Abstract
The effectiveness of placebo treatments depends on the recipient’s expectations, which are at least in part shaped by previous experiences. Thus, positive past experience together with an accordant verbal instruction should enhance outcome expectations and subsequently lead to higher placebo efficacy. This should be reflected in subjective valuation reports and in activation of placebo-related brain structures. We tested this hypothesis in a functional magnetic resonance imaging study, where subjects experienced different levels of pain relief and conforming information about price levels for two placebo treatments during a manipulation phase, thereby establishing a weak and a strong placebo. As expected, both placebos led to a significant pain relief and the strong placebo induced better analgesic efficacy. Individual placebo value estimates reflected treatment efficacy, i.e. subjects were willing to pay more money for the strong placebo even though pain stimulation was completed at this time. On the neural level, placebo effects were associated with activation of the rostral anterior cingulate cortex, the anterior insula, and the ventral striatum and deactivations in the thalamus and secondary somatosensory cortex. However, only placebo-related responses in rostral anterior cingulate cortex were consistent across both the anticipation of painful stimuli and their actual administration. Most importantly, rostral anterior cingulate cortex responses were higher for the strong placebo, thus mirroring the behavioral effects. These results directly link placebo analgesia to anticipatory activity in the ventral striatum, a region involved in reward processing, and highlight the role of the rostral anterior cingulate cortex, as its activity consistently scaled with increasing analgesic efficacy.
Keywords: placebo analgesia, rostral anterior cingulate cortex, pain, subjective value, fMRI
Introduction
Placebo treatments can elicit a variety of physiological responses, such as the release of dopamine in Parkinson’s disease (De la Fuente-Fernández et al., 2001) or the release of endogenous opioids in placebo analgesia (Levine et al., 1978; Amanzio and Benedetti, 1999). In the latter, an inert treatment (e.g. a pharmacologically ineffective cream) causes pain relief (Voudouris et al., 1989; Montgomery and Kirsch, 1997; Price et al., 1999; Amanzio et al., 2001; Colloca et al., 2008). Placebo analgesia is thought to be shaped by expectations, which in turn are influenced by prior experience (Montgomery and Kirsch, 1997; Price et al., 1999; Stewart-Williams and Podd, 2004; Meissner et al., 2011). Manipulations of prior experience and their effects on placebo effects have been studied in terms of learning theory (Voudouris et al., 1989; Colloca et al., 2010; Jensen et al., 2012) and expectancy modulations (Montgomery and Kirsch, 1997). For example, value manipulation, as a means of modulating treatment related expectations, affects the strength of placebo analgesia (Waber et al., 2008).
On the neural level, placebo analgesia has been linked to a brain network including the dorsolateral prefrontal cortex (DLPFC), the rostral anterior cingulate cortex (rACC) and the periaqueductal gray (PAG) (Petrovic et al., 2002; Wager et al., 2004; Bingel et al., 2006; Kong et al., 2006; Eippert et al., 2009). Other regions such as the anterior insula and ventral striatum also seem to play a role (Zubieta et al., 2005; Kong et al., 2006; Scott et al., 2007, 2008). The ventral striatum is also involved in reward processing (Schultz et al., 1997; Yacubian et al., 2007) and it has been suggested that reward processing interacts with endogenous pain modulation (Schweinhardt et al., 2009; Becker et al., 2012). The rACC is thought to be a key player within this antinociceptive network (Bingel and Tracey, 2008), exhibiting complex response patterns to placebo treatment. Stronger rACC activation has been observed when comparing a placebo condition to a control condition during pain stimulation (Bingel et al., 2006; Kong et al., 2006; Eippert et al., 2009), or the anticipation of pain (Wager et al., 2004; Watson et al., 2009). However, some of these studies also reported decreased rACC responses for placebo treatments (Wager et al., 2004; Eippert et al., 2009) during heat pain stimulation. To further investigate the role of the rACC in placebo analgesia, we extended a well-established placebo analgesia paradigm (Price et al., 1999; Wager et al., 2004; Eippert et al., 2009) using functional magnetic resonance imaging (fMRI).
