Abstract
Water homeostasis is crucial to the growth and survival of plants. Plasma membrane intrinsic proteins (PIPs) have been shown to be primary channels mediating water uptake in plant cells. We characterized a novel PIP2 gene, HvPIP2;8 in barley (Hordeum vulgare). HvPIP2;8 shared 72–76% identity with other HvPIP2s and 74% identity with rice OsPIP2;8. The gene was expressed in all organs including the shoots, roots and pistil at a similar level. When HvPIP2;8 was transiently expressed in onion epidermal cells, it was localized to the plasma membrane. HvPIP2;8 showed transport activity for water in Xenopus oocytes, however its interaction with HvPIP1;2 was not observed. These results suggest that HvPIP2;8 plays a role in water homeostasis although further functional analysis is required in future.
Keywords: Hordeum vulgare, Xenopus laevisoocytes, HvPIP2;8, PIP, aquaporin, barley, oocytes, plasma membrane intrinsic protein, water transport
Introduction
Plasma membrane intrinsic proteins (PIPs), members of the plant aquaporin family, have been shown to be primary channels mediating uptake in plant cells.1 Plant PIPs can be classified into two distinct clades, PIP1s and PIP2s, according to their sequence similarity.2-4 PIP1 and PIP2 display different transport activity when expressed alone in Xenopus oocytes. PIP2 increases oocyte membrane water permeability, while PIP1 displays no or low water permeability.5 This differential behavior seems to be related to PIP trafficking; PIP2 is able to reach the plasma membrane whereas PIP1 seems to be retained in the endoplasmic reticulum.6
In the genome of Arabidopsis and rice, there are 13 and 11 PIPs, respectively.7,8 In barley five PIP1s and six PIP2s have been isolated so far.9-11 They showed different expression patterns.11 For example, total expression of PIP1s was comparable between leaf regions, whereas PIP2 expression was up to six times higher in the elongation zone. In the present study, we characterized a novel PIP2 gene, HvPIP2;8 in terms of transport activity, expression pattern and subcellular localization.
Results and Discussion
Search of novel PIPs in barley
A number of aquaporin has been identified in many plant species. In rice genome, there are 33 aquaporin genes.8 Among them 8 OsPIP2s were annotated. In Arabidopsis, there are 8 AtPIP2s among 35 aquaporin genes.7 Since the genome sequence of barley has not been completed, the number of aquaporin in barley is still unknown. So far 6 members belonging to PIP2 (HvPIP2;1 to 2;5, HvPIP2;7) have been isolated in barley.9-11 Recent release of barley full-length cDNAs let us to find more PIPs.12 TBLASTN search by using amino acid sequence of all HvPIPs as a query resulted in 570 hits. On the other hand, BLASTn search by using nucleotide sequnence of whole coding regions of all HvPIPs known as a query yielded 1387 hits. Exclusion of repeated hits from the two merged searches resulted in 17 clones. Among them, 4 clones including AK355531, AK356299, AK373720, and AK361542, have not been reported before. In the present study, we functionally characterized one of them AK356299, which was annotated HvPIP2;8.
Phylogenic analysis of HvPIP2;8
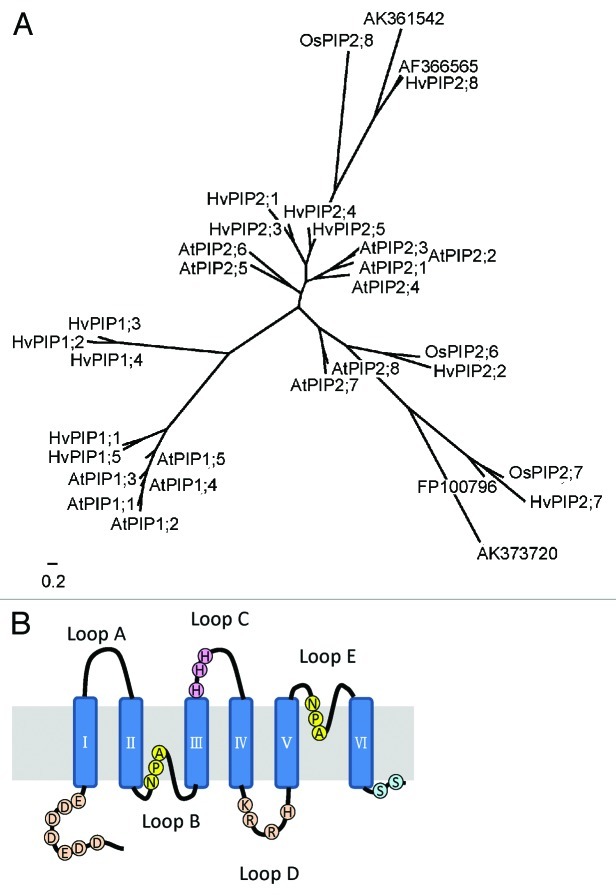
A phylogenic tree analysis was conducted with other PIP members from barley, rice and Arabidopsis. HvPIP2;8 forms a different clade with other PIPs (Fig. 1A). The identity of HvPIP2;8 to other HvPIPs ranged from 72 to 76%. In the same clade, there are four members including HvPIP2;8, OsPIP2;8, AK361542 and AF366565. There are no members from dicots in this clade, suggesting that this clade is specific for monocots.
