Abstract
The establishment of an Arbuscular Mycorrhizal symbiotic interaction (MA) is a successful strategy to substantially promote plant growth, development and fitness. Numerous studies have supported the hypothesis that plant hormones play an important role in the recognition and establishment of symbiosis. Particular attention has been devoted to jasmonic acid (JA) and its derivates, the jasmonates, which are believed to play a major role in AM symbiosis. Jasmonates belong to a diverse class of lipid metabolites known as oxylipins that include other biologically active molecules. Recent transcriptional analyses revealed upregulation of the oxylipin pathway during AM symbiosis in mycorrhizal tomato roots and point a key regulatory feature for oxylipins during AM symbiosis in tomato, particularly these derived from the action of 9-lipoxygenases (9-LOX). In this mini-review we highlight recent progress understanding the function of oxylipins in the establishment of the AM symbiosis and hypothesize that the activation of the 9-LOX pathway might be part of the activation of host defense responses which will then contribute to both, the control of AM fungal spread and the increased resistance to fungal pathogens in mycorrhizal plants.
Keywords: arbuscular mycorrhiza, oxylipins
Arbuscular mycorrhizal (AM) symbiosis is a mutually beneficial interaction among most of the higher plants, including the majority of agricultural crop species, and soil fungi of the phylum Glomeromycota.1 This symbiosis is present in most ecosystems and is of tremendous significance in agricultural ecosystems. Arbuscular mycorrhizal fungus (AMF) play a major role in nutrient cycling, particularly phosphate, and promotes plant growth by improve the nutrient status and the water absorption of their host.1 The plant, in turn, supplies the fungus with carbohydrates. This exchange of nutrients is performed in the cortical cells of the root by fungal structures called arbuscules.2 In addition, the fungus renders the plant more resistant to biotic and abiotic stress.1,3 During mycorrhization, plant cells undergo the structural and functional changes necessary for establishing symbiosis, suggesting that there is a high degree of interaction between both partners at the cellular, molecular and genetic levels. In this regard, plant hormones appear to play an important role in the recognition and establishment of symbiosis.
Plant hormones are essential molecules for signaling changes occurring in the plant’s growth, development and morphological adaptation processes in response to environmental conditions as well as in defense responses to pathogen and abiotic stress factors. In AM, phytohormones play an important role in the recognition and extension of fungus within the roots of host plants. In the first phase, once the host plant recognizes AMF, transcriptional reprogramming occurs in plant cells intended for accommodating the fungus within the cell.4 Subsequently, transcriptional reprogramming is used to control fungal growth inside the roots. This transcriptional reprogramming appears to be mediated by plant hormones. Thus, changes are detected in the relatively abundant plant hormones during mycorrhization, most of which are thought to be involved in the symbiotic process5,6. Salicylic acid (SA) and ethylene (Et) are known to play a negative regulatory role during mycorrhization,7-9 while abscisic acid (ABA) is necessary for the proper functioning of the symbiosis and the formation of arbuscules.10-12 Particular attention has been devoted to jasmonic acid (JA) and its derivatives, jasmonates, which are believed to play a major role in AM symbiosis.13 Experiments involving exogenous application of JA have shown a dose-response effect. Low concentrations of JA boosted colonization,14 while high doses produced inhibitory effects.15 Moreover, mycorrhization experiments with the JA-deficient tomato plant mutant spr2, lacking the chloroplastic fatty acid desaturase involved in JA biosynthesis,16 have shown a reduction in colonization,17,18 while studies with the JA-insensitive tomato mutant jai-1, defective in the function of the tomato homolog of COI1 in Arabidopsis,19 have pointed out an increased colonization respect to wild-type tomato plants,20 suggesting an intricate regulatory role for JA in AM symbiosis. This regulatory role played by JA may be due to the complex biosynthetic pathway and signaling of JA and its derivatives. Jasmonates belong to a diverse class of lipid metabolites known as oxylipins which include other biologically active molecules21,22.