Two placebo treatments of different efficacies induced by value manipulation were employed in this task (Figure 1) while recording fMRI data. Treatment values were manipulated by informing subjects about ostensible high or low price levels, respectively. Regarding such a value modulation of placebo effects (Waber et al., 2008), it is possible that this relationship is bidirectional, i.e. placebo efficacy might affect subjective placebo valuation. In order to relate placebo responses to individual value differences, estimates of subjective placebo value were obtained in a second task, outside the scanner. Behaviorally, we expected the more effective placebo (i) to induce stronger pain relief during the test session and (ii) to receive higher individual value estimates after effective pain relief. As the majority of placebo analgesia imaging studies reported positive rACC responses, we expected the rACC to show greater responses with increasing placebo efficacy. While our main focus lay on the rACC, we also investigated responses from other brain regions implicated in placebo analgesia, such as the secondary somatosensory cortex, dorsal anterior cingulate cortex (dACC), ventral striatum, and thalamus.
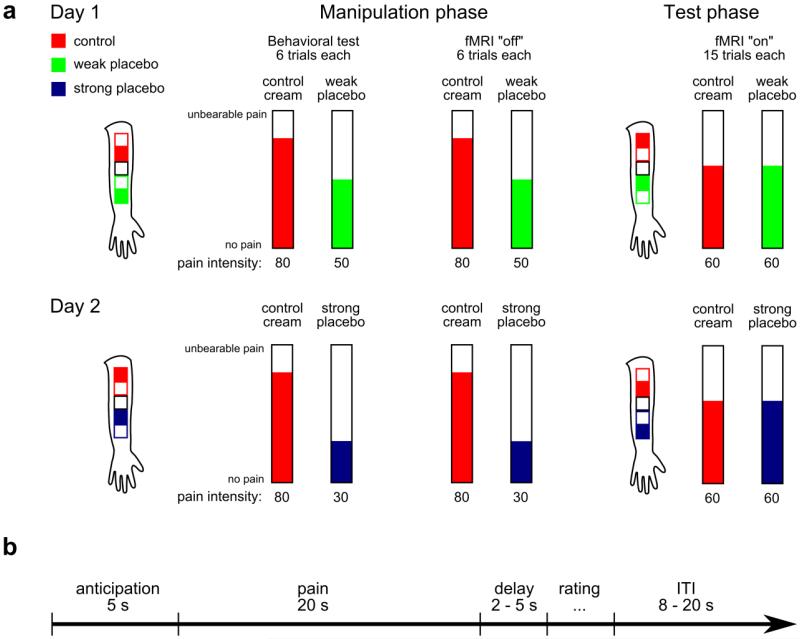
Figure 1.
Experimental design. (a) Illustration of the placebo paradigm. Stimulation sites are indicated by the filled squares on the forearm outline. Placebo creams were introduced as “expensive” and “low priced”, respectively. Subjects underwent two manipulation series on each day, the first one in a medical examination room and the second one inside the MRI scanner without BOLD data acquisition. The temperatures were surreptitiously lowered in the placebo conditions during the manipulation phases. This was done to enhance expectations regarding the placebo treatment efficacy. During test sessions BOLD data were acquired with equal temperatures in placebo and control conditions. Stimulation sites, the order of placebo and control within session and the order of placebos between days were counterbalanced across subjects. (b) Schematic of one trial. Each trial started with a fixed anticipation period of 5 s, followed by 20 s heat pain stimulation, a variable delay, a pain rating and a variable inter trial interval (ITI).