Figure 1. Phylogenetic analysis and predicted protein structure. A. Phylogenetic tree of the plasma membrane intrinsic protein (PIP) from barley (H. vulgare cv Haruna-nijo), Arabidopsis thaliana, and rice (O. sativa cv Nipponbare). The scale bar indicates 0.2 substitutions per site. B. Predicted structure of HvPIP2;8.
Characteristic of the primary structure of HvPIP2;8
HvPIP2;8 exhibits typical MIP motif, six transmembrane domains and two NPA motifs (Fig. 1B). Furthermore, following highly conserved amino acid residues, which are considered to be involved in the regulation of activation, Ser280 and Ser285 via phosphorylation,13 His206, Lys199, Arg200, and Arg203 in Loop D, and Asp,13 Asp,16 Glu,18 Asp27, Asp30, Asp38, and Glu41 in acidic N-terminus, via cytosolic pH,14 are also present in HvPIP2;8 (Fig. 1B). Interestingly, HvPIP2;8 exhibits three histidine residues in Loop C, which are not conserved in other members.
Transport activity in Xenopus oocyte
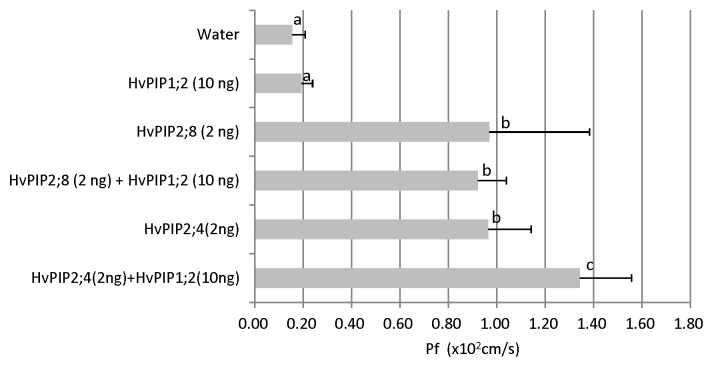
To determine the transport activity for water, HvPIP2;8 was expressed in Xenopus oocytes. Compared with the control injected with water, oocytes expressing HvPIP2;8 showed significantly higher transport activity for water (Fig. 2). It was reported that PIP1 and PIP2 interact each other.15-17 For example, co-expression of ZmPIP1;2 with ether ZmPIP2;1, ZmPIP2;4, ZmPIP2;5 significantly increased the osmotic water permeability coefficient (Pf) of the oocytes compared with the expression of each PIP2 alone.18 Co-expression of HvPIP1;2 with HvPIP2;1, 2;2, 2;3, 2;4, and 2;5 also resulted in increased water permeability coefficient of Xenopus oocytes.10 To investigate whether HvPIP2;8 is able to activate other PIP1 protein, we co-expressed HvPIP2;8 with HvPIP1;2. As a result, the transport activity was not enhanced (Fig. 2). As a positive control, we also co-expressed HvPIP1;2 and HvPIP2;4 to test whether HvPIP1;2 functions in oocytes. The result showed that different from HvPIP2;8, HvPIP2;4 interacted with HvPIP1;2 by further increasing the water permeability coefficient (Fig. 2). This result suggests that the water transport by HvPIP2;8 is relatively constant and there is no need to change dynamically. HvPIP2;8 might be expressed in the cells located inner tissues where water environment is relatively stable.
Figure 2. Water transport activity of HvPIP2;8. The amounts and kind of cRNAs injected were shown on the left of the graph. Pf values are shown. Error bars represent the standard deviation (n = 10). Different letter denotes significant difference (p < 0.05).
