Abstract
Tissue specificity or dramatically different expression levels of transcription factors in different tissue types allows differential regulation of tissue development as well as alternate modes of metabolic regulation. Recently we reported (Rohrmann et al., 2011) the development of a quantitative real-time PCR platform (qRT-PCR) that allows accurate quantification of the expression level of approximately 1000 tomato transcription factors. Application of this platform to samples collected during a ripening time course of wild type tomato and the high pigment mutant hp1 allowed us to identify transcription factors of importance both to ripening per se and to the metabolic shifts that occur during this critical biological process. Here we extend the quantitative real-time PCR analyses to include samples from flower, leaf, stem and root of wild type tomato. Co-expression network analysis to identify both conserved and unique regulatory networks both between individual tissues of tomato and also in cross-species comparisons of specific tissues, suggested some key TF genes which are involved in photosynthesis and fruit development.
Keywords: Solanum lycopersicum, qRT-PCR, tissue specificity, transcription factor, transcriptional regulation
Introduction
Transcription factors (TFs) are master-control proteins in all living cells often exhibiting sequence specific DNA binding. In addition, TFs are being capable of activating or repressing transcription of a broad range of target genes.1 Since the regulation of gene expression is a major control point in many biological processes, it is not surprising, that approximately 7% of genes are TFs.2 That said the aforementioned target genes are often regulated by multiple TFs or even cascades thereof (see example in WD40-bHLH-MYB complex3,4), rendering it important not to study their function in isolation from one another. By means of the interaction patterns of specific TFs this class of protein are able to influence or even control many biological processes including cell cycle progression, metabolism, development and response to the environment.5-12 This regulation occurs both at a general cellular level and in a cell-specific manner. Much of the regulation of TF activity is in itself regulated at the level of transcription,13 The knowledge concerning the location and relative expression of TFs is proving to be highly valuable in elucidating how these proteins interact and ultimately in defining their biological functions. In addition, transcriptional regulation can determine a number of agronomical important traits; therefore, studies of TFs form a major focus in plant biology.2,14
Several different experimental approaches can be used to access the expression of transcription factors. These are either broad-scope or even unbiased such as microarray and RNaseq platforms15 or specifically targeted to TFs such as real-time RT-PCR platform.1,16,17 This addendum describes the comparison of TF networks across different tissues of tomato (Solanum lycopersicum) and between the leaf of tomato and other plant species including Arabidopsis thaliana,1,18 Oryza sativa16,19 and Medicago truncatula.17
For this purpose qRT-PCR analysis was performed according to Rohrmann et al.20 using template cDNA from leaf, stem, flower, root and the 9 different fruit developmental and ripening stages reported previously.20 In this study, it revealed expression of 1057 out of the 1087 tested primer pairs in at least one plant tissue tested. The TFs were regarded as expressed, when they displayed expression in at least two of the three biological replicates. In non-fruit tissue 1051 putative TFs displayed expression (leaf, 968; stem, 989; flower, 1009; root, 942) whereas in fruits 1016 putative TFs were detected (17DAP, 935; 27DAP, 952; MG, 955; B-1, 891; B, 917; B+1, 913; B+5, 816; B+10, 813; B+15, 810). When matched to the latest public available ITAG release 2.3 724 putative TFs for S. lycopersicum were matching to 703 predicted tomato unigenes (Table 1).
Table 1. Expressed ESTs and matching ITAG sequences in tomato.
| Putative TFs EST | ITAG2.3 proteins | |
|---|---|---|
|
Flower
|
1009
|
859
|
|
Leaf
|
968
|
835
|
|
Stem
|
989
|
849
|
|
Root
|
942
|
817
|
|
17DAP
|
935
|
803
|
|
27DAP
|
952
|
811
|
|
MG
|
955
|
818
|
|
Breaker-1
|
891
|
757
|
|
Breaker
|
917
|
784
|
|
Breaker+1
|
913
|
783
|
|
Breaker+5
|
816
|
709
|
|
Breaker+10
|
813
|
704
|
|
Breaker+15
|
810
|
706
|
| total | 1057 | 891 |
EST of TFs of S. lycopersicum were retrieved from the TIGR Transcript Assemblies (53,791 TAs) based on ~250,000 tomato ESTs (release version 5, http://plantta.jcvi.org/cgi-bin/plantta_release.pl) and PlnTFDB database (http://plntfdb.bio.uni-potsdam.de/). Matching ITAG was obtained from SOL Genome Network (http://solgenomics.net) basing on the sequence in PlnTFDB database.
Results
Quantitative gene expression analysis of tomato tissues
Gene sequence encoding transcription factors (TFs) of Solanum lycopersicum were retrieved from the TIGR Transcript Assemblies (53,791 TAs) based on ~250,000 tomato ESTs (release version 5, http://plantta.jcvi.org/cgi-bin/plantta_release.pl) as described by Rohrmann et al.20 The PlnTFDB database (http://plntfdb.bio.uni-potsdam.de/21), was used for identification of TF sequences based on classification rules produced 1090 putative TFs covering 60 TF-families. Design of primer pair sequences were designed tomato EST database.22 The homolog genes in Arabidopsis thaliana were identified by BLAST result in SOL Genome Network (see Supplemental Table S1 of Rohrmann et al.20). To comparison of TF expression in tomato tissues, as an initial experiment samples from different tomato tissues such as complete leaf, stem, flower and were investigated. In non-fruit tissue 1,051 putative TFs were expressed (Flower: 1,009 TFs; root: 942 TFs; leaf: 968 TFs; stem: 989 TFs) whereas in fruits 1,016 putative TFs were detected (17DAP: 935 TFs; 27DAP: 952 TFs; MG: 955 TFs; B-1: 891 TFs; B: 917 TFs; B+1: 913 TFs; B+5: 816 TFs; B+10: 813 TFs; B+15: 810 TFs) (Table 1).
