Abstract
Metasequoia glyptostroboides, a famous relic species of conifer that survived in China, has been successfully planted in large numbers across the world. However, limited information on male cone development in the species is available. In this study, we observed the morphological and anatomical changes that occur during male cone development in M. glyptostroboides using semi-thin sections and scanning electron microscopy. The male cones were borne oppositely on one-year-old twigs that were mainly located around the outer and sunlit parts of crown. Male cones were initiated from early September and shed pollen in the following February. Each cone consisted of spirally arranged microsporophylls subtended by decussate sterile scales, and each microsporophyll commonly consisted of three microsporangia and a phylloclade. The microsporangial wall was composed of an epidermis, endothecium, and tapetum. In mid-February, the endothecium and tapetum layers disintegrated, and in the epidermal layer the cell walls were thickened with inner protrusions. Subsequently, dehiscence of the microsporangia occurred through rupturing of the microsporangial wall along the dehiscence line. These results suggest that the structure, morphology, architecture and arrangement of male cones of M. glyptostroboides are mainly associated with the production, protection and dispersal of pollen for optimization of wind pollination.
Keywords: male cone, Metasequoia glyptostroboides, pollen cone, morphological structure, development, microsporangium wall, microsporangium dehiscence, pollen release, anatomical structure, microstrobili
Introduction
Metasequoia glyptostroboides Hu et Cheng (also known as the dawn redwood, Chinese redwood and water fir), is the sole extant species of Metasequoia, which was widely distributed across the Northern Hemisphere in the Cretaceous and Palaeogene.1,2 The genus Metasequoia was first described as a fossil by Miki in 1941.3 The living species M. glyptostroboides, which survived in China, was identified as a new species of Metasequoia by Hu and Cheng in 1948.4 Since this “living fossil” was discovered, a number of studies have focused on its controversial phylogenetic position. Recent genetic and molecular marker data indicate that Metasequoia has a close relationship with Sequoia and Sequoiadendron, and that this group, previously placed within the Taxodiaceae, should be merged into the larger family Cupressaceae as subfamily Sequoioideae.5-7 This merging is now becoming widely accepted.
Metasequoia glyptostroboides is a monoecious species with male cones (pollen cones or microstrobili) and female cones (seed cones or megastrobili), which are both produced normally on one-year-old twigs of adult trees. Several morphological and anatomical studies on the reproductive structures of M. glyptostroboides have paid greater attention to the female cone than the male cone.8-11 This is probably because many trees produce female cones, but only a few trees produce male cones after its introduction to outside China,12 which renders male cone collection difficult. In addition, although M. glyptostroboides has been planted successfully in large numbers and is now widely grown around the world,13 the frequency of seedling reproduction from most adult trees is very low because of low seed viability largely as a result of inbreeding depression.14,15 However, inbreeding depression can be reversed by outcrossing, and seed production can be improved by increased pollen flow.16-18 Thus pollen flow is an important factor that influences outcrossing and seed production. In M. glyptostroboides, pollen produced by male cones is dispersed by wind to effect pollination (anemophily). Given that male cones are functionally adapted for pollen release and dispersal to facilitate successful pollination, knowledge of the processes of male reproductive development and pollen dispersal are important for understanding the ecology and evolution of wind pollination in M. glyptostroboides. At present, little such information is available for M. glyptostroboides.
In this study we investigated male cone development in M. glyptostroboides. We focused on the morphological and anatomical changes that occur during the development of the male cone, microsporangia and microsporangium wall, and analyzed their adaptive and evolutionary significance in relation to wind pollination in order to provide further insights into male cone development of this relic species.
Results
Arrangement of male cones
Metasequoia glyptostroboides is monoecious and produces unisexual cones on the same tree. As a typical wind-pollinated conifer, M. glyptostroboides produces numerous male cones (microstrobilus) in 50-y-old trees, but very few in 30-y-old trees. Male cones were arranged oppositely in terminal and two lateral sides of one-year-old twigs, and each twig bears more than 100 male cones (Fig. 1A). These male cones were mainly found in the sunlit parts around the outer crown, whereas very few were found in the shaded parts of crown.
Figure 1. Morphological development of male cones in M. glyptostroboides. (A) In early September, oval, bright green male cones were initiated in the axils of opposite leaves, with 2–4 male cones per leaf pair. (B) In mid-October, the male cones had enlarged and changed in color from green to light yellow, and the twigs had turned brown. (C) In early November, the male floral buds had increased in size and their color had changed to tawny. (D–E) In mid-November, the male floral buds continued to enlarge while the leaves had begun to abscise. (F–G) From December to late January, the male floral buds turned brown and all of the leaves had abscised. The male cone cluster was pendulous and spike-like. (H) By mid-February, the microsporophyll was exposed outside the bracts, and male cones shed pollen from the base to the tip of an individual branch. Bars = 2 cm.
