 (Ku) at 1 µM or 5 µM or rapamycin (Rap) at 10 or 100 nM for 2 h in DMEM containing 5 mM glucose, as indicated. Then, the cells were stimulated for 2 h with 5 nM neurotensin (NT) and 10 ng/ml insulin (Ins) and lysed with 2×SDS–PAGE sample buffer. The samples were analyzed by SDS-PAGE and immunoblotting with antibodies that detect the phosphorylated state of S6K at Thr389, S6 at Ser235/236, 4E-BP1 at Thr37/46 and Thr70, Akt at Ser473 and Thr308 and ERK at Thr202 and Tyr204. Immunoblotting with antibodies that recognize total S6K, S6, 4E-BP1, Akt and ERK was used to verify that the cell treatments did not change the total level of these proteins and confirm equal gel loading. Fold increase in ERK phosphorylation was quantified using Multi Gauge V3.0 and plotted as bars. Similar results were obtained in 3 independent experiments.
Figure 2. Differential feedback activation of Akt and ERK phosphorylation by rapamycin and KU63794 in MiaPaCa-2 cells.
Cultures of MiaPaCa-2 cells were incubated in the absence (−) or in the presence of KU63794 (Ku) at 1 µM or 5 µM or rapamycin (Rap) at 10 or 100 nM for 2 h in DMEM containing 5 mM glucose, as indicated. Then, the cells were stimulated for 2 h with 5 nM neurotensin (NT) and 10 ng/ml insulin (Ins) and lysed with 2×SDS–PAGE sample buffer. The samples were analyzed by SDS-PAGE and immunoblotting with antibodies that detect the phosphorylated state of S6K at Thr389 (pS6K), S6 at Ser235/236 (pS6), 4E-BP1 at Thr37/46 and Thr70, Akt at Ser473 and ERK at Thr202 and Tyr204. Immunoblotting with antibodies that recognize total S6K, S6, 4E-BP1, Akt and ERK was used to verify that the cell treatments did not change the total level of these proteins and confirm equal gel loading. Fold increase in ERK phosphorylation was quantified using Multi Gauge V3.0 and plotted as bars. Similar results were obtained in 3 independent experiments.
Differential regulation of 4EBP1 phosphorylation sites in response to mitogenic stimulation, rapamycin and KU63794 in PDAC cells
The phosphorylation of 4EBP1 was also monitored by using site-specific 4E-BP1 antibodies that detect p-Thr37/46 or p-Thr70 in lysayes of PANC-1 ( Fig. 1 ) or MiaPaCa-2 ( Fig. 2 ) cells. These cells displayed a high basal level of 4E-BP1 phosphorylation at Thr37/46 that was not further increased by stimulation with insulin and neurotensin. However, cell stimulation reduced the mobility of 4E-BP1 in SDS/PAGE, a response suggestive of increased phosphorylation at other sites. Indeed, treatment of PANC-1 or MiaPaCa-2 cells with neurotensin and insulin markedly stimulated 4E-BP1phosphorylation on Thr70. The constituve phosphorylation of 4E-BP1 on Thr37/46 was abolished by treatment with KU63794 but was not affected by rapamycin at either 10 or 100 nM, in agreement with reports that rapamycin and its analogs do not inhibit 4E-BP1 phosphorylation at these sites in other cell types. In contrast, the signal responsive phosphorylation of 4E-BP1 on Thr70 was prevented by treatment with either KU63794 or rapamycin at 100 nM. We verified that the total levels of 4E-BP1 did not change in response to the treatments.These results revealed an unappreciated regulation of 4E-BP1 phosphorylation on different residues in response to external signals and demonstrate that rapamycin inhibits inducible but not constitutive 4E-BP1 phosphorylation in PDAC cells whereas active-site mTOR inhibitors suppress phosphorylation of 4E-BP1 at all sites.
Rapamycin and KU63794 induce over-stimulation of different upstream pathways in PDAC cells stimulated with insulin and neurotensin or insulin alone
In order to determine whether allosteric and active-site mTOR inhibitors eliminate feedback loops that restrain the activity of upstream signaling pathways in PDAC cells, we examined the effect of these inhibitors on the phosphorylation of Akt in response to mitogenic signaling in PANC-1 and Mia PaCa-2 cells. Stimulation of these cells with insulin and neurotensin induced a marked increase in Akt phosphorylation on Ser473 ( Figs. 1 and Fig. 2 ). Treatment with either 10 nM or 100 nM rapamycin promoted over-stimulation of Akt phosphorylation on Ser473, consistent with suppression of mTORC1/S6K axis feedback loops. In contrast, prior exposure to the active-site mTOR inhibitor KU63794, which inhibits both mTORC1 and mTORC2, blocked Akt phosphorylation on Ser473 in PANC-1 ( Fig. 1 ) and MiaPaCa-2 cells ( Fig. 2 ), in line with the notion that mTORC2 is the major protein kinase that phosphorylates Akt on Ser473 in PDAC cells. KU63794 did not prevent Akt phosphorylation at Thr308.
The ERK/RSK pathway, which plays a pivotal role in PDAC cell proliferation also leads to mTORC1 activation [5], [62]. In breast and bladder cancer cells, inhibition of the mTORC1/S6K axis by rapamycin induced feedback activation of ERK [63]. Consequently, we examined the effects of rapamycin and KU63794 on ERK activation in PDAC cells. In agreement with previous studies [16], [64], [65], stimulation of either PANC-1 or MiaPaCa-2 cells with insulin and neurotensin markedly activated ERK (ERK phosphorylated on Thr202 and Tyr204), as illustrated in Figs. 1 and 2 . In contrast to the results obtained in other cell types [63], treatment with either 10 or 100 nM rapamycin for 2 h did not alter the basal or the stimulated level of ERK phosphorylation in PANC-1 and MiaPaCa-2 cells. Similar results were obtained when these PDAC cells were treated with rapamycin for 4 or 24 h (results not shown). In contrast, exposure to KU63794 (1–5 µM) increased the basal level of ERK phosphorylation and strikingly enhanced the stimulation of ERK phosphorylation induced by insulin and neurotensin in either PANC-1 or MiaPaCa-2 cells. Quantification of the results with ERK is illustrated in the lower panels of Figs. 1 and 2 (bars). These results demonstrate that rapamycin, an allosteric inhibitor of mTORC1, and KU63794, an active-site inhibitor of mTOR, lead to over-activation of different upstream pro-oncogenic pathways in PDAC cells.