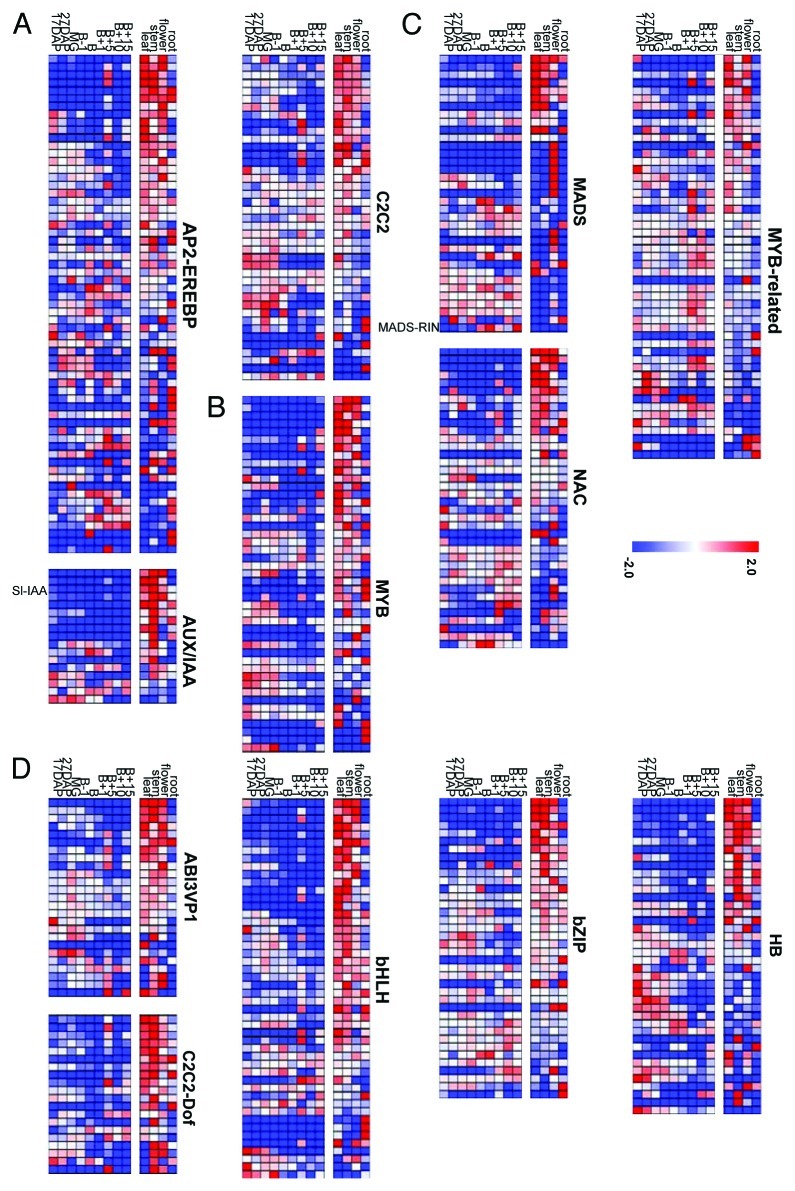
The Gene expression of TFs visualized in heat map (Fig. 1) facilitated classification of seven TF families as early response in fruit development stage (auxin-responsive protein / indoleacetic acid-induced, AP2-EREBP; AUXin/Indole-3-Acetic-Acid response, AUX/IAA; C2C2, Figure 1A), changed between early stage and B+15(tm) (MYB, Figure 1B) and late response in fruit maturing stage (tm) (MADS, NAC, MYB-related, Figure 1C) described by Rohrmann et al. (2011), and five TF families (ABscisic-acid-Insensitive3-Viviparous1, ABI3-VP1; C2C2 dof-type zinc finger, C2C2-Dof; basic-Helix-Loop-Helix, bHLH; basic-region/leucine-ZIPper-motif, bZIP; Homeo-Box, HB) which were found as major TF families in which mostly of genes were highly expressed in photosynthetic tissues (Fig. 1D and Supplemental Table S1). The heat map revealed that mostly of TF genes expressing in photosynthetic tissues such as leaf, stem and flower, were not significantly expressed in fruit ripening stages. On the other hand, the TFs involving fruit developing stages were not observed high expressions in photosynthetic tissue. Such significant difference of tissue specificities between non-fruit tissues and fruit samples was observed in AP2-EREBP, AUX/IAA, MADS, bHLH and bZIP families. Tissue specific TF expression such as flower specific MADS genes (four MADS genes, Solyc05g051830, J0636; Solyc06g059970, J0659; Solyc11g005120, J0682; Solyc08g067230, J0673) and root specific genes (AP2-EREBP gene, Solyc05g050830, J0093; two MYB genes, Solyc04g077260, J0694; Solyc06g074910, J0728; three bHLH genes, Solyc07g052670, J0187; Solyc06g051550, J0204; Solyc05g009640, J0218), were also observed. To compare the TF expression profiles of different tissues and different developmental fruit stages, principal component analysis (PCA) was performed (Fig. 2). The PCA scores plot revealed that the TF expression profiles separated between samples of green tomato fruits (17DAP, 27DAP and MG), breaker stage fruits (B-1, Breaker and B+1), ripe red fruits (B+1, B+10 and B+15) and other non-fruit tissues (leaf, stem, flower and root). Furthermore, clusters of expression profiles in non-fruit tissues clearly were separated from fruit samples. Among the TF families displaying the strongest differences between fruit and non-fruit tissues are AP2-EREBP, AUX/IAA, bHLH, bZIP, HB, HSF, MADS, NAC and WRKY families. While most of the TF-families display a heterogeneous profile for the single TF, some of the TF-families behave homogeneously. For example, TF genes in the families such as AUX/IAA, bHLH and MYB which are particularly expressed in non-fruit tissues, are very lowly expressed in the tested fruit tissues (Fig.A 1). On the other hand genes in AP2-EREBP, MADS and NAC- factors display both kinds of behavior when comparing the expression in fruit and non-fruit tissue. The expression of the AUX/IAA gene named Sl_IAA9 (Solyc01g097290, J0141), which have been characterized to play an important role in fruit development23 particularly expressed in before breaker stages in fruits and photosynthetic tissues (Fig.A 1). The ortholog gene of Solyc10g006880(J0824) in Arabidopsis thaliana named AtNAC056 (At3g15510) have been described as developmentally relevant gene in tomato as well20,24 was not observed expression in non-fruit tissues. The MADS-RIN (ripening inhibition factor, Solyc05g012020, J0650) which is also known to play an important role in fruit ripening,25 was not expressed in any non-fruit tissues. The heat map data for AUX/IAA genes revealed that beside the high expression of these genes in non-fruit tissue, most of the factors were more lowly expressed in fruit samples.