Plant Oxylipins
Oxylipins are a group of biologically active molecules with different structures and functions which are derived from the oxidative metabolism of fatty acids. They are generated by the coordinated action of lipases, lipoxygenases (LOXs) and a group of cytochrome P450 (CYP74)
specialized in metabolizing hydroperoxy fatty acids.23 The synthesis of most plant oxylipins is initiated by the addition of an oxygen molecule at carbon 9 or 13 of the linoleic or linolenic acid.24 This reaction is catalyzed by LOXs (non-heme iron dioxygenases) and generates either a 9-LOX or 13-LOX pathway, depending on where the oxygenation takes place. The LOX-derived hydroperoxy acids can be metabolized by the following enzymes: allene oxide synthase (AOS), divinyl ether synthase (DES), hydroperoxide lyase (HPL), alkyl hydroperoxide reductase, peroxygenase, epoxy alcohol synthase or LOX itself, thus giving rise to different groups of oxylipins.25,26 One the most commonly studied group is the AOS branch of the 13-LOX pathway that leads to the family of jasmonates including JA/MeJA and its precursor, 12-OPDA. However, in recent years, the 9-LOX pathway has been studied due to its essential role in plant defense against microbial pathogens.27,28
13-LOX Oxylipins are involved in several physiological plant development processes, such as growth, fertility29,30 and tuberization in potato.31 Additionally, these oxylipins play an important role in plant adaptation to adverse environmental conditions32 and in defensive responses to fungal and bacterial pathogens.33,34 In this regard, others oxylipins, apart from the 13-LOX derived, are known to play an antimicrobial role in relation to bacteria, oomycetes and fungal pathogens,35,36 and are capable of activate the expression of genes involved in pathogenesis28 and regulate cell death37. It is therefore not surprising that oxylipins are important in relation to regulating symbiotic processes, particularly AM symbiosis.
Jasmonates and Arbuscular Mycorrhiza
In general, much research on oxylipins has focused on the jasmonate family of molecules. It is therefore understandable that, until recently, the study of the regulatory role played by oxylipins in mycorrhization has also focused on jasmonates. AM colonization appears to be related to endogenous JA levels. In this respect, Hause et al. observed an induction of genes involved in JA biosynthesis in cells harboring arbuscules in barley roots.38 Similar results were obtained by Isayenkov et al. in relation to Medicago truncatula.39 They silenced the gene coding for the enzyme allene oxide cyclase of the 13-LOX pathway in hairy roots of M. truncatula, observing a reduction in JA levels and consequently in the degree of mycorrhization, mainly in relation to arbuscular mycorrhizal formation. However, this reduction in the level of arbuscules did not affect their structure. It is possible that one of the mechanisms by which JA regulates the mycorrhization process and the formation of arbuscules is through the regulation of the carbohydrate metabolism and transport in the plant. In this regard, the degree of mycorrhizal colonization in tomato plants correlated with changes in the transcriptional regulation of genes involved in sucrose hydrolysis and transport, cell wall invertase activity and mycorrhizal-specific fatty acid content in roots.17 These results were confirmed in tomato plants deficient in JA synthesis (spr-2), which showed a reduced level of colonization. Exogenous application of JA enabled a partial recovery in the level of mycorrhization and the expression of genes involved in carbon partitioning in the plant.17 Moreover, induction of genes involved in the biosynthesis of JA has been observed in sink tissues.40,41 Cells harbouring arbuscules act in fact as sink tissues for carbohydrates in mycorrhizal plants. Thus, a model has been proposed in which the induced biosynthesis of JA in mycorrhizal roots is linked to the sink phenomenon38. In addition, the expression of sink-specific and defense-related genes is a characteristic of sink tissue42 which may contribute to the enhanced defense status of the plant.
Jasmonates were labeled as secondary metabolites, but now it has become clear that they themselves act as elicitors of the production of secondary metabolites across the plant kingdom, from angiosperms to gymnosperms. Among the classes of metabolites that are induced by JAs are free and conjugated forms of polyamines, quinones, terpenoids, alkaloids, phenylpropanoids, glucosinolates, and antioxidants.43,44 Therefore JA involvement in the mycorrhization process may be also mediated through the induction of the biosynthesis of secondary metabolites such as flavonoids and terpenes, which are known to play a significant role in mycorrhizal symbioses.45 In this sense, it is well known that application of jasmonates leads to increases in phenylalanine ammonia lyase (PAL) mRNA accumulation46 and in PAL enzyme activity,47 suggesting that JA may play a role in regulating flavonoids involved in the process of mycorrhization.