Expression patterns of HvPIP2;8
The organ-dependent expression of HvPIP2;8 was investigated with semi-quantitative RT PCR. HvPIP2;8 was expressed in all organs tested including the shoots, roots and pistil (Fig. 3). In the shoots, both mature and young leaf showed similar expression level (Fig. 3). This expression pattern is different form that of HvPIP2;7 (Fig. 3), which was specifically expressed in the leaves.
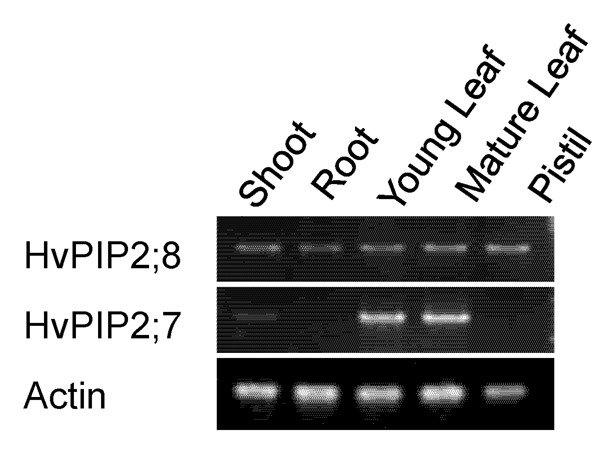
Figure 3. Organ-specific expression of HvPIP2;8 and HvPIP2;7 in barley plants. Semi quantitative RT-PCR analysis in the shoot of 5-d-old seedling, root and young leaves of 10-week-old plants, mature leaves, and pistil were used. ACT1 was used as an internal standard.
Subcellular localization
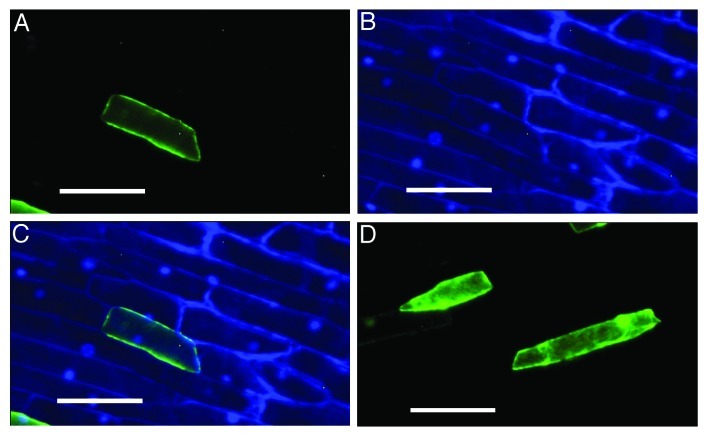
The subcellular localization of HvPIP2;8 was investigated by transiently introducing its fusion with EGFP into the onion epidermal cells. Observation with a fluorescence microscope showed the EGFP signal was localized out of the nuclei, stained with DAPI (Fig. 4A-C), indicating that HvPIP2;8 was localized to the plasma membrane. By contrast, the signal was observed in the cytosol and nuclei in the cells expressing EGFP alone.
Figure 4. Subcellular localization of EGFP:HvPIP2;8 in onion epidermal cells. EGFP:HvPIP2;8 (A), DAPI stained (B), the merged image of A and B (C), and EGFP alone (D) as a control. The white scale bars represent 0.1 mm.
As a conclusion, HvPIP2;8 is a plasma membrane-localized water channel. It is universally expressed in all tissues. Although the exact role of HvPIP2;8 remains to be further examined, our results suggest that it may be involved in water homeostasis.
Materials and Methods
Phylogenetic analysis
A Blast search using the query of new HvPIPs hits AF366565 from wheat and FP100796 from bamboo. The amino acid sequences used for analysis are as follows: all barley and Arabidopsis PIPs, OsPIP2;6, OsPIP2;7, OsPIP2;8, AF366565, and FP100796. After the gaps were eliminated the phylogenetic tree was constructed at Phylogeny.fr (http://www.phylogeny.fr/version2_cgi/index.cgi).19
RNA extraction and analysis of gene expression by RT-PCR
Hordeum vulgare cv Haruna-nijo was used. Samples including mature leaves and pistils for RNA extraction were taken from barley plants grown in fields of Kurashiki, Japan. Samples of the roots and shoots were excised from 4-d-old seedlings and young leaves were excised from one-week-old seedlings. Growth conditions were the same as described previously.9 Total RNA was extracted with the RNeasy Plant Mini Kit (Qiagen, Tokyo, Japan) after grounded with a mortar and pestle. First-strand cDNA synthesis was performed using the High Capacity cDNA Archive Kit (Applied Biosystems). PCR was performed using primer sets in Table 1 according to Utsugi et al.20
Table 1. Gene-specific primer pairs used in the RT–PCR experiments.
