Key Points
CD34+ progenitor cell frequency is reduced in older subjects, and is influenced by environmental factors such as smoking and statin use.
CD34+ progenitor cell frequency is highly heritable and associated with common genetic variants at several loci.
Abstract
Circulating blood CD34+ cells consist of hematopoietic stem/progenitor cells, angiogenic cells, and endothelial cells. In addition to their clinical use in hematopoietic stem cell transplantation, CD34+ cells may also promote therapeutic neovascularization. Therefore, understanding the factors that influence circulating CD34+ cell frequency has wide implications for vascular biology in addition to stem cell transplantation. In the present study, we examined the clinical and genetic characteristics associated with circulating CD34+ cell frequency in a large, community-based sample of 1786 Framingham Heart Study participants. Among subjects without cardiovascular disease (n = 1595), CD34+ frequency was inversely related to older age, female sex, and smoking. CD34+ frequency was positively related to weight, serum total cholesterol, and statin therapy. Clinical covariates accounted for 6.3% of CD34+ variability. CD34+ frequency was highly heritable (h2 = 54%; P < .0001). Genome-wide association analysis of CD34+ frequency identified suggestive associations at several loci, including OR4C12 (chromosome 11; P = 6.7 × 10−7) and ENO1 and RERE (chromosome 1; P = 8.8 × 10−7). CD34+ cell frequency is reduced in older subjects and is influenced by environmental factors including smoking and statin use. CD34+ frequency is highly heritable. The results of the present study have implications for therapies that use CD34+ cell populations and support efforts to better understand the genetic mechanisms that underlie CD34+ frequency.
Introduction
Circulating blood CD34+ cells are a heterogeneous population of hematopoietic stem/progenitor cells, angiogenic cells, and endothelial cells. The CD34+ phenotype is an indicator of progenitor cell activity, and CD34+ cells are widely used clinically in stem/progenitor cell transplantation in hematology and oncology.1–3 Circulating CD34+ progenitor cells may contribute to vascular health,4,5 as suggested by experimental studies indicating that CD34+ or CD34+/CD133+ cells have endothelial progenitor cell or vasculogenic capacity,6,7 although the relative contributions of endothelial differentiation versus paracrine signaling remains controversial. Furthermore, administration of autologous CD34+ cells promotes neovascularization in both animals and humans and may ameliorate refractory angina8 and limb ischemia9 in patients with vascular disease. Therefore, a greater understanding of the role of CD34+ populations in vascular diseases is warranted.
Numerous studies have attempted to correlate CD34+ and CD34+/KDR+ cell frequency with vascular states in the context of cardiovascular disease (CVD),10 autoimmune and inflammatory disorders,11,12 pulmonary disease,13,14 and cancer.15,16 Data from smaller studies in selected populations have reported an inverse association of circulating angiogenic cell phenotypes with cardiovascular risk factors,17 subclinical markers of endothelial dysfunction,18,19 and incident cardiovascular events.20,21 However, these studies varied in the inclusion of patients at known high risk of CVD, which could have altered the function of BM or stem cell compartments. There are few data on the clinical correlates of CD34+ frequency from community-based cohorts. Furthermore, little is known about the heritability, genetic, and environmental factors that influence interindividual variation in circulating CD34+ concentrations and such information may have both biologic and clinical relevance. To clarify these issues, in the present study, we investigated the correlates of circulating CD34+ frequency in PBMCs from participants in a large, community-based ambulatory sample.
Methods
Study sample
The Framingham Heart Study was initiated in 1948, when 5209 residents of Framingham, MA, were enrolled in a prospective cohort study to identify risk factors for CVD.22 In 1971, 5124 additional participants (offspring of the original cohort subjects, and their spouses) were enrolled in the Framingham Offspring Study.23 All offspring study participants receive routine examinations approximately every 4 years. Of the 3021 participants who attended the eighth offspring examination cycle (2005-2008), 1786 had assessment of CD34+. Of the total sample with CD34+ assessed, the majority (n = 1595) of participants did not have known CVD defined as history of myocardial infarction, coronary insufficiency, stroke, or heart failure. All participants gave written informed consent in accordance with the Declaration of Helsinki. The institutional review board of the Boston University School of Medicine approved all study protocols.
Clinical assessment
Participants underwent a standardized medical examination and laboratory assessment of cardiovascular risk factors at the Framingham Heart Study examination. Systolic and diastolic blood pressures were the average of 2 physician-measured readings. Body mass index was calculated as weight divided by height squared (kg/m2). Blood was drawn for glucose, total and high-density lipoprotein (HDL) cholesterol, and triglycerides after an overnight fast. Use of lipid-lowering, antihypertensive, and hormone replacement therapies and cigarette smoking (regular smoker within the past year) were self-reported. Diabetes was defined as having a fasting glucose ≥ 126 mg/dL or use of medications to treat diabetes.
