Abstract
Hemolysis occurs in many hematologic and nonhematologic diseases. Extracellular hemoglobin (Hb) has been found to trigger specific pathophysiologies that are associated with adverse clinical outcomes in patients with hemolysis, such as acute and chronic vascular disease, inflammation, thrombosis, and renal impairment. Among the molecular characteristics of extracellular Hb, translocation of the molecule into the extravascular space, oxidative and nitric oxide reactions, hemin release, and molecular signaling effects of hemin appear to be the most critical. Limited clinical experience with a plasma-derived haptoglobin (Hp) product in Japan and more recent preclinical animal studies suggest that the natural Hb and the hemin-scavenger proteins Hp and hemopexin have a strong potential to neutralize the adverse physiologic effects of Hb and hemin. This includes conditions that are as diverse as RBC transfusion, sickle cell disease, sepsis, and extracorporeal circulation. This perspective reviews the principal mechanisms of Hb and hemin toxicity in different disease states, updates how the natural scavengers efficiently control these toxic moieties, and explores critical issues in the development of human plasma–derived Hp and hemopexin as therapeutics for patients with excessive intravascular hemolysis.
Introduction
When hemoglobin (Hb) bursts from RBCs because of hemolysis, the naked Hb, devoid of its antioxidant sentries that are normally available within the RBC, can wreak oxidative havoc in the vasculature and in exposed tissues.1 To neutralize Hb and its reactive ferric protoporphyrin-IX group (hemin), specialized plasma scavenger proteins sequester the toxic moieties and transit them to compartments where heme-oxygenases can break down hemin into less toxic metabolites. Other molecules and reducing substances contribute to this protective physiology. However, when these clearance and detoxifying systems are overwhelmed by intravascular hemolysis, such as during sickle cell disease, blood transfusion, malaria, or sepsis, Hb and hemin trigger vascular and organ dysfunction that leads to adverse clinical effects (Figure 1). This perspective reviews the mechanisms of Hb toxicity in different disease states, updates how haptoglobin (Hp) and hemopexin (Hpx) efficiently handle free Hb and hemin, and explores why the time has come to consider these proteins as therapeutics in patients with excessive intravascular hemolysis.
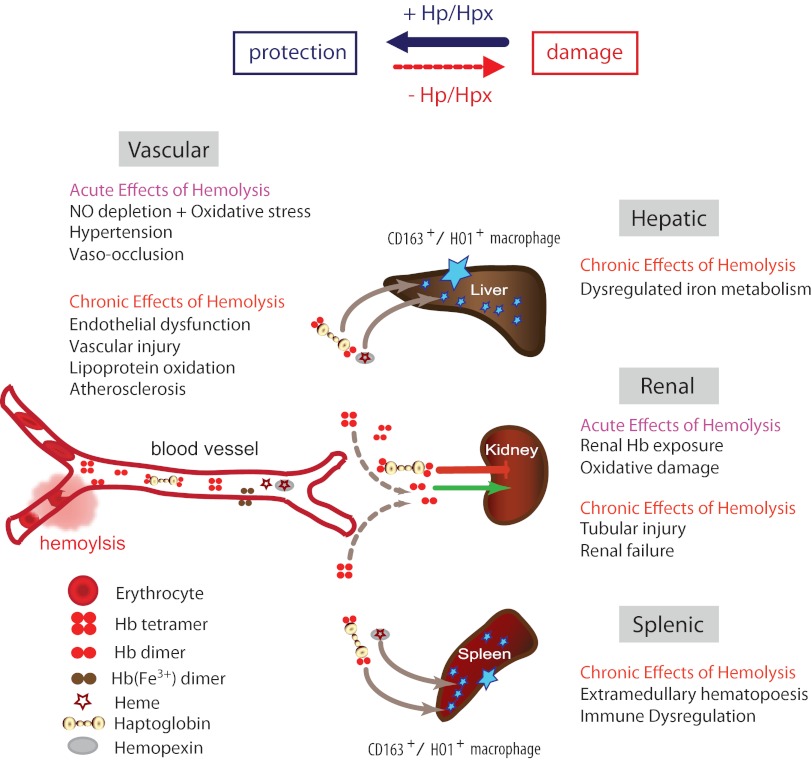
Figure 1.
Schematic summary of the Hb clearance compartments and the main acute and chronic pathologies that can be associated with intravascular hemolysis. The availability of the Hb and hemin scavenger proteins Hp and Hpx shifts the physiologic balance from tissue damage toward protection.
Extracellular Hb and hemin are multicomponent triggers of disease processes
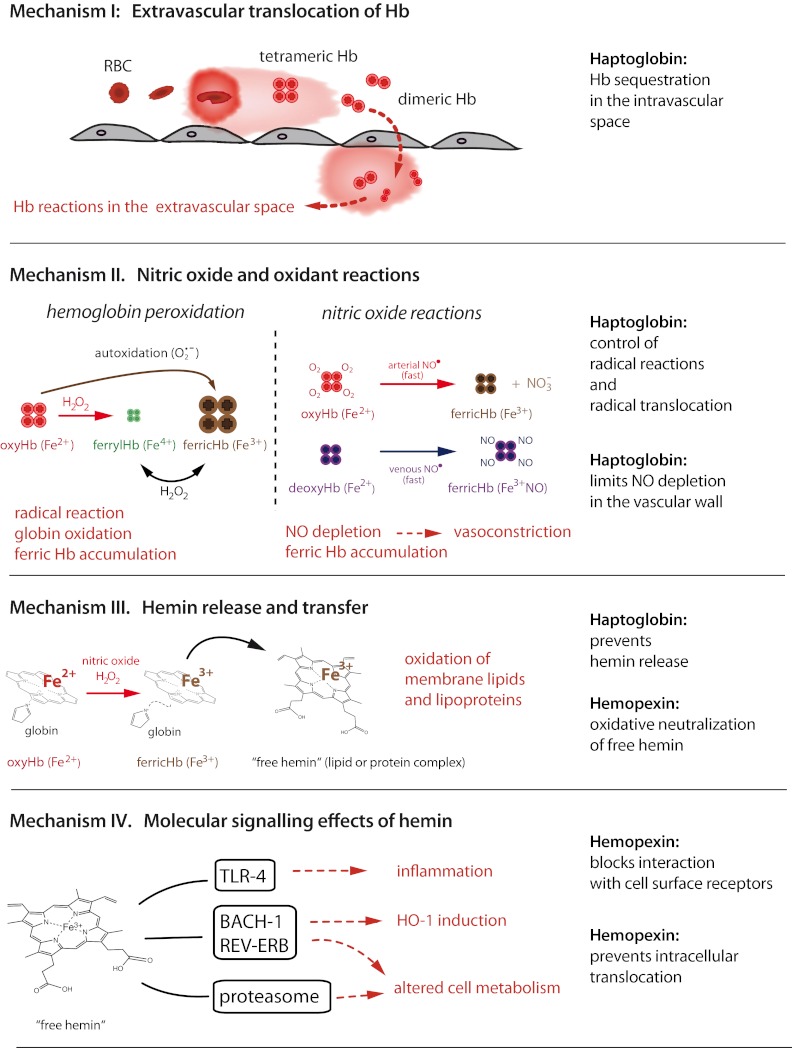
Adverse clinical effects associated with excessive free Hb can be attributed to several specific structural and biochemical properties of the Hb molecule and are caused by the following 4 mutually interacting mechanisms: (1) extravascular translocation of Hb, which is a principal requirement that Hb and hemin can unleash their adverse reactivity in tissues; (2) nitric oxide and oxidative reactions; (3) release of free hemin; and (4) molecular-signaling effects of hemin. These mechanisms are outlined in the subsequent sections and are summarized in Figure 2.
Figure 2.
Schematic summary of the principal mechanisms of Hb toxicity and protection by the plasma scavenger proteins Hp and Hpx.
Mechanism 1: extravascular translocation of Hb
After hemolysis, Hb exists in a dynamic equilibrium of tetramer and αβ-subunit heterodimers, with a predominant dimer state at low plasma Hb concentrations. αβ-Dimers are of a relatively small molecular size (32 kDa), allowing for protein translocation and access to vulnerable anatomic sites (eg, the kidneys and vascular wall). Tissue exposure to Hb is most evident in cases of overt hemoglobinuria after massive intravascular hemolysis, but Hb is also capable of translocating across endothelial barriers, entering the subendothelial and perivascular spaces and the lymph fluid.2,3
Mechanism 2: NO and oxidant reactions
A second mechanism of Hb toxicity is the prooxidative reactivity of Hb in plasma or within tissues after extravasation. The Hb reactions with nitric oxide (NO) and with physiologic oxidants (eg, hydrogen peroxide and lipid peroxides) are the most extensively studied. NO consumption and subsequent Hb oxidation occurs via 2 reactions: (1) NO dioxygenation of oxy-Hb, which generates nitrate (NO3−) and ferric Hb (Hb-Fe3+), and (2) iron nitrosylation of deoxy-Hb, which occurs by direct iron binding of NO to nonliganded ferrous Hb (Hb-Fe2+).
