Abstract
NIMA-related kinase 6 (NEK6) regulates cellular expansion and morphogenesis through microtubule organizaiton in Arabidopsis thaliana. Loss-of-function mutations in NEK6 (nek6/ibo1) cause ectopic outgrowth and microtubule disorganization in epidermal cells. We recently found that NEK6 forms homodimers and heterodimers with NEK4 and NEK5 to destabilize cortical microtubules possibly by direct binding to microtubules and the β-tubulin phosphorylation. Here, we identified a new allele of NEK6 and further analyzed the morphological phenotypes of nek6/ibo1 mutants, along with alleles of nek4 and nek5 mutants. Phenotypic analysis demonstrated that NEK6 is required for the directional growth of roots and hypocotyls, petiole elongation, cell file formation, and trichome morphogenesis. In addition, nek4, nek5, and nek6/ibo1 mutants were hypersensitive to microtubule inhibitors such as propyzamide and taxol. These results suggest that plant NEKs function in directional cell growth and organ development through the regulation of microtubule organization.
Keywords: NIMA-related kinase, microtubule, tubulin, directional growth, epidermis, Arabidopsis
The development of multicellular organisms depends on cellular growth and morphogenesis. The directional growth of plant cells depends on the cortical array of microtubules.1-3 However, the mechanism of microtubule regulation remains to be elucidated. Recent genetic analyses suggested the involvement of protein phosphorylation in microtubule organization and directional cell expansion.4-6
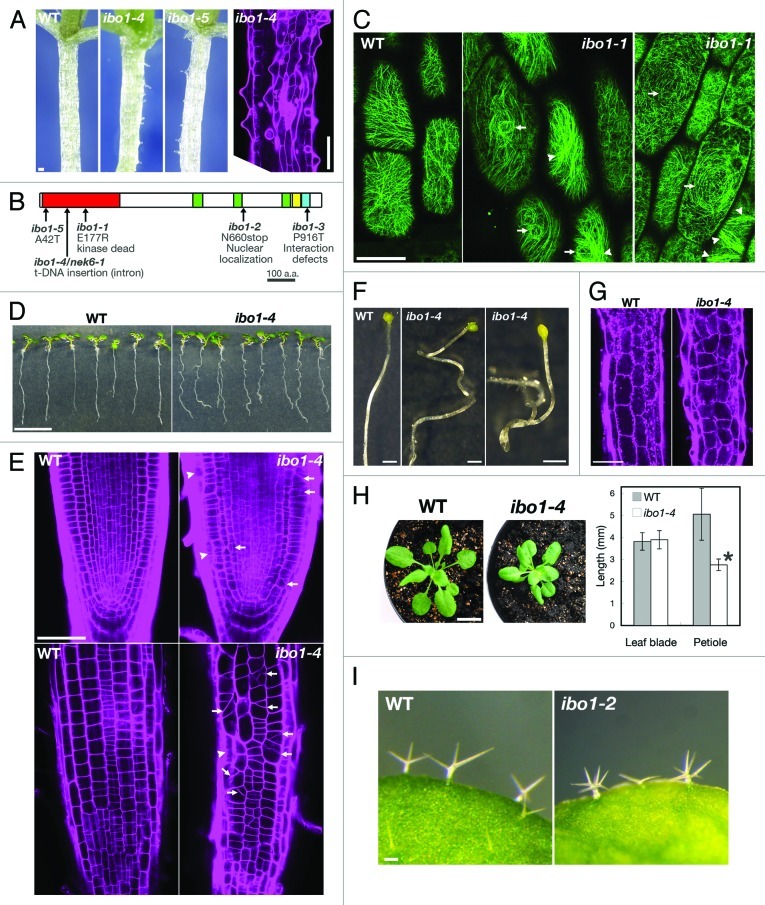
Never in mitosis A (NIMA) is a Ser/Thr protein kinase, which was first discovered from a mitotic mutant nimA of Aspergillus nidulans.7,8 NIMA-related kinases (NEKs) have been found in various fungi and animals, and they comprise a family of mitotic kinases conserved in eukaryotes. In fungi and animals, NEKs regulate various mitotic events including mitotic initiation, centrosome separation, spindle formation, and cytokinesis.7,8 Plants have 6–9 NEK genes but their function is not clearly understood. NEK6 has been found to function in epidermal cell expansion and morphogenesis in Arabidopsis thaliana.9,10 NEK6 has also been identified as an interacting protein with armadillo repeat-containing kinesins (ARKs).9 The loss-of-function mutant of NEK6, ibo1/nek6, exhibits ectopic protuberances in epidermal cells of hypocotyls and petioles (Fig. 1A and B), indicating that NEK6 suppresses ectopic outgrowth in epidermal cells.9,10 A single ectopic protrusion is formed in the middle of the cell of the non-stomatal cell file in hypocotyls, suggesting that the protrusion might be a trichome-like structure.10 The ectopic outgrowth of ibo1/nek6 mutants is strongly promoted by ethylene signaling.10 Genetic and biochemical analyses revealed that the kinase activity of NEK6 and the microtubule localization of NEK6 are essential for suppressing ectopic outgrowth.10