Figure 1. Transcription Factors showing a significant difference between fruit and non-fruit tissue. A) early responding TFs, (B) early responding from early stages until the B+15 stage TFs and (C) late responding TFs during fruit ripening (Rohrmann et al., 2011), and (D) TF families expressing in non-fruit tissues. In total 12 TF gene families showed a significant difference in their expression in fruit and non-fruit tissue. Relative TF contents of fruits harvested from 17DAP until B+15 as well as four non-fruit tissues (leaf, stem, flower and root). TF contents are shown as I"I"CT relative to average expression in all studied tissues = 0. Value shows the median of three biological replicates. Changes are shown from -2CT (blue) up to 2CT (red) values. Value shows median of three biological replicates. B, breaker stage; B+5, 5d after breaker; B+10, 10d after breaker; B+15, 15d after breaker; DAP, days after pollination; MG, mature green.
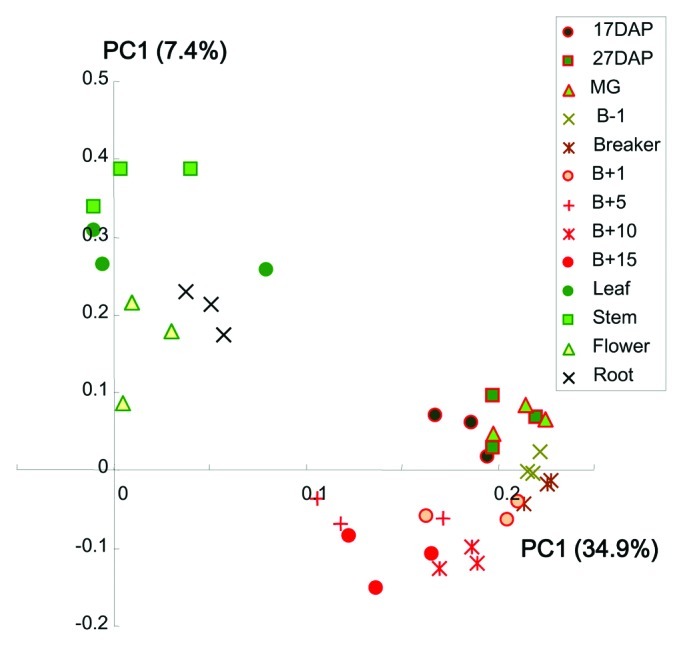
Figure 2. Principal component analysis of qRT-PCR expression profiles between different tomato tissues and fruit developmental stages.PCA score scatter plot of qRT-PCR expression profiles of 13 different tissues and fruits stages with three biological replicates. B, breaker stage; B+5, 5d after breaker; B+10, 10d after breaker; B+15, 15d after breaker; DAP, days after pollination; MG, mature green.
TF genes highly expressed in photosynthetic tissues
To identify TFs expressing in photosynthetic tissues, expression behavior of TFs were represented as Venn diagrams in FigureA 3A. The tissue comparison of TF expressions between leaf, stem, flower and root, resulted the Venn diagrams denoted the TFs expressing in photosynthetic tissues (common in leaf, stem and flower; 178 genes), and those that were leaf- (45 genes), stem- (42 genes), flower- (54 genes) or root-specific (45 genes). In spite of the fact that AP2-EREBP, MADS, NAC and MYB-related genes are major TF families expressing during fruit development and/or ripening stages, bHLH, ABI3-VP1, AP2-EREBP and C2H2-Dof genes were also observed higher expression of TF gene families within photosynthetic tissues (Fig. 3B). Of these putative photosynthesis related genes (178 genes), ortholog genes of Arabidopsis light related TFs such as AB11 (At5g67030, ortholog genes of Solyc02g090890 in tomato), IAA26 (At3g16500, Solyc09g020000) and PAT (At5g48150, Solyc07g063940), shoot meristem related TFs BUM1 (At1g62360, Solyc02g081120) and wax related SHINE2 (At5g25390, Solyc06g053230 and Solyc06g053240) were identified (Supplemental Table S1). On the other hand, TF genes which are significantly expressed in fruits stages, are known TFs which play a important role in tomato fruit development, such as, TAGL1 (Solyc07g055920), FAS (Solyc11g071810) and RIN (Solyc05g01202), and the ortholog gene of Arabidopsis TFs involving cell cycle (AtCDC5, At1g09770) and ethylene related (ETR2, At3g23150; EIN4, At3g04580) genes.
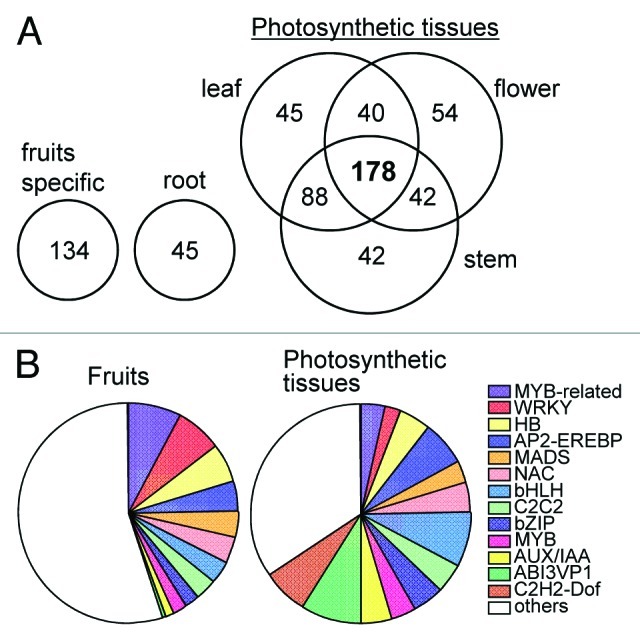
Figure 3. Venn Diagram to show high expressed TFs for each of the four different tissue types. (A) Venn Diagram of TFs which are expressed remarkably in each tissues. (B) Classification of fruit and photosynthetic specific TF genes.