Phenology of male cone development
The phenology of male cone development in Yangzhou was observed for 2 y. Male floral buds were first visible in early September. Normally, 1–2 small, green male cones were borne in the leaf axils and thus 2–4 male cones per leaf pair were borne in an opposite arrangement (Fig. 1A). In mid-October, the buds enlarged and turned light yellow, and the color of the twigs changed from yellow to brown (Fig. 1B). By early November, the size of the male floral buds increased and their color changed from light yellow to tawny (Fig. 1C). From mid to late November, the buds continued to enlarge in size and became deep in brown. At that time, the leaves were tawny in color and began to abscise (Fig. 1D and E). From early December to January, the male cones changed slowly in color and size under the chilly weather for about 2 mo. With complete abscission of all the leaves, the male cone cluster was up to 20 cm long, pendulous and spike-like forming a panicle (Fig. 1F and G). In early February, the male cones enlarged gradually and the microsporophylls protruded outside the scales. In mid-February, the male cones began to shed pollen. Although the pollen dispersal period could last 30 d for an individual tree, peak blooming occurred from February 15th to 28th. Microsporangia dehiscence took place from the base to the tip of an individual branch. The cluster of mature male cones during pollen disposal was pendulous and spike-like (Fig. 1H).
Developmental sequence of the male cone
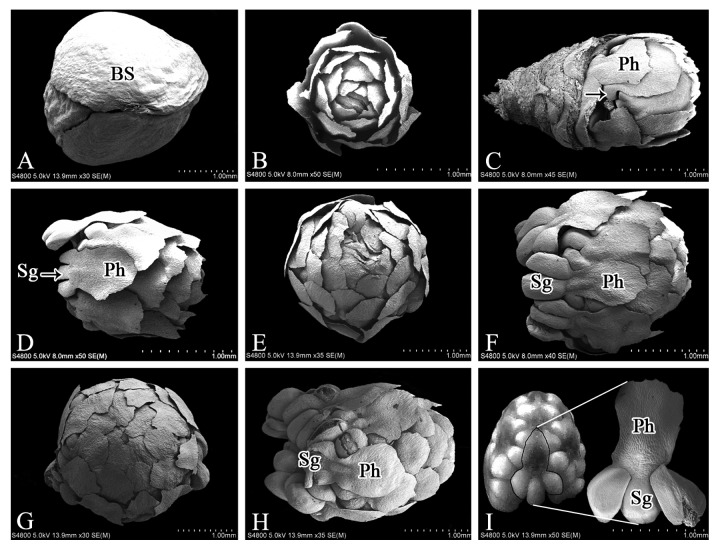
The male cone of M. glyptostroboides consists of the outer scales and the inner microsporophylls. In early September, the male cone was bright green, 1–2 mm in diameter and arranged in the opposite leaf axils on individual twigs. All microsporophylls were subtended normally by four layers of decussate sterile scales (Fig. 2A). Removal of the bud-scales showed that the microsporophylls were arranged spirally (helically) on the main axis and were undergoing differentiation (Fig. 2B). The microsporophyll consists of the phylloclade at the apex and the microsporangia at the base. By late September, the phylloclade had already formed, whereas the microsporangium primordia were only just visible (Fig. 2C), thus differentiation of the phylloclade preceded that of the microsporangia. The microsporangia developed progressively in early October (Fig. 2D). At that time, the microsporophylls were arranged tightly around the main axis and the size of the microsporophylls increased gradually, which resulted in male cone enlargement to 2–3 mm in length and 1.5–2 mm in width (Fig. 2E). In mid-November, the male cone enlarged rapidly (to 3–3.5 mm long and 2–2.5 mm wide) and was elliptical in shape (Fig. 2F and G). Both the microsporangia and the phylloclade enlarged considerably (Fig. 2F), and the microsporophylls were in close contact with each other (Fig. 2G). Subsequently, the male cone underwent a period of only gradual morphological development for about 2 mo (from early December to the end of January). During this period the scales turned brown and underwent lignification, and they concealed the inner microsporophylls (Figs. 1F and 2H). After this period, the outer scales began to unfold. As the microsporangia and phylloclade developed further, the microsporophyll extended outwards ultimately beyond the scales (Figs. 1H and 2I). The number of microsporangia per microsporophyll varied from one to five, but most commonly three were observed (Fig. 2I).