Figure 1. The nek6/ibo1 mutants exhibit disorganized cell growth. (A) Morphology of hypocotyls of 10-d-old seedlings of the wild type (WT) and ibo1. The right part shows a hypocotyl of ibo1–4 stained with propidium iodide. (B) Structure of NEK6. PEST sequence (green), coiled-coil domain (yellow), plant NEK C-terminal motif (blue) and mutation sites are shown. (C) Cortical microtubules were visualized with GFP-TUB6 in hypocotyl epidermal cells of the wild type (WT) and ibo1–1. Arrows and arrowheads in (C) indicate whirled arrays of microtubules and microtubule bundles, respectively. (D and E) Morphology of the wild type (WT) and ibo1–4 seedlings grown vertically for 7 d. (E) Seedlings were stained with propidium iodide and root tips were observed under a confocal microscope. Median longitudinal optical section (upper parts in E) and epidermis (lower parts in E) of root tip were shown. Arrows and arrowheads indicate aberrant cell plates and irregular cell files, respectively. (F) Morphology of hypocotyls of the wild type (WT) and ibo1–4 grown in the dark for 7 d. (G) Hypocotyl cortex of the wild type (WT) and ibo1–4. Seedlings grown in the light for 7 d were stained with propidium iodide and observed under a confocal microscope. (H) Morphology of 4-week-old wild type (WT) and ibo1–4 plants (left parts) and quantification of lengths of leaf blades and petioles of 14-d-old seedlings (right parts). Values are means ± SD (n = 10). Asterisk indicates significant difference from the wild type (Student t-test, p < 0.01). (I) Trichomes of the wild type (WT) and ibo1–4. Bars = 100 µm (A, G and I), 50 µm (C and E), 10 mm (D and H) or 1 mm (F).
Recently, we showed that NEK6 interacts with other NEK members, directly binds to microtubules, phosphorylates β-tubulins, and regulates cortical microtubule organization during epidermal cell expansion.11 The functional NEK6–green fluorescent protein fusion was concentrated in particles exhibiting dynamic movement along microtubules. The nek6/ibo1 mutants showed disturbance in the cortical microtubule array at the site of ectopic protrusions in epidermal cells (Fig. 1C). The quantitative analysis of microtubule dynamics indicated excessive stabilization of cortical microtubules in ibo1/nek6. In addition, NEK6 directly bound to microtubules and phosphorylated β-tubulin in vitro. The interaction of NEK6 with NEK4 and NEK5, which is affected by the ibo1–3 mutation, was shown to be required for the ectopic outgrowth phenotype of ibo1/nek6.11 These results suggest that NEK6 regulates cortical microtubule organization by interacting with other NEKs and the phosphorylation of β-tubulin.