Gene expression of photosynthesis related TFs during fruit ripening and hp-1 mutant
In order to understand TF-TF networks in fruit photosynthesis were evaluated across wild type (AC) and mutant ripening using the hp1 mutant. The hp1 mutant is a photomorphogenic mutant which is characterized by an increased light response26 illustrated by its elevated accumulation of fruit carotenoids, flavonoids27,28 and ripening metabolites.20,29 The hp1 gene carries a mutation in the tomato homologs of the Arabidopsis gene encoding UV-damaged-DNA-binding-protein-1 (DDB1)30,31 which interacts with De-Etiolated-1 (DET1, hp2) to regulate the activity of photomorphogenesis related genes.32 Using K-means clustering analysis, the gene expression profile of photosynthesis related genes during fruit ripening was analyzed in FigureA 4. In total, eight clusters were created by Pearson correlation following a crude rule of thumb(tm) as described by Rohrmann et al.20 In the clusters (a, b, c and d), TF genes which showed lower expression in hp-1 mutant at fruit ripening stages were clustered, including nine AP2-EREBP (Solyc03g120840, Solyc04 g014530, Solyc08g078410, Solyc08g078420, Solyc09g007260, Solyc06 g053230, Solyc06g053240, Solyc06g068570, Solyc03g117130) and seven bHLH TFs (Solyc03g034000, Solyc03g114720, Solyc05g006650, Solyc10g009290, Solyc12g100140 Solyc03g118310, Solyc05g050560). Although the expression pattern of TF genes in the clusters e and f showed slightly increased during fruit development and ripening in AC, these TF genes were expressed lower in fruit developmental stages and higher expressed in fruit ripening stages in hp-1 mutant. The GRAS gene (Solyc07g065270) which is ortholog gene of Arabidopsis thaliana, phytochrome A signal transduction 1 (AtPAT1, At5g48150), was clustered to cluster e.
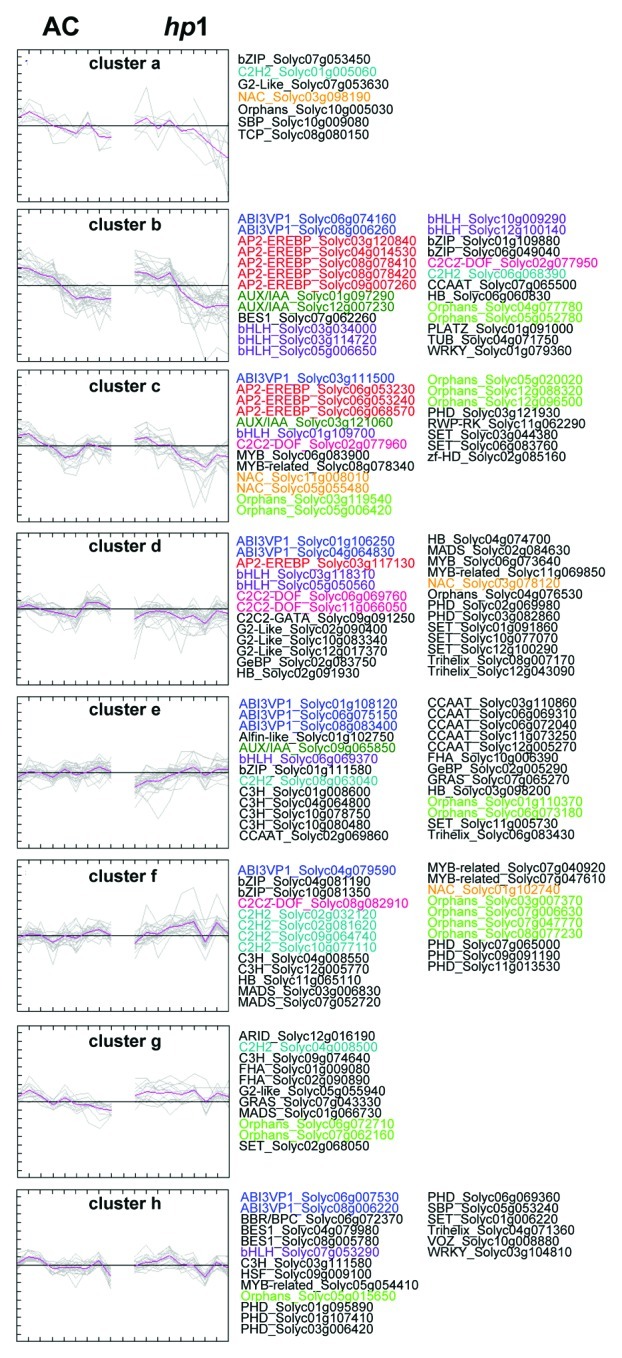
Figure 4.K-means clustering of gene expression of photosynthetic related TFs in AC and hp-1 mutant. Putative photosynthesis related TFs were classifies to eight clusters by gene expression profiles of AC and hp-1 mutant during fruit ripening.20 Colors of text indicates gene family of TF. k-means clustering was performed by MeV software (http://www.tm4.org/mev/).