Figure 2. SEM images of male cones of M. glyptostroboides at different developmental stages. (A) In early September, the male cone was subtended by decussate bud-scales. (B) In mid-September, the spirally arranged microsporophylls underwent differentiation. (C) By late September, microsporangium primordia were visible (arrow). (D) In early October, the microsporophyll was composed of the phylloclade and microsporangia. (E) The microsporophylls enlarged and were tightly arranged around the main axis. (F–G) In November, the microsporangia and phylloclade had enlarged significantly; (H) In December, the enlarged microstrobilus grew slowly; (I) In early February, mature microsporophyll contained 2–3 microsporangia and an phylloclade. BS, bud scales; Ph, phylloclade; Sg, sporangia. Bars = 1 mm.
Development of microsporangium wall
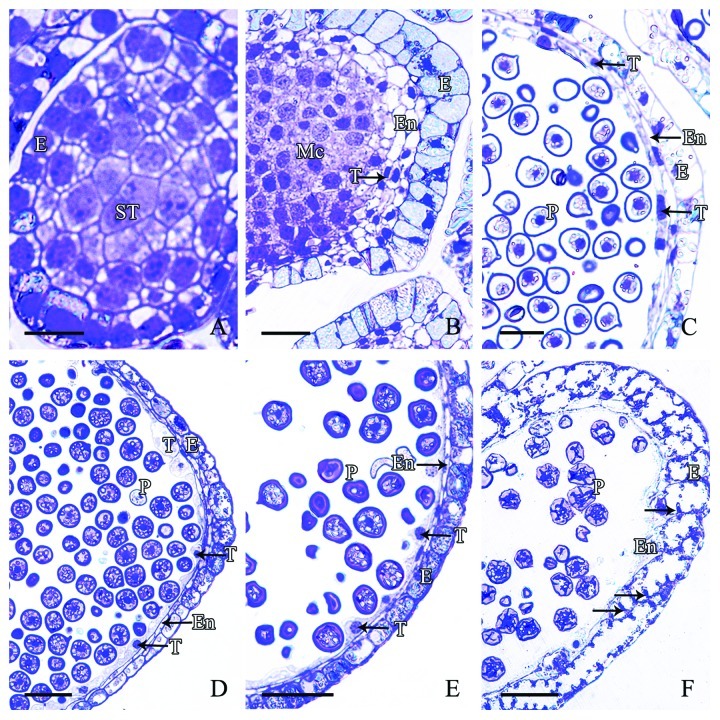
Microsporangia wall of microspore in M. glyptostroboides originated from the sporogenous cells that differentiated from the surface cells of the microsporophyll primordial. This differentiation occurs prior to the formation of the microspore mother cells. In early stage, the sporogonia underwent several periclinal and anticlinal divisions to give rise to the microsporangial wall and the sporogenous cells. The microsporangium wall was thin and composed of 3–4 layers of cells, namely an outermost epidermis, 1–2 layer(s) of the endothecium, and an inner tapetum layer. In mid-September, the epidermal cells were large, rectangular and closely arranged. No tapetum layer existed at this stage (Fig. 3A). In early October, with development of the microspore mother cells, the outermost epidermal cells underwent anticlinal division, which increased the number of cells and broadened the volume and inner space of the microsporangium. At this time, the 2–3 layers of cells to the inside of the epidermis were small, linear in shape, tightly arranged and contained lightly stained cytoplasm and a conspicuous nucleus. These cells differentiated into the early tapetum and obscure endothecium (Fig. 3B). By early November, with development of the microspores, the epidermal cells were stretched radially and became elongated, and the nucleus and some starch grains were apparent in these cells (Fig. 3C). The endothecium and tapetum were tightly appressed to the epidermis and each comprised a thin layer of cells. The endothecium cells were irregularly polygonal in shape and compactly arranged. The tapetum cells were elongated radially and contained a circular or oblong nucleus (Fig. 3C). In early December, the epidermal cells continued to anticlinal divided. The number of starch grains in the epidermal cells increased markedly and some epidermal cells stained darkly. At this stage, the endothecium cells were linear in shape and in most endothecium cells the nucleus was not visible. However, the tapetum cells enlarged and some protruded inwards into the microsporangium. These cells contained a large and circular nucleus (Fig. 3D). By early January, the microsporangium wall cells showed little significant difference in appearance. The cell inclusions increased in density in the epidermal cells, and some tapetum cells still showed vigorous metabolic activity (Fig. 3E). In mid-February, with the decrease in cytoplasm density of the epidermal cells, the cell wall became thickened and some place around the cell wall formed inner protrusion. The cells of endothecium and tapetum layers disintegrated, leaving vestigial cell remnants to the inner of the epidermal cells (Fig. 3F).