CD34+ cell phenotyping
All blood specimens for cell phenotyping were collected from fasting participants in the morning between 8 and 9 am. Each blood sample underwent initial centrifugation and the resulting buffy coat was further processed for cell phenotyping within 4 hours of specimen collection as described previously1,24 with modifications. Specifically, buffy coat samples were diluted to 10.5 mL with PBS (Invitrogen) and layered over 5 mL of Ficoll (Amersham Pharmacia Biotech). Samples were centrifuged at 800g for 15 minutes at 10°C. PBMCs were isolated from the buffy coat using Ficoll density-gradient centrifugation, incubated for 15 minutes at room temperature with Fc block (Miltenyi Biotec), and then incubated for 25 minutes on ice with anti-CD34 FITC human antibody (BD Pharmingen). Stained cells were fixed in 2% paraformaldehyde and stored at 4°C for no longer than 3 days to maintain fluorescence intensity. Samples were batched, and expression of the surface markers was evaluated by FACS analysis; the number of positive cells was quantified using a FACSCalibur flow cytometer (BD Biosciences) equipped with a high-throughput 96-well sample plate with fluorochrome-matched IgG isotype controls. FACS plot analysis was blinded. Data were analyzed via FlowJo Version 8.8.7 software (TreeStar) using population gating on CD34+ events as a percentage and frequency of total nucleated events (see supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Genotyping
In the Framingham Heart Study, genotyping was performed by Affymetrix using their 500K GeneChip array with a custom-designed gene-centric 50K molecular inversion probe. The BRLMM algorithm was used to call Affymetrix 500K genotypes.25 After excluding individual participants for prespecified criteria (ie, call rates ≤ 97%, per subject heterozygosity ± 5 SD from the mean, or excess Mendelian errors), the sample included 8481 genotyped subjects. Of the 534 982 genotyped autosomal single nucleotide polymorphisms (SNPs) in the Framingham Heart Study (based on Affymetrix 500K and MIPS 50K combined), we used 378 163 SNPs in the imputation of HapMap SNPs after filtering out 15 586 SNPs because of Hardy-Weinberg P < 10−6, 64 511 SNPs (call rate < 97%), 45 361 SNPs (mishap P < 10−9), 4857 SNPs (> 100 Mendelian errors), 67 269 SNPs (frequency < 0.01), 2 SNPs (because of strandedness issues on merging with HapMap data), and a further 13 394 SNPs not present on HapMap. Imputation of approximately 2.5 million autosomal SNPs in HapMap was conducted to maximize coverage of the genome using the algorithm implemented in MACH Version 1.0.15 (HapMap CEU release 22, build 36).26 A total of 200 biologically unrelated subjects with high-quality genotype data (missingness < 0.011 and low Mendelian errors) were used to infer model parameters first, and then the model was applied to all 8481 subjects who were genotyped.
Statistical analyses
Because of skewed distribution, CD34+ and triglycerides were natural logarithmically transformed before analyses. In the total sample, we examined the relations of CD34+ with prevalent CVD. We analyzed clinical correlates of CD34+ in 1595 participants free of CVD at the time of examination. Multiple linear regression was used to model the relations of CD34+ with the following covariates: age, sex, systolic blood pressure, diastolic blood pressure, body mass index, fasting glucose, total cholesterol, HDL cholesterol, LDL cholesterol, log-triglycerides, hypertension (systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg or taking antihypertensive medications), diabetes, smoking status, and specific medications (ACE inhibitors, angiotensin receptor blockers, β-blockers, calcium-channel blockers, diuretics, hormone replacement therapy, and statins). Stepwise regression models were used to associate CD34+ with variables with P < .10 in individual models. We also performed analyses relating CD34+ to the Framingham Risk Score27 in the sample free of CVD. Our sample size, with N = 1595 independent observations and a fixed significance level α = 0.05, afforded 80% power to detect a significant result for a variable accounting for 0.49% of the variance in CD34+.
In secondary analyses, we repeated analyses using generalized estimating equations28 to account for correlations among related subjects (siblings) in the study sample (SAS PROC GENMOD). All analyses were performed using SAS Version 9.1.3. statistical software. P < .10 was the criterion for covariates to enter and remain in stepwise regression models, and a 2-tailed P < .05 was considered statistically significant.
Heritability
For each of the transformed progenitor cell phenotypes, we obtained age- and sex-specific models; the residuals of each model were percentiled (ranked) and the equivalent percentile value from a normal distribution was substituted to fit a normal distribution (ie, the residuals were rank normalized). The normalized variables for each cell phenotype were then used as the dependent variables for estimation of heritability (h2). The heritability estimates, defined as the proportion of total variation explained by additive genetic effect, were computed using the Sequential Oligogenic Linkage Analysis Routines (SOLAR) Version 2.1.4.29
Genetic determinants
We performed the genetic association analyses in the total study sample, including participants with previous CVD, to maximize statistical power. We created age- and sex-adjusted residuals of log-transformed CD34+. These residuals were tested for association with each imputed (approximately 2.5 million) SNP under an additive genetic model using the linear mixed effects model in the R-GWAF package to account for relatedness.30 We selected a P threshold of 5 × 10−7 for genome-wide statistical significance (and 5 × 10−6 for associations of moderate statistical significance), which is the threshold used by the Wellcome Trust Case–Control Consortium and accounts for approximately 1 million independent tests given the known high correlation among the majority of identifiable SNPs.31 For 2.5 million tests, this threshold provides an expectation of < 1.25 false-positive results across the genome. The threshold for moderate genome-wide statistical significance (5 × 10−6) was considered to identify all potential loci of interest.31 Given the uniqueness of measuring CD34+ in a large, community-based cohort, a replication sample was not available for the present study.
Results
Clinical characteristics of the total sample and the subset without prevalent CVD are shown in Table 1. The mean (± SD) age was 66 ± 9 years and 54% of the subjects were women. The median CD34+ count was 0.076% (interquartile range, 0.057), with a corresponding median CD34+ frequency of 7.6 × 10−4.
Table 1.