The current understanding of the biochemistry and pathophysiology of these reactions have largely been derived from the study of Hb-based oxygen carriers (HBOCs) and their well-documented adverse effects on hemodynamics.4 Targeted mutagenesis of Hb aimed at limiting interactions with NO or chemical modifications to limit access to sites of NO bioavailability within the vascular wall (eg, by chemical cross-linking of Hb into large polymers or surface-decorated conjugates) attenuate vasoconstriction and hypertension.5,6 Therefore, NO depletion by extracellular Hb is now a widely accepted hypothesis to explain the acute hypertensive response that occurs during massive hemolysis (reaching moderate to high plasma levels of free Hb) or during HBOC infusion.6,7 In addition to vasodilator depletion, another result of Hb-NO reactions might be the generation of Hb-Fe3+ within tissue parenchyma. Accumulation of Hb-Fe3+ within tissues may promote hemin release and/or transfer of hemin to other proteins/lipids with secondary toxicity driven by the free hemin.
The biochemistry of the Hb reaction with peroxides has been scrutinized over the past 40 years,8 but the significance of these reactions for Hb- and hemin-driven pathophysiology is still poorly defined. The assumption that oxidative Hb side reactions could be an important determinant of Hb toxicity was based on observations that peroxides are formed and released into the extracellular space in relatively large quantities during inflammation and ischemia-reperfusion. Under in vitro conditions, the Hb reaction with peroxide results in the formation of Hb-Fe3+, higher oxidation iron species such as ferryl Hb (Hb-Fe4+), and associated radicals (Figure 2 mechanism II). It has been suggested that globin-chain free radicals are available for localized amino acid oxidations (eg, within Hb) or radical transfer to non-Hb molecules (eg, lipoproteins).9,10 The proposed final outcome of these reactions is Hb self-destruction, hemin loss, and globin chain cross-linking/precipitation, which can ultimately lead to tissue damage.11 It should be noted that the putative impact of these reactions on disease conditions is based on limited and indirect experimental evidence, so it remains uncertain whether significant quantities of Hb-Fe4+ and radicals are generated during in vivo hemolysis and if they contribute to disease. The only oxidized Hb species that can be consistently quantified in vivo are Hb-Fe3+ and hemichrome, a structurally distorted form of Hb-Fe3+. The disparity between in vitro biochemical observations and in vivo findings may be because of the shifted balance between oxidation and reduction reactions in in vivo conditions.12 The availability of large quantities of small-molecule and enzymatic reductants may reduce the stability of higher-oxidation-state Hb species and Hb-derived radicals to undetectable levels. Intramolecular Hb cross-links, porphyrin-globin covalent adducts, and globin chain amino acid oxidations have been defined as surrogate markers for Hb-Fe4+ formation. Such modifications have been found in Hb recovered from the spinal fluid after subarachnoid hemorrhage and from the urine, suggesting that peroxidative reactions may contribute to Hb toxicity in vivo.13,14
Mechanism 3: hemin release and transfer
A third mechanism of Hb toxicity is through release of hemin from Hb-Fe3+, which is the main product of the oxidative reactions described in the previous section. Hemin release allows for transfer of the reactive porphyrin to cell membranes or soluble plasma proteins and lipids and provides free hemin as a ligand for molecular signaling interactions. As a hydrophobic molecule, it is unlikely that significant quantities of free, monomeric hemin can be present in the plasma. Therefore, transferred hemin in the form of low-affinity hemin protein (eg, hemin-albumin) or hemin-lipid complexes are the most likely physiologic end products of hemin release. Depending on the protein or lipid environment, free iron-protoporphyrin can function as an intermediate and transform the recipient molecule into a reactive end product. The most identifiable toxic end product of hemin release is oxidized low-density lipoprotein (oxLDL).15 LDL oxidation and the associated inflammatory and cytotoxic activities represents a critical example of the ability of Hb to induce vascular injury.16,17
Mechanism 4: molecular signaling effects of hemin
Hemin can selectively bind to several receptors, transcription factors, and enzymes and thereby alter cell activation state, gene transcription, and metabolism. The most well-defined interaction is the binding of hemin to the transcriptional repressor Bach-1, which regulates transcription of heme-oxygenase 1 (HO-1) and other antioxidant enzymes essential for the adaptive response to enhanced intracellular hemin levels.18 Hemin is also a ligand of the nuclear hormone receptor REV-ERB, which regulates circadian rhythm, glucose metabolism, and adipogenesis.19 Inhibition of the proteasome by hemin and by some designed hydrophilic porphyrins has thus far been documented in biochemical assays only.20,21 However, if confirmed in biologic systems, this activity may help explain aspects of hemin toxicity. In addition, activation of TLRs and downstream inflammatory signaling, particularly of the TLR-4 pathway, can be triggered by free hemin in some models.22–24
In summary, the definitive pathophysiology of extracellular Hb is dependent on timing, quantity, and tissue localization of Hb/hemin exposure in a specific clinical condition and may result from cumulative effects largely described by the 4 mechanisms discussed in the sections above. For example, systemic and, to some extent, pulmonary hypertension is the most apparent and readily measurable effect of free Hb after intravascular hemolysis. The mechanisms driving acute elevations in blood pressure are Hb translocation into subendothelial spaces, local NO depletion within the vessel wall, and subsequent vasoconstriction. Vascular complications of chronic and/or intermittent Hb exposure are likely to be more complicated, involving inflammation, localized oxidative reactions, thrombosis, vascular remodeling, and renal impairment.25–28 As outlined in the following sections, all 4 mechanisms of Hb and hemin toxicity are specifically attenuated by the natural scavenger proteins Hp and Hpx, explaining the extraordinary protective function of these molecules.
Biochemistry and physiology of Hb and hemin-scavenger proteins
During mild to moderate hemolysis, a network of scavenger proteins, receptors, and enzymes accomplishes clearance and detoxification of extracellular Hb and hemin. The primary clearance pathway involves transport to the liver parenchyma or macrophages, breakdown of the porphyrin by the heme-oxygenases into bilirubin, carbon monoxide and iron, and recovery of the iron for de novo erythropoiesis. Scavenger proteins attenuate the toxicity of Hb and hemin so that these potentially toxic molecules are captured and transported in an inert form. The plasma proteins with the most promising therapeutic potential are Hp and Hpx. These proteins are discussed in more detail in the following sections.
