Abstract
The proper development of fruits is important for the sexual reproduction and propagation of many plant species. The fruit of Arabidopsis derives from the fertilized gynoecium, which initiates at the center of the flower and obtains its final shape, size, and functional tissues through progressive stages of development. Hormones, specially auxins, play important roles in gynoecium and fruit patterning. Cytokinins, which act as counterparts to auxins in other plant tissues, have been studied more in the context of ovule formation and parthenocarpy. We recently studied the role of cytokinins in gynoecium and fruit patterning and found that they have more than one role during gynoecium and fruit patterning. We also compared the cytokinin response localization to the auxin response localization in these organs, and studied the effects of spraying cytokinins in young flowers of an auxin response line. In this addendum, we discuss further the implications of the observed results in the knowledge about the relationship between cytokinins and auxins at the gynoecium.
Keywords: gynoecium, fruit, patterning and development, auxin, cytokinin
Fruits are plant organs that nurture, protect, and facilitate seed dispersal and are therefore very important for successful plant propagation through sexual reproduction in many species. Most fruits originate from the female reproductive organ, the gynoecium, after ovules inside become fertilized by the pollen. From top to bottom, the Arabidopsis gynoecium is formed by a stigma, style, ovary, and gynophore (Fig. 1A). Internally, the ovary contains the ovules that become seed when fertilized and is divided in two by an internal tissue: the septum (Fig. 1B). Externally, the ovary consists of two valves separated by the replum, the external continuation of the septum.1 After fertilization, the Arabidopsis gynoecium produces a long, dry, dehiscent fruit named silique. In siliques, a specialized tissue, the ¨valve margin¨ develops between the valves and replum, and is the site where a mature silique opens to release the seed.2 The gynoecium is the last organ to be formed from the floral meristem, and, in Arabidopsis, it starts as a short tube at the center of the developing flower. This “hollow tube” elongates while two internal ridges (medial ridges) grow toward each other until they fuse and give rise to septum and placenta.3-7 Different internal and external tissues of the gynoecium and later the fruit, such as the ovules, placenta, transmitting tract, septum and replum, style, and stigma also derive from the medial tissues.4,5 Different factors guide the processes that exquisitely shape the gynoecium and fruit, and hormones are an important part of these factors. In particular, auxin is known to be relevant for fruit patterning. The current model involves an apical-basal auxin gradient that specifies the different parts of the gynoecium and later fruit.8 On the other hand, both the lack of auxin and the presence of gibberellin have been reported to be required for proper valve margin formation.9,10 Recently, brassinosteroids have also been implicated in the development of the reproductive tract through which pollen tubes grow to reach the ovules.11,12
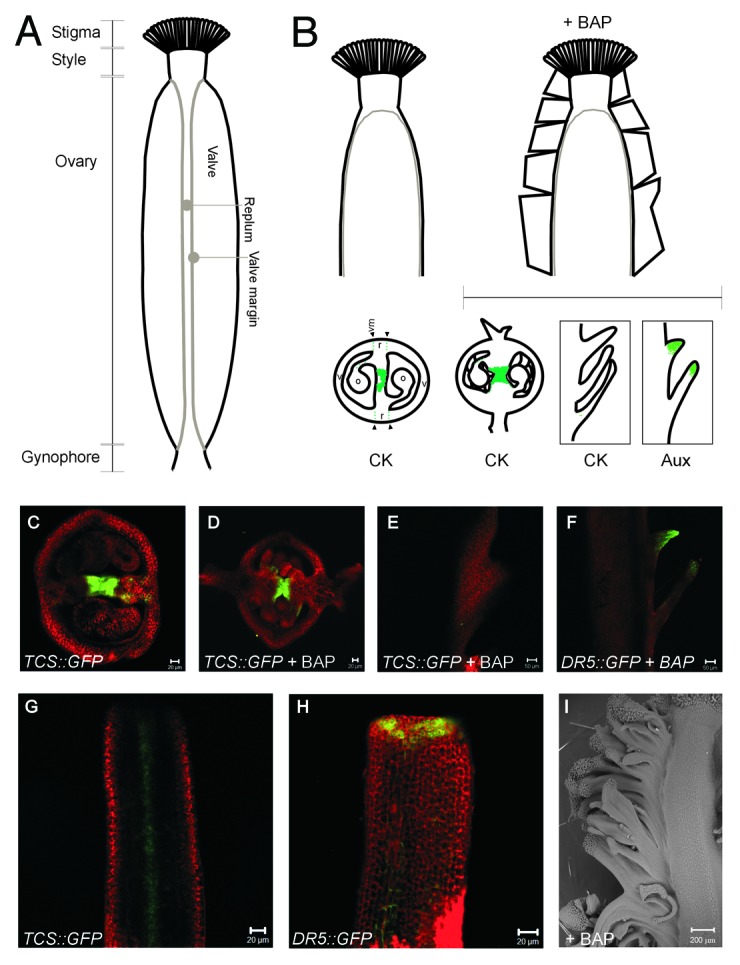
Figure 1. Cytokinin and auxin response in gynoecia and fruits. A) Parts of the Arabidopsis gynoecium. B) Drawings of the side view of a control (left) and BAP sprayed (right) gynoecium. The schemes below represent the localization of the cytokinin (CK) or auxin (Aux) response as indicated. Single plane confocal photographs showing the response to each hormone are also shown: C) transverse section of control TCS::GFP, D) and E) transverse (D) and longitudinal optical (E) sections of BAP sprayed TCS::GFP, F) longitudinal optical section of sprayed DR5rev::GFP. G) and H) Longitudinal confocal photographs (single plane) of young TCS::GFP (G) or DR5rev::GFP (H) gynoecia. I) Scanning electron micrograph of a BAP-sprayed gynoecium, where the ectopic tissue can be observed at the right side. v, valve; o, ovule; r, replum; vm, valve margin.