Figure 3. Stages of microsporangial wall development in M. glyptostroboides (A) In mid-September, the outermost epidermal cells differentiated and contained a large conspicuous nucleus. (B) In late September, the number of epidermal cells increased and the inner 2–3 layers of cells formed the early endothecium and tapetum layers, which showed no distinct differences. (C) In November, the linear-shaped tapetum and endothecium cell layers differentiated. (D–E) From early December to late January, the tapetum cells were well-developed and protruded inwards. (F) In mid-February, expansion and wall thickening of the epidermal cells occurred with protrusion inwards on cell walls, and the tapetum disintegrated. E, Epidermis; En, endothecium; Mc, mother cell; P, pollen; ST, sporogenous tissue; T, tapetum. Scale bars = 50 μm.
Microsporangium dehiscence and pollen release
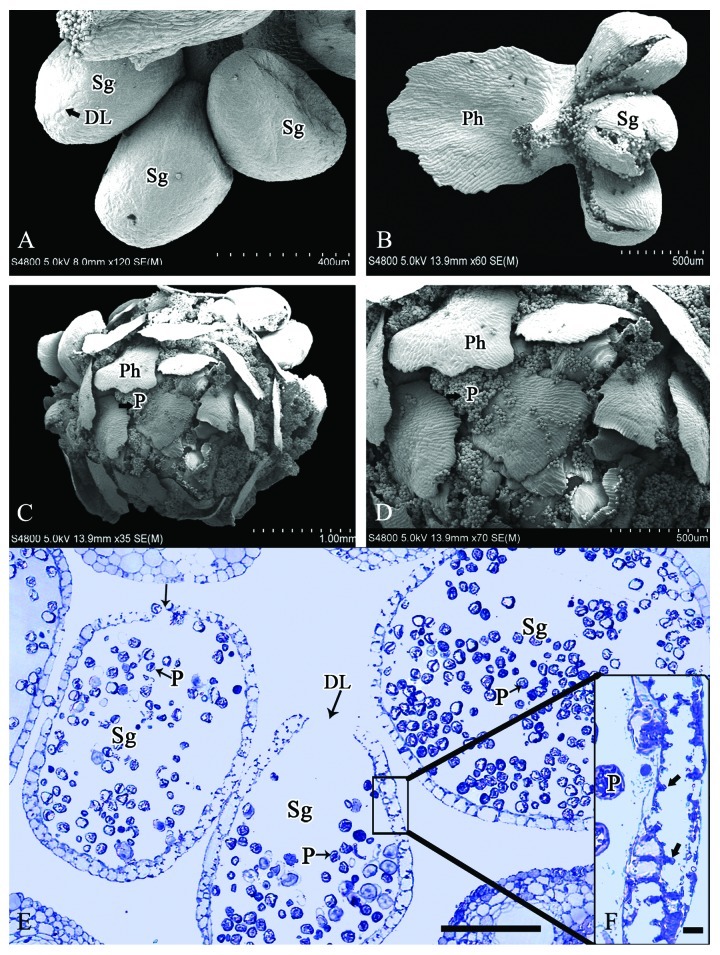
Formation of the dehiscence line on the microsporangium occurred in mid-October (Fig. 4A). In the following February, many internal protrusions were evident along the inner tangential wall of the microsporangial wall cells (Fig. 4E and F). Dehiscence of the microsporangium occurred soon after the thickening of the inner tangential wall. At this stage, the microsporangium extended beyond the scales and was exposed to the air, and the outer wall cells dehydrated. This dehydration increased the unbalanced stress tension between internal and external sides of the microsporangium, leading to the rupture of the microsporangial wall along the dehiscence line. The microsporangial wall then bent outwards along the dehiscence line to promote dispersal of the pollen grains (Fig. 4B and E). All microsporangia within a cone dehisced almost simultaneously and the mature pollen grains were released spirally in the space between the phylloclades (Fig. 4C and D).
Figure 4. Microsporangia at the pollen dispersal stage in M. glyptostroboides. (A) Pollen grains are released through the dehiscence line, which formed early in microsporangium development in mid-October. (B) Microsporangial wall ruptured along the dehiscence line in February. (C–D) Mature pollen grains are dispersed spirally in the space between the phylloclades. (E) Transverse section of dehisced microsporangia in mid-February. (F) Thickening of microsporangial wall with many internal protrusions (arrows). DL, Dehiscence line; P, pollen; Ph, phylloclade; Sg, sporangia. Scale bars (A) = 400 μm, (B, D) = 500 μm, (C) = 1 mm, (E) = 100 μm, (F) = 10 μm.
