Abstract
Plant growth and development is determined by intracellular and intercellular auxin gradients that are controlled at first hand by auxin efflux catalysts of the ABCB/PGP and PIN families. ABCB transport activity was shown to be counter-actively regulated by protein phosphorylation by the AGC protein kinase, PINOID (PID), that is coordinated by interaction with the immunophilin-like FKBP42, TWISTED DWARF1 (TWD1). In contrast, PID was shown to determine PIN polarity, however, the direct impact of PID on PIN activity has yet not been tested. Co-expression in yeast indicates that PID had no effect on PIN1,2 alone but specifically inhibits interactive ABCB1-PIN1/PIN2 auxin efflux in an action that is dependent on its kinase activity. PIN1-PID co-transfection in N. benthamiana revealed that PID blocks PIN1-mediated auxin efflux without changing PIN1 location. In summary, these data provide evidence that PID phosphorylation does not only determine PIN polarity but also has a direct impact on transport activity of the activity of the binary PIN-ABCB1 complex.
Keywords: polar auxin transport, PIN, ABCB, PINOID, AGC protein kinase
Protein phosphorylation plays a key role in the regulation of polar auxin transport.1 The AGC protein kinase, PINOID (PID),2 has been shown to phosphorylate the hydrophilic loop of PIN proteins in vivo leading to apical PIN targeting.3 It appears that PID together with protein phosphatase PP2A/RCN1 act as a binary switch to control the balance of phosphorylation and dephosphorylation determining PIN polarity and controlling auxin flows.4,5
Employing different phospho-proteomics approaches, plant ABCB proteins have recently shown to be phosphorylated in a so-called regulatory linker domain in analogy to their mammalian orthologs.6-9 Subsequently, the photoreceptor kinase, PHOTROPIN1 (phot1), was shown to phosphorylate ABCB19 and inhibit its auxin efflux activity.10 Recently, PID was identified as a valid interaction partner with TWD1 by co-immunoprecipitation and shotgun LC-MS/MS analysis.11 In-vitro and yeast expression data indicated that PID specifically modulates ABCB1-mediated auxin efflux, which is dependent on its kinase activity. ABCB1/PID co-transfection in N. benthamiana revealed that PID phosphorylates at S634 of the linker in the absence of TWD1 enhancing ABCB1-mediated auxin efflux. PID had a negative impact on ABCB1 in triple ABCB1/PID/TWD1 co-transfection. This suggests that PID determines ABCB1 activity by means of protein phosphoryation in an action that is controlled by TWD1-PID interaction.
However, it is an open question if altered PIN polarity is indeed directly caused by PIN phosphorylation or not simply the consequence of altered PIN activity canalizing auxin streams, which upregulate PIN expression leading to enhanced PIN capacity. Altered PIN activity by PIN phosphorylation has been suggested for D6 protein kinase.12 Therefore, we tested if PID had an effect on PIN1- or PIN2-mediated auxin transport by co-expressing them in S. cerevisiae. The ratio to choose yeast was that ABCB1 was shown in this system to functionally interact with PIN1 and PIN2,13 allowing to monitor PIN-mediated transport as well as transport of interactive PIN-ABCB1 transport.13 PID co-expression did not significantly influence PINa-mediated transport alone. As published recently,13 PIN1 had a stimulating effect on ABCB1-mediated auxin export, while the opposite was found with PIN2 (Fig. 1A). Surprisingly, PID blocked ABCB1-PIN1- and ABCB1-PIN2-mediated efflux drastically to vector control (background) level. In analogy to the effect of PID on ABCB1,11 such an inhibition was not seen with an inactive kinase version, MPID (Fig. 1A). Remarkably, PID-induced inhibition on ABCB1/PIN combinations was specific for IAA transport, as it was not found for the unspecific, diffusion control, benzoic acid (BA) (Fig. 1B).