Stimulation of PANC-1 cells or MiaPaCa-2 with insulin alone induced robust increase in PI3K/Akt/mTORC1 but does not induce significant increase in ERK phosphorylated on Thr202 and Tyr204 ( Fig. 3 ). Consequently, we determined whether the differential effects of rapamycin and KU63794 depicted in PDAC stimulated with the combination of insulin and neurotensin (Figs. 1 and 2) can also be produced when PANC-1 and MiaPaCa-2 cells are challenged with insulin alone. Cultures of these cells were incubated for 2 h in the absence or presence of rapamycin (10–100 nM) or KU63794 (1–5 µM) and then stimulated with insulin (10 ng/ml). We monitored phosphorylation of S6K on Thr389, S6 on Ser235/236, Akt on Ser473 and Thr308 and ERK on Thr202 and Tyr204. Prior exposure to either rapamycin or KU63794 abolished the increase in the phosphorylation of S6K and S6 in response to insulin in either PANC-1 or MiaPaCa-2 cells ( Fig. 3 ). Exposure to rapamycin over-activated whereas treatment with KU63794 abolished Akt phosphorylation on Ser473 in the insulin-stimulated PDAC cells. Rapamycin did not produce any detectable effect on ERK activation in un-stimulated or insulin-treated cells. A salient feature of the results shown in Fig. 3 is that exposure to KU63794 induced a marked increase in the phosphorylation of ERK on Thr202 and Tyr204. These results corroborated that the allosteric inhibitor of mTORC1 and the active-site site inhibitor of mTOR promote over-activation of different upstream pathways in PDAC cells challenged with insulin or insulin and neurotensin, a combination that elicits crosstalk between insulin/IGF and GPCR signaling systems.
Figure 3. Differential feedback activation of Akt and ERK phosphorylation by rapamycin and KU63794 in insulin-stimulated MiaPaCa-2 and PANC-1 cells.
The cultures of PANC-1 (upper panels) and MiaPaCa-2 (lower panels) were incubated in the absence (−) or in the presence of KU63794 (Ku) at 1 µM or 5 µM or rapamycin (Rap) at 10 or 100 nM for 2 h in DMEM containing 5 mM glucose, as indicated. Then, the cells were stimulated for 2 h with 10 ng/ml insulin and lysed with 2×SDS–PAGE sample buffer. The samples were analyzed by SDS-PAGE and immunoblotting with antibodies that detect the phosphorylated state of S6K at Thr389, S6 at Ser235/236, Akt at Ser473 and Thr308 and ERK at Thr202 and Tyr204. Immunoblotting with total S6K, S6, Akt and ERK was used to verify equal gel loading. Fold increase in ERK phosphorylation was quantified using Multi Gauge V3.0 and plotted as bars. Similar results were obtained in 3 independent experiments.
PP242, like KU63794, enhances ERK activation in PANC-1 cells stimulated with insulin and neurotensin
Subsequently, we determined whether the striking over-activation of ERK by the active-site mTOR inhibitor KU63794 could be also produced by a structurally unrelated active-site mTOR inhibitor. Cultures of PANC-1 were incubated for 2 h in the absence or presence of PP242 (1–5 µM), a recently identified active-site mTOR inhibitor [37], and stimulated for 2 h with insulin and neurotensin. We monitored phosphorylation of S6K on Thr389, S6 on Ser235/236, 4EBP1 on Thr37/46, Akt on Ser473 and ERK on Thr202 and Tyr204. As shown in Fig. 4 A , prior exposure to PP242 abolished the phosphorylation of S6K, S6, 4EBP1 and Akt in PANC-1 cells. The key feature of the results is that PP242, like KU63794, induced a marked increase in the phosphorylation of ERK on Thr202 and Tyr204 ( Fig. 4A and quantification in Fig. 4B ). Because PP242 is a less selective mTOR inhibitor [66], we determined whether the concentrations of PP242 that promoted ERK activation coincide with those that inhibit mTORC1 activity. As shown in Fig. 4C , PP242 enhanced ERK activation and inhibited S6 phosphorylation at almost identical concentrations.
Figure 4. Feedback activation of ERK phosphorylation by PP242: role of PI3K.
A , Cultures of PANC-1 cells were incubated in the absence (−) or in the presence of PP242 at 1 µM or 5 µM for 2 h in DMEM containing 5 mM glucose, as indicated. Then, the cells were stimulated for 2 h with 5 nM neurotensin (NT) and 10 ng/ml insulin (Ins) and lysed with 2×SDS–PAGE sample buffer. The samples were analyzed by SDS-PAGE and immunoblotting with antibodies that detect the phosphorylated state of S6K at Thr389, S6 at Ser235/236, 4E-BP1 at Thr37/46, Akt at Ser473 and ERK at Thr202 and Tyr204. Immunoblotting with antibodies that recognize total S6K, S6, 4E-BP1, Akt and ERK was used to verify that the cell treatments did not change the total level of these proteins and confirm equal gel loading. Similar results were obtained in 3 independent experiments. B , The bars represent the increase in ERK phosphorylation induced by insulin (Ins) and neurotensin (NT) in cells without or with prior exposure to PP242. Quantification was performed using Multi Gauge V3.0 C, Cultures of PANC-1 cells were incubated as in panel A but in the presence of increasing concentrations of PP242. The samples were analyzed to detect the phosphorylated state of S6 at Ser235/236 and ERK at Thr202 and Tyr204. Immunoblotting with total ERK and S6 (not shown) was used to verify equal gel loading. Quantification was performed using Multi Gauge V3.0. D , Cultures of PANC-1 cells were incubated in the absence (−) or in the presence of either KU63794 (Ku) or PP242 at 5 µM for 2 h. Then, the cells were stimulated for 2 h with 5 nM neurotensin (NT) and 10 ng/ml insulin (Ins) and lysed with 2×SDS–PAGE sample buffer. The samples were analyzed by SDS-PAGE and immunoblotting with antibodies that detect the phosphorylated state of ERK at Thr202 and Tyr204, Akt at Ser473 and Thr308. Immunoblotting with total Akt and ERK was used to verify equal gel loading.