In other plant tissues, auxins and cytokinins are closely related and together guide the development of different organs.13-17 In the gynoecium, cytokinins promote the growth of the placenta and ovules.18,19 In different species, exogenous application or altered levels of cytokinins in developing flowers and gynoecia can promote the formation of extra floral organs, trichomes in valves, or trigger parthenocarpy.20-23 However, there was little experimental evidence about the natural localization of cytokinin signaling in fruits, its role in fruit patterning, and the positional relationship to auxin signaling. We recently explored these questions in Arabidopsis, where cytokinin appears to fulfill at least two roles during gynoecium and fruit development: Initially, a role in promoting proliferation at the medial region during gynoecium development, and later, an unexpected role at the valve margin during fruit development.25 Here, we speculate further about the relationship and implications of the localization of cytokinin and auxin during gynoecium development.
We compared the patterns of both hormones using the reporter lines TCS::GFP for cytokinin15 and DR5rev::GFP for auxin signaling.24 Complementary patterns were observed, particularly at specific locations and developmental stages such as: a) cytokinin response at the center of the gynoecium, and auxin response around it at early stages of development, and b) presence of cytokinin response and absence of auxin response at the valve margins in mature gynoecia and developing fruits.25 However, while auxin has been proposed to act as a gradient to define the stigma-style, ovary and gynophore regions in the developing gynoecia,8 a clear apical-basal gradient as revealed by the reporter line was not directly evident at the stages and tissues we analyzed: DR5rev::GFP gynoecia showed high fluorescence at the top and low fluorescence below (Fig. 1H). This localization of auxin at the top, as revealed by the synthetic reporter, has been reported before.24,26,27 Based on this, Østergaard (2009) noticed that the auxin localization indicated a two-zone division of the gynoecium.9 Therefore, he complemented the auxin gradient model by proposing that another morphogen, in combination with auxin, could provide positional information to define the stigma-style, ovary and gynophore regions, and suggested that cytokinin, in an inverted gradient, could be this second morphogen.28 In our study, when gynoecia of the TCS::GFP cytokinin signaling marker were visualized longitudinally, an apical-basal gradient of fluorescence was not directly evident at the stages and tissues analyzed (an example of cytokinin localization in a young gynoecium can be observed in Fig. 1G). There appears to be a complementary pattern of both hormones at least in some of the specific tissues and developmental stages visualized, but, as far as we could determine, the marker lines did not reveal evident apical-basal gradients that converged at the same tissue at the same stage. It may still be that the current marker lines used do not allow the proper detection of the gradients in these tissues; that the gradients can only be observed at the earliest stages of development, for only a brief time; or that the gradients are so steep or subtle, that more detailed analysis are required to detect them. In any case, the lack of visualization of gradients keeps open the question of whether the patterning of the gynoecium and fruit is guided through these gradients or whether the hormones analyzed, very relevant for gynoecium development and related to each other, are not necessarily organized as gradients to perform their functions in gynoecium and fruit patterning.