Discussion
Male cones of extant conifer species exhibit only small morphological changes throughout conifer evolution, whereas female cones have undergone more dramatic morphological evolution.19,20 This is because female cones are subject to stronger biotic selective pressures, such as seed predators and seed dispersers, which have contributed to their evolution over time. In contrast, male cones essentially have a single function, i.e., pollen dispersal, which is driven mainly by abiotic wind.19,20 In Metasequoia, although abundant fossils of female cones exist, the fossil record of male cones is sparse. Several fossils show that male cones were arranged oppositely in the leaf axils; the male cone shape, size and arrangement are all similar to those of the extant species M. glyptostroboides,10,21 which indicates that no significant morphological changes in the male cone have occurred over a long evolutionary time scale in M. glyptostroboides. This result is consistent with the above-mentioned evolutionary history of male cones in other conifers, and that the evolution of the male cone in M. glyptostroboides may be shaped mainly by wind pollination.
The distinct decussate arrangement of the female cone scales and leaves in Metasequoia was the taxonomic basis for establishment of the genus.3 This arrangement is unique in the Sequoioideae. The male cones also have a decussate-opposite arrangement in the leaf axils. However, only several layers of bud scales (bracts) that subtend the male cone have a decussate arrangement (Fig. 2A), whereas all of the microsporophylls have a spiral (helical) arrangement (Fig. 2B). In general, the male cones of most extant conifers are interpreted as strobili,22 in which the cone scales and microsporophylls are regarded to be modified leaves. Thus, the arrangement of microsporophylls should accord with the original arrangement of leaves. However, the different arrangement of the microsporophylls and leaves in Metasequoia indicates that the arrangement pattern of either the microsporophylls or the leaves might have changed during their evolution. There is no fossil record of the arrangement of microsporophylls in the original male cone of the ancestral Metasequoia, thus it is difficult to know whether the decussate pattern is a primitive or derived trait. However, the spiral pattern in the extant male cone enables a higher number of microsporophylls to be borne along the cone axis than that of the decussate pattern, meaning higher quantities of pollen can be produced. Furthermore, this type of architecture with increased layers of tightly imbricate microsporophylls favors resistance to freezing in winter and to potential predators for protection of the developing microsporangia (pollen sacs). The development of male cones from their first visible appearance to maturity requires about 6 mo (from September to February). During this period, the unprotected male cones, especially in winter after the leaves have abscised, might be subject to certain selective pressures, such as predation and freezing injury. Indeed, in the present study, a considerable proportion of male cones were lost between their appearance and maturity (Fig. 1). Therefore, in Metasequoia, the spiral pattern with tightly packed scales in the male cone, which differs from the decussate pattern of the leaves and scales in the female cones, may be associated with adaption for pollen production and protection of the microsporangia, in addition to pollen dispersal.
Metasequoia has a close phylogenetic relationship with Sequoia and Sequoiadendron as indicated by molecular evidence.5-7 With regard to male cone morphology, the microsporophyll of Metasequoia consists of the phylloclade at the apex and commonly three microsporangia at the base; these characteristics are consistent with those of Sequoia and Sequoiadendron,23 which represents a phylogenetic homology among these three genera. However, apart from the opposite arrangement of male cones in the leaf axils, there are several other notable differences in male cone morphology between Metasequoia and the other two genera, including lateral cone attachment on specialized shoots vs terminal on regular shoots,23 and production of cones in clusters vs solitary. In M. glyptostroboides, male cones are borne on long, pendulous and slender twigs that form panicle-like shoots. This cluster type is entirely different from that of male cones in Sequoia and Sequoiadendron, but similar to that of male cones in Taxodium. Because Metasequoia is more distantly related to Taxodium than Sequoia and Sequoiadendron, the divergence in the production of male cones singly or in pendulous clusters within Sequoioideae suggests that male cone evolution in the different species has been driven by different selective pressures. On the other hand, the similar pendulous, panicle-like shoots bearing male cones in Metasequoia and Taxodium apparently have been shaped by convergent evolution and could be architecturally advantageous for pollen dispersal by wind, because a pendulous cluster of male structures is common in many deciduous, anemophilous plant taxa, e.g., alder, aspen, birch, hazel, oak and walnut.24
In wind pollination of gymnosperms, pollen dispersal from male cones may be affected by the positional relationship between male cones and the surrounding structures, which could reduce pollen filtration and wastage caused by the leaves.24,25 In the present study, the male cones on 50-y-old trees of M. glyptostroboides were located mainly in the outside of the crown. This location would obviously expose the male cones to the maximal wind and therefore is advantageous for pollen dispersal by wind. Furthermore, male cones are only borne in sunlit parts of the crown, where more photosynthetic products from leaves are available for the cone development. Similarly, cone development may have also been facilitated by a possible photosynthetic function of the green cones themselves in the early stage. However, the majority of artificial populations of M. glyptostroboides are planted in mass groups with a high plant density. At maturity, most trees are shaded under each other, and lower part of the crowns of these trees is often deadwood due to lack of light, thereby greatly limiting male cone production and pollen output. In addition, male cones are abundant in 50-y-old trees but few in 30-y-old trees meaning a late maturing age of reproduction of this species. However, the age of current artificial populations seldom reaches 50 y. Therefore, in addition to low genetic variation in the artificial population,14 poor male cone production and immature age may be important contributors to low seed quantity and quality in artificially planted populations.