We verified that the active-site mTOR inhibitors KU63794 and PP242, at concentrations that markedly enhanced ERK activation and inhibited Akt phosphorylation on Ser473 did not prevent Akt phosphorylation at Thr308 in PDAC cells ( Fig. 4D ). In fact, the specific mTOR inhibitor KU63794 slightly enhanced Akt phosphorylation at Thr308, consistent with suppression of feedback loops that restrain PI3K activity ( Fig. 4D ). PP242 was less effective than KU63794 in enhancing Akt phosphorylation at Thr308, most likely reflecting off-target inhibition of PI3K [66]. Thus, the specific active-site site mTOR inhibitors KU63794 and PP242 suppressed Akt phosphorylation on Ser473, did not decrease Akt phosphorylation on Thr308 and stimulated over-activation of ERK phosphorylation at Thr202 and Tyr204 in PDAC cells.
The mechanism by which active-site inhibitors enhance ERK activation is not well understood. Our results do not support the existence in PDAC cells of a putative mTORC1/S6K/PI3K/ERK feedback loop, proposed in other cell types [63], since potent inhibition of the mTORC1/S6K axis by either rapamycin or everolimus did not produce overstimulation of ERK in PDAC cells. To substantiate this conclusion, PANC-1 cells were treated with KU63794 or PP242 and stimulated with insulin and neurotensin in the absence or presence of A66 [67], a selective inhibitor of the 110α catalytic subunit of PI3K. As shown in Fig. 4D , exposure to A66 did not prevent enhancement of ERK activation in response to exposure to either KU63794 or PP242. We corroborated that A66, at the concentration used, potently inhibited PI3K within PANC-1 cells since it prevented insulin-induced Akt phosphorylation at Thr308, the key residue in the Akt activation loop phosphorylated by PI3K-dependent PDK1.
In order to obtain further insight of the mechanism by which treatment with KU63794 induces over-activation of ERK we also determined the effect of this active-site mTOR inhibitor on the activation of MEK, the upstream kinase that phosphorylates ERK. MEK activation was scored by assessing the phosphorylation of Ser217 and Ser221 in its activation loop. As shown in Fig. S1, treatment of PANC-1 or MiaPaCa-2 cells with KU63794 markedly enhanced MEK phosphorylation induced by insulin and neurotensin. Collectively, the results demonstrate that active-site mTOR inhibitors led to MEK/ERK hyper-activation through a PI3K/S6K-independent feedback loop in PDAC cells.
Differential patterns of Akt and ERK activation in response to rapamycin, everolimus, KU63794 and PP242 in PANC-1 cells stimulated with serum
The preceding results were obtained with PDAC cells stimulated with defined mitogens that act through specific receptors. To extend further these findings we also tested whether differential patterns of Akt and ERK activation are produced when the cells are stimulated with fetal bovine serum. Cultures of PANC-1 cells were incubated for 2 h in the absence or presence of rapamycin (100 nM), everolimus (100 nM), KU63794 (1 µM) or PP242 (1 µM) and stimulated with medium containing fetal bovine serum. We monitored phosphorylation of S6 on Ser235/236, Akt on Ser473 and ERK on Thr202 and Tyr204. Prior exposure to rapamycin, everolimus, KU63794 or PP242 abolished the increase in the phosphorylation of S6 in response to serum ( Fig. 5 ). Exposure to rapamycin or everolimus over-activated whereas treatment with KU63794 or PP242 abolished Akt phosphorylation on Ser473 in serum-stimulated PDAC cells. Rapamycin or everolimus did not produce any detectable effect on ERK activation whereas exposure to KU63794 or PP242 induced a marked increase in the phosphorylation of ERK on Thr202 and Tyr204 in serum-treated cells ( Fig. 5 ). These results corroborated that allosteric and active-site site inhibitors of mTOR promote over-activation of different upstream pathways in PDAC cells under a variety of experimental conditions, including cells challenged with insulin, insulin and the GPCR agonist neurotensin or with fresh fetal bovine serum.
Figure 5. Differential feedback activation of Akt and ERK by rapamycin, everolimus, KU63794 and PP242 in serum-stimulated PANC-1 cells.
The cultures were incubated in the absence (−) or in the presence of 5 µM KU63794 (Ku), 5 µM PP242, 100 nM rapamycin (Rap) or 100 nM everolimus for 2 h in DMEM containing 5 mM glucose, as indicated. Then, the cells were stimulated for 2 h with fetal bovine at a final dilution of 2% (SERUM) and lysed with 2×SDS–PAGE sample buffer. The samples were analyzed by SDS-PAGE and immunoblotting with antibodies that detect the phosphorylated state of Akt at Ser473, S6 at Ser235/236, and ERK at Thr202 and Tyr204. Immunoblotting with total Akt, S6 and ERK was used to verify equal gel loading. Similar results were obtained in 3 independent experiments.
Metformin, in contrast to allosteric and active-site mTOR inhibitors, inhibits ERK activation and does not induce over-stimulation of Akt in PDAC cells
Like rapamycin and active-site mTOR inhibitors, metformin also inhibits stimulation of the mTORC1/S6K axis but its effects on feedback loops regulating Akt and ERK activation have not been examined in PDAC cells. Recently, we demonstrated that the sensitivity of PDAC cells to the inhibitory effects of metformin are markedly enhanced by culturing PDAC cells in medium containing physiological (5 mM) rather than supra-physiological (25 mM) concentrations of glucose [68]. In order to determine the effect of metformin on Akt and ERK signaling in PDAC cells, PANC-1 and MiaPaCa-2 cells grown in medium containing 5 mM glucose were treated with or without metformin (1 mM) and then stimulated with insulin and the GPCR agonist neurotensin. mTORC1 activity was determined by phosphorylation of S6K at Thr389 and phosphorylation of S6 (Ser235/236) and ERK activation by detecting ERK phosphorylated on Thr202 and Tyr204. Metformin, like rapamycin, virtually abolished mTORC1 activation induced by insulin and neurotensin in PANC-1 and MiaPaCa-2 cells (pS6K, pS6 in Fig. 6, A and B ) without changing the total levels of either S6K or S6. However, metformin did not over-stimulated Akt phosphorylation on Ser473 in the PDAC cells (p-Akt473 in Fig. 6, A and B ), a result strikingly different from that obtained with rapamycin and everolimus. The salient feature of the results in Fig. 6 A and B is that metformin, in sharp contrast to the effects of active-site mTOR inhibitors, prevented ERK activation in PANC-1 and MiaPaCa-2 cells in multiple independent experiments (depicted by the bars) but did not alter the level of total ERK. We verified that under these experimental conditions, metformin markedly induced AMPK activation, as shown by the phosphorylation of acetyl-CoA carboxylase (ACC) at Ser79, a residue directly phosphorylated by AMPK and used as a biomarker of its activity within intact cells.