Sample characteristics
| Characteristic | Total sample (N = 1786) | Non-CVD sample (n = 1595) |
|---|---|---|
| Age, y | 66 ± 9 | 66 ± 9 |
| Female, % | 54 | 56 |
| Systolic blood pressure, mmHg | 128 ± 17 | 128 ± 17 |
| Diastolic blood pressure, mmHg | 74 ± 10 | 74 ± 10 |
| Body mass index, kg/m2 | 28 ± 5 | 28 ± 5 |
| Fasting glucose, mg/dL | 107 ± 24 | 106 ± 23 |
| Total cholesterol, mg/dL | 186 ± 38 | 189 ± 36 |
| LDL cholesterol, mg/dL | 105 ± 31 | 107 ± 31 |
| HDL cholesterol, mg/dL | 57 ± 18 | 58 ± 18 |
| Triglycerides, mg/dL | 118 ± 71 | 116 ± 69 |
| Hypertension, % | 63 | 59 |
| Diabetes mellitus, % | 14 | 12 |
| Cigarette smoking, % | 8 | 8 |
| Medications, % | ||
| ACE inhibitors | 25 | 22 |
| Angiotensin receptor blockers | 8 | 7 |
| Beta-blockers | 27 | 22 |
| Calcium channel blockers | 14 | 12 |
| Diuretics | 23 | 21 |
| Hormone replacement | 4 | 5 |
| Statins | 41 | 37 |
| Framingham risk score | 9 ± 4 | 8 ± 4 |
| Prevalent cardiovascular disease, % | 11 |
Values shown are means ± SD or percentages.
Clinical correlates
CD34+ cell frequency was log-transformed before analyses, because of right-skewed distribution. (Henceforth, we will refer to log CD34+ frequency as simply CD34+ frequency.) In age- and sex-adjusted analyses performed in subjects without prevalent CVD, CD34+ was inversely associated with age, female sex, HDL cholesterol, and cigarette smoking, and positively associated with body mass index, weight, log triglycerides, hypertension, and statin therapy (Table 2). In stepwise multivariable-adjusted analyses, CD34+ was inversely associated with older age (P < .0001), female sex (P < .0001), and cigarette smoking (P = .009), and positively associated with weight (P = .003), total cholesterol (P = .001), and statin therapy (P < .001). Statin therapy remained significantly associated with CD34+ in multivariable analyses even after adjusting for total cholesterol level. In additional analyses adjusting for age, sex, weight, smoking status, total cholesterol, and statin therapy, a multiplicative term representing the interaction between total cholesterol and statin use was not significant (P = .37). We observed an inverse relation of CD34+ with the Framingham Risk Score that was statistically significant in men (coefficient = −0.37; P = .004), but not in women (coefficient = 0.08; P = .59). The relationship of CD34+ frequency with the Framingham Risk Score was largely dependent on age, as evidenced by lack of a statistically significant association between CD34+ frequency with the risk score when excluding the contribution of age in both men (P = .60) and women (P = .29). In analyses of the total sample, CD34+ was not associated with prevalent CVD (P = .69). When these analyses were repeated using generalized estimating equations, the results were similar (data not shown). Overall, the clinical covariates included in multivariable analyses accounted for 6.3% of the variability in CD34+.
Table 2.
Clinical correlates of CD34+
| Covariate | Age- and sex-adjusted |
Multivariable-adjusted |
||
|---|---|---|---|---|
| Regression coefficient (SE) | P | Regression coefficient (SE) | P | |
| Age, y | −0.072 (0.013) | < .0001 | −0.072 (0.013) | < .0001 |
| Female sex | −0.150 (0.025) | < .0001 | −0.129 (0.029) | < .0001 |
| Body mass index | 0.045 (0.013) | < .001 | ||
| Height | −0.019 (0.019) | .333 | ||
| Weight | 0.048 (0.014) | .001 | 0.044 (0.015) | .003 |
| Systolic blood pressure | 0.010 (0.013) | .442 | ||
| Diastolic blood pressure | 0.023 (0.013) | .080 | ||
| Fasting glucose | 0.012 (0.013) | .365 | ||
| Total cholesterol | 0.024 (0.013) | .075 | 0.048 (0.014) | .001 |
| LDL cholesterol | 0.017 (0.013) | .173 | ||
| HDL cholesterol | −0.028 (0.014) | .039 | ||
| Log triglycerides | 0.058 (0.012) | < .0001 | ||
| Hypertension | 0.064 (0.027) | .018 | ||
| Diabetes mellitus | 0.036 (0.039) | .365 | ||
| Cigarette smoking | −0.135 (0.046) | .004 | −0.122 (0.046) | .009 |
| Medications | ||||
| ACE inhibitors | 0.034 (0.031) | .272 | ||
| Angiotensin receptor blockers | 0.023 (0.048) | .631 | ||
| Beta-blockers | 0.042 (0.031) | .174 | ||
| Calcium channel blockers | 0.028 (0.039) | .461 | ||
| Diuretics | 0.063 (0.031) | .047 | ||
| Hormone replacement | −0.013 (0.060) | .833 | ||
| Statins | 0.067 (0.026) | .011 | 0.102 (0.029) | < .001 |
Data are from the subset of subjects without known cardiovascular disease (n = 1595). The CD34+ variable is log transformed. Coefficients (SE) represent change logCD34+ per increase in the value of the covariates shown (by 1 SD for continuous variables). In age- and sex-adjusted models, the association with age is adjusted for sex and the association with sex is adjusted for age.
Heritability
We then estimated the heritability of CD34+ cell frequency using variance component methods implemented in SOLAR on sibship-pair and spouse-pair data. The heritability of CD34+ was estimated at 53.9% ± 9.2% (mean ± SE) in age- and sex-adjusted analyses, and was highly statistically significant (P < .0001). There were a total of 516 sibling pairs and 277 spousal pairs in the total sample of 1786 participants with CD34+ frequency phenotyped. In this sample, the between-sibling correlation of CD34+ was 0.29 ± 0.05 (P < .0001) and between-spouse correlation was 0.10 ± 0.07 (P = .16).