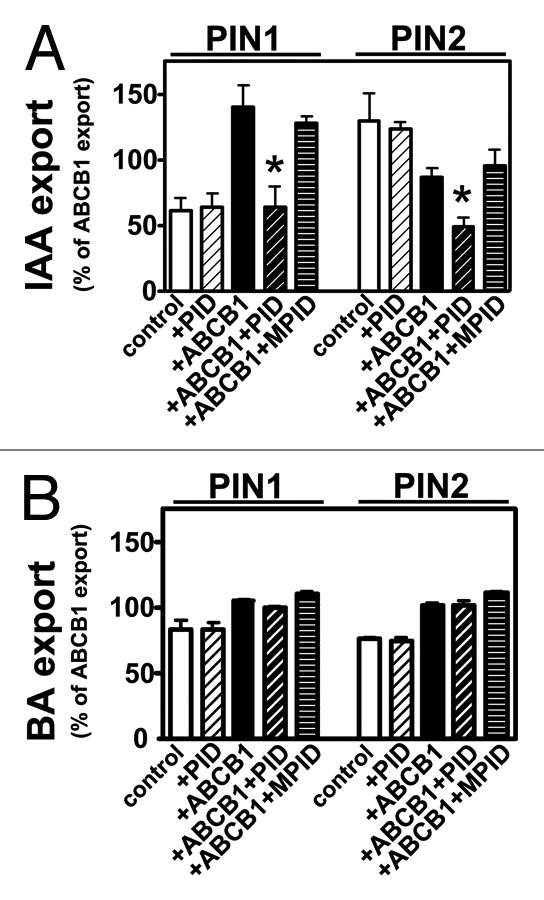
Figure 1. PID modulates PIN-mediated auxin efflux in yeast. (A) PID has no significant impact on PIN-mediated auxin (IAA) export but specifically inhibits ABCB1/PIN1- and ABCB1/PIN2-mediated IAA export not seen with MPID. Note that PIN1 in the absence of ABCB1 is inactive in the yeast, S. cerevisiae.13(B) PID modulation of ABCB and PIN-mediated IAA export is specific. PID and a mutated, inactive PID (MPID) have no significant influence on PIN1-, PIN2-, ABCB1/PIN1- or ABCB1/PIN2-mediated benzoic acid (BA) export. Reduction of IAA and BA retention (efflux) were calculated as relative export of initial export where ABCB1 was set to 100% (mean ± SE; n = 4–10). Significant differences (unpaired t-test with Welch’s correction, p < 0.05) between –PID controls are indicated by asterisks.
In order to test if PID had an effect on PIN1-mediated auxin transport alone we employed N. benthamiana leaf infiltration as heterologous plant expression system. PIN1 expression in tobacco cells resulted in a high auxin efflux, which was roughly twice of what was found for ABCB1 (Fig. 2A),11 verifying previous results using tobacco BY2 cells14 or S. pombe.15 However, co-expression with PID resulted in a drastic inhibition of PIN1-catalyzed IAA and NAA export to vector control level, while PID expression alone had a slightly stimulating effect on background levels as reported previously.11 In analogy to the yeast system, PID expression did not alter significantly PIN1 location or expression on the plasma membrane as monitored by co-localization analysis of GFP-tagged PID and YFP-tagged PIN1 (Fig. 2B). In some cases we noticed that PIN1-YFP was showing some internal locations in addition to the PM signal. In summary, together with the yeast data, these results provide evidence that PID negatively regulates PIN1-mediated auxin efflux in planta in an action that requires its kinase activity.
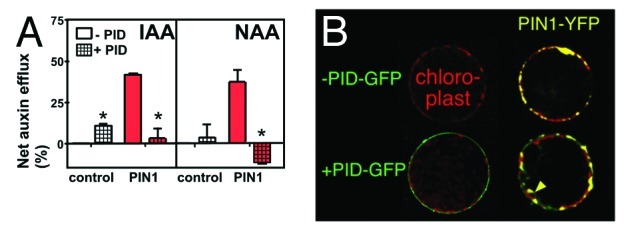
Figure 2. PID negatively regulates PIN1-mediated auxin efflux in planta(A) Co-transfection of N. benthamiana protoplasts with PID strongly blocks PIN1 activity (mean ± SE; n = 4). Significant differences (unpaired t-test with Welch’s correction, p < 0.05) between –PID controls are indicated by asterisks. (B) Co-transfection of N. benthamiana protoplasts with PID-GFP does not significantly alter PIN1-YFP location and expression. Arrow marks partially intracellular membrane locations of PIN1-YFP in the presence of PID-GFP.