Tomato-Arabidopsis cross species co-expression analysis
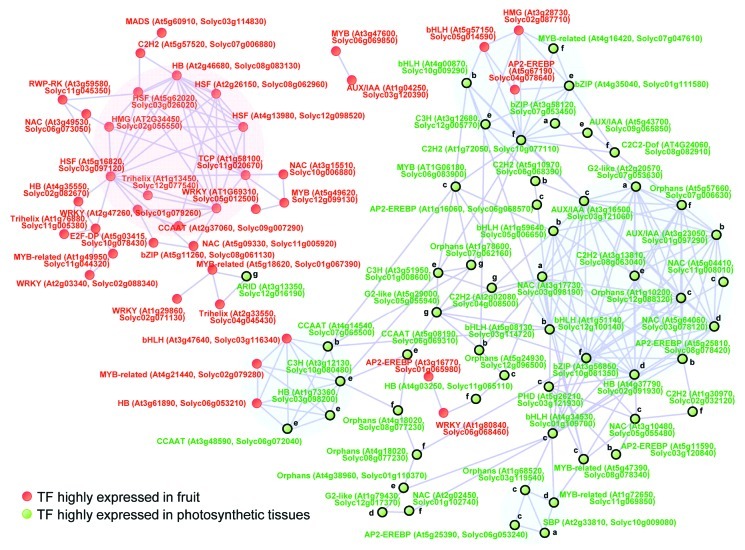
The depiction of co-expression gene network analysis is better than standard single gene analyses, because it can easily give the means to the understanding of gene functions of interest by biologists.33,34 However since the gene expression level of TFs is generally lower detection intensity in comparison to other genes, gene expression profile with highly sensitivity and quantitatively such as qRT-PCR analysis is a very powerful tool for studying transcriptional TF networks. In addition, given that the knowledge and characterization of tomato TF functions are correctly in sufficient for constructing a global framework with functional connections, we next conducted network interactions based on TF expression with cross species comparison of tomato and Arabidopsis thaliana. The TFs of photosynthetic related (178 TFs) and fruit specific (134 TFs) genes described in FigureA 3A, were used for co-expression network analysis with the connection between ortholog genes of tomato and Arabidopsis thaliana (http://solgenomics.net/). The qRT-PCR data of 101 TFs overlapping the Arabidopsis qRT-PCR platform1,18 were combined with our qRT-PCR expression data of tomato into a single matrix for Pearson coefficient analysis (Fig.A 5). The correlation network analysis revealed two sub-cluster of photosynthesis related genes and one sub-cluster of fruit specific genes and two common subclusters. Interestingly, four photosynthesis related genes (CCAAT, At4g14540, Solyc07g065500; C3H, At3g12130, Solyc10g080480; HB, At2g35940, Solyc01g007070; CCAAT, At3g48590, Solyc06g072040) and three fruit TFs (bHLH, At3g47640, Solyc03g116340; MYB-related, At4g21440, Solyc02g079280; HB, At3g61890, Solyc06g053210) were correlated in the sub-cluster (Fig.A 5). In these four photosynthesis related genes, three TFs were classified to cluster e in Figure 4. In addition, five photosynthesis related genes (MYB-related, At4g16420, Solyc07g047610; bZIP, At4g35040, Solyc01g111580; bZIP, At3g58120, Solyc07g053450; C2H2, At1g72050, Solyc10g077110; C3H, At3g12680, Solyc12g005770; bHLH, At4g00870, Solyc10g009290) and three fruit TFs (bHLH, At5g57150, Solyc05g14590; AP2-EREBP, At5g67190, Solyc04g078640; HMG, At3g28730, Solyc02g087710) were correlated in the sub-cluster. These networks revealed that some of cell growth related genes such as nuclear factor Y subunit B3 (At4g14540), nuclear factor Y subunit C1 (At3g48590) and nucleosome/chromatin assembly factor D (At3g28730) showing slightly increased during fruit development and ripening in AC, were correlated with fruits related genes in tomato. The network analysis results reported here are intriguing in that they provide transcription factor functions that putatively explain regulatory connections between photosynthetic tissues and non-photosynthetic tissues to understanding fruit development and maturing. However, it is clear that experimental confirmation of these putative functions is required.
Figure 5. Network interaction of TF expressions with cross species comparison of tomato and Arabidopsis. Gene expression profile of tomato ripening stages,20 tomato tissues (in this study), Arabidopsis18 were combined based on the best BLAST hit gene (SOL Genome Network, SGN, http://solgenomics.net/). In total 91 TF genes were found in the correlation network with coefficient value (r > 0.75). Correlation network was determined using Pearson's correlation. Nodes; green indicates photosynthesis related TFs, red indicates TF genes highly expressed in fruit developmental stages. a)-h) indicates classification by K-mean clustering described in Figure 4.
Discussion
Since a tomato TF profiling platform based on approximately 1000 putative TFs capable of both sensitive and accurate quantification using qRT-PCR platform have been described (Rohrmann et al., 2011), we applied TF expression profile of tomato developing fruits and several tissues to this platform. We compared TF expression levels in tomato tissues to discuss about tissue specificities and putative photosynthesis related TF genes using homology-based inference of experimentally characterized TFs from Arabidopsis. Given putative photosynthesis related TF genes were used in comparison to TF expression profile during fruit ripening to gain insights into TF transcriptional control during tomato fruit development. In comparison to TF expression of tissue specific genes, such as flower specific MADS (Solyc05g051830, Solyc06g059970, Solyc11 g005120, Solyc08g067230) and root specific genes (Solyc05g050830, Solyc04g077260, Solyc06g074910, Solyc07g052670, Solyc06g051550, Solyc05g009640), understating of tissue specificity of TF expression profile elucidates 178 TFs which are highly expressed to photosynthetic tissues. In addition, Venn Diagram and the k-mean clustering revealed putative TFs which may play important roles in photosynthesis during fruits development such as AB11(Solyc02g090890), IAA26 (Solyc09g020000) and PAT(Solyc07g063940). Furthermore, nine AP2-EREBP (Solyc03g120840, Solyc04g014530, Solyc08g078410, Solyc08g078420, Solyc09g007260, Solyc06g053230, Solyc06g053240, Solyc06g068570, Solyc03g117130) know as ethylene-responsive element binding proteins, showed lower gene expression in hp-1 mutant at fruit ripening stages. This result suggested that these photosynthesis related AP2-EREBP genes were suppressed in the high pigmented fruits mutant. The across species TF network analysis of putative photosynthesis related TFs in this article results that tissue specific TF-TF network is conserved connection of coexpression network. In thee gene expression network, two sub-cluster showed clear connection between photosynthesis related genes (Solyc07g065500, Solyc10g080480, Solyc01g007070, Solyc06g072040 Solyc07g04761, Solyc01g111580, Solyc07g053450, Solyc10g077110, Solyc12g005770, Solyc10g009290) and fruit specific genes (Solyc03g116340, Solyc02g07928, Solyc06g053210, Solyc05g14590, Solyc04g078640, Solyc02g087710) including TF genes which are putatively involved in cell growth related genes such as nuclear factor subunits and nucleosome/chromatin assembly factor. This may provide transcription factor functions that putatively explain regulatory connections between well documented physiological and metabolic aspects of tissue development and fruit ripening.
Materials and Methods
Plant materials
The Solanum lycopersicum L. cv Ailsa Craig obtained from the Boyce Thompson Institute for Plant Research (New York, USA), was cultivated in the green house under 16h light/8h dark condition as described in Alba et al.35 Each tomato tissues were harvested and frozen in liquid N2, and stored at -80°C.