Genetic correlates
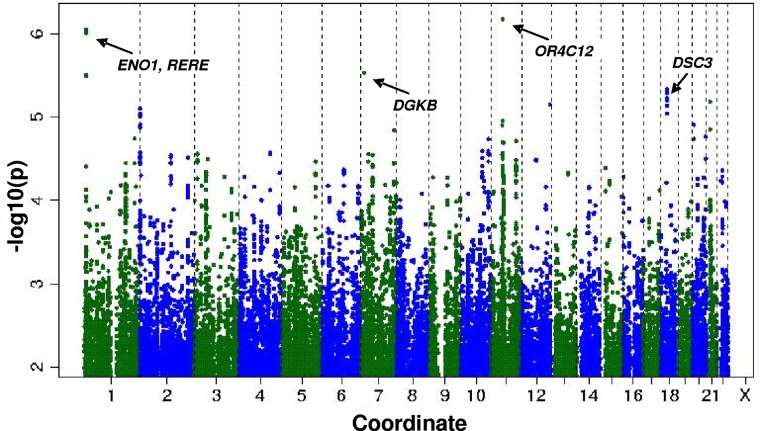
The heritability estimation described in the preceding section raised the possibility that individual CD34+ frequency might be associated with polymorphisms at defined genetic loci. Therefore, we performed a genome-wide association study for CD34+ frequency in the entire sample. The genomic inflation factor (lambda) was small at 1.0003, which is consistent with minimal population stratification. Figure 1 shows the P values (−log base 10 scale) for the associations of the individual SNPs with CFU plotted against chromosomal position. The individual variants with the strongest associations are listed in Table 3. Common variants at 4 loci had suggestive associations with CD34+: rs2183383 (P = 6.67 × 10−7) at chromosome position 11p11 (near the OR4C12 gene), rs11121242 (P = 8.81 × 10−7) at chromosome position 1p36 (near ENO1 and RERE), rs976760 (P = 2.86 × 10−6) at chromosome position 7p21 (within DGKB), and rs1943535 (P = 4.61 × 10−6) at chromosome position 18q12 (near DSC3).
Figure 1.
Distribution of P values for the association of individual SNPs with CD34+ according to chromosome number and position.
Table 3.
SNPs associated with log CD34+ after adjustment for age and sex
| Candidate gene(s) | Loci | SNP | Position, bp | Location relative to gene | MAF | A1-A2 | Estimated regression coefficient (SE)* | P |
|---|---|---|---|---|---|---|---|---|
| OR4C12 | 11p11.1 | rs2183383 | 50194788 | Downstream | 0.20 | C-T | −0.22 (0.04) | 6.67 × 10−7 |
| ENO1, RERE | 1p36 | rs11121242 | 8828888 | Intergenic | 0.48 | G-A | −0.19 (0.04) | 8.81 × 10−7 |
| rs6577536 | 8832697 | Intergenic | 0.47 | G-A | −0.18 (0.04) | 9.78 × 10−7 | ||
| rs11121245 | 8838811 | Downstream | 0.49 | C-T | −0.17 (0.04) | 3.10 × 10−6 | ||
| rs11590606 | 8840256 | Downstream | 0.49 | T-C | −0.17 (0.04) | 3.17 × 10−6 | ||
| rs10864368 | 8840900 | Downsteram | 0.49 | C-T | −0.17 (0.04) | 3.18 × 10−6 | ||
| DGKB | 7p21 | rs976760 | 14240178 | Intronic | 0.26 | C-T | 0.25 (0.05) | 2.86 × 10−6 |
| DSC3 | 18q12 | rs1943535 | 26620710 | Intergenic | −0.25 | G-T | −0.25 (0.05) | 4.61 × 10−6 |
| rs2591120 | 26621355 | Intergenic | −0.25 | A-T | −0.25 (0.05) | 4.62 × 10−6 | ||
| rs2591119 | 26621746 | Intergenic | −0.25 | A-G | −0.25 (0.05) | 4.65 × 10−6 | ||
| rs2591118 | 26621891 | Intergenic | −0.25 | T-C | −0.25 (0.05) | 4.65 × 10−6 | ||
| rs2733151 | 26624082 | Intergenic | −0.23 | T-A | −0.23 (0.05) | 4.89 × 10−6 | ||
| rs2733150 | 26624049 | Intergenic | −0.23 | C-G | −0.23 (0.05) | 4.90 × 10−6 | ||
| rs2591117 | 26624221 | Intergenic | −0.23 | A-T | −0.23 (0.05) | 4.93 × 10−6 | ||
| rs2591116 | 26624582 | Intergenic | −0.23 | A-T | −0.23 (0.05) | 4.94 × 10−6 | ||
| rs2105759 | 26626223 | Intergenic | −0.23 | A-T | −0.23 (0.05) | 4.99 × 10−6 | ||
| rs2733152 | 26627615 | Intergenic | −0.23 | A-G | −0.23 (0.05) | 4.99 × 10−6 |
MAF indicates minor allele frequency; A1, major allele; and A2, minor allele.
The regression coefficients shown can be used to estimate the magnitude of association between the SNP and CD34+ frequency. For example, the presence of the OR4C12 polymorphism is associated with an e(−0.22) = 0.80, or 20% lower CD34+ frequency. Therefore, for a subject with the median value of CD34+ frequency (ie, 0.076% or 7.6 × 10−4), the presence of this polymorphism would be associated with a 1.5 × 10−4 lower CD34+ frequency.