Our data suggest that PID, beside its function as a molecular switch of PIN polarity, has an impact on PIN-mediated auxin efflux transporter activity. Interestingly, in contrast to ABCB1, PIN1 export was drastically blocked by PID co-expression in planta, which is of interest because PIN1 (root-ward) and ABCB1 (shoot-ward) were described to contribute to opposite auxin flows in the root stele and epidermis, respectively.16,17 However, lack of PID effect on PIN2 activity in yeast suggests that PIN activity regulation by PID might need additional factors that are absent in yeast but present in plant systems. Emerging candidates are ABCBs that are absent in yeast16 but present in tobacco leaf protoplasts. This concept, which is depicted in Figure 3., is indirectly supported by the finding that PID enhanced likewise vector control and ABCB1 auxin efflux (Fig. 2A).11 A further confirmation comes from the finding that PID has a strong, negative regulatory effect on PIN1 and PIN2 when co-expressed with ABCB1 (Fig. 1A). Interestingly, PID inhibition on PIN/ABCB1 combinations in yeast was much stronger than on ABCB1 alone11 arguing further for the idea that PID acts predominantly on the ABCB/PIN complex than on PINs alone. Therefore, these data also further underline the relevance of functional ABCB/PIN interactions and provide a mechanistic explanation for inverse effects of PIN1 and PIN2 on ABCB-mediated transport,13 which might be caused by differential PID phosphorylation.
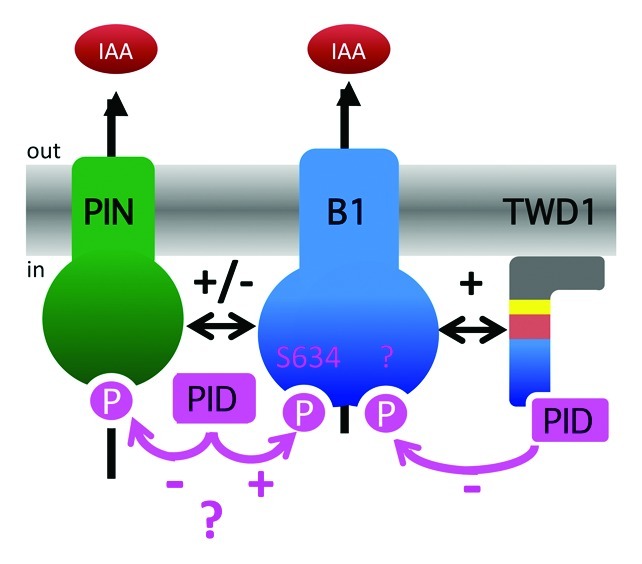
Figure 3. Model of PIN-ABCB-TWD1 interaction. In this hypothetical model, TWD1 functions in recruiting PID for ABCB1 linker phosphorylation resulting in inhibition of auxin efflux (-). In the absence of TWD1, PID is thought to phosphorylate ABCB1 at an unknown site resulting in activation (+). The opposite is found for PIN/ABCB-mediated efflux (-) but at the moment it is unclear if this is the consequence of PIN or ABCB phosphorylation. Functional domains of TWD1 are in blue (FKBD), red (TPR), yellow (calmodulin-binding domain) and gray (in-plane membrane anchor). Question marks indicate uncertainties that are currently under investigation.