Microsporangium dehiscence is the final stage of male cone development, of which the ultimate goal is pollen dispersal and output. Although microsporangium dehiscence is essential for wind pollination in gymnosperms, few studies have investigated the process of dehiscence.25 In contrast, anther dehiscence in angiosperms has been studied extensively on a number of species, such as Arabidopsis, Lilium, rice, maize and members of the Solanaceae.26 The basic processes of anther dehiscence in angiosperms are similar and involve secondary thickening in the anther endothecium, breakdown of the septum, splitting of the stomium and subsequent opening of the anther.26 Results from the present study show that, during the development of the microsporangial wall and the process of microsporangium dehiscence, M. glyptostroboides displays the following two characteristics.
(1) The thickening of cell walls occurs in the epidermis (Figs. 3E and F, 4E and F). In angiosperms,26 all localized secondary thickenings occur in the endothecium and the splitting of the stomium results in anther opening. In Ginkgo,25 the microsporangial wall is of the eusporangiate form, consisting of 9–11 layers of cells, and the thickening occurs in the endothecium before sporangium dehiscence. In cycads,27 the thick-walled cells occur in the apex of sporangia. However, in M. glyptostroboides only the epidermal cell layer undergoes thickening and cell expansion, whereas the other layers within the microsporangial wall disintegrate gradually. This epidermis thickening is similar to that of other conifer,28 e. g. Pinus, Cedrus, Cupressus and Cephalotaxus. The epidermis thickening develops around the microsporangial wall except in the stomium, which facilitates ultimate opening along the stomium. This opening mechanism is identical to that of another dehiscence in angiosperms but differs in the specific cell layer that experiences wall thickening. This difference is apparently associated with the microsporangial wall structure in M. glyptostroboides, which contains three cell layers of the leptosporangiate form, rather than the higher number of cell layers characteristic of the eusporangiate form.
(2) Breakdown of the septum does not occur during sporangium dehiscence. In angiosperms, the septum separates the two locules of the anther. After enzymatic lysis of the septum, and the likely induction of programmed cell death, the septum ruptures to form a single locule and is followed by subsequent opening of the anther.26 Although septum breakdown is absent in Ginkgo, the sporangium opens on the facing sides of two adjacent microsporangia,25 which is similar to anther opening in angiosperms. However, in M. glyptostroboides, each microsporangium opens directly and independently along the dehiscence line. This is likely related to the separated microsporangia on a microsporophyll, and might be an adaptation for coordination of pollen disposal with male cone architecture in M. glyptostroboides.