Discussion
In the present study, we report the clinical and genetic correlates of circulating CD34+ cells in a community-based sample of predominantly healthy men and women. Our principal findings are 3-fold. First, we observed inverse associations between CD34+ and a subset of traditional cardiovascular risk factors (eg, age and smoking). In addition, lower CD34+ was related to higher overall risk factor burden in men, as reflected by the Framingham Risk Score, although this was largely attributable to the inverse relationship of CD34+ with age. Second, the CD34+ trait was highly heritable, but was also associated with environmental factors such as smoking (negative association) and statin use (positive association). Third, we identified potential novel associations between CD34+ and genetic variants at several loci that were deemed “suggestive” at a genome-wide level. Our data indicate that variation in circulating CD34+ progenitor cell frequency is dependent on both acquired and heritable attributes.
To our knowledge, the present study is the largest to date to assess the relationship of the CD34+ phenotype with cardiovascular risk factors using a well-defined community cohort with a low frequency of prevalent CVD. To measure CD34+ cell frequency, we used a flow cytometric protocol originally developed to study CD34+ progenitor cell response to antiangiogenic therapy in cancer patients1 and adapted it to enable high-throughput analysis of large numbers of samples. Age, weight, and female sex were the strongest clinical determinants of circulating CD34+ frequency. Interestingly, prior stem cell transplantation studies have shown that similar clinical factors are inversely correlated with the mobilization of peripheral blood CD34+ cells in response to G-CSF.32 This suggests that factors contributing to steady-state CD34+ frequency may also contribute to CD34+ mobilization. Our data did indicate some clinical associations that were not in the expected direction. In age- and sex-adjusted analyses, higher CD34+ frequency was associated with prevalent hypertension, higher triglycerides, and lower HDL; lack of significance for these associations in multivariable analyses suggests the presence of confounding by other clinical covariates such as body size. The association of higher CD34+ frequency with greater weight remained significant after multivariable adjustment and is consistent with previous studies,32 suggesting a positive effect of body size on CD34+ mobilization and/or stores.
Our present data also demonstrate that CD34+ frequency was positively correlated with both higher total cholesterol and statin therapy in multivariable-adjusted analyses. Total cholesterol remained significantly associated with CD34+ even after adjusting for statin use, suggesting an independent relationship that could be related to body size and/or as-yet-unidentified factors. The association of statin use with higher CD34+ also remained significant even after adjusting for total cholesterol and lipid subfractions. While interpreting our data with caution, these findings support the hypothesis that levels of circulating progenitor cells may be influenced therapeutically. Prior studies in selected populations have measured directly the effect of statins on progenitor cells in patients with CVD. For example, short-term treatment with rosuvastatin increased the 24-hour mobilization of CD34+/KDR+ and CD34+/CD133+/KDR+ cells in patients with congestive heart failure.33 Moderate-dose atorvastatin increased CD34+ cells in patients with myocardial infarction34 and intensive (compared with low-dose) atorvastatin significantly increased CD34+/KDR+ frequency in patients undergoing angioplasty.35 Although atorvastatin therapy has also been associated with increased circulating progenitor cells in patients with stable coronary artery disease,36,37 the longer-term effects of statin therapy on progenitor cell quantity have yet to be established.38 In contrast to the effect of statins, we observed in the present study an inverse correlation between tobacco use and CD34+ frequency, a finding that is consistent with results of a study of circulating CD34+ cells in young, healthy women.39 These data suggest the potential of pharmacologic or other external stimuli to either positively or negatively influence progenitor populations. Further research into the mechanisms by which statins influence circulating CD34+ progenitor cell frequency is warranted.
Therapeutic interventions designed to augment CD34+ cell quantity via BM transplantation or peripheral blood cell mobilization have been used for some time in the treatment of malignant hematologic disease. Accumulating data now suggest a possible role for stem/progenitor cells in the treatment of CVD. Martin-Rendon et al performed a meta-analysis of 13 randomized controlled trials of BM-derived stem cells for the treatment of acute myocardial infarction.40 Although CD34+ purification was not performed, data from these heterogeneous trials demonstrated benefits of BM-derived stem cells with respect to improved left ventricular ejection fraction and myocardial lesion area compared with controls; improvements were noted particularly within subgroups in which cells were infused < 7 days after myocardial infarction and in which dose of cells was > 108. Quyyumi et al have reported the use of CD34+-enriched cells for the treatment of ST elevation myocardial infarction41; these data demonstrated increased myocardial perfusion when high doses (> 106) of CD34+ cells were infused. Recently, Losordo et al reported on a phase 2 randomized trial of intramyocardial, autologous CD34+ cell therapy for refractory angina. In that study, CD34+ cellular therapy led to decreased angina frequency and increased exercise tolerance.8 Therefore, elucidating factors associated with CD34+ frequency and identifying potential pharmacologic modifiers are relevant to both cardiovascular and hematologic disease.
We observed herein that clinical covariates accounted for less than 10% of CD34+ cell variability, suggesting that genetic factors might affect CD34+ cell frequency. We therefore performed a heritability analysis, which suggested that additive genetic factors accounted for more than 50% of the unexplained variation in CD34+ cell number. To our knowledge, this is the first demonstration in humans that peripheral blood CD34+ cell frequency is predominantly associated with heritable rather than clinical factors. Our approach to estimating the genetic effects on CD34+ variation does not account for all of the possible shared environmental factors that could contribute to the heritability estimate. Nonetheless, we did observe that CD34+ frequency was significantly correlated between siblings, but not between spouses, in our study sample.