Hp
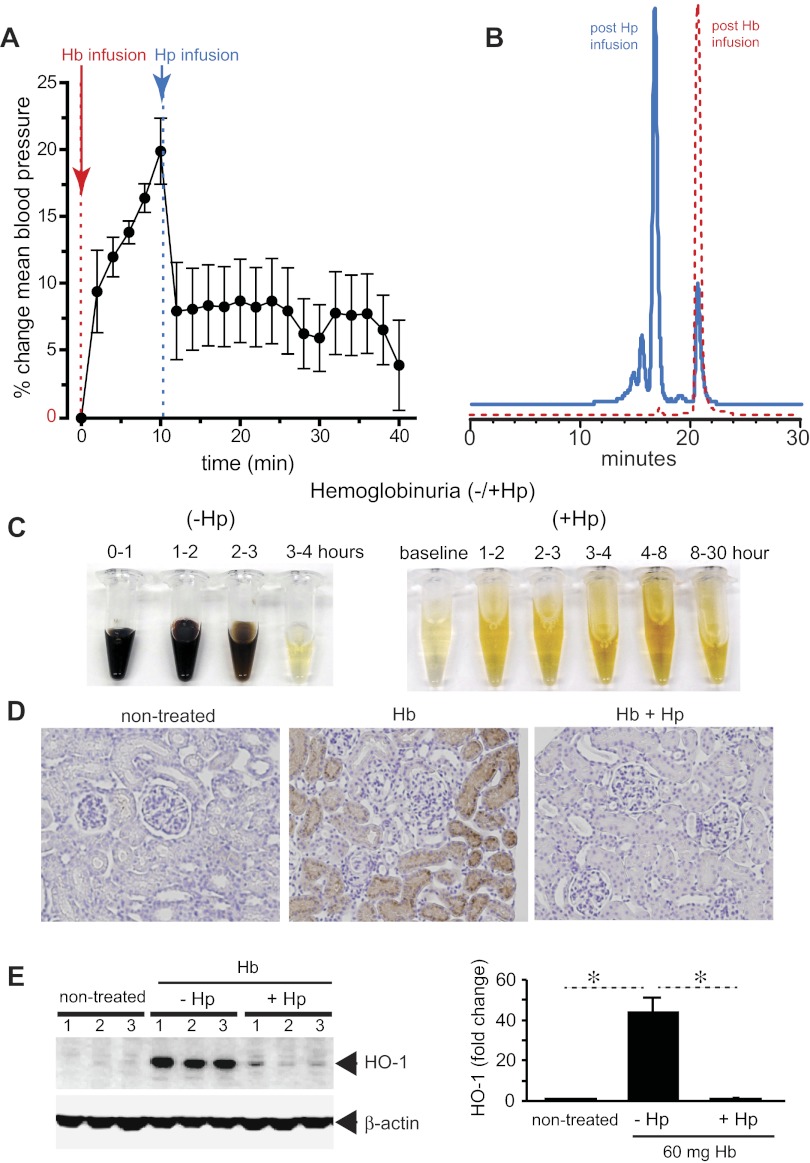
Hp fundamentally changes the biochemical and physiologic profile of free Hb.29 When bound within the large molecular size Hb:Hp complex (> 150 kDa), Hb remains sequestered within the intravascular space and its translocation into the kidney and across the endothelial layer appears to be prevented. This simple mechanism keeps potentially adverse biochemical reactions of the free Hb with NO and/or peroxides away from the most susceptible anatomic sites such as the vascular wall. Intravascular sequestration appears to be the most effective way by which Hp prevents Hb-induced hypertension and renal damage (Figure 3). In addition, Hb remains contained within the reducing (ie, antioxidant-rich) environment of the blood plasma until monocyte and macrophage clearance is complete.
Figure 3.
Hp sequestration of Hb. Guinea pigs were infused with stroma-free Hb (peak plasma Hb concentration, 150μM heme) and, after 10 minutes, a treatment group of animals was infused with human plasma–derived Hp to match an equimolar Hb:Hp concentration. (A) Mean arterial blood pressure response before and after Hp treatment. (B) Unbound plasma Hb (red) before and after Hp administration. The left shift in the chromatogram indicates the large molecular size Hb:Hp complex with approximately 90% Hp bound and 10% unbound Hb. (C) Hemoglobinuria after Hb infusion (150μM heme) without (−Hp, left) and with Hp (+Hp, right). (D) Iron deposition (brown staining) in normal kidney renal cortex (left), Hb infusion (middle), and Hb infusion plus Hp (right). (E) HO-1 expression in kidneys after 24 hours after Hb exposure with and without Hp infusion (left). Densitometry is shown to the right of the HO-1 Western blot. All data are presented as means ± SEM.
Recent in vitro experiments suggest that Hp may also alter Hb's oxidative reactions. Hp decreases the redox potential of bound Hb-Fe3+, stabilizes the higher oxidation state of Hb-Fe4+, and prevents radical transfer to non-Hb molecules in the presence of oxidants.30,31 The recently resolved crystal structure of the porcine Hb:Hp complex provided some structural basis for the protection of critical amino acids that are primary targets of globin oxidation.32 As a result of this protection, globin oxidation with subsequent protein degradation does not occur when Hb is sequestered in the Hb:Hp complex.33 The structural stability of the complex may prevent accumulation of proinflammatory Hb-degradation products that can evade clearance by scavenger receptors.14,34 In addition, hemin resides firmly in the Hb:Hp complex, it cannot transfer to hemin acceptors such as Hpx, lipoproteins, and albumin.35 Therefore, the prevention of hemin transfer is another essential mechanism by which Hp protects against Hb-driven oxidation of membrane lipids and plasma lipoproteins, preventing the accumulation of free hemin.
Genetic heterogeneity of the Hp α-subunit composition in humans allows for the existence of a structural polymorphism with a dimeric Hp 1-1 (α2β2) and heterogeneous multimeric Hp 2-2 and Hp 2-1 (α > 2β > 2) phenotypes.36 Each Hp β chain can bind one Hb αβ dimer, allowing for 2 binding sites in Hp 1-1 and multiple binding sites in Hp 2-2. The protein-binding capacity and the basic biochemical functions of Hp 1-1 and Hp 2-2 are equal because they relate to Hb clearance and detoxification (D.J.S. and P.W.B., submitted manuscript). The molecular basis for the apparent association of the Hp 2-2 genotype with an enhanced risk for cardiovascular diseases in some high-risk patient populations remains unexplained.36,37
A human plasma–derived Hp product was developed by the Japanese Green Cross (now Benesis Corporation) and was approved in 1985 for the treatment of hemolysis due to extracorporeal circulation, burn injuries, and trauma with massive blood transfusions (Table 1). Subsequent findings of several small-scale studies indicated that Hp supplementation could prevent renal tubulus damage during open-heart surgery with cardiopulmonary bypass, HELLP (hemolysis elevated liver enzymes low platelets) syndrome, and after hemolysis complicating endoscopic injection sclerotherapy of esophageal varices with ethanolamine-oleate (Table 1). Case reports and studies in a limited number of patients further support the successful use of Hp in patients with hemolytic anemias (ie, thalassemia and glucose-6-phosphate dehydrogenase deficiency), severe burn injury, in patients with hemolysis resulting from accidental ABO-incompatible RBC transfusion, and as a prophylaxis or treatment for hemolysis after ABO-mismatched BM transplantation. The primary clinical criteria for Hp administration and dosing in these studies were the appearance and reversal of hemoglobinuria with the intent of limiting renal injury. The reported doses of Hp that were administered for treating acute hemolytic conditions were approximately 2 g to > 10 g per patient, with dosing schedules of repeated 2 g (children) or 4 g (adults) boluses. The cost for the Hp therapeutic in Japan is currently at $540 USD per 2-g bolus. To our knowledge, there is so far no reported use of Hp in chronic hemolytic conditions in which repeated dosing over a longer treatment period might be necessary to effectively suppress free plasma Hb levels.
Table 1.
Literature-cited clinical use of haptoglobin in Japan
| Clinical condition | Subjects/controls | Haptoglobin dose | Primary finding (intention of treatment) | Reference |
|---|---|---|---|---|
| Cardiopulmonary bypass surgery | 16/21 | 6 g | Prevention of acute renal failure | Hashimoto et al87 |
| 14/0 | 4 g | Tanaka et al88 | ||
| Coronary artery bypass grafting in a patient with β-thalassemia and severe hemolysis | Single case report | Horai et al89 | ||
| Peripheral blood stem cell transplantation | Single case report | 3 g | Prevention of acute renal failure | Tsuda et al90 |
| Hemolytic transfusion reaction | Single case report | 6 g | Prevention of acute renal failure | Homann et al91 |
| Massive transfusion | 16/34 | 6 g | Undetermined benefit | Gando et al92 |
| Extracorporeal circulation | 10/10 | 6 g | Prevention of acute renal failure | Kanamori et al93 |
| HELLP syndrome | 17/17 | 3 g | Prevention of acute renal failure | Yamamoto et al94 |
| ABO-incompatible BM transplantation | Two patient case reports | 11 g | Prevention of acute renal failure | Ito et al95 |
| Thermal injury | Single case report (repeated, large-dose therapy) | > 20 g | Improved outcome | Imaizumi et al96 |
| Prevention of hemoglobinuria and acute renal failure | Yoshioka et al97 | |||
| Glucose-6-phosphate dehydrogenase deficiency | Single patient case report (24-mo-old boy) | 8.5 g | Prevention of hemoglobinuria | Ohga et al98 |
| Adult patent ductus arteriosus, coil embolization | Single patient case report | 45 g | Prevention of hemoglobinuria | Eda et al99 |
Hpx
Hpx is a hemin-binding plasma glycoprotein that forms the second line of defense against intravascular hemolysis associated with hemin release from Hb-Fe3+. Multiple proteins bind hemin, including albumin (Kd, approximately 10−8M) and the lipoprotein particles LDL/HDL (Kd, 10−10M to 10−11M). However, Hpx (Kd < 10−13M) is the most effective hemin-binding protein that sequesters porphyrin in an oxidatively inert hexacoordinate conformation in a complex with a 1:1 stoichiometry.38–42 The most relevant function of Hpx in plasma and tissues is likely the protection of susceptible lipoproteins against oxidative modifications and limiting hemin interactions with cell-surface receptors such as TLR-4.24,38,43 In hemin exposure models using the Hpx-knockout mouse, the scavenger protein demonstrated attenuation of hemin-induced endothelial cell activation, vasoocclusion in the liver, and renal damage.27,44 In that same model, data suggested that Hpx shifts plasma hemin to endocytosis and decomposition in hepatocytes.