Figure 6. Metformin inhibits mTORC1 and ERK signaling without over-activating Akt in PDAC cells incubated in medium containing a physiological glucose concentration.
A) Cultures of MiaPaca-2 (A) and PANC-1 (B) cells were incubated in the absence (−) or in the presence of 1 mM metformin (Met) for 16 h in DMEM containing 5 mM glucose, as indicated. Then, the cells were stimulated for 2 h with 5 nM neurotensin and 10 ng/ml insulin (NT+Ins) and lysed with 2×SDS–PAGE sample buffer. The samples were analyzed by SDS-PAGE and immunoblotting with antibodies that detect the phosphorylated state of S6K at Thr389, S6 at Ser235/236, ACC at Ser79, Akt at Ser473 and ERK at Thr202 and Tyr204. Immunoblotting with antibodies that recognize total S6K, S6, Akt, ERK and ACC was used to verify that the cell treatments did not change the total level of these proteins and confirm equal gel loading. Similar results were obtained in 3 independent experiments. The bars in panels A and B represent the % of the maximal ERK phosphorylation (mean ±SEM) induced by insulin (Ins) and neurotensin (NT) in cells without or with prior treatment with 1 mM metformin. The results of ERK phosphorylation were obtained in multiple independent experiments (N = 12 for PANC-1 and N = 8 for MiaPaca-2) Quantification was performed using Multi Gauge V3.0 C). Mia PaCa-2 cells were incubated with DMEM containing 5 mM glucose either in absence or presence of 0.05 mM or 0.1 mM metformin for 16 h. Then, the cells were treated with NT+Ins, as above, and lysates analyzed by immunoblotting. Similar results were obtained in 6 independent experiments. D) The experiment presented in panel C was representative of 6 independent experiments. Quantification of these experiments was performed using Multi Gauge V3.0. Results are expressed as the percentage of maximum mean ±SEM, n = 6. P values were determined using the t-test (Sigma Plot 12.) *p<0.05; **p<0.001; n = 6.
We next determined whether metformin inhibits ERK activation at concentrations (0.05–0.1 mM) that are close to the therapeutic range. As shown in Fig. 6 C , metformin dose-dependently inhibited phosphorylation S6K at Thr389 and ERK activation at concentrations as low as 0.05–0.1 mM. Metformin, at these concentrations, also induced AMPK activation, as shown by ACC phosphorylation at Ser79. Quantification of the immunoblotting results is illustrated in Fig. 6 D . Our results demonstrate that the effects of metformin on Akt and ERK activation are strikingly different from those elicited by allosteric or active-site mTOR inhibitors, though all these agents potently inhibited the mTORC1/S6K axis.
Effects of metformin, KU63974 and rapamycin on the proliferation of PANC-1 cells
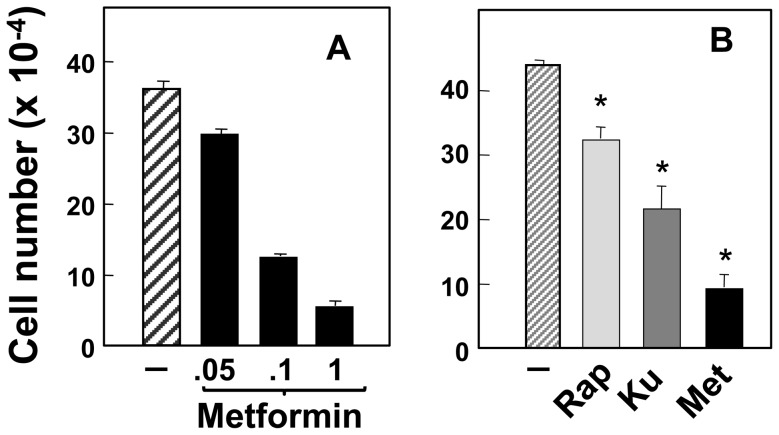
The differential effects of metformin, KU63794 and rapamycin on the activation of PI3K/Akt and MEK/ERK in PDAC cells, prompted us to determine the effects of these agents on the proliferation of these cells. Initially, we assessed the effect of increasing concentrations of metformin on the increase in the number of PANC-1 cells induced by stimulation with neurotensin and insulin in the presence of 0.25% serum for 4 days ( Fig. 7, A ). Metformin prevented the increase in the number of PANC-1 cells in a dose-dependent manner. A marked inhibitory effect was induced by metformin at a concentration as low as 0.1 mM and complete suppression of cell proliferation was achieved by metformin at 1 mM. The concentrations of metformin that inhibited PANC-1 cell proliferation coincided with the concentration of metfornin that prevented mTORC1 and ERK signaling in these cells.
Figure 7. Metformin inhibits PANC-1 cell proliferation more potently than rapamycin or KU63794.
A, Single-cell suspensions of PANC-1 cells were plated at a density of 105 cells per dish. After 24 h, the cultures were shifted to media containing 0.25% FBS without (−) or with 10 nM neurotensin and 10 ng/ml insulin (NT+Ins) in the absence (open bars) or presence (closed bars) of increasing concentrations of metformin, as indicated. After 4 days, cell numbers were determined from 6 plates per condition. Results are presented as mean ± SEM. Similar results were obtained in two independent experiments. p values compared to control were all <0.05 n = 6. B, Single-cell suspensions of PANC-1 cells were plated at a density of 105 cells per dish. After 24 h, the cultures were shifted to media containing 2.5% FBS without (−) or with 1 mM metformin, 5 µM KU63794 (Ku) or 100 nM rapamycin (Rap) as indicated. After 4 days, cell numbers were determined from 6–8 plates per condition. Results are presented as mean ± SEM. Similar results were obtained in two independent experiments. * All p values compared to control were <0.05 n = 6. Anova analysis showed that metformin inhibition of cell proliferation was statistically significant (<p<0.05) from either rapamycin or KU63794. In turn, KU63794 was statistically different from rapamycin (p<0.05).
Next, we examined the effect of 1 mM metformin, 5 µM KU63794 and 100 nM rapamycin on the proliferation of PANC-1 incubated in medium containing serum. Each agent was tested at a concentration that produced maximal inhibition of the mTORC1/S6K axis in PDAC cells. As seen in Fig. 7 B , the agents inhibited PANC-1 cell proliferation but with important differences in their efficacy. Metformin induced a more pronounced inhibition of proliferation than either KU63794 or rapamycin while, the active-site mTOR inhibitor was more effective than rapamycin (all these differences were statistically significative). The results suggest that the effects of inhibiting mTORC1/S6K by the allosteric or active-site inhibitors is compensated by over-activation of Akt (rapamycin) or ERK (KU63794). The comparatively stronger inhibition of PDAC cell proliferation by metformin could be attributed, at least in part, to inhibition of ERK signaling.