We also reported that cytokinin treatments induce overproliferation in the replum of developing gynoecia (Fig. 1I), and analyzed the pattern of the TCS::GFP and DR5rev::GFP markers in treated gynoecia. Interestingly, the cytokinin signaling pattern as revealed by the TCS::GFP marker showed a change in intensity at the center of the gynoecium, and was less evident at the new, external tissue (Fig. 1B to E). In contrast, DR5rev::GFP gynoecia showed intense fluorescence at the tips of the new ectopic proliferating tissue induced by cytokinin (Fig. 1B and F). Recently, Yoshida and collaborators showed that the light environment controls lateral organ initiation in the shoot apical meristem through the regulation of cytokinin and auxin, and proposed that “cytokinin is required for meristem propagation, while auxin redirects cytokinin-inducible meristem growth toward organ formation.”29 The medial tissues, the region where cytokinin signaling was observed in developing gynoecia, are considered to have meristematic properties.4-6,30 Are we observing in the cytokinin-treated young gynoecia, a similar process to what Yoshida et al. propose to naturally occur in the shoot apical meristem? The external growth of the medial tissue could be explained by the cytokinin-inducible promotion of the meristematic activity of the medial tissue, which is then re-directed by auxin to produce a new ectopic “lateral organ.” We can then speculate whether a similar cross-talk is naturally relevant for the development of the gynoecium.
Conclusions
In conclusion, the analysis of auxin and cytokinin response localization patterns and the effects that cytokinin treatments have on the auxin response marker suggest that, as has been observed in other tissues, these pathways are related, and possibly, we could speculate that their cross-talk might be shaping the gynoecium. More detailed experiments can help to better understand the mechanisms by which both pathways communicate, and the relevance of this communication in the patterning of the gynoecium and fruit.
Acknowledgments
IRO, PLS, and VZM were supported by Mexican National Council of Science and Technology (CONACyT) fellowships (210085, 219883, and 210100, respectively). This work was financed by the CONACyT grant 82826.