Like Hp levels, Hpx plasma levels are generally depleted in conditions with intravascular hemolysis, suggesting that supplementation of the plasma protein might provide some benefit to patients.45 However, no Hpx product is available at a quality that would allow more extensive in vivo studies beyond murine models. Therefore, expanded studies with Hpx treatment may not be possible in the near future for larger animal models of relevant clinical conditions such as blood transfusion or coronary artery bypass grafting and extracorporeal circulation.
Hb and hemin scavenger receptors
Endocytic receptors clear the biochemically inert circulating Hb:Hp and hemin:Hpx complexes from the circulation. In vitro and ex vivo experiments have demonstrated that macrophages and peripheral blood monocytes, respectively, are the primary cell types that clear Hb and Hb:Hp complexes via binding and internalization of the scavenger receptor CD163.46–49 The strong induction of CD163 by glucocorticoid treatment in patients with autoimmune diseases identified the Hb scavenger receptor as a potential therapeutic target.50 Clearance of “endogenous” Hb:Hp is a rapid process after mild hemolysis; however, data obtained in animal models of Hb:Hp infusion or intravascular hemolysis with therapeutic Hp administration suggest slower clearance depending on the level of Hb:Hp accumulation and on the species evaluated. We observed a long circulation half-life of approximately 12 hours for human and canine Hb:Hp complexes in beagle dogs with starting plasma concentrations of approximately 150μM (heme). The half-life of a human Hb:Hp complex was approximately 16 hours at the same plasma concentration in guinea pigs (D.J.S. and P.W.B., unpublished data). Recent work indicates that the CD163 binding specificity for Hb and the Hb:Hp complex, respectively, has considerable interspecies variability, so the threshold for saturation of clearance mechanisms in humans and some laboratory animals might differ significantly.51 It will therefore be critical for early human trials to define the pharmacokinetics of the Hb:Hp complex at different intravascular concentrations that match conditions of single or repeated Hp administration during acute, intermittent, and chronic hemolysis. A clearance concept similar to that of the Hb:Hp complex has been proposed for clearance of the hemin-Hpx complex, which is bound and internalized by the LDL receptor-related protein (LRP)/CD91 on hepatocytes, where hemin is degraded by HO-1.52,53
Depletion of Hp and Hpx complexes via their cognate receptors during acute and chronic intravascular hemolysis promotes the pathophysiologic consequences outlined in Figure 1, which we hypothesize could be interrupted by supplementation.
The role of Hb, heme, and their scavenger proteins in specific human diseases
Sickle cell disease and other hematologic diseases.
Hemolysis and vasoocclusion are the hallmarks of sickle cell disease. Intravascular hemolysis accounts for one-third of RBC destruction leading to increases in plasma free Hb and hemin. In the 1960s, it was recognized that plasma levels of free Hb can be as high as 25μM during sickle cell crisis, with basal plasma Hb levels at 5-10μM in sickle cell patients. Hp and Hpx levels were found to be depleted.45 Low Hp and increased plasma free Hb levels were associated with increased protein carbonyl and nitrotyrosine levels in sickle cell anemia.54 Clinically, uncomplicated pain episodes were found to be associated with increases in plasma hemoglobin levels,55,56 and low levels of Hp were correlated with pulmonary hypertension in sickle cell anemia.57 Although the reported plasma Hb levels in sickle cell patients are much lower than the plasma Hb levels that have been explored in hemodynamic studies of HBOC administration and some animal models of severe hemolysis, these data suggest that free Hb could be a strong pathophysiologic component of the vascular complications of sickle cell disease.
Pathophysiologic hypotheses have focused on the causal relationship among Hb-mediated NO consumption, vascular complications, and increased pulmonary artery pressure. In these critical studies, an increased tricuspid valve jet regurgitation (TRV) of > 2.5 m/s was defined as a surrogate marker of pulmonary hypertension in sickle cell patients. Increased TRV was found in 30% of patients with sickle cell disease and was well correlated with markers of hemolysis.58 Generally, plasma Hb levels in sickle cell patients are orders of magnitude lower than the levels that were studied in classic experimental models of Hb- or HBOC-mediated NO depletion and vasoconstriction. However, free Hb concentrations of 6μM impaired the vasodilatory response to nitroprusside infusion, suggesting that the low free plasma Hb concentrations in sickle cell patients could still critically limit NO bioavailability and promote vasoconstriction, platelet activation, and coagulopathy.59–61 Cumulatively, these effects were hypothesized to be causative for pulmonary hypertension and other vascular complications. More recently, 2 independent studies from France62 and Brazil63 used right heart catheterization (the gold standard for diagnosing pulmonary hypertension) in addition to echocardiography for the evaluation of sickle cell patients. These French and Brazilian studies confirmed that a large proportion of sickle patients have increased TRV, but in only 6% and 10% of the patients, respectively, was pulmonary hypertension confirmed by right heart catheterization. Even fewer patients (2.75% and 3.75%) had a precapillary pattern of pulmonary hypertension, which would be expected as the typical hemodynamic pattern if NO depletion and vasoconstriction within the pulmonary circulation were the primary pathophysiologic mechanism. In both studies, the levels of lactate dehydrogenase were significantly higher in patients with confirmed pulmonary hypertension. It was therefore concluded that pulmonary hypertension in sickle cell patients is caused by a complex, multifactorial pathophysiology that may involve NO depletion, vasoconstriction, thromboembolic vascular occlusion, left ventricular impairment, chronic vascular remodeling, and secondary hemodynamic effects caused by the hyperdynamic circulation that occurs in many patients with more severe or chronic anemia.64,65 The quantitative contributions of these individual factors and the specific role of free Hb and hemin in the pathophysiology of vascular complication in sickle cell disease remain an important area for further mechanistic evaluation in clinical settings.
Murine models of sickle cell disease continue to provide support for a critical role of hemolysis and free Hb and hemin, respectively, in vasoocclusive complications.66 Sickle mice have intravascular hemolysis, as evidenced by elevated plasma Hb, decreased Hp and Hpx, and elevated reticulocytes and plasma bilirubin. In studies using a model of vascular stasis, induction or overexpression of HO-1 inhibited the vascular stasis induced by hypoxia.67–69 Furthermore, infusion of hemoglobin or heme into sickle mice induced vascular stasis that could be inhibited by Hp or Hpx supplementation.70 In the prooxidative environment of sickle cell anemia, Hb-Fe2+ may react with NO or other oxidants to generate Hb-Fe3+, from which hemin is released.69 In the absence of Hp and Hpx, plasma heme can interact with albumin or other plasma proteins and/or lipoproteins, but ultimately heme is distributed to cells in the vasculature, including endothelial cells and leukocytes. The hydrophobicity of heme allows it to enter cell membranes and activate TLR-4–signaling pathways that trigger proinflammatory and prothrombotic responses, thereby promoting blood cell adhesion and vasoocclusion.24,70
The epidemiologic and experimental data support a concept whereby substitution of Hp and/or Hpx could attenuate or prevent complications associated with accelerated intravascular hemolysis in sickle cell disease.
There are many other hematologic diseases in which hemolysis constitutes a primary disease process (eg, thalassemia, paroxysmal nocturnal hemoglobinuria, glucose-6-phosphate dehydrogenase deficiency) or a secondary phenomenon (eg, hemolytic uremic syndrome or thrombotic thrombocytopenic purpura). Although the contribution of free Hb and hemin toxicity to disease outcome has not been explored in this wider arena of hemolytic disorders, the broader availability of Hp and Hpx will provide us with the required tools to systematically explore the therapeutic potential of Hb/hemin neutralization in these multifactorial diseases.
Blood transfusion.
Retrospective data analyses suggest that transfusion of older stored blood (approaching 42 days) results in increased morbidity and mortality compared with newer blood.71 Increased morbidity has typically been associated with immune suppression, infection, cardiovascular complications, and acute/chronic kidney injury. One of the most essential consequences of the so-called “RBC storage lesion” is related to reduced deformability, enhanced in vivo fragility, and susceptibility of RBCs to attack by components of the immune system. These changes may be accompanied by increased hemolysis of older storage RBCs.