RNA extraction, DNA digestion and cDNA synthesis
Tomato samples from the four different tissues (leaf, stem, fower and root) were used for extraction of total RNA by phenol separation and LiCl precipitation as described by Rohrmann et al.20 Ground frozen material (500mg) were transferred into an microfuge tube containing 2ml extraction buffer (100mM TRIS-HCl pH 9.0, 200mM NaCl, 15mM EDTA, 0.5% (w/v) N-lauryl-sarcosine, 0.8% (v/v) 2-mercaptoethanol) After adding 2ml phenol, 0.4ml Chl:IAA (24:1 v/v) and 140I1/4l sodium acetate (3M), it was vortexed and stored on ice for 15 min. The samples were subsequently centrifuged for 10 min at 4000 g and the supernatant was transferred into a fresh microfuge tube. After adding 2ml Phenol:Chl:IAA (25:24:1 v/v/v), vortexing and centrifugation (4000 g, 10 min) the aqueous phase was transferred into a new tube. One volume isopropanol was added and the sample incubated at -80°C for 20 min. RNA was concentrated by centrifugation (4,000 g for 10 min), washed with 1 ml of 80% ethanol and centrifuged again and then air-dried for approximately 10 min. The pellet was suspended in one ml autoclaved MilliQ-water, transferred to a 2ml microfuge tube and precipitated with 500I1/4l LiCl (8M) over night at 4°C. The next day, the RNA was spin down, washed with one ml ethanol (80%) and suspended in 100I1/4l autoclaved MilliQ-water.
DNA digestion by DNase I was performed by mixing 30I1/4l RNA, 1I1/4l DNAase I and 3I1/4l DNase buffer. The digestion mix was incubated for 60 min at 37°C. The digestion was stopped by adding 3.5I1/4l inactivation buffer, mixing and incubating at room temperature for 2 min. To confirm the absence of genomic DNA contamination, a quantitative PCR analysis using three primer pairs was performed. The sequence of primer pairs were designed by amplification of intron sequences of three tomato genes;
tDET1 (AJ224356) forward 5′-CGAAGCAAGCGTGAACAAAT-3′
reverse 5′-TGCGGAGATT AGGATGGACA-3′
LEACO1 (X58273) forward 5′-TCTCTTCTTTTCGTCGCTCTTG-3′
reverse 5′-TTGCAACTTGGCAGTTGAATTA-3′
LEACO2 (Y00478) forward 5′- TGCGAACTTCATTCAACAGC-3′
reverse 5′-GC TCCACCATCGATCAAAAC-3′
The qRT-PCR reaction was performed using 0.5I1/4l RNA, 2.5I1/4l SYBR Green Master Mix and 2 I1/4l primer mix (forward and reverse, 0.5I1/4M each). The qRT-PCR reactions were performed following the recommended thermal profile: 50°C for 2 min, 95°C for 10 min, followed by 40 cycles of 95°C for 15 sec and 60°C for one minute. After 40 cycles, the specificity of the amplifications was tested by heating from 60°C to 95°C at an increment of 1.9°C per minute, resulting in melting curves. The absence of genomic DNA was ensured by the absence of amplification signal after 40 cycles.
The quality of RNA was checked on 1% w/v agarose gels. The RNA concentration was scored using a Nanodrop ND-1000 spectrophotometer (http://www.nanodrop.com/) before and after DNase I digestion. The cDNA synthesis was conducted starting from 2 I1/4g of total RNA using Superscript III reverse transcriptase (Invitrogen, http://www.invitrogen.com/), by mixing up to 12I1/4l RNA, 1I1/4l oligo(I"T)18 primer (0,5I1/4g/I1/4l), 1I1/4l dNTPs (10mM each) and MilliQ-water to 14I1/4l total volume. The mix was incubated at 65°C for 5 min and chilled on ice before 4I1/4l Superscript III buffer, 1I1/4l DTT (0.1M) and 1I1/4l Superscript III reverse transcriptase (200U) were added. The mix was incubated 50 min at 50°C followed by 15 min at 70°C. The cDNA synthesis efficiency was evaluated using two sets of primer pairs amplifying the 5′ and 3′ regions of glyceraldehyde 3-phosphate dehydrogenase (GAPDH, AB110609, forward primer 5′-GATATCCCATGGGGTGAAGC-3′/reverse primer 5′-CACAACCTTCTTGGCACCAC-3′ and forward primer 5′-GGCTGCAATCAAGGAGGAAT-3′/reverse primer 5′-CAGCCTTGGCATCAAAAATG-3′). Additionally the primer pairs for the three reference genes ubiquitin, cyclophilin and phosphoglycerate kinase were tested with the cDNA.
Design and validation of qRT-PCR primers
The primers were designed by the TIGR Transcript Assemblies (53791 TAs) based on ~250.000 ESTs using the QuantPrime software22 as described by Rohrmann et al.20 Gene sequence encoding TF of Solanum lycopersicum were retrieved from the TIGR Transcript Assemblies (53,791 TAs) based on ~250,000 tomato ESTs (release version 5, http://plantta.jcvi.org/cgi-bin/plantta_release.pl) as described by Rohrmann et al.20 The PlnTFDB database (http://plntfdb.bio.uni-potsdam.de/21), was used for identification of TF sequences based on classification rules produced 1090 putative TFs covering 60 TF-families. Design of primer pair sequences used the QuantPrime software.22 Gene identifications (IDs) were integrated via GenBank ID conversion with a gene table downloaded from tomato gene databases, National Center for Biotechnology Information (NCBI, http://www.ncbi.nlm.nih.gov/guide/), SOL Genome Network (SGN, http://solgenomics.net/) and Tomato Transcription Factor Database (TFDB, http://planttfdb.cbi.pku.edu.cn:9010/web/index.php?sp=le).