To determine whether specific common genetic variants are associated with CD34+ cell frequency, we performed a genome-wide scan of possible contributory polymorphisms. We identified 4 loci demonstrating suggestive associations with CD34+ (OR4C12, ENO1/RERE, DGKB, and DSC3). ENO1 codes for α-enolase and c-myc binding protein (MBP-1), which are involved in glucose metabolism and cellular growth regulation.42 Modulation of ENO1-encoded proteins is implicated in cellular survival in the setting of hypoxia, suggesting that the ENO1 locus could influence the viability of CD34+ cell development in the relatively hypoxic environment of the BM. RERE is notable for interactions with genes involved in hematopoiesis. This gene encodes a transcriptional repressor during embryonic development with putative roles in cell survival. In neuroblastoma cell lines, RERE colocalizes with the promyelocytic leukemia protein and overexpression of RERE recruits BAX to the nucleus, triggering cell death.43 Although these connections are intriguing, the SNPs we identified await validation in an independent cohort. Additional associations may exist between CD34+ cell frequency and polymorphisms that did not meet our threshold for statistical significance.
We recently reported the clinical and genetic correlates for an alternative “endothelial progenitor cell” phenotype, CFU, in the same community-based cohort.44 Although some clinical correlations for CFU and CD34+ frequency are shared (including inverse associations with age and female sex and positive associations with statin use), overall, there was no correlation between CFU and CD34+ frequency (r = 0.04). It has been suggested that a comparison of genetic factors associated with each phenotype would be helpful in determining whether these 2 phenotypes are biologically distinct or similar.45 Our genome-wide association study analyses of the CFU and CD34+ phenotypes identified distinct sets of nonoverlapping genetic loci. Therefore, measuring 2 well-defined progenitor cell phenotypes in the same community sample clearly illustrates how the CD34+ frequency and CFU reflect different cell populations and thus distinct biologic activities.
Several limitations of our study merit consideration. Although we analyzed CD34+ cells, we cannot completely rule out a contribution to variation by changes in non-CD34+ populations. Moreover, in the absence of markers specific to circulating progenitor cells with angiogenic capacity, available methods are recognized as having limited ability to differentiate true vascular precursor cells from other cell types with proangiogenic and vasculogenic properties.46,47 However, CD34+ quantity is believed to represent both cellular and paracrine factors with angiogenic capacity and therefore provides a reasonable representation of angiogenic potential. The prevalence of active smoking was low in our study sample, which may have been at least partly because of underreporting. We expect that any imprecision regarding the ascertainment of smoking status would have biased the results of smoking analyses to the null. As in all heritability analyses, we cannot exclude the possibility that some of the heritability is attributable to unmeasured, shared environmental factors. To our knowledge, CD34+ frequency has not been assessed in other large, community-based cohorts, likely because of the technical requirements of specimen handling and the specialized assays required. Therefore, incorporation of standardized protocols for obtaining samples suitable for CD34+ and related cell-based phenotyping in future prospective cohorts appears warranted. Lastly, our study was limited to white subjects of European ancestry, so the generalizability of our main findings to other racial/ethnic groups is unknown.
In summary, the present study of clinical and genetic correlates of circulating CD34+ cell frequency represents the largest to date in a community-based cohort. We have shown that CD34+ frequency is associated with a subset of traditional cardiovascular risk factors and is higher in subjects taking statin medications. Furthermore, we observed that heritable factors contribute substantially more to variability in the CD34+ phenotype than identifiable environmental or clinical factors. Although our genome-wide association study findings remain to be replicated, our data demonstrate the feasibility of using large, community-based cohorts to study stem and progenitor cell populations in humans. Such data will be highly relevant to both cardiovascular risk assessment and hematopoietic stem/progenitor cell research. Indeed, a further understanding of these processes could be used to develop strategies to ameliorate vascular ischemia or to enhance the collection of CD34+ stem/progenitor cell products for use in allogeneic stem cell transplantations for therapeutic interventions.
Supplementary Material
Acknowledgments
The authors thank Jennifer Yamamoto for statistical assistance with the heritability analyses.
This work was supported in part by the National Heart, Lung, and Blood Institute's Framingham Heart Study (contract number N01-HC-25195, grant R01-HL083197 to T.J.W., and grant R01-HL93328 to R.S.V.). S.C. is supported in part by National Institutes of Health grant K99HL107642 and the Ellison Foundation.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: K.S.C. and S.C. designed and performed the research, analyzed the data, and wrote the manuscript; M.G.L. designed the research, analyzed the data, and edited the manuscript; L.A.C., E.L.M., J.S.N., and Y.A.W. analyzed the data and edited the manuscript; R.P.M. and R.J.K. performed the research, analyzed the data, and edited the manuscript; B.H., Y.G., and C.J.O. analyzed the data and edited the manuscript; R.S.V. designed the research and edited the manuscript; and S.Y.S. and T.J.W. designed and performed the research, analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: Y.A.W. is currently an employee and stockholder of Novartis. R.P.M. is currently an employee of Genzyme. The remaining authors declare no competing financial interests.
Correspondence: Thomas J. Wang, MD, Cardiology Division, GRB-800, Massachusetts General Hospital, 55 Fruit St, Boston, MA 02114; e-mail: tjwang@partners.org; or Stanley Y. Shaw, MD, PhD, Center for Systems Biology, Simches Research Center CPZN-5226, 185 Cambridge St, Boston, MA 02114; e-mail: shaw.stanley@mgh.harvard.edu; or Kenneth S. Cohen, MD, Section of Hematology/Oncology, University of Chicago Medical Center, 5841 S Maryland Ave, MC 2115, Chicago, IL, 60637; e-mail: kcohen@medicine.bsd.uchicago.edu.