Although the clinical impact of blood storage duration on clinical outcome remains controversial, numerous models have been developed to evaluate the effects of hemolysis and Hb toxicity.72–75 Guinea pigs subjected to massive transfusion (the equivalent of > 10 units) of maximally stored guinea pig blood demonstrated increased plasma Hb concentrations over a 24-hour period, which resulted from posttransfusion hemolysis.76 This was associated with increased hemoglobinuria, acute renal failure, hypertension, and vascular injury. Hp coinfusion with older storage blood resulted in attenuation of adverse renal and vascular effects to resemble that of new blood transfusion. These observations are supported by several studies suggesting that blood transfusion in mice, rats, and sheep are associated with increased blood pressure, NO depletion, and increased plasma Hb concentrations.74,75,77 Hb-related transfusion complications in humans have also been associated with ABO mismatch and massive transfusion (> 10 units/24 hours).
Clinically, Hp has been used to treat adverse transfusion outcomes in Japan, and the primary reported outcome of therapeutic Hp treatment appears to be prevention of renal tubular injury (Table 1).
Sepsis and malaria.
Hemolysis can also occur during severe bacterial infection and sepsis, and depletion of Hpx has been associated with more severe disease and fatal outcome.78 Within these complex disease processes, hemin could act as an activator of innate immunity via pattern recognition receptor activation (eg, TLR-4), via synergistic effects with microbial products or endogenous pro-inflammatory ligands (eg, HMGB1), or via peroxidative tissue damage.24,79,80 Studies in mice suggested that the net effect of free hemin in polymicrobial sepsis could be detrimental and that neutralization of hemin by Hpx administration ameliorates this effect.78
The protective action of Hpx in sepsis may involve the control of excessive hemin peroxidative activity, inhibition of innate immunity receptor interactions, and redirection of free hemin away from susceptible tissues toward clearance in the liver. Restriction of hemin-iron access by Hb and hemin scavenger proteins could be another protective mechanism against severe infections by some bacterial strains.81,82 The in vivo activity of Hpx may also involve Hpx activities that are independent of its primary hemin scavenger function. The outcome of such activities has been noted as anti-inflammatory in some cases (eg, Hpx attenuates inflammatory cytokine secretion by lipopolysaccharide-stimulated macrophages83), whereas impaired bacterial clearance was found as a consequence of suppressed neutrophil migration in other mouse studies.84 At this point, it is unknown whether such non-hemin–related effects of Hpx are an inherent activity of the protein itself or if they are side effects caused by impurities or degradation products in some preparations of plasma-derived Hpx.85
In mouse models of malaria, Hb/hemin-driven oxidative damage and NO depletion were linked to the evolution of cerebral malaria.86 However, although we suspect that this pathophysiology could be attenuated by Hp and/or Hpx treatment, the efficacy of scavenger proteins has not yet been tested for this specific condition.
Conclusion: is there an unmet need for scavenger protein-based therapeutics?
Multiple hematologic and nonhematologic disease states are associated with RBC lysis, and the adverse effects of free Hb and hemin potentially complicate clinical disease outcome. There is currently no viable therapy designed to attenuate the adverse effects of free Hb and hemin. Replacement or supraphysiologic dosing of Hp and/or Hpx to match acute or ongoing hemolysis may be of therapeutic benefit by attenuating many of the pathophysiologies that we discuss in this perspective and several clinical situations in which Hb contributes to acute (eg, acute kidney injury) or chronic (eg, vascular remodeling) sequelae. Plasma-purified Hp has been marketed in Japan since 1985, with primary indications for use in conjunction with extracorporeal circulation, massive transfusion, and thermal injury. The primary therapeutic effect in these disease states is protection of the kidneys from Hb-induced toxicity.87 Thus far, no therapeutic experience exists for Hpx in a clinical setting. However, several United States– and European-based pharmaceutical companies have commenced development projects to fractionate Hp and Hpx from human plasma for use as therapeutics during hemolytic diseases. In 2011, a human plasma–derived Hp was given orphan drug status for the treatment of sickle cell disease in the European Union. However, to date, preclinical proof of concept for Hp and Hpx has been studied within a general context of Hb- and hemolysis-driven pathophysiology and not with the intention of treating a specific disease. Within most countries, drugs/biologics are approved based on indication-specific improvement in disease outcome and acceptable safety. Therefore, rational selection of potential indications, dose schedules, and clinical trial design to achieve measurable outcomes and acceptable safety profiles will be critical for the concepts presented herein to successfully progress toward improved patient care. These and other questions will pose exciting basic scientific, clinical, and regulatory challenges in the development process of Hb/hemin-scavenging therapeutics.
Acknowledgments
This work was supported by the Swiss National Science Foundation (grants 310030/120658 and 31003A/138500), the University of Zurich Research Priority Program Integrative Human Physiology, and the Swiss Federal Commission for Technology and Innovation. Additional funding was received from the National Heart, Lung and Blood Institute, National Institutes of Health (P01 HL55552 and R01 HL115467-01 to J.D.B. and G.M.V.), the National Institutes of Health (HL110900 to A.I.A.), and the US Food and Drug Administration (to P.W.B. and A.I.A.).
Authorship
Contribution: D.J.S., P.W.B., A.I.A., J.D.B., and G.M.V. contributed to the writing and editing of this article.
Conflict-of-interest disclosure: J.D.B. and G.M.V. receive research funding from Sangart Inc. The remaining authors declare no competing financial interests.
Correspondence: Dominik J. Schaer, MD, Division of Internal Medicine, University Hospital, CH-8091 Zurich, Switzerland; e-mail: dominik.schaer@usz.ch; or Gregory M. Vercellotti, MD, FACP, Division of Hematology, Oncology, and Transplantation, University of Minnesota Medical School, D495 Mayo Memorial Bldg, MMC 480, 420 Delaware St SE, Minneapolis, MN 55455; e-mail: verce001@umn.edu.
References
- 1.Balla J, Vercellotti GM, Jeney V, et al. Heme, heme oxygenase, and ferritin: how the vascular endothelium survives (and dies) in an iron-rich environment. Antioxid Redox Signal. 2007;9(12):2119–2137. doi: 10.1089/ars.2007.1787. [DOI] [PubMed] [Google Scholar]
- 2.Nakai K, Sakuma I, Ohta T, et al. Permeability characteristics of hemoglobin derivatives across cultured endothelial cell monolayers. J Lab Clin Med. 1998;132(4):313–319. doi: 10.1016/s0022-2143(98)90045-2. [DOI] [PubMed] [Google Scholar]
- 3.Matheson B, Razynska A, Kwansa H, Bucci E. Appearance of dissociable and cross-linked hemoglobins in the renal hilar lymph. J Lab Clin Med. 2000;135(6):459–464. doi: 10.1067/mlc.2000.106458. [DOI] [PubMed] [Google Scholar]
- 4.Buehler PW, D'Agnillo F, Schaer DJ. Hemoglobin-based oxygen carriers: From mechanisms of toxicity and clearance to rational drug design. Trends Mol Med. 2010;16(10):447–457. doi: 10.1016/j.molmed.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 5.Doherty DH, Doyle MP, Curry SR, et al. Rate of reaction with nitric oxide determines the hypertensive effect of cell-free hemoglobin. Nat Biotechnol. 1998;16(7):672–676. doi: 10.1038/nbt0798-672. [DOI] [PubMed] [Google Scholar]