| Gene Primera |
|---|
|
HvPIP2;8 Forward: 5′ 213ACACAAGCGCCAGACCGACG232 3′ Reverse: 5′ 870GGCGGCATCGTAGTTGGACCG850 3′ HvPIP2;7 Forward: 5′ 200AGAGCCAGTCCTCCGCCCAG219 3′ Reverse: 5′ 907GGAGCTTCGACCGATCACACG887 3′ ACT1 Forward: 5′ 1ATGGCTGACGGTGAGGACATCC22 3′ Reverse: 5′ 422ACGGCCTGAATAGCGACGTAC402 3′ |
a Numbers for each primer correspond to nucleotide positions from the ATG initiation codon.
Transient expression in onion epidermis cells
The coding sequence of HvPIP2;8 was inserted into pBI221-EGFP21 to be fused in-frame to the C terminus of EGFP coding sequence. The fusion construct was introduced into onion epidermal cells using the particle bombardment according to the procedure described by Utsugi et al.20 After overnight incubation at 23þC, epidermises were peeled and observed with a fluorescence microscope (BZ-8000, Keyence, Osaka, Japan) using a filter set for GFP (OP-66836, Keyence, Osaka, Japan). DAPI (final concentration 2μM, NACALAI TESQUE INC, Kyoto, Japan) was used as necessary to stain nuclei. DAPI staining images were observed using a filter set for DAPI (OP-66834, Keyence, Osaka, Japan) merged with the corresponding EGFP image.
Water transport activity assay in Xenopus laevis oocytes
The coding regions of HvPIP2;8 cDNA was subcloned into the pXβGev1 expression vector. The construct was linearlized with NotI, and capped cRNA was synthesized using the mMESSAGE mMACHINE T3 in vitro transcription kit (Ambion). Oocytes were isolated from adult female Xenopus laevis frogs and maintained as described previously.28 Oocytes were injected with 50 nl of a cRNA solution containing 2 ng of HvPIP2;8 and/or 10 ng of HvPIP1;2, 2 ng of HvPIP2;4 and 10 ng of HvPIP1;2 as a positive control. As a negative control, water-injected oocytes were used. The osmotic water permeability coefficient of oocytes was measured according to the procedures described previously.22
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Dr. D. Saisho for his helpful discussion.
Glossary
Abbreviations:
- EGFP
enhanced green fluorescence protein
- Pf
osmotic water permeability coefficient
- PIPs
plasma membrane intrinsic proteins
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/22294
REFERENCES
- 1.Maurel C, Verdoucq L, Luu DT, Santoni V. Plant aquaporins: membrane channels with multiple integrated functions. Annu Rev Plant Biol. 2008;59:595–624. doi: 10.1146/annurev.arplant.59.032607.092734. [DOI] [PubMed] [Google Scholar]
- 2.Chaumont F, Barrieu F, Wojcik E, Chrispeels MJ, Jung R. Aquaporins constitute a large and highly divergent protein family in maize. Plant Physiol. 2001;125:1206–15. doi: 10.1104/pp.125.3.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tyerman SD, Bohnert HJ, Maurel C, Steudle E, Smith JA. Plant aquaporins: their molecular biology, biophysics and significance for plant water relations. J Exp Bot. 1999;50:1055–71. [Google Scholar]
- 4.Weig A, Deswarte C, Chrispeels MJ. The major intrinsic protein family of Arabidopsis has 23 members that form three distinct groups with functional aquaporins in each group. Plant Physiol. 1997;114:1347–57. doi: 10.1104/pp.114.4.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaumont F, Barrieu F, Jung R, Chrispeels MJ. Plasma membrane intrinsic proteins from maize cluster in two sequence subgroups with differential aquaporin activity. Plant Physiol. 2000;122:1025–34. doi: 10.1104/pp.122.4.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zelazny E, Miecielica U, Borst JW, Hemminga MA, Chaumont F. An N-terminal diacidic motif is required for the trafficking of maize aquaporins ZmPIP2;4 and ZmPIP2;5 to the plasma membrane. Plant J. 2009;57:346–55. doi: 10.1111/j.1365-313X.2008.03691.x. [DOI] [PubMed] [Google Scholar]