Materials and Methods
Plant material
Male cones were collected at weekly intervals from 50-y-old trees of M. glyptostroboides growing on the Yangzhou University campus, Jiangsu province, China (32°20′ N, 119°30′ E), from early September 2010 to the end of February 2011. During the pollen dispersal period, the cones were collected daily. After dissection of the bracts under a Motic SMZ-168 dissecting microscope, the samples were fixed separately in 2.5% glutaraldehyde and FAA solution (formalin: acetic acid: 70% alcohol, 1:1:18) at 4°C until use. Male strobilus clusters and individual cones selected at different developmental stages were photographed against a background of black cloth using a DSC-H7 Sony digital camera
Semi-thin sections
Sporangial wall development and microsporangial dehiscence were examined using light microscopy. The samples were prefixed in 2.5% glutaraldehyde for 20 h at 4°C and postfixed with 1% osmium tetroxide for 6 h at room temperature. After rinsing three times in phosphate buffer (pH 7.2), the samples were dehydrated in an alcohol series (30, 50, 70, 80, 90, 95 and 100% v/v, 15 min each), treated with propylene oxide (twice for 30 min), and infiltrated in Spurr resin. Sections (1 µm thick) were cut with a glass knife using a Leica EM UC6 (Leica Co., Austria) ultramicrotome and stained with 1% Toluidine Blue O before examination. The sections were observed under a Zeiss Axioskop 40 microscope (Carl Zeiss, Germany) and digital images were captured with an Axiocam MRC camera
Scanning electron microscopy (SEM)
Morphological development of the male cone was observed using scanning electron microscopy. The samples fixed in FAA solution were rinsed in 0.1 M phosphate buffer (pH 7.2, three times, 15 min each), dehydrated in a graded ethanol series (30, 50, 80, 90, 95 and 100% v/v, 15 min each) and treated with a series of acetone and isoamyl acetate (acetone:isoamyl acetate, 1:1, 1:2, 0:1, 15 min each). After drying at critical point, the samples were mounted on specimen stubs. The samples were coated with gold using a sputter coater (SCD500) and examined under a S-4800 scanning electron microscopy (Hitachi, Japan) at 15.0 kV
Authors’ contributions
B.J. designed the project; Y.L. collected the samples; L.T., Y.L., D.W., M.Z. and J.M. participated in SEM and semi-thin sectioning experiments; BJ drafted the manuscript and all co-authors participated in its editing.
Acknowledgments
This work was financially supported by the Natural Science Found of Jiangsu Province No. BK2011444, and the National Natural Science Foundation of China No. 31200145.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/22898
References
- 1.LePage BA, Yang H, Matsumoto M. The evolution and biogeographic history of Metasequoia. In: LePage BA, Williams C, Yang C eds. The Geobiology and Ecology of Metasequoia. New York: Springer. 2005:3-114. [Google Scholar]
- 2.Wolfe AP, Csank AZ, Reyes AV, McKellar RC, Tappert R, Muehlenbachs K. Pristine early eocene wood buried deeply in kimberlite from northern Canada. PLoS One. 2012;7:e45537. doi: 10.1371/journal.pone.0045537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miki S. On the change of flora in eastern Asia since Tertiary period (I). The clay or lignite beds flora in Japan with special reference to the Pinus trifolia beds in Central Hondo. Jap J Bot. 1941;11:237–304. [Google Scholar]
- 4.Hu HH, Cheng WC. On the new family Metasequoiaceae and on Metasequoia glyptostroboides, a living species of the genus Metasequoia found in Szechuan and Hupen. The Bulletin of the Fan. Memorial Institute of Biology. New Series. 1948;1:153–61. [Google Scholar]
- 5.Brunsfeld SJ, Soltis PS, Soltis DE, Gadek PA, Quinn CJ, Strenge DD, et al. Phylogenetic relationships among the genera of Taxodiaecae and Cupressaceae: evidence from rbcL sequence. Syst Bot. 1994;19:253–62. doi: 10.2307/2419600. [DOI] [Google Scholar]
- 6.Tsumura Y, Yoshimura K, Tomaru N, Ohba K. Molecular phylogeny of conifers using RFLP analysis of PCR-amplified specific chloroplast genes. Theor Appl Genet. 1995;91:1222–36. doi: 10.1007/BF00220933. [DOI] [PubMed] [Google Scholar]
- 7.Gadek PA, Alpers DL, Heslewood MM, Quinn CJ. Relationships within Cupressaceae sensu lato: a combined morphological and molecular approach. Am J Bot. 2000;87:1044–57. doi: 10.2307/2657004. [DOI] [PubMed] [Google Scholar]