On the other hand, it has also been suggested that JA is involved in regulating AM fungus colonization mediated by the induction of the expression of genes coding for defense-related proteins. Mutant JA-insensitive (jai-1) tomato plants showed increased susceptibility to AM colonization.20 Jai-1 plant mutants are deficient in systemic wound-inducible expression of proteinase inhibitor (PI) genes and also lack PI expression in response to MeJA application.48 Exogenous application of MeJA to tomato plants reduced mycorrhization and mainly affected the fungal phosphate metabolism and arbuscular formation, showing that AM colonization may be controlled by the JA signaling pathway.20
9-LOX Oxylipin Pathway and Arbuscular Mycorrhiza
Few studies are available on the changes that occur in the 9-LOX oxylipin pathway during AM formation. Two recent microarray analyses of mycorrhizal tomato roots showed significant upregulation of genes involved in the metabolism of 9-LOX oxylipins.49,50 However, in the case of M. truncatula, the analysis of fatty acid profiles of non-mycorrhizal roots and in roots colonized by G. intraradices did not show significant differences between the 9-LOX and 13-LOX products of linoleic and α-linolenic acid, except in relation to JA (a 13-LOX oxylipin) which reached high levels in mycorrhizal roots.51 Therefore, it would be plausible to assert that the 9-LOX pathway plays a more important role in Solanaceae plants than in other plant families.
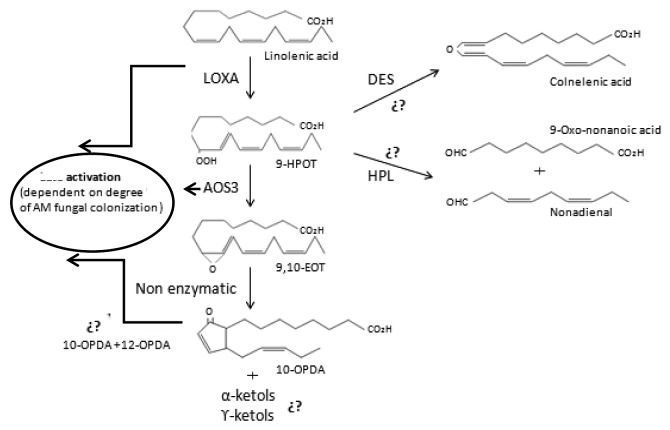
The LOXA and AOS3 genes involved in the 9-LOX metabolism were induced in tomato roots with a well-established colonization by G. intraradices, and their expression appears to be dependent on a certain degree of AM fungal colonization.18 In addition, the induction of these genes in mycorrhizal tomato roots only occurred on the colonized part of the mycorrhizal split-root plants. Moreover, the 9-LOX pathway is known to play a defensive role in relation to microbial pathogens in plants.27,28,52 This suggests that the activation of the 9-LOX pathway could be a mechanism for controlling AMF development in the roots of Solanaceae plants. However, tomato plants deficient in the synthesis and/or perception of JA (spr-2, def-1 and jai-1) did not show an upregulation of LOXA and AOS3 genes, indicating that this strategy for controlling fungal spread in roots is at least partly dependent on JA pathway activation.18 Unlike M. truncatula, the content of OPDA (a mixture of 10- and 12-OPDA) in mycorrhizal tomato plants has been shown to increase compared with non-mycorrhizal plants and was not accompanied by any increase in free JA.51 Thus, it is possible that OPDA and other oxylipins, though nor free JA, play an important role in orchestrating plant responses to AM fungi in Solanaceae.51 12-OPDA is known to play a role in plant defense signaling,53 with a similar role being suggested for 10-OPDA.54 These results show that 9-LOX pathway may be involved in regulating the mycorrhization process.