- 6.Olson JS, Foley EW, Rogge C, Tsai AL, Doyle MP, Lemon DD. NO scavenging and the hypertensive effect of hemoglobin-based blood substitutes. Free Radic Biol Med. 2004;36(6):685–697. doi: 10.1016/j.freeradbiomed.2003.11.030. [DOI] [PubMed] [Google Scholar]
- 7.Minneci PC, Deans KJ, Zhi H, et al. Hemolysis-associated endothelial dysfunction mediated by accelerated NO inactivation by decompartmentalized oxyhemoglobin. J Clin Invest. 2005;115(12):3409–3417. doi: 10.1172/JCI25040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reeder BJ. The redox activity of hemoglobins: from physiologic functions to pathologic mechanisms. Antioxid Redox Signal. 2010;13(7):1087–1123. doi: 10.1089/ars.2009.2974. [DOI] [PubMed] [Google Scholar]
- 9.Jia Y, Buehler PW, Boykins RA, Venable RM, Alayash AI. Structural basis of peroxide-mediated changes in human hemoglobin: a novel oxidative pathway. J Biol Chem. 2007;282(7):4894–4907. doi: 10.1074/jbc.M609955200. [DOI] [PubMed] [Google Scholar]
- 10.Miller YI, Altamentova SM, Shaklai N. Oxidation of low-density lipoprotein by hemoglobin stems from a heme-initiated globin radical: antioxidant role of haptoglobin. Biochemistry. 1997;36(40):12189–12198. doi: 10.1021/bi970258a. [DOI] [PubMed] [Google Scholar]
- 11.Vallelian F, Pimenova T, Pereira CP, et al. The reaction of hydrogen peroxide with hemoglobin induces extensive alpha-globin crosslinking and impairs the interaction of hemoglobin with endogenous scavenger pathways. Free Radic Biol Med. 2008;45(8):1150–1158. doi: 10.1016/j.freeradbiomed.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 12.Buehler PW, D'Agnillo F, Hoffman V, Alayash AI. Effects of endogenous ascorbate on oxidation, oxygenation, and toxicokinetics of cell-free modified hemoglobin after exchange transfusion in rat and guinea pig. J Pharmacol Exp Ther. 2007;323(1):49–60. doi: 10.1124/jpet.107.126409. [DOI] [PubMed] [Google Scholar]
- 13.Reeder BJ, Sharpe MA, Kay AD, Kerr M, Moore K, Wilson MT. Toxicity of myoglobin and haemoglobin: oxidative stress in patients with rhabdomyolysis and subarachnoid haemorrhage. Biochem Soc Trans. 2002;30(4):745–748. doi: 10.1042/bst0300745. [DOI] [PubMed] [Google Scholar]
- 14.Buehler PW, Abraham B, Vallelian F, et al. Haptoglobin preserves the CD163 hemoglobin scavenger pathway by shielding hemoglobin from peroxidative modification. Blood. 2009;113(11):2578–2586. doi: 10.1182/blood-2008-08-174466. [DOI] [PubMed] [Google Scholar]
- 15.Balla G, Jacob HS, Eaton JW, Belcher JD, Vercellotti GM. Hemin: a possible physiological mediator of low density lipoprotein oxidation and endothelial injury. Arterioscler Thromb. 1991;11(6):1700–1711. doi: 10.1161/01.atv.11.6.1700. [DOI] [PubMed] [Google Scholar]
- 16.Jeney V, Balla J, Yachie A, et al. Pro-oxidant and cytotoxic effects of circulating heme. Blood. 2002;100(3):879–887. doi: 10.1182/blood.v100.3.879. [DOI] [PubMed] [Google Scholar]
- 17.Nagy E, Eaton JW, Jeney V, et al. Red cells, hemoglobin, heme, iron, and atherogenesis. Arterioscler Thromb Vasc Biol. 2010;30(7):1347–1353. doi: 10.1161/ATVBAHA.110.206433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ogawa K, Sun J, Taketani S, et al. Heme mediates derepression of Maf recognition element through direct binding to transcription repressor Bach1. EMBO J. 2001;20(11):2835–2843. doi: 10.1093/emboj/20.11.2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raghuram S, Stayrook KR, Huang P, et al. Identification of heme as the ligand for the orphan nuclear receptors REV-ERBalpha and REV-ERBbeta. Nat Struct Mol Biol. 2007;14(12):1207–1213. doi: 10.1038/nsmb1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanaka K, Ichihara A. Autodegradation of rat liver proteasomes (large multicatalytic proteinase complexes). Biochem Biophys Res Commun. 1989;158(2):548–554. doi: 10.1016/s0006-291x(89)80084-1. [DOI] [PubMed] [Google Scholar]
- 21.Santoro AM, Lo Giudice MC, D'Urso A, Lauceri R, Purrello R, Milardi D. Cationic porphyrins are reversible proteasome inhibitors. J Am Chem Soc. 2012;134(25):10451–10457. doi: 10.1021/ja300781u. [DOI] [PubMed] [Google Scholar]
- 22.Lin S, Yin Q, Zhong Q, et al. Heme activates TLR4-mediated inflammatory injury via MyD88/TRIF signaling pathway in intracerebral hemorrhage. J Neuroinflammation. 2012;9(1):46. doi: 10.1186/1742-2094-9-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fortes GB, Alves LS, de Oliveira R, et al. Heme induces programmed necrosis on macrophages through autocrine TNF and ROS production. Blood. 2012;119(10):2368–2375. doi: 10.1182/blood-2011-08-375303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Figueiredo RT, Fernandez PL, Mourao-Sa DS, et al. Characterization of heme as activator of Toll-like receptor 4. J Biol Chem. 2007;282(28):20221–20229. doi: 10.1074/jbc.M610737200. [DOI] [PubMed] [Google Scholar]
- 25.Buehler PW, Baek JH, Lisk C, et al. Free hemoglobin induction of pulmonary vascular disease: evidence for an inflammatory mechanism. Am J Physiol Lung Cell Mol Physiol. 2012;303(4):L312–L326. doi: 10.1152/ajplung.00074.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wagener FA, Eggert A, Boerman OC, et al. Heme is a potent inducer of inflammation in mice and is counteracted by heme oxygenase. Blood. 2001;98(6):1802–1811. doi: 10.1182/blood.v98.6.1802. [DOI] [PubMed] [Google Scholar]
- 27.Vinchi F, Gastaldi S, Silengo L, Altruda F, Tolosano E. Hemopexin prevents endothelial damage and liver congestion in a mouse model of heme overload. Am J Pathol. 2008;173(1):289–299. doi: 10.2353/ajpath.2008.071130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Socie G, Mary JY, de Gramont A, et al. Paroxysmal nocturnal haemoglobinuria: long-term follow-up and prognostic factors. French Society of Haematology. Lancet. 1996;348(9027):573–577. doi: 10.1016/s0140-6736(95)12360-1. [DOI] [PubMed] [Google Scholar]
- 29.Boretti FS, Buehler PW, D'Agnillo F, et al. Sequestration of extracellular hemoglobin within a haptoglobin complex decreases its hypertensive and oxidative effects in dogs and guinea pigs. J Clin Invest. 2009;119(8):2271–2280. doi: 10.1172/JCI39115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cooper CE, Schaer DJ, Buehler PW, et al. Haptoglobin binding stabilizes hemoglobin ferryl iron and the globin radical on tyrosine beta145 [published online ahead of print August 2012]. Antioxid Redox Signal. doi: 10.1089/ars.2012.4547. doi: 10.1089/ars.2012.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Banerjee S, Jia Y, Parker Siburt CJ, et al. Haptoglobin alters oxygenation and oxidation of hemoglobin and decreases propagation of peroxide-induced oxidative reactions. Free Radic Biol Med. 2012;53(6):1317–1326. doi: 10.1016/j.freeradbiomed.2012.07.023. [DOI] [PubMed] [Google Scholar]
- 32.Andersen CB, Torvund-Jensen M, Nielsen MJ, et al. Structure of the haptoglobin-haemoglobin complex. Nature. 2012;489(7416):456–459. doi: 10.1038/nature11369. [DOI] [PubMed] [Google Scholar]
- 33.Pimenova T, Pereira CP, Gehrig P, Buehler PW, Schaer DJ, Zenobi R. Quantitative mass spectrometry defines an oxidative hotspot in hemoglobin that is specifically protected by haptoglobin. J Proteome Res. 2010;9(8):4061–4070. doi: 10.1021/pr100252e. [DOI] [PubMed] [Google Scholar]
- 34.Buehler PW, Vallelian F, Mikolajczyk MG, et al. Structural stabilization in tetrameric or polymeric hemoglobin determines its interaction with endogenous antioxidant scavenger pathways. Antioxid Redox Signal. 2008;10(8):1449–1462. doi: 10.1089/ars.2008.2028. [DOI] [PubMed] [Google Scholar]
- 35.Bunn HF, Jandl JH. Exchange of heme among hemoglobins and between hemoglobin and albumin. J Biol Chem. 1968;243(3):465–475. [PubMed] [Google Scholar]
- 36.Levy AP, Asleh R, Blum S, et al. Haptoglobin: basic and clinical aspects. Antioxid Redox Signal. 2010;12(2):293–304. doi: 10.1089/ars.2009.2793. [DOI] [PubMed] [Google Scholar]