Quantitative real time polymerase chain reaction (qRT PCR) settings
PCR reactions were conducted in an ABI PRISM 7900 HT sequence detection system (Applied Biosystems). A 5 I1/4l reaction mix containing 0.5 I1/4l of cDNA (1.25 ng/I1/4l), 200 nM of each gene-specific primer and 2.5 I1/4l of SYBR Green master mix (Applied Biosystems Applera, Darmstadt, Germany), was used to monitor double-strand DNA synthesis. The qRT-PCR reactions were performed following the recommended thermal profile: 50°C for 2 min, 95°C for 10 min, followed by 40 cycles of 95°C for 15 sec and 60°C for 1 min. After 40 cycles, the specificity of the amplifications was tested by heating from 60°C to 95°C with a ramp speed of 1.9°C min-1, to produce in melting curves. The SDS 2.2.1 software (Applied Biosystems) was used for data analysis. All amplification curves were analyzed with a normalized reporter (Rn: the ratio of the fluorescence emission intensity of SYBR Green to the fluorescence signal of the passive reference dye) threshold of 0.2 to obtain the CT values (threshold cycle). The reference control genes (elongation-factor-α1, X14449; α-tubulin, TC115716; GAPDH, TC206735; actin, TC124219; ubiquitin, TC115896, phosphoglycerate kinase, TC116028; DNAJ-like protein, TC201130; cyclophilin, TC115937; Clathrin Adaptor Complex medium subunit, CAC, SGN-U314153; SAND family protein, SGN-U316474; expressed sequence, SGN-U346908; Ribosomal Protein L8, X64562) were measured in two replicates for each PCR run, and their average CT value was used for relative expression analyses. TF expression data were normalized by subtracting the mean reference gene CT value from their CT value (I"CT).
Correlation analysis
Pearson correlation was performed using the statistical software package R. TF expression data of leaf, stem, flower, root and 9 fruit developmental stages with 3 biological replicates were normalized by average of TF expressions and used for the heatmap and correlation analysis. The criteria for correlation candidates were set to a correlation value of 0.70. Correlation networks were described with Pajek software (http://vlado.fmf.uni-lj.si/pub/networks/pajek/) and presented as described in Tohge et al.36
Supplementary Material
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
Funding from the Max-Planck-Society (to JR, ARF and TT) is gratefully acknowledged. Research activity of TT is supported by the Alexander von Humboldt Foundation. We are most grateful to the BMBF for funding in the Genome Analysis of the Plant Biological System Initiative (GABI) and for funding to JJG from National Science Foundation Grants DBI-0820612 and DBI-0923312.
Supplementary Materials
Supplementary materials may be found at: www.landesbioscience.com/journals/psb/article/22264.
Reference
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/22264
References
- 1.Czechowski T, Bari RP, Stitt M, Scheible WR, Udvardi MK. Real-time RT-PCR profiling of over 1400 Arabidopsis transcription factors: unprecedented sensitivity reveals novel root- and shoot-specific genes. Plant J 2004; 38: 366-379; PMID: 15078338; 10.1111/j.1365-313X.2004.02051.x. [DOI] [PubMed]
- 2.Udvardi MK, Kakar K, Wandrey M, Montanari O, Murray J, Andriankaja A, et al. Legume transcription factors: global regulators of plant development and response to the environment. Plant Physiol. 2007;144:538–49. doi: 10.1104/pp.107.098061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li XX, Duan XP, Jiang HX, Sun YJ, Tang YP, Yuan Z, et al. Genome-wide analysis of basic/helix-loop-helix transcription factor family in rice and Arabidopsis. Plant Physiol. 2006;141:1167–84. doi: 10.1104/pp.106.080580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosinski JA, Atchley WR. Molecular evolution of the Myb family of transcription factors: evidence for polyphyletic origin. J Mol Evol. 1998;46:74–83. doi: 10.1007/PL00006285. [DOI] [PubMed] [Google Scholar]
- 5.Jin HL, Martin C. Multifunctionality and diversity within the plant MYB-gene family. Plant Mol Biol. 1999;41:577–85. doi: 10.1023/A:1006319732410. [DOI] [PubMed] [Google Scholar]
- 6.Stracke R, Werber M, Weisshaar B. The R2R3-MYB gene family in Arabidopsis thaliana. Curr Opin Plant Biol. 2001;4:447–56. doi: 10.1016/S1369-5266(00)00199-0. [DOI] [PubMed] [Google Scholar]
- 7.Ito M. Conservation and diversification of three-repeat Myb transcription factors in plants. J Plant Res. 2005;118:61–9. doi: 10.1007/s10265-005-0192-8. [DOI] [PubMed] [Google Scholar]
- 8.Zhong R, Ye ZH. Regulation of cell wall biosynthesis. Curr Opin Plant Biol. 2007;10:564–72. doi: 10.1016/j.pbi.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Rushton PJ, Somssich IE, Ringler P, Shen QJ. WRKY transcription factors. Trends Plant Sci. 2010;15:247–58. doi: 10.1016/j.tplants.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 10.Dubos C, Stracke R, Grotewold E, Weisshaar B, Martin C, Lepiniec L. MYB transcription factors in Arabidopsis. Trends Plant Sci. 2010;15:573–81. doi: 10.1016/j.tplants.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Antoni R, Rodriguez L, Gonzalez-Guzman M, Pizzio GA, Rodriguez PL. News on ABA transport, protein degradation, and ABFs/WRKYs in ABA signaling. Curr Opin Plant Biol. 2011;14:547–53. doi: 10.1016/j.pbi.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 12.Puranik S, Sahu PP, Srivastava PS, Prasad M. NAC proteins: regulation and role in stress tolerance. Trends Plant Sci. 2012;17:369–81. doi: 10.1016/j.tplants.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 13.Chen W, Provart NJ, Glazebrook J, Katagiri F, Chang HS, Eulgem T, et al. Expression profile matrix of Arabidopsis transcription factor genes suggests their putative functions in response to environmental stresses. Plant Cell. 2002;14:559–74. doi: 10.1105/tpc.010410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Richardt S, Lang D, Reski R, Frank W, Rensing SA. PlanTAPDB, a phylogeny-based resource of plant transcription-associated proteins. Plant Physiol. 