Glossary
Abbreviations:
- TCS
Two Component System
- GFP
GREEN FLUORESCENT PROTEIN
- CK
cytokinin
- Aux
auxin
- BAP
benzylaminopurine
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/22422
References
- 1.Sundberg E, Ferrándiz C. Gynoecium Patterning in Arabidopsis: A Basic Plan behind a Complex Structure. In: Østergaard L, ed. Annual Plant Reviews Volume 38: Fruit Development and Seed Dispersal Oxford, UK: Blackwell Publishing Ltd, 2010:doi: 10.1002/9781444314557.ch2. [Google Scholar]
- 2.Ferrándiz C. Regulation of fruit dehiscence in Arabidopsis. J Exp Bot. 2002;53:2031–8. doi: 10.1093/jxb/erf082. [DOI] [PubMed] [Google Scholar]
- 3.Roeder AHK, Ferrándiz C, Yanofsky MF. The role of the REPLUMLESS homeodomain protein in patterning the Arabidopsis fruit. Curr Biol. 2003;13:1630–5. doi: 10.1016/j.cub.2003.08.027. [DOI] [PubMed] [Google Scholar]
- 4.Girin T, Sorefan K, Ostergaard L. Meristematic sculpting in fruit development. J Exp Bot. 2009;60:1493–502. doi: 10.1093/jxb/erp031. [DOI] [PubMed] [Google Scholar]
- 5.Balanzá V, Navarrete M, Trigueros M, Ferrándiz C. Patterning the female side of Arabidopsis: the importance of hormones. J Exp Bot. 2006;57:3457–69. doi: 10.1093/jxb/erl188. [DOI] [PubMed] [Google Scholar]
- 6.Alonso-Cantabrana H, Ripoll JJ, Ochando I, Vera A, Ferrándiz C, Martínez-Laborda A. Common regulatory networks in leaf and fruit patterning revealed by mutations in the Arabidopsis ASYMMETRIC LEAVES1 gene. Development. 2007;134:2663–71. doi: 10.1242/dev.02864. [DOI] [PubMed] [Google Scholar]
- 7.Smyth DR, Bowman JL, Meyerowitz EM. Early flower development in Arabidopsis. Plant Cell. 1990;2:755–67. doi: 10.1105/tpc.2.8.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nemhauser JL, Feldman LJ, Zambryski PC. Auxin and ETTIN in Arabidopsis gynoecium morphogenesis. Development. 2000;127:3877–88. doi: 10.1242/dev.127.18.3877. [DOI] [PubMed] [Google Scholar]
- 9.Arnaud N, Girin T, Sorefan K, Fuentes S, Wood TA, Lawrenson T, et al. Gibberellins control fruit patterning in Arabidopsis thaliana. Genes Dev. 2010;24:2127–32. doi: 10.1101/gad.593410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sorefan K, Girin T, Liljegren SJ, Ljung K, Robles P, Galván-Ampudia CS, et al. A regulated auxin minimum is required for seed dispersal in Arabidopsis. Nature. 2009;459:583–6. doi: 10.1038/nature07875. [DOI] [PubMed] [Google Scholar]
- 11.Poppenberger B, Rozhon W, Khan M, Husar S, Adam G, Luschnig C, et al. CESTA, a positive regulator of brassinosteroid biosynthesis. EMBO J. 2011;30:1149–61. doi: 10.1038/emboj.2011.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crawford BC, Yanofsky MF. HALF FILLED promotes reproductive tract development and fertilization efficiency in Arabidopsis thaliana. Development. 2011;138:2999–3009. doi: 10.1242/dev.067793. [DOI] [PubMed] [Google Scholar]
- 13.Ruzicka K, Simásková M, Duclercq J, Petrásek J, Zazímalová E, Simon S, et al. Cytokinin regulates root meristem activity via modulation of the polar auxin transport. Proc Natl Acad Sci U S A. 2009;106:4284–9. doi: 10.1073/pnas.0900060106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pernisová M, Klíma P, Horák J, Válková M, Malbeck J, Soucek P, et al. Cytokinins modulate auxin-induced organogenesis in plants via regulation of the auxin efflux. Proc Natl Acad Sci U S A. 2009;106:3609–14. doi: 10.1073/pnas.0811539106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Müller B, Sheen J. Cytokinin and auxin interaction in root stem-cell specification during early embryogenesis. Nature. 2008;453:1094–7. doi: 10.1038/nature06943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao Z, Andersen SU, Ljung K, Dolezal K, Miotk A, Schultheiss SJ, et al. Hormonal control of the shoot stem-cell niche. Nature. 2010;465:1089–92. doi: 10.1038/nature09126. [DOI] [PubMed] [Google Scholar]