- 7.Johanson U, Karlsson M, Johansson I, Gustavsson S, Sjövall S, Fraysse L, et al. The complete set of genes encoding major intrinsic proteins in Arabidopsis provides a framework for a new nomenclature for major intrinsic proteins in plants. Plant Physiol. 2001;126:1358–69. doi: 10.1104/pp.126.4.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sakurai J, Ishikawa F, Yamaguchi T, Uemura M, Maeshima M. Identification of 33 rice aquaporin genes and analysis of their expression and function. Plant Cell Physiol. 2005;46:1568–77. doi: 10.1093/pcp/pci172. [DOI] [PubMed] [Google Scholar]
- 9.Katsuhara M, Akiyama Y, Koshio K, Shibasaka M, Kasamo K. Functional analysis of water channels in barley roots. Plant Cell Physiol. 2002;43:885–93. doi: 10.1093/pcp/pcf102. [DOI] [PubMed] [Google Scholar]
- 10.Horie T, Kaneko T, Sugimoto G, Sasano S, Panda SK, Shibasaka M, et al. Mechanisms of water transport mediated by PIP aquaporins and their regulation via phosphorylation events under salinity stress in barley roots. Plant Cell Physiol. 2011;52:663–75. doi: 10.1093/pcp/pcr027. [DOI] [PubMed] [Google Scholar]
- 11.Besse M, Knipfer T, Miller AJ, Verdeil JL, Jahn TP, Fricke W. Developmental pattern of aquaporin expression in barley (Hordeum vulgare L.) leaves. J Exp Bot. 2011;62:4127–42. doi: 10.1093/jxb/err175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsumoto T, Tanaka T, Sakai H, Amano N, Kanamori H, Kurita K, et al. Comprehensive sequence analysis of 24,783 barley full-length cDNAs derived from 12 clone libraries. Plant Physiol. 2011;156:20–8. doi: 10.1104/pp.110.171579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Törnroth-Horsefield S, Wang Y, Hedfalk K, Johanson U, Karlsson M, Tajkhorshid E, et al. Structural mechanism of plant aquaporin gating. Nature. 2006;439:688–94. doi: 10.1038/nature04316. [DOI] [PubMed] [Google Scholar]
- 14.Tournaire-Roux C, Sutka M, Javot H, Gout E, Gerbeau P, Luu DT, et al. Cytosolic pH regulates root water transport during anoxic stress through gating of aquaporins. Nature. 2003;425:393–7. doi: 10.1038/nature01853. [DOI] [PubMed] [Google Scholar]
- 15.Zelazny E, Borst JW, Muylaert M, Batoko H, Hemminga MA, Chaumont F. FRET imaging in living maize cells reveals that plasma membrane aquaporins interact to regulate their subcellular localization. Proc Natl Acad Sci U S A. 2007;104:12359–64. doi: 10.1073/pnas.0701180104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Otto B, Uehlein N, Sdorra S, Fischer M, Ayaz M, Belastegui-Macadam X, et al. Aquaporin tetramer composition modifies the function of tobacco aquaporins. J Biol Chem. 2010;285:31253–60. doi: 10.1074/jbc.M110.115881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sorieul M, Santoni V, Maurel C, Luu DT. Mechanisms and effects of retention of over-expressed aquaporin AtPIP2;1 in the endoplasmic reticulum. Traffic. 2011;12:473–82. doi: 10.1111/j.1600-0854.2010.01154.x. [DOI] [PubMed] [Google Scholar]
- 18.Fetter K, Van Wilder V, Moshelion M, Chaumont F. Interactions between plasma membrane aquaporins modulate their water channel activity. Plant Cell. 2004;16:215–28. doi: 10.1105/tpc.017194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dereeper A, Guignon V, Blanc G, Audic S, Buffet S, Chevenet F, et al. Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Res. 2008;36(Web Server issue):W465-9. doi: 10.1093/nar/gkn180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Utsugi S, Nakamura S, Noda K, Maekawa M. Structural and functional properties of Viviparous1 genes in dormant wheat. Genes Genet Syst. 2008;83:153–66. doi: 10.1266/ggs.83.153. [DOI] [PubMed] [Google Scholar]
- 21.Kwon C, Chung IK. Interaction of an Arabidopsis RNA-binding protein with plant single-stranded telomeric DNA modulates telomerase activity. J Biol Chem. 2004;279:12812–8. doi: 10.1074/jbc.M312011200. [DOI] [PubMed] [Google Scholar]
- 22.Mahdieh M, Mostajeran A, Horie T, Katsuhara M. Drought stress alters water relations and expression of PIP-type aquaporin genes in Nicotiana tabacum plants. Plant Cell Physiol. 2008;49:801–13. doi: 10.1093/pcp/pcn054. [DOI] [PubMed] [Google Scholar]