Here, we identified a new allele of NEK6 (ibo1–5), which had a point mutation in the kinase domain (Ala to Thr substitution at position 42). The ibo1–5 mutant exhibited ectopic protrusions from epidermal cells, which were identical to those of other nek6/ibo1 mutants (Fig. 1A). This result supports that the kinase domain is essential for the function of NEK6. We further analyzed the developmental phenotypes of nek6 mutants. The nek6–1/ibo1–4 seedlings showed aberrant root waving or skewing pattern when grown vertically (Fig. 1D). In the root tip of the nek6–1/ibo1–4 mutant, cell files were disorganized and abnormal cell plates were formed (Fig. 1E), indicating that NEK6 is required for organized cell division and expansion, leading to regular cell file formation. The hypocotyl of a dark-grown nek6–1/ibo1–4 seedling exhibited twisted growth (Fig. 1F). In the hypocotyl cortex of the ibo1–4 mutant, reduced cell length and less organized cell files were observed (Fig. 1G). In addition, the nek6–1/ibo1–4 mutant had short petioles (Fig, 1H). Furthermore, trichomes in ibo1–2 had more branches than in the wild type (Fig. 1I). These results indicate that NEK6 is required for directional growth, organized cell division and expansion, petiole elongation and trichome branching.
To study the involvement of NEK4, NEK5 and NEK6 in the process of microtubule-dependent growth, we analyzed the effects of a microtubule-depolymerizing drug, propyzamide, and a microtubule-stabilizing drug, taxol, on the root growth in nek4, nek5 and nek6/ibo1 mutants. The nek4–1, nek5–1, and nek6–1/ibo1–4 mutants were hypersensitive to propyzamide and taxol (Fig. 2A-C). In the presence of microtubule inhibitors, the mutant roots were shorter and slanted rightward more severely than those of the wild type. This result implies that NEK4, NEK5, and NEK6 are involved in the regulation of microtubule organization during root growth.
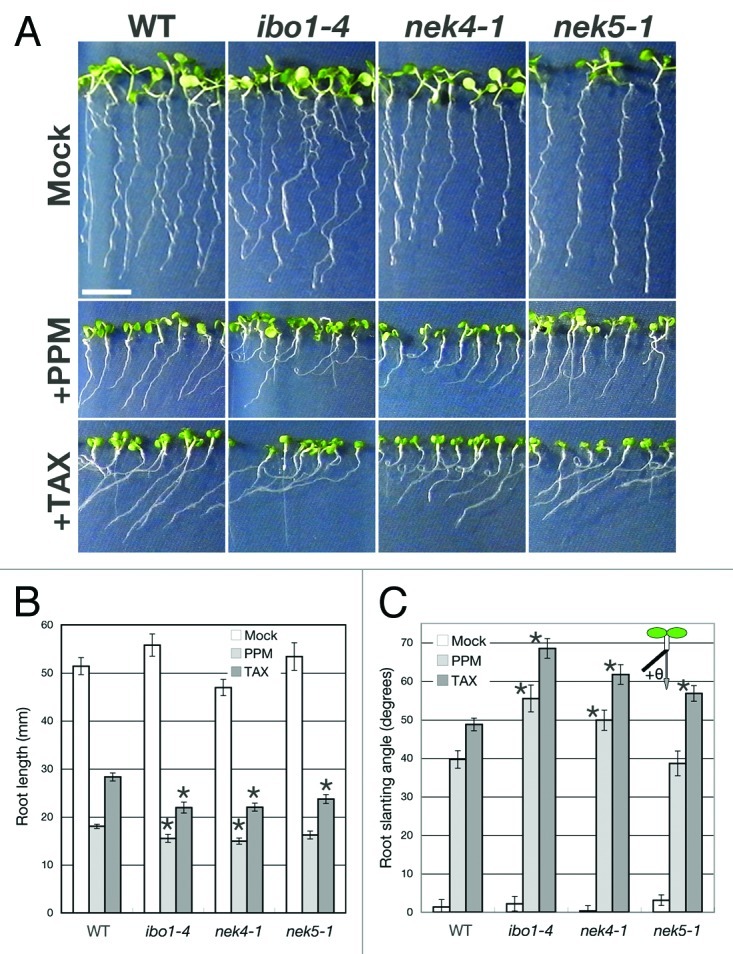
Figure 2. The nek4, nek5 and nek6/ibo1 mutants are hypersensitive to microtubule inhibitors. (A) Morphology of the wild type (WT) and nek mutant seedlings grown for 8 d on the MS agar medium in the absence (Mock) or presence of 3 µM propyzamide (+PPM) or 1 µM taxol (+TAX). Bar = 10 mm. (B) Root length of the wild type (WT) and nek mutants grown for 10 d in the absence (Mock) or presence of 3 µM propyzamide (PPM) or 1 µM taxol (TAX). (C) Root slanting angles (θ) of 8-d-old seedlings of the wild type (WT) and nek mutants grown as described above. Rightward- and leftward-slanting angles (viewed from the shoot apex) are expressed as positive and negative values, respectively. In (B) and (C), values are means ± SE (n ≥ 22). Asterisks indicate significant difference from the wild type (Student t-test, p < 0.02).