The expression pattern during AM formation of LeDES, a gene coding for a 9-LOX desaturase which catalyzes the biosynthesis of colnelenic and colneleic acids, is the subject of some controversy. Although LeDES has been described by López-Ráez et al. as a mycorrhizal upregulated tomato gene in a microarray of tomato roots,50 two other transcriptional analyses did not demonstrate any upregulation.49,55 The transcriptional regulation of LeDES during mycorrhization may not be directly dependent on the degree of colonization, as seems to be the case with LOXA and AOS3.
It has been suggested that the bioprotective effect of mycorrhization against fungal pathogens and the autoregulation of mycorrhization possibly are two sides of the same medal.56 It seems plausible that mycorrhizal plants develop only one mechanism to repulse further colonization by fungi, not discriminating between AMF and soil-borne pathogenic fungi, and the activation of 9-LOX metabolism take part of this mechanism. As reported with respect to Phytophthora parasitica57 and other fungal pathogens,25,28 it is conceivable that the 9-LOX pathway plays a defensive role and restricts fungal spread in roots. In several studies a local bioprotectional effect has been linked with a high degree of AM root colonization, whereas intermediate and low levels of AM root colonization showed no bioprotectional effect. Apparently a critical level of AM root colonization is needed to provide bioprotection for mycorrhizal plants, and similarly the upregulation of the expression of genes involved in the 9-LOX metabolism in mycorrhizal roots seems to depend on a particular degree of AM-fungal colonization. It is therefore conceivable that the activation pathway of 9-LOX oxylipins could be responsible for the increased resistance to fungal pathogens in mycorrhizal plants.
Conclusions
Research to date shows that oxylipins play an important role in the colonization and establishment of AM. In particular, JA clearly seems to play a key role in arbuscular formation, possibly through the regulation of the carbohydrate metabolism and transport in the plant, the induction of secondary metabolites such as flavonoids and/or through the activation of genes coding for defense-related proteins. However, not much is known about the regulatory role of 9-LOX pathway in the process of mycorrhization (Fig. 1). Recent studies have shown a late activation of the 9-LOX pathway during the establishment of AM symbiosis in Solanaceae, which could be related to a mechanism for controlling AMF development in roots, although we cannot rule out an involvement of the 9-LOX metabolism in the bioprotective effect of mycorrhization against fungal pathogens. Further research is required in areas such as the use of plant mutants in the biosynthesis and perception of oxylipins in order to clarify the role played by the 9-LOX pathway.
Figure 1. The 9-LOX metabolism of linolenic acid in response to AM fungal colonization in tomato roots. LOXA and AOS3 are induced when the AM colonization is well-established in roots. OPDA (a mixture of 10- and 12-OPDA) increased in response to AM colonization at this stage. The role of DES is controversial and its induction no dependent directly on the degree of colonization. The role of HPL and ketols are not known during AM colonization. LOX, lipoxygenase; AOS, allene oxide synthase; DES, divinyl ether synthase; HPL, hydroperoxide lyase; 9-HPOT, 9-hydroperoxy linolenic acid; 9–10-EOT, 9–10-epoxy octadecatrienoic acid; 10-OPDA, 10-oxo phytodienoic acid.