Discussion
Aberrant stimulation of the mTOR pathway in many cancer cells, including PDAC, is eliciting intense interest for targeting this pathway [1]. However, it is increasingly appreciated that the mTORC1/S6K axis also mediates negative feedback loops that attenuate signaling via insulin/IGF receptor and other tyrosine kinase receptors. Suppression of these feed-back loops unleashes over-activation of upstream pathways that potentially counterbalance the anti-proliferative effects of mTOR inhibitors. Consequently, the identification of negative feedback loops by either allosteric or active-site mTOR inhibitors has emerged as an area of major interest in cancer therapy. Because the operation of these complex feedback loops is cell-context specific, we examined the patterns of Akt and ERK feedback activation in response to mTORC1 inhibition by rapamycin, active-site mTOR inhibitors and metformin in human PDAC cells. PDAC is one of the most lethal human diseases, with overall 5-year survival rate of only 3–5% and a median survival period of 4–6 months. The incidence of this disease in the US has increased to more than 44,000 new cases in 2011 and is now the fourth leading cause of cancer mortality in both men and women [69]. As the current therapies offer very limited survival benefits, novel molecular therapeutic targets and strategies are urgently needed to treat this aggressive disease.
Our results demonstrate that treatment of PDAC cells with allosteric mTORC1 inhibitors (rapamycin, everolimus) augmented Akt phosphorylation at Ser473 while the active-site inhibitors of mTOR (KU63794 and PP242) completely abrogated Akt phosphorylation at this site consistent with the notion that mTORC2 is the major kinase that phosphorylates Akt at Ser473. A salient feature of our results is that active-site inhibitors of mTOR promoted a marked increase in ERK activation in PDAC cells stimulated with insulin, insulin and neurotensin or serum. These results indicate that first and second generations of mTOR inhibitors promote over-activation of different upstream pro-oncogenic pathways in PDAC cells.
While augmentation of Akt phosphorylation at Ser473 by rapalogs is well known in other cell types [30], [33]–[35], the enhancing effect of active-site mTOR inhibitors on ERK has been much less explored. In order to understand the mechanism by which active-site mTOR inhibitors promote ERK activation in PDAC cells, we determined here the role of a feedback loop involving mTORC1/S6K/PI3K/ERK, proposed to mediate ERK activation in other cell types [63]. Several lines of evidence dissociated this feedback loop from the enhancement of ERK activation induced by active-site mTOR inhibitors in PDAC cells. Firstly, neither rapamycin nor everolimus, at concentrations that completely blocked the mTORC1/S6K axis, produced any detectable enhancement of ERK activation in PDAC cells under a variety of experimental conditions. Secondly, KU63794 induced ERK hyper-activation even in PDAC cells treated with A66, a potent and selective inhibitor of the 110α catalytic subunit of PI3K [67]. These results, indicating that active-site inhibitors enhance ERK through a PI3K-independent pathway, are in agreement with a recent report using PP242 in multiple myeloma cells [70]. However, PP242 inhibits a number of protein kinases in vitro, including MEK, whereas KU63794 did not inhibit any protein kinase other than mTOR [66]. The possibility that PP242 could induce ERK via off-target effects is an important consideration. Our results demonstrate, for the first time, that the highly selective inhibitor of mTOR KU63794 enhances MEK/ERK activation through a PI3K-independent pathway. Given that active-site mTOR inhibitors are increasingly considered for clinical use [1], the findings presented here imply that suppression of feed-back loops by these inhibitors should be a major consideration in the use of these inhibitors for PDAC therapy.
Many epidemiological studies have linked obesity and long-standing type 2 diabetes mellitus (T2DM), with increased risk for developing PDAC and other clinically aggressive cancer [71], [72]. Obesity and T2DM are multifaceted but characterized by peripheral insulin resistance and compensatory overproduction of insulin by the β cells of the islet leading to increase circulating levels of insulin and enhanced bioavailability of IGF-1. Further, epidemiological studies are linking administration of metformin, the most widely used drug in the treatment of T2DM, with reduced incidence, recurrence and mortality of a variety of cancers in T2DM patients [20], [47]–[56], including PDAC. Indeed, T2DM patients who had taken metformin had a 62% lower adjusted incidence of PDAC compared with those who had not taken metformin [54], a result recently substantiated in a different patient population [56]. Here we demonstrate that metformin abolishes mTORC1/S6K activation in PDAC cells but in contrast to rapamycin, metformin treatment did not overactivate Akt phosphorylation on Ser473. We verified that, under our conditions, metformin stimulated AMPK in PDAC cells. In this context it is relevant that AMPK not only blocks mTORC1 activation but also mediates IRS-1 phosphorylation at Ser794, an inhibitory site that attenuates PI3K/Akt activation [29], [73]. We confirmed that metformin (but not rapamycin) induced IRS-1 phosphorylation at Ser794 in PANC-1 cells (our unpublished results). Consequently, it is plausible that metformin, via AMPK-mediated phosphorylation of IRS-1 at Ser794, attenuates PI3K overstimulation caused by interrupting feedback loops mediated by the mTOR/S6K axis and thereby avoids hyper-activation of Akt phosphorylation on Ser473. More importantly, we found that metformin inhibited rather than enhanced ERK activation (like active-site mTOR inhibitors) in PDAC cells. The inhibitory effects of metformin on ERK were elicited at low concentrations when the cells were cultured in medium containing physiological concentrations of glucose [68]. Thus, the effects of metformin on Akt phosphorylation and ERK activation are strikingly different from allosteric or active-site mTOR inhibitors, though all these agents potently inhibited the mTORC1/S6K axis. In line with differential effects on feedback loops, further findings revealed that metformin inhibited cell proliferation of PDAC cells more efficiently than allosteric or active-site mTOR inhibitors in PDAC cells. Although the elucidation of the precise mechanism(s) involved requires further experimental work, it is tempting to speculate that the favorable effects of metformin on preventing over-activation of pro-oncogenic pathways, such as Akt and ERK, may contribute to its antiproliferative effects in vitro and ultimately explain its beneficial anticancer effects.