Taken together, the findings of the present study reveals that NEK6 regulates multiple developmental processes, including the directional growth of roots and hypocotyls, cell file formation, petiole elongation, and epidermal cell morphogenesis. Because NEK6 participates in the destabilization of microtubules, possibly through the phosphoryation of β-tubulin,11 the regulation of microtubule organization by NEK6 may be important for directional cell growth and an organized pattern of cell division during organ development. The hypersensitivity of nek4, nek5 and nek6 mutants to microtubule inhibitors indicates that NEK4 and NEK5 also regulate microtubule-dependent cellular growth in concert with NEK6. The pleiotropic phenotype of nek6/ibo1, together with the mild phenotypes of nek4 and nek5, suggests that NEK6 plays central roles in NEK-related regulatory pathways to control cell growth. In consonance with this, our previous study implied that NEK6 regulates the activity and localization of NEK4 and NEK5.11 This is also consistent with other reports of multiple functions of NEK6.9-12 In addition, we demonstrated the involvement of other NEK members in microtubule function, as was recently reported for another NEK member, namely, AtNek2 in A. thaliana.13 Fungal and animal NEKs mainly regulate mitotic cell division7,8 whereas plant NEKs control directional cell expansion9-11 and also participate in stress response and seed germination.12,14 This might reflect that plant NEKs have evolved to acquire novel functions for the adaptation of plants to changing environmental conditions. Recent genetic analyses suggest that plant mitotic regulators might be recruited for the regulation of cell growth (e.g., endocycle) and environmental responses.15,16 Besides β-tubulin and ARKs, we identified novel proteins interacting with NEK6 and substrates phosphorylated by NEK6. Further analysis of the plant NEK family and its downstream factors will provide novel insights into the regulation of organized cell growth and the underlying microtubule functions.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Drs. Takahiro Hamada, Takehide Kato, Takashi Murata, Mitsuyasu Hasebe, Takashi Hashimoto, Yuichiro Watanabe, and Tatsuya Sakai for helpful advices, and the Arabidopsis Biological Resource Center for providing seeds. This work was supported by the Ministry of Education, Culture, Sports, Science and Technology (MEXT) in Japan [Grants in Aid for Scientific Research (22770043 and 23119513 to H.M., 22370021 and 23012032 to T.T.)] and Grant in Aid from the Ryobi Teien Memory Foundation.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/22412
References
- 1.Barlow PW, Baluška F. Cytoskeletal perspectives on root growth and morphogenesis. Annu Rev Plant Physiol Plant Mol Biol. 2000;51:289–322. doi: 10.1146/annurev.arplant.51.1.289. [DOI] [PubMed] [Google Scholar]