Acknowledgments
This study was supported by FEDER founds, through the Spanish Ministry of Economy and competitiveness (MICINN-AGL2008–00742; AGL-2011–25930). R León Morcillo is supported by a fellowship from the JAE-pre CSIC program. We would also like to thanks Michel O’Shea for proof-reading the document.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/22098
References
- 1.Smith SE, Read DJ. Mycorrhizal symbiosis 2008; 3rd edn. London: Academic Press. [Google Scholar]
- 2.Parniske M. Arbuscular mycorrhiza: the mother of plant root endosymbioses. Nat Rev Microbiol. 2008;6:763–75. doi: 10.1038/nrmicro1987. [DOI] [PubMed] [Google Scholar]
- 3.Pozo MJ, Azcón-Aguilar C. Unraveling mycorrhiza-induced resistance. Curr Opin Plant Biol. 2007;10:393–8. doi: 10.1016/j.pbi.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 4.Genre A, Chabaud M, Faccio A, Barker DG, Bonfante P. Prepenetration apparatus assembly precedes and predicts the colonization patterns of arbuscular mycorrhizal fungi within the root cortex of both Medicago truncatula and Daucus carota. Plant Cell. 2008;20:1407–20. doi: 10.1105/tpc.108.059014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hause B, Mrosk C, Isayenkov S, Strack D. Jasmonate in arbuscular mychorrhizal interactions. Phytochemistry. 2007;8:101–10. doi: 10.1016/j.phytochem.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 6.Ludwing-Müller J. Hormonal responses in host plants triggered by arbuscular mycorrhizal fungi. Koltai H, Kapunlnik Y, (Eds) Arbuscular mycorrhizas: physiology and fuction Springer Science+Business B.V. 2010; 169-190. [Google Scholar]
- 7.Herrera-Medina MJ, Gagnon H, Pinché Y, Ocampo JA, García-Garrido JM, Vierhelig H. Root colonization by arbuscular mycorrhizal fungi is affected by the salicylic acid content of the plant. Plant Sci. 2003;164:993–8. doi: 10.1016/S0168-9452(03)00083-9. [DOI] [Google Scholar]
- 8.Riedel T, Groten K, Baldwin IT. Symbiosis between Nicotiana attenuata and Glomus intraradices: ethylene plays a role, jasmonic acid does not. Plant Cell Environ. 2008;31:1203–13. doi: 10.1111/j.1365-3040.2008.01827.x. [DOI] [PubMed] [Google Scholar]
- 9.Gutjahr C, Paszkowski U. Weights in the balance: jasmonic acid and salicylic acid signaling in root-biotroph interactions. Mol Plant Microbe Interact. 2009;22:763–72. doi: 10.1094/MPMI-22-7-0763. [DOI] [PubMed] [Google Scholar]
- 10.Herrera-Medina MJ, Steinkellner S, Vierheilig H, Ocampo Bote JA, García Garrido JM. Abscisic acid determines arbuscule development and functionality in the tomato arbuscular mycorrhiza. New Phytol. 2007;175:554–64. doi: 10.1111/j.1469-8137.2007.02107.x. [DOI] [PubMed] [Google Scholar]
- 11.Martín-Rodriguez JA, León-Morcillo RL, Vierheilig H, Ocampo JA, Ludwig-Müller J, García-Garrido JM. Mycorrhization of the notabilis and sitiens tomato mutants in relation to abscisic acid and ethylene contents. J Plant Physiol. 2010;167:606–13. doi: 10.1016/j.jplph.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 12.Martín-Rodriguez JA, León-Morcillo RJ, Vierheilig H, Ocampo Bote JA, Ludwig-Müller J, García-Garrido JM. Ethylene-dependent/ethylene-indenpendent ABA regulation of tomato plants colonized by arbuscular mycorrhiza fungi. New Phytol. 2011;190:193–205. doi: 10.1111/j.1469-8137.2010.03610.x. [DOI] [PubMed] [Google Scholar]
- 13.Hause B, Schaarschmidt S. The role of jasmonates in mutualistic symbioses between plants and soil-born microorganisms. Phytochemistry. 2009;70:1589–99. doi: 10.1016/j.phytochem.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 14.Regvar M, Gogala N, Zalar P. Effects of jasmonic acid on mycorrhizal Allium sativum. New Phytol. 1996;134:703–7. doi: 10.1111/j.1469-8137.1996.tb04936.x. [DOI] [PubMed] [Google Scholar]