Methods
Participants
Fifty-two healthy, male, right-handed subjects participated in this study and were tested on two days. Twelve subjects had to be excluded. One subject was excluded because of an incidental neurological finding, and another subject because of analgesic medication on the second day. Furthermore, two subjects had to be excluded because of technical problems with the thermal stimulation device, four subjects were excluded because of movement artifacts during scanning (volume to volume translation > 1.5 mm or rotation > 1.5°) and four subjects bid zero Euros for all creams in the value estimation experiment (details see below) which prohibited analysis of the value models. All of the remaining forty subjects (mean age: 26 years, range: 19 – 40 years) passed drug tests (e.g. amphetamines, benzodiazepines, tetrahydrocannabinol and opiates) on both test days (see study design). The Ethics committee of the Medical Chamber Hamburg approved the study and all subjects gave written consent. The consent form explained that subjects would be treated with powerful analgesics “which have been shown to relief pain in several studies”, referring to the repeated demonstration of analgesia induced by placebo treatments (Levine et al., 1978; Price et al., 1999; Amanzio and Benedetti, 1999; Petrovic et al., 2002; Bingel et al., 2006; Colloca et al., 2008; Eippert et al., 2009).
Study Design
Experiments took place in a medical examination room adjacent to the MR facilities. We used a within-subject design to investigate the analgesic efficacy of two placebos on two days (Figure 1), by extending an established placebo analgesia paradigm (Price et al., 1999; Wager et al., 2004; Colloca et al., 2008; Eippert et al., 2009) through the introduction of a second placebo cream. This paradigm involves both expectation and conditioning manipulations to elicit placebo responses. Subjects were informed that the study investigated pain modulation by two powerful analgesics that differed in market price, i.e. one “expensive” and one “low priced” cream. All creams consisted solely of acrylate gel without any active agent and were identical, except for the label. One placebo cream was labeled “analgesic A”, the other “analgesic B”, and the control was labeled “acrylate gel”. On each test day, one of the two placebos was tested against the control cream. Test days were approximately one week apart. Heat pain stimuli were applied on a total of five patches on the volar side of the left forearm. Two patches were used during the manipulation phase (for the placebo and the control, respectively), two were used during the test phase (to avoid habituation or sensitization effects) and the middle patch was used on day 1 for calibration only.
Prior to the pain calibration on day 1, we applied the creams to “allow the analgesic to take effect during the time of calibration”. Temperature calibration was carried out on the cream-free middle patch, using a series of 16 heat stimuli (20s duration) presented in a pseudorandom order. After each stimulus, subjects rated their pain intensity on a computerized visual analogue scale (VAS) ranging from 0 to 100. The scale was anchored with “no pain” and “unbearable pain”. Subjects were instructed to rate “unbearable pain” only in case they had to lift the thermode because of too intense pain. A linear interpolation (i.e. regression) was used to calculate temperatures corresponding to 30, 50, 60 and 80 on the VAS. After calibration, a first series of 6 stimuli was administered outside the scanner on patches treated with the placebo or control cream (VAS 80), respectively. Importantly, subjects expected the VAS 80 temperature on both sites, but the temperature on the placebo patch was surreptitiously lowered to a temperature corresponding to VAS 30 for the strong and VAS 50 for the weak placebo. This was done to provide subjects with an experience congruent with their expectations (“expensive” vs. “low priced”). We used a combination of efficacy manipulation and verbal value instructions as the combination of both procedures has been shown to support placebo analgesia (Montgomery and Kirsch, 1997; Price et al., 1999; Waber et al., 2008). The same stimulation series was repeated inside the scanner (with the scanner being turned off). Subjects then left the scanner for cream application on the remaining two patches and waited for 10 min to allow the creams to take effect (completing the State-trait-anxiety-inventory while waiting). During the test phase, 30 identical stimuli (VAS 60) were applied on placebo and control patches, respectively (15 on each patch), and BOLD data were recorded. After each pain stimulus, subjects had to rate their pain intensity on a VAS. After each test phase, subjects were asked to retrospectively rate the analgesic efficacy of the creams used in that session on a six-point scale anchored “no effect” and “extremely effective”.
On the second day, subjects underwent the same pain stimulation series, but calibration was omitted and temperatures during the manipulation phase corresponded to the respective placebo condition (i.e. VAS 30 for the strong or VAS 50 for the weak placebo). During all sessions, subjects rated the perceived pain intensity on a VAS after each pain stimulus. The order of stimulation (placebo or control first) and patch positions (placebo or control above the middle patch) were counterbalanced across subjects. We also recorded skin conductance responses during test runs.