- 37.Purushothaman KR, Purushothaman M, Levy AP, et al. Increased expression of oxidation-specific epitopes and apoptosis are associated with haptoglobin genotype: possible implications for plaque progression in human atherosclerosis. J Am Coll Cardiol. 2012;60(2):112–119. doi: 10.1016/j.jacc.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 38.Miller YI, Smith A, Morgan WT, Shaklai N. Role of hemopexin in protection of low-density lipoprotein against hemoglobin-induced oxidation. Biochemistry. 1996;35(40):13112–13117. doi: 10.1021/bi960737u. [DOI] [PubMed] [Google Scholar]
- 39.Seery VL, Muller-Eberhard U. Binding of porphyrins to rabbit hemopexin and albumin. J Biol Chem. 1973;248(11):3796–3800. [PubMed] [Google Scholar]
- 40.Tolosano E, Fagoonee S, Morello N, Vinchi F, Fiorito V. Heme scavenging and the other facets of hemopexin. Antioxid Redox Signal. 2010;12(2):305–320. doi: 10.1089/ars.2009.2787. [DOI] [PubMed] [Google Scholar]
- 41.Hanson MS, Piknova B, Keszler A, et al. Methaemalbumin formation in sickle cell disease: effect on oxidative protein modification and HO-1 induction. Br J Haematol. 2011;154(4):502–511. doi: 10.1111/j.1365-2141.2011.08738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paoli M, Anderson BF, Baker HM, Morgan WT, Smith A, Baker EN. Crystal structure of hemopexin reveals a novel high-affinity heme site formed between two beta-propeller domains. Nat Struct Biol. 1999;6(10):926–931. doi: 10.1038/13294. [DOI] [PubMed] [Google Scholar]
- 43.Gutteridge JM, Smith A. Antioxidant protection by haemopexin of haem-stimulated lipid peroxidation. Biochem J. 1988;256(3):861–865. doi: 10.1042/bj2560861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tolosano E, Hirsch E, Patrucco E, et al. Defective recovery and severe renal damage after acute hemolysis in hemopexin-deficient mice. Blood. 1999;94(11):3906–3914. [PubMed] [Google Scholar]
- 45.Muller-Eberhard U, Javid J, Liem HH, Hanstein A, Hanna M. Concentrations of hemopexin, haptoglobin and heme in patients with various hemolytic diseases. Blood. 1968;32(5):811–815. [PubMed] [Google Scholar]
- 46.Kristiansen M, Graversen JH, Jacobsen C, et al. Identification of the haemoglobin scavenger receptor. Nature. 2001;409(6817):198–201. doi: 10.1038/35051594. [DOI] [PubMed] [Google Scholar]
- 47.Schaer CA, Vallelian F, Imhof A, Schoedon G, Schaer DJ. CD163-expressing monocytes constitute an endotoxin-sensitive Hb clearance compartment within the vascular system. J Leukoc Biol. 2007;82(1):106–110. doi: 10.1189/jlb.0706453. [DOI] [PubMed] [Google Scholar]
- 48.Schaer DJ, Schaer CA, Buehler PW, et al. CD163 is the macrophage scavenger receptor for native and chemically modified hemoglobins in the absence of haptoglobin. Blood. 2006;107(1):373–380. doi: 10.1182/blood-2005-03-1014. [DOI] [PubMed] [Google Scholar]
- 49.Schaer CA, Schoedon G, Imhof A, Kurrer MO, Schaer DJ. Constitutive endocytosis of CD163 mediates hemoglobin-heme uptake and determines the noninflammatory and protective transcriptional response of macrophages to hemoglobin. Circ Res. 2006;99(9):943–950. doi: 10.1161/01.RES.0000247067.34173.1b. [DOI] [PubMed] [Google Scholar]
- 50.Vallelian F, Schaer CA, Kaempfer T, et al. Glucocorticoid treatment skews human monocyte differentiation into a hemoglobin-clearance phenotype with enhanced heme-iron recycling and antioxidant capacity. Blood. 2010;116(24):5347–5356. doi: 10.1182/blood-2010-04-277319. [DOI] [PubMed] [Google Scholar]
- 51.Etzerodt A, Kjolby M, Nielsen MJ, Maniecki M, Svendsen P, Moestrup SK. Plasma clearance of hemoglobin and haptoglobin in mice and effect of CD163 gene targeting disruption [published online ahead of print August 29, 2012]. Antioxid Redox Signal. doi: 10.1089/ars.2012.4605. doi: 10.1089/ars.2012.4605. [DOI] [PubMed] [Google Scholar]
- 52.Hvidberg V, Maniecki MB, Jacobsen C, Hojrup P, Moller HJ, Moestrup SK. Identification of the receptor scavenging hemopexin-heme complexes. Blood. 2005;106(7):2572–2579. doi: 10.1182/blood-2005-03-1185. [DOI] [PubMed] [Google Scholar]
- 53.Smith A, Morgan WT. Haem transport to the liver by haemopexin. Receptor-mediated uptake with recycling of the protein. Biochem J. 1979;182(1):47–54. doi: 10.1042/bj1820047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kupesiz A, Celmeli G, Dogan S, Antmen B, Aslan M. The effect of hemolysis on plasma oxidation and nitration in patients with sickle cell disease. Free Radic Res. 2012;46(7):883–890. doi: 10.3109/10715762.2012.686037. [DOI] [PubMed] [Google Scholar]
- 55.Ballas SK. Sickle cell anemia with few painful crises is characterized by decreased red cell deformability and increased number of dense cells. Am J Hematol. 1991;36(2):122–130. doi: 10.1002/ajh.2830360211. [DOI] [PubMed] [Google Scholar]
- 56.Ballas SK, Marcolina MJ. Hyperhemolysis during the evolution of uncomplicated acute painful episodes in patients with sickle cell anemia. Transfusion. 2006;46(1):105–110. doi: 10.1111/j.1537-2995.2006.00679.x. [DOI] [PubMed] [Google Scholar]
- 57.Yuditskaya S, Tumblin A, Hoehn GT, et al. Proteomic identification of altered apolipoprotein patterns in pulmonary hypertension and vasculopathy of sickle cell disease. Blood. 2009;113(5):1122–1128. doi: 10.1182/blood-2008-03-142604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gladwin MT, Sachdev V, Jison ML, et al. Pulmonary hypertension as a risk factor for death in patients with sickle cell disease. N Engl J Med. 2004;350(9):886–895. doi: 10.1056/NEJMoa035477. [DOI] [PubMed] [Google Scholar]
- 59.Reiter CD, Wang X, Tanus-Santos JE, et al. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nat Med. 2002;8(12):1383–1389. doi: 10.1038/nm1202-799. [DOI] [PubMed] [Google Scholar]
- 60.Rother RP, Bell L, Hillmen P, Gladwin MT. The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: a novel mechanism of human disease. JAMA. 2005;293(13):1653–1662. doi: 10.1001/jama.293.13.1653. [DOI] [PubMed] [Google Scholar]
- 61.Villagra J, Shiva S, Hunter LA, Machado RF, Gladwin MT, Kato GJ. Platelet activation in patients with sickle disease, hemolysis-associated pulmonary hypertension, and nitric oxide scavenging by cell-free hemoglobin. Blood. 2007;110(6):2166–2172. doi: 10.1182/blood-2006-12-061697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Parent F, Bachir D, Inamo J, et al. A hemodynamic study of pulmonary hypertension in sickle cell disease. N Engl J Med. 2011;365(1):44–53. doi: 10.1056/NEJMoa1005565. [DOI] [PubMed] [Google Scholar]
- 63.Fonseca GH, Souza R, Salemi VM, Jardim CV, Gualandro SF. Pulmonary hypertension diagnosed by right heart catheterisation in sickle cell disease. Eur Respir J. 2012;39(1):112–118. doi: 10.1183/09031936.00134410. [DOI] [PubMed] [Google Scholar]
- 64.Bunn HF, Nathan DG, Dover GJ, et al. Pulmonary hypertension and nitric oxide depletion in sickle cell disease. Blood. 2010;116(5):687–692. doi: 10.1182/blood-2010-02-268193. [DOI] [PubMed] [Google Scholar]
- 65.Hebbel RP. Reconstructing sickle cell disease: a data-based analysis of the ‘hyperhemolysis paradigm’ for pulmonary hypertension from the perspective of evidence-based medicine. Am J Hematol. 2011;86(2):123–154. doi: 10.1002/ajh.21952. [DOI] [PubMed] [Google Scholar]
- 66.Belcher JD, Bryant CJ, Nguyen J, et al. Transgenic sickle mice have vascular inflammation. Blood. 2003;101(10):3953–3959. doi: 10.1182/blood-2002-10-3313. [DOI] [PubMed] [Google Scholar]