- 15.Ludwig-Müller J, Bennett RR, Garcia-Garrido JM, Piché Y, Vierheilig H. Reduced arbuscular mycorrhizal root colonization in Tropaeolum majus and Carica papaya after jasmonic acid application cannot be attributed to increased glucosinolate. J Plant Physiol. 2002;159:517–23. doi: 10.1078/0176-1617-00731. [DOI] [Google Scholar]
- 16.Li Ch, Liu G, Xu Ch, Lee GI, Bauer P, Ling H-Q, et al. The tomato suppressor of prosystemin-mediated responses2 gene encodes a fatty acid desaturase required for the biosynthesis of jasmonic acid and the production of a systemic wound signal for defense gene expression. Plant Cell. 2003;15:1646–61. doi: 10.1105/tpc.012237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tejeda-Sartorius M, Martínez de la Vega O, Délano-Frier JP. Jasmonic acid influences mycorrhizal colonization in tomato plants by modifying the expression of genes involved in carbohydrate partitioning. Physiol Plant. 2008;133:339–53. doi: 10.1111/j.1399-3054.2008.01081.x. [DOI] [PubMed] [Google Scholar]
- 18.León-Morcillo RJ, Angel J, Martín-Rodríguez JA, Vierheilig H, Ocampo JA, García-Garrido JM. Late activation of the 9-oxylipin pathway during arbuscular mycorrhiza formation in tomato and its regulation by jasmonate signalling. J Exp Bot. 2012;63:3545–58. doi: 10.1093/jxb/ers010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feys BJF, Benedetti CE, Penfold CN, Turner JG. Arabidopsis mutants selected for resistance to the phytotoxin coronatine are male-sterile, insensitive to methyl jasmonate, and resistant to a bacterial pathogen. Plant Cell. 1994;6:751–9. doi: 10.1105/tpc.6.5.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herrera-Medina MJ, Tamayo M, Vierheilig H, Ocampo JA, García-Garrido JM. The jasmonic acid signalling pathway restricts the develpment and functionality in the tomato arbuscular mycorrhiza. J Plant Growth Regul. 2008;175:554–64. [Google Scholar]
- 21.Wasternack C. Jasmonates: an update on biosynthesis, signal transduction and action in plant stress response, growth and development. Ann Bot. 2007;100:681–97. doi: 10.1093/aob/mcm079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mosblech A, Feussner I, Heilmann I. Oxylipins: structurally diverse metabolites from fatty acid oxidation. Plant Physiol Biochem. 2009;47:511–7. doi: 10.1016/j.plaphy.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 23.Howe GA, Schilmiller AL. Oxylipin metabolism in response to stress. Curr Opin Plant Biol. 2002;5:230–6. doi: 10.1016/S1369-5266(02)00250-9. [DOI] [PubMed] [Google Scholar]
- 24.Siedow JN. Plant lipoxygenase: structure and function. Annu Rev Plant Physiol Plant Mol Biol. 1991;42:145–88. doi: 10.1146/annurev.pp.42.060191.001045. [DOI] [Google Scholar]
- 25.Blée E. Phytooxylipins and plant defense reactions. Prog Lipid Res. 1998;37:33–72. doi: 10.1016/S0163-7827(98)00004-6. [DOI] [PubMed] [Google Scholar]
- 26.Feussner I, Kühn H, Wasternack C. Lipoxygenase-dependent degradation of storage lipids. Trends Plant Sci. 2001;6:268–73. doi: 10.1016/S1360-1385(01)01950-1. [DOI] [PubMed] [Google Scholar]
- 27.Blée E. Impact of phyto-oxylipins in plant defense. Trends Plant Sci 2002; 7: 315:322. [DOI] [PubMed]
- 28.Vellosillo T, Martínez M, López MA, Vicente J, Cascón T, Dolan L, et al. Oxylipins produced by the 9-lipoxygenase pathway in Arabidopsis regulate lateral root development and defense responses through a specific signaling cascade. Plant Cell. 2007;19:831–46. doi: 10.1105/tpc.106.046052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Creelman RA, Mullet JE. Biosynthesis and action of jasmonates in plants. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:355–81. doi: 10.1146/annurev.arplant.48.1.355. [DOI] [PubMed] [Google Scholar]