- 17.Jones B, Gunnerås SA, Petersson SV, Tarkowski P, Graham N, May S, et al. Cytokinin regulation of auxin synthesis in Arabidopsis involves a homeostatic feedback loop regulated via auxin and cytokinin signal transduction. Plant Cell. 2010;22:2956–69. doi: 10.1105/tpc.110.074856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ashikari M, Sakakibara H, Lin S, Yamamoto T, Takashi T, Nishimura A, et al. Cytokinin oxidase regulates rice grain production. Science. 2005;309:741–5. doi: 10.1126/science.1113373. [DOI] [PubMed] [Google Scholar]
- 19.Bartrina I, Otto E, Strnad M, Werner T, Schmülling T. Cytokinin regulates the activity of reproductive meristems, flower organ size, ovule formation, and thus seed yield in Arabidopsis thaliana. Plant Cell. 2011;23:69–80. doi: 10.1105/tpc.110.079079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vivian-Smith A, Koltunow AM. Genetic analysis of growth-regulator-induced parthenocarpy in Arabidopsis. Plant Physiol. 1999;121:437–51. doi: 10.1104/pp.121.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greenboim-Wainberg Y, Maymon I, Borochov R, Alvarez J, Olszewski N, Ori N, et al. Cross talk between gibberellin and cytokinin: the Arabidopsis GA response inhibitor SPINDLY plays a positive role in cytokinin signaling. Plant Cell. 2005;17:92–102. doi: 10.1105/tpc.104.028472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gordon SP, Chickarmane VS, Ohno C, Meyerowitz EM. Multiple feedback loops through cytokinin signaling control stem cell number within the Arabidopsis shoot meristem. Proc Natl Acad Sci U S A. 2009;106:16529–34. doi: 10.1073/pnas.0908122106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lindsay DL, Sawhney VK, Bonham-Smith PC. Cytokinin-induced changes in CLAVATA1 and WUSCHEL expression temporally coincide with altered floral development in Arabidopsis. Plant Sci. 2006;170:1111–7. doi: 10.1016/j.plantsci.2006.01.015. [DOI] [Google Scholar]
- 24.Benková E, Michniewicz M, Sauer M, Teichmann T, Seifertová D, Jürgens G, et al. Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell. 2003;115:591–602. doi: 10.1016/S0092-8674(03)00924-3. [DOI] [PubMed] [Google Scholar]
- 25.Marsch-Martínez N, Ramos-Cruz D, Irepan Reyes-Olalde J, Lozano-Sotomayor P, Zúñiga-Mayo VM, de Folter S. The role of cytokinin during Arabidopsis gynoecia and fruit morphogenesis and patterning. Plant J. 2012;72:222–234. doi: 10.1111/j.1365-313X.2012.05062.x. [DOI] [PubMed] [Google Scholar]
- 26.Aloni R, Aloni E, Langhans M, Ullrich CI. Role of auxin in regulating Arabidopsis flower development. Planta. 2006;223:315–28. doi: 10.1007/s00425-005-0088-9. [DOI] [PubMed] [Google Scholar]
- 27.Girin T, Paicu T, Stephenson P, Fuentes S, Körner E, O’Brien M, et al. INDEHISCENT and SPATULA interact to specify carpel and valve margin tissue and thus promote seed dispersal in Arabidopsis. Plant Cell. 2011;23:3641–53. doi: 10.1105/tpc.111.090944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Østergaard L. Don’t ‘leaf’ now. The making of a fruit. Curr Opin Plant Biol. 2009;12:36–41. doi: 10.1016/j.pbi.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 29.Yoshida S, Mandel T, Kuhlemeier C. Stem cell activation by light guides plant organogenesis. Genes Dev. 2011;25:1439–50. doi: 10.1101/gad.631211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alvarez J, Smyth DR. CRABS CLAW and SPATULA genes regulate growth and pattern formation during gynoecium development in Arabidopsis thaliana. Int J Plant Sci. 2002;163:17–41. doi: 10.1086/324178. [DOI] [Google Scholar]