- 8.Sterling C. Some features in the morphology of Metasequoia. Am J Bot. 1949;36:461–71. doi: 10.2307/2438080. [DOI] [Google Scholar]
- 9.Takaso T, Tomlinson PB. Seed cone and ovule ontogeny in Metasequoia, Sequoia and Sequoiadendron (Taxodiaceae-coniferales) Bot J Linn Soc. 1992;109:15–37. doi: 10.1111/j.1095-8339.1992.tb00256.x. [DOI] [Google Scholar]
- 10.Liu YJ, Li CS, Wang YF. Studies on fossil Metasequoia from north-east China and their taxonomic implications. Bot J Linn Soc. 1999;130:267–97. [Google Scholar]
- 11.Dörken VM. Proliferating seed cones in Metasequoia glyptostroboides HU & CHENG (Cupressaceae s.l., Coniferales) elucidate the evolution of seed cones and ovules in Cupressaceae s.l. Feddes Repert. 2011;122:409–20. doi: 10.1002/fedr.201200004. [DOI] [Google Scholar]
- 12.Johnson LC, Ward MF. Male cone production in Metasequoia glyptostroboides growing at the Dominican College of San Rafael, California. California Horticultural Journal. 1972;33:98–100. [Google Scholar]
- 13.Ma JS. A worldwide survey of cultivated Metasequoia glyptostroboides Hu & Cheng (Taxodiaceae: Cupressaceae) from 1947 to 2007. Bulletin of the Peabody Museum of Natural History. 2007;48:235–53. doi: 10.3374/0079-032X(2007)48[235:AWSOCM]2.0.CO;2. [DOI] [Google Scholar]
- 14.Li YY, Chen XY, Zhang X, Wu TY, Lu HP, Cai YW. Genetic differences between wild and artificial populations of Metasequoia glyptostroboides: implications for species recovery. Conserv Biol. 2005;19:224–31. doi: 10.1111/j.1523-1739.2005.00025.x. [DOI] [Google Scholar]
- 15.Li YY, Tsang EPK, Cui MY, Chen XY. Too early to call it success: An evaluation of the natural regeneration of the endangered Metasequoia glyptostroboides. Biol Conserv. 2012;150:1–4. doi: 10.1016/j.biocon.2012.02.020. [DOI] [Google Scholar]
- 16.Allison TD. Pollen production and plant density affect pollination and seed production in Taxus canadensis. Ecology. 1990;71:516–22. doi: 10.2307/1940305. [DOI] [Google Scholar]
- 17.Burczyk J, Lewandowski A, Chalupka W. Local pollen dispersal and distant gene flow in Norway spruce (Picea abies [L.] Karst.) For Ecol Manage. 2004;197:39–48. doi: 10.1016/j.foreco.2004.05.003. [DOI] [Google Scholar]
- 18.Smouse PE, Sork VL. Measuring pollen flow in forest trees: an exposition of alternative approaches. For Ecol Manage. 2004;197:21–38. doi: 10.1016/j.foreco.2004.05.049. [DOI] [Google Scholar]
- 19.Leslie AB. Shifting functional roles and the evolution of conifer pollen-producing and seed-producing cones. Paleobiology. 2011;37:587–602. doi: 10.1666/10049.1. [DOI] [Google Scholar]
- 20.Leslie AB. Predation and protection in the macroevolutionary history of conifer cones. Proc Biol Sci. 2011;278:3003–8. doi: 10.1098/rspb.2010.2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rothwell GW, Basinger JF. Metasequoia milleri n. sp., anatomically preserved pollen cones from the Middle Eocene (Allenby Formation) of British Columbia. Can J Bot. 1979;57:958–70. doi: 10.1139/b79-118. [DOI] [Google Scholar]
- 22.Mundry I, Mundry M. Male cones in Taxaceae s. l. – an example of Wettstein's pseudanthium concept. Plant Biol. 2001;3:405–16. doi: 10.1055/s-2001-16466. [DOI] [Google Scholar]
- 23.Hernandez-Castillo GR, Stockey RA, Beard G. Taxodiaceous pollen cones from the early Tertiary of British Columbia, Canada. Int J Plant Sci. 2005;166:339–46. doi: 10.1086/427485. [DOI] [Google Scholar]
- 24.Niklas KJ. The aerodynamics of wind pollination. Bot Rev. 1985;51:328–86. doi: 10.1007/BF02861079. [DOI] [Google Scholar]
- 25.Lu Y, Wang L, Wang D, Wang Y, Zhang M, Jin B, et al. Male cone morphogenesis, pollen development and pollen dispersal mechanism in Ginkgo biloba L. Can J Plant Sci. 2011;91:971–81. doi: 10.4141/cjps2011-036. [DOI] [Google Scholar]
- 26.Wilson ZA, Song J, Taylor B, Yang C. The final split: the regulation of anther dehiscence. J Exp Bot. 2011;62:1633–49. doi: 10.1093/jxb/err014. [DOI] [PubMed] [Google Scholar]
- 27.Smith FG. Morphology of trunk and development of the microsporangium of cycads. Bot Gaz. 1907;43:187–204. doi: 10.1086/329141. [DOI] [Google Scholar]
- 28.Biswas C, Johri BM. The Gymnosperms. Berlin: Springer-Verlag Press, 1997. [Google Scholar]