- 30.Stintzi A, Browse J. The Arabidopsis male-sterile mutant, opr3, lacks the 12-oxophytodienoic acid reductase required for jasmonate synthesis. Proc Natl Acad Sci U S A. 2000;97:10625–30. doi: 10.1073/pnas.190264497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kolomiets MV, Hannapel DJ, Chen H, Tymeson M, Gladon RJ. Lipoxygenase is involved in the control of potato tuber development. Plant Cell. 2001;13:613–26. doi: 10.1105/tpc.13.3.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Creelman RA, Mullet JE. Jasmonic acid distribution and action in plants: regulation during development and response to biotic and abiotic stress. Proc Natl Acad Sci U S A. 1995;92:4114–9. doi: 10.1073/pnas.92.10.4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vijayan P, Shockey J, Lévesque CA, Cook RJ, Browse J. A role for jasmonate in pathogen defense of Arabidopsis. Proc Natl Acad Sci U S A. 1998;95:7209–14. doi: 10.1073/pnas.95.12.7209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Farmer EE, Alméras E, Krishnamurthy V. Jasmonates and related oxylipins in plant responses to pathogenesis and herbivory. Curr Opin Plant Biol. 2003;6:372–8. doi: 10.1016/S1369-5266(03)00045-1. [DOI] [PubMed] [Google Scholar]
- 35.Weber H, Chételat A, Caldelari D, Farmer EE. Divinyl ether fatty acid synthesis in late blight-diseased potato leaves. Plant Cell. 1999;11:485–94. doi: 10.1105/tpc.11.3.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prost I, Dhondt S, Rothe G, Vicente J, Rodriguez MJ, Kift N, et al. Evaluation of the antimicrobial activities of plant oxylipins supports their involvement in defense against pathogens. Plant Physiol. 2005;139:1902–13. doi: 10.1104/pp.105.066274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De León IP, Sanz A, Hamberg M, Castresana C. Involvement of the Arabidopsis α-DOX1 fatty acid dioxygenase in protection against oxidative stress and cell death. Plant J. 2002;29:61–2. doi: 10.1046/j.1365-313x.2002.01195.x. [DOI] [PubMed] [Google Scholar]
- 38.Hause B, Maier W, Miersch O, Kramell R, Strack D. Induction of jasmonate biosynthesis in arbuscular mycorrhizal barley roots. Plant Physiol. 2002;130:1213–20. doi: 10.1104/pp.006007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Isayenkov S, Mrosk C, Stenzel I, Strack D, Hause B. Suppression of allene oxide cyclase in hairy roots of Medicago truncatula reduces jasmonate levels and the degree of mycorrhization with Glomus intraradices. Plant Physiol. 2005;139:1401–10. doi: 10.1104/pp.105.069054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maucher H, Hause B, Feussner I, Ziegler J, Wasternack C. Allene oxide synthases of barley (Hordeum vulgare cv. Salome): tissue specific regulation in seedling development. Plant J. 2000;21:199–213. doi: 10.1046/j.1365-313x.2000.00669.x. [DOI] [PubMed] [Google Scholar]
- 41.Hause B, Stenzel I, Miersch O, Maucher H, Kramell R, Ziegler J, et al. Tissue-specific oxylipin signature of tomato flowers: allene oxide cyclase is highly expressed in distinct flower organs and vascular bundles. Plant J. 2000;24:113–26. doi: 10.1046/j.1365-313x.2000.00861.x. [DOI] [PubMed] [Google Scholar]
- 42.Roitsch T. Source-sink regulation by sugar and stress. Curr Opin Plant Biol. 1999;2:198–206. doi: 10.1016/S1369-5266(99)80036-3. [DOI] [PubMed] [Google Scholar]
- 43.Chen H, Jones AD, Howe GA. Constitutive activation of the jasmonate signaling pathway enhances the production of secondary metabolites in tomato. FEBS Lett. 2006;580:2540–6. doi: 10.1016/j.febslet.2006.03.070. [DOI] [PubMed] [Google Scholar]
- 44.De Geyter N, Gholami A, Goormachtig S, Goossens A. Transcriptional machineries in jasmonate-elicited plant secondary metabolism. Trends Plant Sci. 2012;17:349–59. doi: 10.1016/j.tplants.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 45.Strack D, Fester T, Hause B, Schliemann W, Walter MH. Arbuscular mycorrhiza: biological, chemical, and molecular aspects. J Chem Ecol. 2003;29:1955–79. doi: 10.1023/A:1025695032113. [DOI] [PubMed] [Google Scholar]