Supporting Information
Treatment with KU63794 causes over-activation of MEK and ERK phosphorylation in PANC-1 and MiaPaCa-2 cells. The cultures of PANC-1 and MiaPaCa-2 were incubated in the absence (−) or in the presence of KU63794 (Ku) at 5 mM for 2 h in DMEM containing 5 mM glucose, as indicated. Then, the cells were stimulated with 10 ng/ml insulin and 5 nM neurotensin (NT+Ins) for 2 h and lysed with 2×SDS–PAGE sample buffer. The samples were analyzed by SDS-PAGE and immunoblotting with antibodies that detect the phosphorylated state of MEK at Ser217/221, ERK at Thr202 and Tyr204 and Akt at Ser473 Immunoblotting with total MEK, ERK and Akt was used to verify equal gel loading.
(TIF)
Funding Statement
This study was supported by National Institutes of Health Grants P30DK41301, P01CA163200 and R01DK55003, Department of Veterans Affair Grant 1I01BX001473 and funds from the endowed Ronald S. Hirshberg Chair of Pancreatic Cancer Research all to ER. (http://www.nih.gov/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Laplante M, Sabatini DM (2012) mTOR signaling in growth control and disease. Cell 149: 274–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ma XM, Blenis J (2009) Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol 10: 307–318. [DOI] [PubMed] [Google Scholar]
- 3. Garami A, Zwartkruis FJ, Nobukuni T, Joaquin M, Roccio M, et al. (2003) Insulin activation of Rheb, a mediator of mTOR/S6K/4E-BP signaling, is inhibited by TSC1 and 2. Mol Cell 11: 1457–1466. [DOI] [PubMed] [Google Scholar]
- 4. Zhang Y, Gao X, Saucedo LJ, Ru B, Edgar BA, et al. (2003) Rheb is a direct target of the tuberous sclerosis tumour suppressor proteins. Nat Cell Biol 5: 578–581. [DOI] [PubMed] [Google Scholar]
- 5. Foster KG, Fingar DC (2010) Mammalian Target of Rapamycin (mTOR): conducting the cellular signaling symphony. J Biol Chem 285: 14071–14077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wullschleger S, Loewith R, Hall MN (2006) TOR signaling in growth and metabolism. Cell 124: 471–484. [DOI] [PubMed] [Google Scholar]
- 7. Inoki K, Guan KL (2006) Complexity of the TOR signaling network. Trends Cell Biol 16: 206–212. [DOI] [PubMed] [Google Scholar]
- 8. Taniguchi CM, Emanuelli B, Kahn CR (2006) Critical nodes in signaling pathways: insights into insulin action. Nat Rev Mol Cell Biol 7: 85–96. [DOI] [PubMed] [Google Scholar]
- 9. Zoncu R, Efeyan A, Sabatini DM (2011) mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol 12: 21–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kornmann M, Maruyama H, Bergmann U, Tangvoranuntakul P, Beger HG, et al. (1998) Enhanced expression of the insulin receptor substrate-2 docking protein in human pancreatic cancer. Cancer Res 58: 4250–4254. [PubMed] [Google Scholar]
- 11. Kolb S, Fritsch R, Saur D, Reichert M, Schmid RM, et al. (2007) HMGA1 controls transcription of insulin receptor to regulate cyclin D1 translation in pancreatic cancer cells. Cancer Res 67: 4679–4686. [DOI] [PubMed] [Google Scholar]
- 12. Kwon J, Stephan S, Mukhopadhyay A, Muders MH, Dutta SK, et al. (2009) Insulin receptor substrate-2 mediated insulin-like growth factor-I receptor overexpression in pancreatic adenocarcinoma through protein kinase Cdelta. Cancer Res 69: 1350–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stoeltzing O, Liu W, Reinmuth N, Fan F, Parikh AA, et al. (2003) Regulation of hypoxia-inducible factor-1alpha, vascular endothelial growth factor, and angiogenesis by an insulin-like growth factor-I receptor autocrine loop in human pancreatic cancer. Am J Pathol 163: 1001–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dong X, Javle M, Hess KR, Shroff R, Abbruzzese JL, et al. (2010) Insulin-like growth factor axis gene polymorphisms and clinical outcomes in pancreatic cancer. Gastroenterology 139: 464–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng Z (2010) p53 Regulation of the IGF-1/AKT/mTOR Pathways and the Endosomal Compartment. Cold Spring Harb Perspect Biol 2, a001057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kisfalvi K, Guha S, Rozengurt E (2005) Neurotensin and EGF induce synergistic stimulation of DNA synthesis by increasing the duration of ERK signaling in ductal pancreatic cancer cells. J Cell Physiol 202: 880–890. [DOI] [PubMed] [Google Scholar]
- 17. Kisfalvi K, Rey O, Young SH, Sinnett-Smith J, Rozengurt E (2007) Insulin potentiates Ca2+ signaling and phosphatidylinositol 4,5-bisphosphate hydrolysis induced by Gq protein-coupled receptor agonists through an mTOR-dependent pathway. Endocrinology 148: 3246–3257. [DOI] [PubMed] [Google Scholar]
- 18. Kisfalvi K, Eibl G, Sinnett-Smith J, Rozengurt E (2009) Metformin disrupts crosstalk between G protein-coupled receptor and insulin receptor signaling systems and inhibits pancreatic cancer growth. Cancer Res 69: 6539–6545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Young SH, Rozengurt E (2010) Crosstalk between insulin receptor and G protein-coupled receptor signaling systems leads to Ca(2+) oscillations in pancreatic cancer PANC-1 cells. Biochem Biophys Res Commun 401: 154–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rozengurt E, Sinnett-Smith J, Kisfalvi K (2010) Crosstalk between Insulin/Insulin-like growth factor-1 receptors and G protein-coupled receptor signaling systems: a novel target for the antidiabetic drug metformin in pancreatic cancer. Clin Cancer Res 16: 2505–2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Asano T, Yao Y, Shin S, McCubrey J, Abbruzzese JL, et al. (2005) Insulin receptor substrate is a mediator of phosphoinositide 3-kinase activation in quiescent pancreatic cancer cells. Cancer Res 65: 9164–9168. [DOI] [PubMed] [Google Scholar]
- 22. Asano T, Yao Y, Zhu J, Li D, Abbruzzese JL, et al. (2005) The rapamycin analog CCI-779 is a potent inhibitor of pancreatic cancer cell proliferation. Biochem Biophys Res Commun 331: 295–302. [DOI] [PubMed] [Google Scholar]