References
- 1.Duda DG, Cohen KS, Scadden DT, Jain RK. A protocol for phenotypic detection and enumeration of circulating endothelial cells and circulating progenitor cells in human blood. Nat Protoc. 2007;2(4):805–810. doi: 10.1038/nprot.2007.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Batchelor TT, Betensky RA, Esposito JM, et al. Age-dependent prognostic effects of genetic alterations in glioblastoma. Clin Cancer Res. 2004;10(1 Pt 1):228–233. doi: 10.1158/1078-0432.ccr-0841-3. [DOI] [PubMed] [Google Scholar]
- 3.Willett CG, Boucher Y, di Tomaso E, et al. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med. 2004;10(2):145–147. doi: 10.1038/nm988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lev EI, Leshem-Lev D, Mager A, et al. Circulating endothelial progenitor cell levels and function in patients who experienced late coronary stent thrombosis. Eur Heart J. 2010;31(21):2625–2632. doi: 10.1093/eurheartj/ehq184. [DOI] [PubMed] [Google Scholar]
- 5.Beijk MA, Klomp M, Verouden NJ, et al. Genous endothelial progenitor cell capturing stent vs. the Taxus Liberte stent in patients with de novo coronary lesions with a high-risk of coronary restenosis: a randomized, single-centre, pilot study. Eur Heart J. 2010;31(9):1055–1064. doi: 10.1093/eurheartj/ehp476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275(5302):964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 7.Peichev M, Naiyer AJ, Pereira D, et al. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000;95(3):952–958. [PubMed] [Google Scholar]
- 8.Losordo DW, Henry TD, Davidson C, et al. Intramyocardial, autologous CD34+ cell therapy for refractory angina. Circ Res. 2011;109(4):428–436. doi: 10.1161/CIRCRESAHA.111.245993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta R, Losordo DW. Cell therapy for critical limb ischemia: moving forward one step at a time. Circ Cardiovasc Interv. 2011;4(1):2–5. doi: 10.1161/CIRCINTERVENTIONS.110.960716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shantsila E, Watson T, Lip GY. Endothelial progenitor cells in cardiovascular disorders. J Am Coll Cardiol. 2007;49(7):741–752. doi: 10.1016/j.jacc.2006.09.050. [DOI] [PubMed] [Google Scholar]
- 11.Zavada J, Kideryova L, Pytlik R, Hruskova Z, Tesar V. Reduced number of endothelial progenitor cells is predictive of early relapse in anti-neutrophil cytoplasmic antibody-associated vasculitis. Rheumatology (Oxford) 2009;48(10):1197–1201. doi: 10.1093/rheumatology/kep130. [DOI] [PubMed] [Google Scholar]
- 12.Westerweel PE, Verhaar MC. Endothelial progenitor cell dysfunction in rheumatic disease. Nat Rev Rheumatol. 2009;5(6):332–340. doi: 10.1038/nrrheum.2009.81. [DOI] [PubMed] [Google Scholar]
- 13.Fadini GP, Avogaro A, Ferraccioli G, Agostini C. Endothelial progenitors in pulmonary hypertension: new pathophysiology and therapeutic implications. Eur Respir J. 2010;35(2):418–425. doi: 10.1183/09031936.00112809. [DOI] [PubMed] [Google Scholar]
- 14.Fadini GP, Schiavon M, Cantini M, et al. Circulating progenitor cells are reduced in patients with severe lung disease. Stem Cells. 2006;24(7):1806–1813. doi: 10.1634/stemcells.2005-0440. [DOI] [PubMed] [Google Scholar]
- 15.Taylor M, Rossler J, Geoerger B, et al. High levels of circulating VEGFR2+ Bone marrow-derived progenitor cells correlate with metastatic disease in patients with pediatric solid malignancies. Clin Cancer Res. 2009;15(14):4561–4571. doi: 10.1158/1078-0432.CCR-08-2363. [DOI] [PubMed] [Google Scholar]
- 16.Zheng PP, Hop WC, Luider TM, Sillevis Smitt PA, Kros JM. Increased levels of circulating endothelial progenitor cells and circulating endothelial nitric oxide synthase in patients with gliomas. Ann Neurol. 2007;62(1):40–48. doi: 10.1002/ana.21151. [DOI] [PubMed] [Google Scholar]
- 17.Vasa M, Fichtlscherer S, Aicher A, et al. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001;89(1):E1–E7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- 18.Heiss C, Keymel S, Niesler U, Ziemann J, Kelm M, Kalka C. Impaired progenitor cell activity in age-related endothelial dysfunction. J Am Coll Cardiol. 2005;45(9):1441–1448. doi: 10.1016/j.jacc.2004.12.074. [DOI] [PubMed] [Google Scholar]
- 19.Murphy C, Kanaganayagam GS, Jiang B, et al. Vascular dysfunction and reduced circulating endothelial progenitor cells in young healthy UK South Asian men. Arterioscler Thromb Vasc Biol. 2007;27(4):936–942. doi: 10.1161/01.ATV.0000258788.11372.d0. [DOI] [PubMed] [Google Scholar]
- 20.Werner N, Kosiol S, Schiegl T, et al. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med. 2005;353(10):999–1007. doi: 10.1056/NEJMoa043814. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt-Lucke C, Rossig L, Fichtlscherer S, et al. Reduced number of circulating endothelial progenitor cells predicts future cardiovascular events: proof of concept for the clinical importance of endogenous vascular repair. Circulation. 2005;111(22):2981–2987. doi: 10.1161/CIRCULATIONAHA.104.504340. [DOI] [PubMed] [Google Scholar]
- 22.Dawber TR, Meadors GF, Moore FE., Jr Epidemiological approaches to heart disease: the Framingham Study. Am J Public Health Nations Health. 1951;41(3):279–281. doi: 10.2105/ajph.41.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol. 1979;110(3):281–290. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- 24.Hill JM, Zalos G, Halcox JP, et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348(7):593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 25.Rabbee N, Speed TP. A genotype calling algorithm for affymetrix SNP arrays. Bioinformatics. 2006;22(1):7–12. doi: 10.1093/bioinformatics/bti741. [DOI] [PubMed] [Google Scholar]