- 2.Sedbrook JC, Kaloriti D. Microtubules, MAPs and plant directional cell expansion. Trends Plant Sci. 2008;13:303–10. doi: 10.1016/j.tplants.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 3.Wasteneys GO, Ambrose JC. Spatial organization of plant cortical microtubules: close encounters of the 2D kind. Trends Cell Biol. 2009;19:62–71. doi: 10.1016/j.tcb.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 4.Camilleri C, Azimzadeh J, Pastuglia M, Bellini C, Grandjean O, Bouchez D. The Arabidopsis TONNEAU2 gene encodes a putative novel protein phosphatase 2A regulatory subunit essential for the control of the cortical cytoskeleton. Plant Cell. 2002;14:833–45. doi: 10.1105/tpc.010402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Naoi K, Hashimoto T. A semidominant mutation in an Arabidopsis mitogen-activated protein kinase phosphatase-like gene compromises cortical microtubule organization. Plant Cell. 2004;16:1841–53. doi: 10.1105/tpc.021865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ben-Nissan G, Cui W, Kim DJ, Yang Y, Yoo BC, Lee JY. Arabidopsis casein kinase 1-like 6 contains a microtubule-binding domain and affects the organization of cortical microtubules. Plant Physiol 2008; 148:1897-1907; 10.1104/pp.108.129346. [DOI] [PMC free article] [PubMed]
- 7.O’Connell MJ, Krien MJE, Hunter T. Never say never. The NIMA-related protein kinases in mitotic control. Trends Cell Biol. 2003;13:221–8. doi: 10.1016/S0962-8924(03)00056-4. [DOI] [PubMed] [Google Scholar]
- 8.O’regan L, Blot J, Fry AM. Mitotic regulation by NIMA-related kinases. Cell Div. 2007;2:25–36. doi: 10.1186/1747-1028-2-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sakai T, Honing H, Nishioka M, Uehara Y, Takahashi M, Fujisawa N, et al. Armadillo repeat-containing kinesins and a NIMA-related kinase are required for epidermal-cell morphogenesis in Arabidopsis. Plant J. 2008;53:157–71. doi: 10.1111/j.1365-313X.2007.03327.x. [DOI] [PubMed] [Google Scholar]
- 10.Motose H, Tominaga R, Wada T, Sugiyama M, Watanabe Y. A NIMA-related protein kinase suppresses ectopic outgrowth of epidermal cells through its kinase activity and the association with microtubules. Plant J. 2008;54:829–44. doi: 10.1111/j.1365-313X.2008.03445.x. [DOI] [PubMed] [Google Scholar]
- 11.Motose H, Hamada T, Yoshimoto K, Murata T, Hasebe M, Watanabe Y, et al. NIMA-related kinases 6, 4, and 5 interact with each other to regulate microtubule organization during epidermal cell expansion in Arabidopsis thaliana. Plant J. 2011;67:993–1005. doi: 10.1111/j.1365-313X.2011.04652.x. [DOI] [PubMed] [Google Scholar]
- 12.Zhang B, Chen HW, Mu RL, Zhang WK, Zhao MY, Wei W, et al. NIMA-related kinase NEK6 affects plant growth and stress response in Arabidopsis. Plant J. 2011;68:830–43. doi: 10.1111/j.1365-313X.2011.04733.x. [DOI] [PubMed] [Google Scholar]
- 13.Agueci F, Rutten T, Demidov D, Houben A. Arabidopsis AtNek2 kinase is essential and associates with microtubules. Plant Mol Biol Rep. 2012;30:339–48. doi: 10.1007/s11105-011-0342-1. [DOI] [Google Scholar]
- 14.Lee SJ, Cho DI, Kang JY, Kim MD, Kim SY. AtNEK6 interacts with ARIA and is involved in ABA response during seed germination. Mol Cells. 2010;29:559–66. doi: 10.1007/s10059-010-0070-7. [DOI] [PubMed] [Google Scholar]
- 15.De Veylder L, Larkin JC, Schnittger A. Molecular control and function of endoreplication in development and physiology. Trends Plant Sci. 2011;16:624–34. doi: 10.1016/j.tplants.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 16.Komaki S, Sugimoto K. Control of the plant cell cycle by developmental and environmental cues. Plant Cell Physiol. 2012;53:953–64. doi: 10.1093/pcp/pcs070. [DOI] [PubMed] [Google Scholar]