- 67.Belcher JD, Mahaseth H, Welch TE, Otterbein LE, Hebbel RP, Vercellotti GM. Heme oxygenase-1 is a modulator of inflammation and vaso-occlusion in transgenic sickle mice. J Clin Invest. 2006;116(3):808–816. doi: 10.1172/JCI26857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Belcher JD, Vineyard JV, Bruzzone CM, et al. Heme oxygenase-1 gene delivery by Sleeping Beauty inhibits vascular stasis in a murine model of sickle cell disease. J Mol Med (Berl) 2010;88(7):665–675. doi: 10.1007/s00109-010-0613-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Belcher JD, Beckman JD, Balla G, Balla J, Vercellotti G. Heme degradation and vascular injury. Antioxid Redox Signal. 2010;12(2):233–248. doi: 10.1089/ars.2009.2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Belcher JD, Nguyen J, Chen CS, et al. Plasma hemoglobin and heme trigger Weibel Palade body exocytosis and vaso-occlusion in transgenic sickle mice. Blood. 2011;118(21):896. [Google Scholar]
- 71.Wang D, Sun J, Solomon SB, Klein HG, Natanson C. Transfusion of older stored blood and risk of death: a meta-analysis. Transfusion. 2012;52(6):1184–1195. doi: 10.1111/j.1537-2995.2011.03466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hod EA, Brittenham GM, Billote GB, et al. Transfusion of human volunteers with older, stored red blood cells produces extravascular hemolysis and circulating non-transferrin-bound iron. Blood. 2011;118(25):6675–6682. doi: 10.1182/blood-2011-08-371849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hod EA, Zhang N, Sokol SA, et al. Transfusion of red blood cells after prolonged storage produces harmful effects that are mediated by iron and inflammation. Blood. 2010;115(21):4284–4292. doi: 10.1182/blood-2009-10-245001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Donadee C, Raat NJ, Kanias T, et al. Nitric oxide scavenging by red blood cell microparticles and cell-free hemoglobin as a mechanism for the red cell storage lesion. Circulation. 2011;124(4):465–476. doi: 10.1161/CIRCULATIONAHA.110.008698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Baron DM, Yu B, Lei C, et al. Pulmonary hypertension in lambs transfused with stored blood is prevented by breathing nitric oxide. Anesthesiology. 2012;116(3):637–647. doi: 10.1097/ALN.0b013e318246ef77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Baek JH, D'Agnillo F, Vallelian F, et al. Hemoglobin-driven pathophysiology is an in vivo consequence of the red blood cell storage lesion that can be attenuated in guinea pigs by haptoglobin therapy. J Clin Invest. 2012;122(4):1444–1458. doi: 10.1172/JCI59770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yu B, Lei C, Baron DM, Steinbicker AU, Bloch KD, Zapol WM. Diabetes augments and inhaled nitric oxide prevents the adverse hemodynamic effects of transfusing syngeneic stored blood in mice. Transfusion. 2012;52(7):1410–1422. doi: 10.1111/j.1537-2995.2011.03473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Larsen R, Gozzelino R, Jeney V, et al. A central role for free heme in the pathogenesis of severe sepsis. Sci Transl Med. 2010;2(51):51ra71. doi: 10.1126/scitranslmed.3001118. [DOI] [PubMed] [Google Scholar]
- 79.Lin T, Kwak YH, Sammy F, et al. Synergistic inflammation is induced by blood degradation products with microbial Toll-like receptor agonists and is blocked by hemopexin. J Infect Dis. 2010;202(4):624–632. doi: 10.1086/654929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lin T, Sammy F, Yang H, et al. Identification of hemopexin as an anti-inflammatory factor that inhibits synergy of hemoglobin with HMGB1 in sterile and infectious inflammation. J Immunol. 2012;189(4):2017–2022. doi: 10.4049/jimmunol.1103623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rocha ER, Smith A, Smith CJ, Brock JH. Growth inhibition of Bacteroides fragilis by hemopexin: proteolytic degradation of hemopexin to overcome heme limitation. FEMS Microbiol Lett. 2001;199(1):73–78. doi: 10.1111/j.1574-6968.2001.tb10653.x. [DOI] [PubMed] [Google Scholar]
- 82.Torres VJ, Pishchany G, Humayun M, Schneewind O, Skaar EP. Staphylococcus aureus IsdB is a hemoglobin receptor required for heme iron utilization. J Bacteriol. 2006;188(24):8421–8429. doi: 10.1128/JB.01335-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liang X, Lin T, Sun G, Beasley-Topliffe L, Cavaillon JM, Warren HS. Hemopexin down-regulates LPS-induced proinflammatory cytokines from macrophages. J Leukoc Biol. 2009;86(2):229–235. doi: 10.1189/jlb.1208742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Spiller F, Costa C, Souto FO, et al. Inhibition of neutrophil migration by hemopexin leads to increased mortality due to sepsis in mice. Am J Respir Crit Care Med. 2011;183(7):922–931. doi: 10.1164/rccm.201002-0223OC. [DOI] [PubMed] [Google Scholar]
- 85.Mauk MR, Smith A, Mauk AG. An alternative view of the proposed alternative activities of hemopexin. Protein Sci. 2011;20(5):791–805. doi: 10.1002/pro.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gramaglia I, Sobolewski P, Meays D, et al. Low nitric oxide bioavailability contributes to the genesis of experimental cerebral malaria. Nat Med. 2006;12(12):1417–1422. doi: 10.1038/nm1499. [DOI] [PubMed] [Google Scholar]
- 87.Hashimoto K, Nomura K, Nakano M, Sasaki T, Kurosawa H. Pharmacological intervention for renal protection during cardiopulmonary bypass. Heart Vessels. 1993;8(4):203–210. doi: 10.1007/BF01744743. [DOI] [PubMed] [Google Scholar]
- 88.Tanaka K, Kanamori Y, Sato T, et al. Administration of haptoglobin during cardiopulmonary bypass surgery. ASAIO Trans. 1991;37(3):M482–M483. [PubMed] [Google Scholar]
- 89.Horai T, Tanaka K, Takeda M. Coronary artery bypass grafting under cardiopulmonary bypass in a patient with beta-thalassemia: report of a case. Surg Today. 2006;36(6):538–540. doi: 10.1007/s00595-006-3184-y. [DOI] [PubMed] [Google Scholar]
- 90.Tsuda H, Shirono K, Shimizu K. Is prophylactic haptoglobin infusion in peripheral blood stem cell transplantation clinically useful? Eur J Haematol. 1995;55(3):214–215. doi: 10.1111/j.1600-0609.1995.tb00257.x. [DOI] [PubMed] [Google Scholar]
- 91.Homann B, Kult J, Weis KH. [On the use of concentrated haptoglobin in the treatment of a haemolytic transfusion accident of the ABO-system (author's transl)]. Anaesthesist. 1977;26(8):485–488. [PubMed] [Google Scholar]
- 92.Gando S, Tedo I. The effects of massive transfusion and haptoglobin therapy on hemolysis in trauma patients. Surg Today. 1994;24(9):785–790. doi: 10.1007/BF01636307. [DOI] [PubMed] [Google Scholar]
- 93.Kanamori Y, Tanabe H, Shimono T, et al. The effects of administration of haptoglobin for hemolysis by extracorporeal circulation [article in Japanese]. Rinsho Kyobu Geka. 1989;9(5):463–467. [PubMed] [Google Scholar]
- 94.Yamamoto H, Nishikawa S, Yamazaki K, Kudo R. Efficacy of haptoglobin administration in the early postoperative course of patients with a diagnosis of HELLP syndrome. J Obstet Gynaecol. 2000;20(6):610–611. doi: 10.1080/01443610020001459. [DOI] [PubMed] [Google Scholar]
- 95.Ito M, Imoto S, Nakagawa T, Kai S, Hara H. [Administration of haptoglobin in ABO incompatible bone marrow transplantation]. Rinsho Ketsueki. 1990;31(10):1716–1720. [PubMed] [Google Scholar]
- 96.Imaizumi H, Tsunoda K, Ichimiya N, Okamoto T, Namiki A. Repeated large-dose haptoglobin therapy in an extensively burned patient: case report. J Emerg Med. 1994;12(1):33–37. doi: 10.1016/0736-4679(94)90009-4. [DOI] [PubMed] [Google Scholar]
- 97.Yoshioka T, Sugimoto T, Ukai T, Oshiro T. Haptoglobin therapy for possible prevention of renal failure following thermal injury: a clinical study. J Trauma. 1985;25(4):281–287. doi: 10.1097/00005373-198504000-00001. [DOI] [PubMed] [Google Scholar]
- 98.Ohga S, Higashi E, Nomura A, et al. Haptoglobin therapy for acute favism: a Japanese boy with glucose-6-phosphate dehydrogenase Guadalajara. Br J Haematol. 1995;89(2):421–423. doi: 10.1111/j.1365-2141.1995.tb03322.x. [DOI] [PubMed] [Google Scholar]
- 99.Eda K, Ohtsuka S, Seo Y, et al. Conservative treatment of hemolytic complication following coil embolization in two adult cases of patent ductus arteriosus. Jpn Circ J. 2001;65(9):834–836. doi: 10.1253/jcj.65.834. [DOI] [PubMed] [Google Scholar]