The major question that emerges now is if PID alters activity of the PIN/ABCB complex by phosphorylating PINs or ABCB1. Although, we cannot exclude entirely at the moment that PIN phosphorylation is the key event altering PIN/ABCB activity, our yeast data make it more likely that ABCB phosphorylation might alter PIN activity. Future work will show if these alterations are perceived by interfering with PIN/ABCB interaction as suggested for TWD1/ABCB interaction.10,11 At the moment this option, however, is less likely as PIN2 has a negative impact on ABCB1, and therefore PIN2/ABCB1 disruption by PID phosphorylation would be expected to result in an activation.
Material and Methods
Auxin transport assays
ABCB1/PGP1, PIN1 and PIN2 were expressed from pNEV-PGP1/ABCB1, pAD4M-PIN1 and pADE1-PIN2.13,16 PID cDNA was PCR amplified from pGEX4T-1-PID18 and inserted BamHI/SalI into pRS314CUP, resulting in pRS314CUP-PID. Yeast IAA transport was assayed with the unspecific benzoic acid (BA) as control in parallel and performed as in Henrichs et al.11
IAA export from N. benthamiana mesophyll protoplasts was analyzed 4 dai agrobacterium-mediated co-transfection of 35S:PIN1-YFP and 35S:PID-GFP (pGREEN0179-PID-GFP); for details see Henrichs et al.11 35S:PIN1-YFP was constructed by gateway recombination (Invitrogen Inc.) of PIN1 cDNA into pBIN19 vector.19 Presented are average values from 6–8 independent experiments (yeast: independent transformations; protoplasts: infiltrations of independent agrobacterium transformants).
Transfected protoplasts were analyzed by confocal laser scanning microscopy (Leica, DMIRE2) equipped with argon lasers, 488 nm for GFP, 514 nm for YFP). Images were electronically colored and merged with Photoshop 10.0.1. (Adobe Systems).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank V. Vincenzetti for outstanding technical support and E. Martinoia for help and mentorship. This work was supported by grants from the Pool de Recherche of the University of Fribourg (to M.G.), from the Danish Research School for Biotechnology, FOBI (to M.G.) and from the Swiss National Funds (to M.G.)
aNote that PIN1 was shown to be non-functional as transporter in S. cerevisiae but able to enhance ABCB1-mediated auxin efflux.13
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/22093
References
- 1.Delbarre A, Muller P, Guern J. Short-Lived and Phosphorylated Proteins Contribute to Carrier-Mediated Efflux, but Not to Influx, of Auxin in Suspension-Cultured Tobacco Cells. Plant Physiol. 1998;116:833–44. doi: 10.1104/pp.116.2.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Galván-Ampudia CS, Offringa R. Plant evolution: AGC kinases tell the auxin tale. Trends Plant Sci. 2007;12:541–7. doi: 10.1016/j.tplants.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 3.Michniewicz M, Zago MK, Abas L, Weijers D, Schweighofer A, Meskiene I, et al. Antagonistic regulation of PIN phosphorylation by PP2A and PINOID directs auxin flux. Cell. 2007;130:1044–56. doi: 10.1016/j.cell.2007.07.033. [DOI] [PubMed] [Google Scholar]
- 4.Friml J, Yang X, Michniewicz M, Weijers D, Quint A, Tietz O, et al. A PINOID-dependent binary switch in apical-basal PIN polar targeting directs auxin efflux. Science. 2004;306:862–5. doi: 10.1126/science.1100618. [DOI] [PubMed] [Google Scholar]
- 5.Grunewald W, Friml J. The march of the PINs: developmental plasticity by dynamic polar targeting in plant cells. EMBO J. 2010;29:2700–14. doi: 10.1038/emboj.2010.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benschop JJ, Mohammed S, O’Flaherty M, Heck AJ, Slijper M, Menke FL. Quantitative phosphoproteomics of early elicitor signaling in Arabidopsis. Mol Cell Proteomics. 2007;6:1198–214. doi: 10.1074/mcp.M600429-MCP200. [DOI] [PubMed] [Google Scholar]