2007;143:1452–66. doi: 10.1104/pp.107.095760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matas AJ, Yeats TH, Buda GJ, Zheng Y, Chatterjee S, Tohge T, et al. Tissue- and cell-type specific transcriptome profiling of expanding tomato fruit provides insights into metabolic and regulatory specialization and cuticle formation. Plant Cell. 2011;23:3893–910. doi: 10.1105/tpc.111.091173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caldana C, Scheible WR, Mueller-Roeber B, Ruzicic S. A quantitative RT-PCR platform for high-throughput expression profiling of 2500 rice transcription factors. Plant Methods. 2007;3:9. doi: 10.1186/1746-4811-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kakar K, Wandrey M, Czechowski T, Gaertner T, Scheible WR, Stitt M, et al. A community resource for high-throughput quantitative RT-PCR analysis of transcription factor gene expression in Medicago truncatula. Plant Methods. 2008;4:18. doi: 10.1186/1746-4811-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parlitz S, Kunze R, Mueller-Roeber B, Balazadeh S. Regulation of photosynthesis and transcription factor expression by leaf shading and re-illumination in Arabidopsis thaliana leaves. J Plant Physiol. 2011;168:1311–9. doi: 10.1016/j.jplph.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 19.Narsai R, Castleden I, Whelan J. Common and distinct organ and stress responsive transcriptomic patterns in Oryza sativa and Arabidopsis thaliana. BMC Plant Biol. 2010;10:262. doi: 10.1186/1471-2229-10-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rohrmann J, Tohge T, Alba R, Osorio S, Caldana C, McQuinn R, et al. Combined transcription factor profiling, microarray analysis and metabolite profiling reveals the transcriptional control of metabolic shifts occurring during tomato fruit development. Plant J. 2011;68:999–1013. doi: 10.1111/j.1365-313X.2011.04750.x. [DOI] [PubMed] [Google Scholar]
- 21.Pérez-Rodríguez P, Riaño-Pachón DM, Corrêa LG, Rensing SA, Kersten B, Mueller-Roeber B. PlnTFDB: updated content and new features of the plant transcription factor database. Nucleic Acids Res. 2010;38(Database issue):D822–7. doi: 10.1093/nar/gkp805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arvidsson S, Kwasniewski M, Riaño-Pachón DM, Mueller-Roeber B. QuantPrime - a flexible tool for reliable high-throughput primer design for quantitative PCR. BMC Bioinformatics. 2008;9:15. doi: 10.1186/1471-2105-9-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang H, Schauer N, Usadel B, Frasse P, Zouine M, Hernould M, et al. Regulatory features underlying pollination-dependent and -independent tomato fruit set revealed by transcript and primary metabolite profiling. Plant Cell. 2009;21:1428–52. doi: 10.1105/tpc.108.060830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kunieda T, Mitsuda N, Ohme-Takagi M, Takeda S, Aida M, Tasaka M, et al. NAC family proteins NARS1/NAC2 and NARS2/NAM in the outer integument regulate embryogenesis in Arabidopsis. Plant Cell. 2008;20:2631–42. doi: 10.1105/tpc.108.060160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vrebalov J, Ruezinsky D, Padmanabhan V, White R, Medrano D, Drake R, et al. A MADS-box gene necessary for fruit ripening at the tomato ripening-inhibitor (rin) locus. Science. 2002;296:343–6. doi: 10.1126/science.1068181. [DOI] [PubMed] [Google Scholar]
- 26.Bino RJ, Ric de Vos CH, Lieberman M, Hall RD, Bovy A, Jonker HH, et al. The light-hyperresponsive high pigment-2dg mutation of tomato: alterations in the fruit metabolome. New Phytol. 2005;166:427–38. doi: 10.1111/j.1469-8137.2005.01362.x. [DOI] [PubMed] [Google Scholar]
- 27.Cookson PJ, Kiano JW, Shipton CA, Fraser PD, Romer S, Schuch W, et al. Increases in cell elongation, plastid compartment size and phytoene synthase activity underlie the phenotype of the high pigment-1 mutant of tomato. Planta. 2003;217:896–903. doi: 10.1007/s00425-003-1065-9. [DOI] [PubMed] [Google Scholar]
- 28.Jarret RL, Sayama H, Tigchelaar EC. Pleiotropic Effects Associated with the Chlorophyll Intensifier Mutations High Pigment and Dark Green in Tomato. J Am Soc Hortic Sci. 1984;109:873–8. [Google Scholar]
- 29.Yen H, Shelton A, Howard L, Vrebalov J, Giovanonni JJ. The tomato high-pigment (hp) locus maps to chromosome 2 and influences plastome copy number and fruit quality. Theor Appl Genet. 1997;95:1069–79. doi: 10.1007/s001220050664. [DOI] [Google Scholar]
- 30.Liu Y, Roof S, Ye Z, Barry C, van Tuinen A, Vrebalov J, et al. Manipulation of light signal transduction as a means of modifying fruit nutritional quality in tomato. Proc Natl Acad Sci U S A. 2004; 101: 9897-9902; 15178762; 10.1073/pnas.0400935101. [DOI] [PMC free article] [PubMed]
- 31.Lieberman M, Segev O, Gilboa N, Lalazar A, Levin I. The tomato homolog of the gene encoding UV-damaged DNA binding protein 1 (DDB1) underlined as the gene that causes the high pigment-1 mutant phenotype. Theor Appl Genet. 2004;108:1574–81. doi: 10.1007/s00122-004-1584-1. [DOI] [PubMed] [Google Scholar]
- 32.Waters MT, Langdale JA. The making of a chloroplast. EMBO J. 2009;28:2861–73. doi: 10.1038/emboj.2009.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mao L, Van Hemert JL, Dash S, Dickerson JA. Arabidopsis gene co-expression network and its functional modules. BMC Bioinformatics. 2009;10:346. doi: 10.1186/1471-2105-10-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mutwil M, Klie S, Tohge T, Giorgi FM, Wilkins O, Campbell MM, et al. PlaNet: combined sequence and expression comparisons across plant networks derived from seven species. Plant Cell. 2011;23:895–910. doi: 10.1105/tpc.111.083667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alba R, Payton P, Fei Z, McQuinn R, Debbie P, Martin GB, et al. Transcriptome and selected metabolite analyses reveal multiple points of ethylene control during tomato fruit development. Plant Cell. 2005;17:2954–65. doi: 10.1105/tpc.105.036053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tohge T, Kusano M, Fukushima A, Saito K, Fernie AR. Transcriptional and metabolic programs following exposure of plants to UV-B irradiation. Plant Signal Behav. 2011;6:1987–92. doi: 10.4161/psb.6.12.18240. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.