- 23. Pham NA, Schwock J, Iakovlev V, Pond G, Hedley DW, et al. (2008) Immunohistochemical analysis of changes in signaling pathway activation downstream of growth factor receptors in pancreatic duct cell carcinogenesis. BMC Cancer 8: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ying H, Elpek KG, Vinjamoori A, Zimmerman SM, Chu GC, et al. (2011) PTEN is a major tumor suppressor in pancreatic ductal adenocarcinoma and regulates an NF-kB cytokine network. Cancer Discovery 1: 158–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Efeyan A, Sabatini DM (2010) mTOR and cancer: many loops in one pathway. Curr Opin Cell Biol 22: 169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Um SH, Frigerio F, Watanabe M, Picard F, Joaquin M, et al. (2004) Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature 431: 200–205. [DOI] [PubMed] [Google Scholar]
- 27. Harrington LS, Findlay GM, Gray A, Tolkacheva T, Wigfield S, et al. (2004) The TSC1-2 tumor suppressor controls insulin-PI3K signaling via regulation of IRS proteins. J Cell Biol 166: 213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tzatsos A, Kandror KV (2006) Nutrients suppress phosphatidylinositol 3-kinase/Akt signaling via raptor-dependent mTOR-mediated insulin receptor substrate 1 phosphorylation. Mol Cell Biol 26: 63–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tzatsos A, Tsichlis PN (2007) Energy depletion inhibits phosphatidylinositol 3-kinase/Akt signaling and induces apoptosis via AMP-activated protein kinase-dependent phosphorylation of IRS-1 at Ser-794. J Biol Chem 282: 18069–18082. [DOI] [PubMed] [Google Scholar]
- 30. Carracedo A, Pandolfi PP (2008) The PTEN-PI3K pathway: of feedbacks and cross-talks. Oncogene 27: 5527–5541. [DOI] [PubMed] [Google Scholar]
- 31. Hsu PP, Kang SA, Rameseder J, Zhang Y, Ottina KA, et al. (2011) The mTOR-regulated phosphoproteome reveals a mechanism of mTORC1-mediated inhibition of growth factor signaling. Science 332: 1317–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yu Y, Yoon SO, Poulogiannis G, Yang Q, Ma XM, et al. (2011) Phosphoproteomic analysis identifies Grb10 as an mTORC1 substrate that negatively regulates insulin signaling. Science 332: 1322–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. O'Reilly KE, Rojo F, She QB, Solit D, Mills GB, et al. (2006) mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res 66: 1500–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lane HA, Breuleux M (2009) Optimal targeting of the mTORC1 kinase in human cancer. Curr Opin Cell Biol 21: 219–229. [DOI] [PubMed] [Google Scholar]
- 35. Easton JB, Kurmasheva RT, Houghton PJ (2006) IRS-1: Auditing the effectiveness of mTOR inhibitors. Cancer Cell 9: 153–155. [DOI] [PubMed] [Google Scholar]
- 36. Choo AY, Yoon SO, Kim SG, Roux PP, Blenis J (2008) Rapamycin differentially inhibits S6Ks and 4E-BP1 to mediate cell-type-specific repression of mRNA translation. Proc Natl Acad Sci USA 105: 17414–17419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Feldman ME, Apsel B, Uotila A, Loewith R, Knight ZA, et al. (2009) Active-site inhibitors of mTOR target rapamycin-resistant outputs of mTORC1 and mTORC2. PLoS Biol 7: e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Garcia-Martinez JM, Moran J, Clarke RG, Gray A, Cosulich SC, et al. (2009) Ku-0063794 is a specific inhibitor of the mammalian target of rapamycin (mTOR). Biochem J 421: 29–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Thoreen CC, Kang SA, Chang JW, Liu Q, Zhang J, et al. (2009) An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. J Biol Chem 284: 8023–8032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yu K, Toral-Barza L, Shi C, Zhang WG, Lucas J, et al. (2009) Biochemical, cellular, and in vivo activity of novel ATP-competitive and selective inhibitors of the mammalian target of rapamycin. Cancer Res 69: 6232–6240. [DOI] [PubMed] [Google Scholar]
- 41. Sawyers CL (2003) Will mTOR inhibitors make it as cancer drugs? Cancer Cell 4: 343–348. [DOI] [PubMed] [Google Scholar]
- 42. LoPiccolo J, Blumenthal GM, Bernstein WB, Dennis PA (2008) Targeting the PI3K/Akt/mTOR pathway: effective combinations and clinical considerations. Drug Resist Updat 11: 32–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wolpin BM, Hezel AF, Abrams T, Blaszkowsky LS, Meyerhardt JA, et al. (2009) Oral mTOR inhibitor everolimus in patients with gemcitabine-refractory metastatic pancreatic cancer. J Clin Oncol 27: 193–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Javle MM, Shroff RT, Xiong H, Varadhachary GA, Fogelman D, et al. (2010) Inhibition of the mammalian target of rapamycin (mTOR) in advanced pancreatic cancer: results of two phase II studies. BMC Cancer 10: 368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Liu Q, Chang JW, Wang J, Kang SA, Thoreen CC, et al. (2010) Discovery of 1-(4-(4-propionylpiperazin-1-yl)-3-(trifluoromethyl)phenyl)-9-(quinolin-3-yl)benz o[h][1,6]naphthyridin-2(1H)-one as a highly potent, selective mammalian target of rapamycin (mTOR) inhibitor for the treatment of cancer. J Med Chem 53: 7146–7155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chresta CM, Davies BR, Hickson I, Harding T, Cosulich S, et al. (2010) AZD8055 is a potent, selective, and orally bioavailable ATP-competitive mammalian target of rapamycin kinase inhibitor with in vitro and in vivo antitumor activity. Cancer Res 70: 288–298. [DOI] [PubMed] [Google Scholar]
- 47. Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD (2005) Metformin and reduced risk of cancer in diabetic patients. BMJ 330: 1304–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bowker SL, Majumdar SR, Veugelers P, Johnson JA (2006) Increased cancer-related mortality for patients with type 2 diabetes who use sulfonylureas or insulin. Diabetes Care 29: 254–258. [DOI] [PubMed] [Google Scholar]
- 49. Libby G, Donnelly LA, Donnan PT, Alessi DR, Morris AD, et al. (2009) New users of metformin are at low risk of incident cancer. Diabetes Care 32: 1620–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chong CR, Chabner BA (2009) Mysterious metformin. Oncologist 14: 1178–1181. [DOI] [PubMed] [Google Scholar]