- 7.de la Fuente van Bentem S, Anrather D, Roitinger E, Djamei A, Hufnagl T, Barta A, et al. Phosphoproteomics reveals extensive in vivo phosphorylation of Arabidopsis proteins involved in RNA metabolism. Nucleic Acids Res. 2006;34:3267–78. doi: 10.1093/nar/gkl429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nühse TS, Stensballe A, Jensen ON, Peck SC. Phosphoproteomics of the Arabidopsis plasma membrane and a new phosphorylation site database. Plant Cell. 2004;16:2394–405. doi: 10.1105/tpc.104.023150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peck SC. Phosphoproteomics in Arabidopsis: moving from empirical to predictive science. J Exp Bot. 2006;57:1523–7. doi: 10.1093/jxb/erj126. [DOI] [PubMed] [Google Scholar]
- 10.Christie JM, Yang H, Richter GL, Sullivan S, Thomson CE, Lin J, et al. phot1 inhibition of ABCB19 primes lateral auxin fluxes in the shoot apex required for phototropism. PLoS Biol. 2011;9:e1001076. doi: 10.1371/journal.pbio.1001076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henrichs S, Wang B, Fukao Y, Zhu J, Charrier L, Bailly A, et al. Regulation of ABCB1/PGP1-catalysed auxin transport by linker phosphorylation. EMBO J. 2012;31:2965–80. doi: 10.1038/emboj.2012.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zourelidou M, Müller I, Willige BC, Nill C, Jikumaru Y, Li H, et al. The polarly localized D6 PROTEIN KINASE is required for efficient auxin transport in Arabidopsis thaliana. Development. 2009;136:627–36. doi: 10.1242/dev.028365. [DOI] [PubMed] [Google Scholar]
- 13.Blakeslee JJ, Bandyopadhyay A, Lee OR, Mravec J, Titapiwatanakun B, Sauer M, et al. Interactions among PIN-FORMED and P-glycoprotein auxin transporters in Arabidopsis. Plant Cell. 2007;19:131–47. doi: 10.1105/tpc.106.040782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petrásek J, Mravec J, Bouchard R, Blakeslee JJ, Abas M, Seifertová D, et al. PIN proteins perform a rate-limiting function in cellular auxin efflux. Science. 2006;312:914–8. doi: 10.1126/science.1123542. [DOI] [PubMed] [Google Scholar]
- 15.Yang H, Murphy AS. Functional expression and characterization of Arabidopsis ABCB, AUX 1 and PIN auxin transporters in Schizosaccharomyces pombe. Plant J. 2009;59:179–91. doi: 10.1111/j.1365-313X.2009.03856.x. [DOI] [PubMed] [Google Scholar]
- 16.Geisler M, Blakeslee JJ, Bouchard R, Lee OR, Vincenzetti V, Bandyopadhyay A, et al. Cellular efflux of auxin catalyzed by the Arabidopsis MDR/PGP transporter AtPGP1. Plant J. 2005;44:179–94. doi: 10.1111/j.1365-313X.2005.02519.x. [DOI] [PubMed] [Google Scholar]
- 17.Gälweiler L, Guan CH, Müller A, Wisman E, Mendgen K, Yephremov A, et al. Regulation of polar auxin transport by AtPIN1 in Arabidopsis vascular tissue. Science. 1998;282:2226–30. doi: 10.1126/science.282.5397.2226. [DOI] [PubMed] [Google Scholar]
- 18.Christensen SK, Dagenais N, Chory J, Weigel D. Regulation of auxin response by the protein kinase PINOID. Cell. 2000;100:469–78. doi: 10.1016/S0092-8674(00)80682-0. [DOI] [PubMed] [Google Scholar]
- 19.Subramanian C, Woo J, Cai X, Xu X, Servick S, Johnson CH, et al. A suite of tools and application notes for in vivo protein interaction assays using bioluminescence resonance energy transfer (BRET) Plant J. 2006;48:138–52. doi: 10.1111/j.1365-313X.2006.02851.x. [DOI] [PubMed] [Google Scholar]