- 46.Gundlach H, Müller MJ, Kutchan TM, Zenk MH. Jasmonic acid is a signal transducer in elicitor-induced plant cell cultures. Proc Natl Acad Sci U S A. 1992;89:2389–93. doi: 10.1073/pnas.89.6.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thoma I, Loeffler C, Sinha AK, Gupta M, Krischke M, Steffan B, et al. Cyclopentenone isoprostanes induced by reactive oxygen species trigger defense gene activation and phytoalexin accumulation in plants. Plant J. 2003;34:363–75. doi: 10.1046/j.1365-313X.2003.01730.x. [DOI] [PubMed] [Google Scholar]
- 48.Li L, Zhao Y, McCaig BC, Wingerd BA, Wang J, Whalon ME, et al. The tomato homolog of CORONATINE-INSENSITIVE1 is required for the maternal control of seed maturation, jasmonate-signaled defense responses, and glandular trichome development. Plant Cell. 2004;16:126–43. doi: 10.1105/tpc.017954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Garrido JM, Morcillo RJ, Rodríguez JA, Bote JA. Variations in the mycorrhization characteristics in roots of wild-type and ABA-deficient tomato are accompanied by specific transcriptomic alterations. Mol Plant Microbe Interact. 2010;23:651–64. doi: 10.1094/MPMI-23-5-0651. [DOI] [PubMed] [Google Scholar]
- 50.López-Ráez JA, Verhage A, Fernández I, García JM, Azcón-Aguilar C, Flors V, et al. Hormonal and transcriptional profiles highlight common and differential host responses to arbuscular mycorrhizal fungi and the regulation of the oxylipin pathway. J Exp Bot. 2010;61:2589–601. doi: 10.1093/jxb/erq089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stumpe M, Carsjens JG, Stenzel I, Göbel C, Lang I, Pawlowski K, et al. Lipid metabolism in arbuscular mycorrhizal roots of Medicago truncatula. Phytochemistry. 2005;66:781–91. doi: 10.1016/j.phytochem.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 52.Gao XQ, Starr J, Göbel C, Engelberth J, Feussner I, Tumlinson J, et al. Maize 9-lipoxygenase ZmLOX3 controls development, root-specific expression of defense genes, and resistance to root-knot nematodes. Mol Plant Microbe Interact. 2008;21:98–109. doi: 10.1094/MPMI-21-1-0098. [DOI] [PubMed] [Google Scholar]
- 53.Taki N, Sasaki-Sekimoto Y, Obayashi T, Kikuta A, Kobayashi K, Ainai T, et al. 12-oxo-phytodienoic acid triggers expression of a distinct set of genes and plays a role in wound-induced gene expression in Arabidopsis. Plant Physiol. 2005;139:1268–83. doi: 10.1104/pp.105.067058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Itoh A, Schilmiller AL, McCaig BC, Howe GA. Identification of a jasmonate-regulated allene oxide synthase that metabolizes 9-hydroperoxides of linoleic and linolenic acids. J Biol Chem. 2002;277:46051–8. doi: 10.1074/jbc.M207234200. [DOI] [PubMed] [Google Scholar]
- 55.Fiorilli V, Catoni M, Miozzi L, Novero M, Accotto GP, Lanfranco L. Global and cell-type gene expression profiles in tomato plants colonized by an arbuscular mycorrhizal fungus. New Phytol. 2009;184:975–87. doi: 10.1111/j.1469-8137.2009.03031.x. [DOI] [PubMed] [Google Scholar]
- 56.Vierheilig H, Steinkellner S, Khaosaad T, Garcia-Garrido JM. The biocontrol effect of mycorrhization on soil-borne fungal pathogens and the autoregulation of the AM symbiosis: one mechanism, two effects? Varma A (Ed) MYCORRHIZA: Genetics and Molecular Biology, Eco-Function, Biotechnology, Eco-Physiology, Structure and Systematic (third edition) Springer-Verlag, Berlin Heidelberg, Germany. 2008; 307-320. [Google Scholar]
- 57.Fammartino A, Cardinale F, Göbel C, Mène-Saffrané L, Fournier J, Feussner I, et al. Characterization of a divinyl ether biosynthetic pathway specifically associated with pathogenesis in tobacco. Plant Physiol. 2007;143:378–88. doi: 10.1104/pp.106.087304. [DOI] [PMC free article] [PubMed] [Google Scholar]