- 26.Li Y, Abecasis GR. Mach 1.0: Rapid haplotype reconstruction and missing genotype inferenc. Am J Hum Genet. 2006;S79:2290. [Google Scholar]
- 27.Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97(18):1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 28.SAS Institute. SAS/STAT User's Guide, Version 8. Cary, NC: SAS Institute; 2000. [Google Scholar]
- 29.Almasy L, Blangero J. Multipoint quantitative-trait linkage analysis in general pedigrees. Am J Hum Genet. 1998;62(5):1198–1211. doi: 10.1086/301844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen MH, Yang Q. GWAF: an R package for genome-wide association analyses with family data. Bioinformatics. 2010;26(4):580–581. doi: 10.1093/bioinformatics/btp710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burton PR, Clayton DG, Cardon LR, et al. Wellcome Trust Case Control Consortium. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447(7145):661–678. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vasu S, Leitman SF, Tisdale JF, et al. Donor demographic and laboratory predictors of allogeneic peripheral blood stem cell mobilization in an ethnically diverse population. Blood. 2008;112(5):2092–2100. doi: 10.1182/blood-2008-03-143677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tousoulis D, Andreou I, Tsiatas M, et al. Effects of rosuvastatin and allopurinol on circulating endothelial progenitor cells in patients with congestive heart failure: the impact of inflammatory process and oxidative stress. Atherosclerosis. 2011;214(1):151–157. doi: 10.1016/j.atherosclerosis.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 34.Hong SJ, Choi SC, Kim JS, et al. Low-dose versus moderate-dose atorvastatin after acute myocardial infarction: 8-month effects on coronary flow reserve and angiogenic cell mobilisation. Heart. 2010;96(10):756–764. doi: 10.1136/hrt.2009.182683. [DOI] [PubMed] [Google Scholar]
- 35.Leone AM, Rutella S, Giannico MB, et al. Effect of intensive vs standard statin therapy on endothelial progenitor cells and left ventricular function in patients with acute myocardial infarction: Statins for regeneration after acute myocardial infarction and PCI (STRAP) trial. Int J Cardiol. 2008;130(3):457–462. doi: 10.1016/j.ijcard.2008.05.036. [DOI] [PubMed] [Google Scholar]
- 36.Schmidt-Lucke C, Fichtlscherer S, Rossig L, Kamper U, Dimmeler S. Improvement of endothelial damage and regeneration indexes in patients with coronary artery disease after 4 weeks of statin therapy. Atherosclerosis. 2010;211(1):249–254. doi: 10.1016/j.atherosclerosis.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 37.Vasa M, Fichtlscherer S, Adler K, et al. Increase in circulating endothelial progenitor cells by statin therapy in patients with stable coronary artery disease. Circulation. 2001;103(24):2885–2890. doi: 10.1161/hc2401.092816. [DOI] [PubMed] [Google Scholar]
- 38.Hristov M, Fach C, Becker C, et al. Reduced numbers of circulating endothelial progenitor cells in patients with coronary artery disease associated with long-term statin treatment. Atherosclerosis. 2007;192(2):413–420. doi: 10.1016/j.atherosclerosis.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 39.Ludwig A, Jochmann N, Kertesz A, et al. Smoking decreases the level of circulating CD34+ progenitor cells in young healthy women–a pilot study. BMC Womens Health. 2010;10:20. doi: 10.1186/1472-6874-10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martin-Rendon E, Brunskill SJ, Hyde CJ, Stanworth SJ, Mathur A, Watt SM. Autologous bone marrow stem cells to treat acute myocardial infarction: a systematic review. Eur Heart J. 2008;29(15):1807–1818. doi: 10.1093/eurheartj/ehn220. [DOI] [PubMed] [Google Scholar]
- 41.Quyyumi AA, Waller EK, Murrow J, et al. CD34(+) cell infusion after ST elevation myocardial infarction is associated with improved perfusion and is dose dependent. Am Heart J. 2011;161(1):98–105. doi: 10.1016/j.ahj.2010.09.025. [DOI] [PubMed] [Google Scholar]
- 42.Sedoris KC, Thomas SD, Miller DM. Hypoxia induces differential translation of enolase/MBP-1. BMC Cancer. 2010;10:157. doi: 10.1186/1471-2407-10-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Waerner T, Gardellin P, Pfizenmaier K, Weith A, Kraut N. Human RERE is localized to nuclear promyelocytic leukemia oncogenic domains and enhances apoptosis. Cell Growth Differ. 2001;12(4):201–210. [PubMed] [Google Scholar]
- 44.Shaw SY, Cheng S, Cupples LA, et al. Genetic and clinical correlates of early-outgrowth colony-forming units. Circ Cardiovasc Genet. 2011;4(3):296–304. doi: 10.1161/CIRCGENETICS.110.958470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Finkel T, Quyyumi AA. Genetic links between circulating cells and cardiovascular risk. Circ Cardiovasc Genet. 2011;4(3):218–220. doi: 10.1161/CIRCGENETICS.111.960310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Timmermans F, Plum J, Yoder MC, Ingram DA, Vanderkerckhove B, Case J. Endothelial progenitor cells: identity defined? J Cell Mol Med. 2009;13(1):87–102. doi: 10.1111/j.1582-4934.2008.00598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sirker AA, Astroulakis ZM, Hill JM. Vascular progenitor cells and translational research: the role of endothelial and smooth muscle progenitor cells in endogenous arterial remodelling in the adult. Clin Sci (Lond) 2009;116(4):283–299. doi: 10.1042/CS20080001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.