- 51. Ben Sahra I, Le Marchand-Brustel Y, Tanti JF, Bost F (2010) Metformin in cancer therapy: a new perspective for an old antidiabetic drug? Mol Cancer Ther 9: 1092–1099. [DOI] [PubMed] [Google Scholar]
- 52. Landman GWD, Kleefstra N, van Hateren KJJ, Groenier KH, Gans ROB, et al. (2010) Metformin associated with lower cancer mortality in type 2 diabetes. Diabetes Care 33: 322–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. DeCensi A, Puntoni M, Goodwin P, Cazzaniga M, Gennari A, et al. (2010) Metformin and cancer risk in diabetic patients: a systematic review and meta-analysis. Cancer Prev Res 3: 1451–1461. [DOI] [PubMed] [Google Scholar]
- 54. Li D, Yeung SCJ, Hassan MM, Konopleva M, Abbruzzese JL (2009) Anti-diabetic therapies affect risk of pancreatic cancer. Gastroenterology 137: 482–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Currie CJ, Poole CD, Gale EA (2009) The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia 52: 1766–1777. [DOI] [PubMed] [Google Scholar]
- 56. Lee MS, Hsu CC, Wahlqvist ML, Tsai HN, Chang YH, et al. (2011) Type 2 diabetes increases and metformin reduces total, colorectal, liver and pancreatic cancer incidences in Taiwanese: a representative population prospective cohort study of 800,000 individuals. BMC Cancer 11: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hardie DG (2007) AMP-activated protein kinase as a drug target. Annu Rev Pharmacol Toxicol 47: 185–210. [DOI] [PubMed] [Google Scholar]
- 58. Inoki K, Zhu T, Guan KL (2003) TSC2 mediates cellular energy response to control cell growth and survival. Cell 115: 577–590. [DOI] [PubMed] [Google Scholar]
- 59. Shaw RJ, Bardeesy N, Manning BD, Lopez L, Kosmatka M, et al. (2004) The LKB1 tumor suppressor negatively regulates mTOR signaling. Cancer Cell 6: 91–99. [DOI] [PubMed] [Google Scholar]
- 60. Inoki K, Ouyang H, Zhu T, Lindvall C, Wang Y, et al. (2006) TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell 126: 955–968. [DOI] [PubMed] [Google Scholar]
- 61. Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, et al. (2008) AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell 30: 214–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Carriere A, Romeo Y, Acosta-Jaquez HA, Moreau J, Bonneil E, et al. (2011) ERK1/2 phosphorylate raptor to promote Ras-dependent activation of mTOR Complex 1 (mTORC1). J Biol Chem 286: 567–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Carracedo A, Ma L, Teruya-Feldstein J, Rojo F, Salmena L, et al. (2008) Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. J Clin Invest 118: 3065–3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ryder NM, Guha S, Hines OJ, Reber HA, Rozengurt E (2001) G protein-coupled receptor signaling in human ductal pancreatic cancer cells: Neurotensin responsiveness and mitogenic stimulation. J Cell Physiol 186: 53–64. [DOI] [PubMed] [Google Scholar]
- 65. Guha S, Lunn JA, Santiskulvong C, Rozengurt E (2003) Neurotensin stimulates protein kinase C-dependent mitogenic signaling in human pancreatic carcinoma cell line PANC-1. Cancer Res 63: 2379–2387. [PubMed] [Google Scholar]
- 66. Liu Q, Kirubakaran S, Hur W, Niepel M, Westover K, et al. (2012) Kinome-wide selectivity profiling of ATP-competitive mammalian target of rapamycin (mTOR) inhibitors and characterization of their binding kinetics. J Biol Chem 287: 9742–9752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Jamieson S, Flanagan JU, Kolekar S, Buchanan C, Kendall JD, et al. (2011) A drug targeting only p110 α can block phosphoinositide 3-kinase signalling and tumour growth in certain cell types. Biochem J 438: 53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Sinnett-Smith J, Kisfalvi K, Kui R, Rozengurt E (2012) Metformin inhibition of mTORC1 activation, DNA synthesis and proliferation in pancreatic cancer cells: Dependence on glucose concentration and role of AMPK. Biochem Biophys Res Commun (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Siegel R, Ward E, Brawley O, Jemal A (2011) Cancer statistics, 2011. CACancer J Clin 61: 212–236. [DOI] [PubMed] [Google Scholar]
- 70. Hoang B, Benavides A, Shi Y, Yang Y, Frost P, et al. (2012) The PP242 mammalian target of rapamycin (mTOR) inhibitor activates extracellular signal-regulated kinase (ERK) in multiple myeloma cells via a target of rapamycin complex 1 (TORC1)/eukaryotic translation initiation factor 4E (eIF-4E)/RAF pathway and activation is a mechanism of resistance. J Biol Chem 287: 21796–21805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Arslan AA, Helzlsouer KJ, Kooperberg C, Shu XO, Steplowski E, et al. (2010) Anthropometric measures, body mass index, and pancreatic cancer: a pooled analysis from the pancreatic cancer cohort consortium (PanScan). Arch Intern Med 170: 791–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Giovannucci E, Harlan DM, Archer MC, Bergenstal RM, Gapstur SM, et al. (2010) Diabetes and cancer: a consensus report. Diabetes Care 33: 1674–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ning J, Clemmons DR (2010) AMP-Activated Protein Kinase Inhibits IGF-I Signaling and Protein Synthesis in Vascular Smooth Muscle Cells via Stimulation of Insulin Receptor Substrate 1 S794 and Tuberous Sclerosis 2 S1345 Phosphorylation. Mol Endocrinol 24: 1218–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Treatment with KU63794 causes over-activation of MEK and ERK phosphorylation in PANC-1 and MiaPaCa-2 cells. The cultures of PANC-1 and MiaPaCa-2 were incubated in the absence (−) or in the presence of KU63794 (Ku) at 5 mM for 2 h in DMEM containing 5 mM glucose, as indicated. Then, the cells were stimulated with 10 ng/ml insulin and 5 nM neurotensin (NT+Ins) for 2 h and lysed with 2×SDS–PAGE sample buffer. The samples were analyzed by SDS-PAGE and immunoblotting with antibodies that detect the phosphorylated state of MEK at Ser217/221, ERK at Thr202 and Tyr204 and Akt at Ser473 Immunoblotting with total MEK, ERK and Akt was used to verify equal gel loading.
(TIF)