Each trial consisted of an anticipation phase, the painful stimulation, a pause, a pain rating and an inter-trial-interval (ITI, Figure 1b). Trials started with a white fixation cross which switched color at the beginning of the anticipation phase. The color was different for each condition; red for the control, blue for the strong, and green for the weak placebo. Subjects pressed a button after the fixation cross changed its color and the crosshair turned white again for the remainder of the 5s anticipation period. The subsequent 20 s pain stimulation consisted of ~2 s ramp up, 16 s plateau heat and ~2 s ramp down. During heat stimulation, the crosshair color changed again to the respective color of the current condition. After the heat stimulation a variable delay of 2 – 5 s followed, before the VAS appeared on the screen and subjects rated the perceived pain intensity. A white crosshair was displayed during an ITI of 8 – 20 s duration.
On day 2, after the last pain session, subjects completed a Becker-deGroot-Marschak (BDM) auction (Becker et al., 1964) outside the scanner and a subsequent choice task inside the scanner to identify behavioral and neural estimates of value for both placebos, respectively (results from the choice task will be reported elsewhere). The BDM auction estimates subjective value from the bid on a particular good, using specific rules. Importantly, the auction includes the possibility of a real purchase, and the price is determined by a random number generator. Only if the price is lower than the bid, a purchase is made at the randomly determined price. This ensures that the bid is only influenced by the subjective willingness-to-pay (WTP) and cannot be influenced by strategic bids. Consequently, bids represent WTP estimates. The instructions for the BDM auctions were similar to those used previously (Plassmann et al., 2007) and pre-tested for comprehensibility.
Subjects placed their bids for 21 over-the-counter medical products including the three creams (weak placebo, strong placebo and control). This extended set of goods was used to disguise our experimental hypotheses to the subjects. Bids could be between € 0 and € 5 in steps of € 0.50. Volunteers were informed that one trial of the BDM auction or the purchasing choice task would be randomly drawn and implemented at the end of the experiment (i.e. subjects bought one good depending on their behavior in the selected trial). Hence, they realistically could end up buying one of the products for a maximum of their € 5 cash endowment. By choosing only one random trial to be implemented, subjects did not have to worry about splitting their budget across items and could treat each trial as an independent decision. The procedure lasted about 3 h on each of the two test days.
Data acquisition
Stimulus presentation, response logging and thermode triggering were done using the Cogent Toolbox (v1.29, http://www.vislab.ucl.ac.uk/cogent.php). Thermal stimulation was delivered via a MRI compatible 3 × 3 cm peltier thermode (TSA-II, Medoc, Israel). Skin conductance was recorded from the hypothenar of the left hand. The signal was amplified using a CED 2502 amplifier and digitized at 1000 Hz using a CED micro1401 and downsampled offline to 100 Hz (both by Cambridge Electronic Design, Cambridge, UK). Functional magnetic resonance imaging (fMRI) data were acquired on a Siemens Trio 3 Tesla system equipped with a 32-channel head coil (Siemens, Erlangen, Germany). Forty-two transversal slices (voxel size 2 × 2 × 2 mm, 1 mm gap) were acquired within each volume using a T2* sensitive echo planar imaging (EPI) sequence (TR=2.58s, TE=26ms, flip angle: 80°, field of view: 220 × 220 mm). Slices were tilted about 30° relative to the AC-PC line to improve signal-to-noise ratio in the orbitofrontal cortex (Deichmann et al., 2003). Functional image coverage did not include the uppermost parts of the parietal cortex. Additionally, T1 weighted structural images (1 × 1 × 1 mm resolution) were obtained using a MPRAGE sequence.
Behavioral data analysis
Pain ratings were analyzed using a 2 × 2 repeated measures analysis of variance (ANOVA) with factors treatment (placebo, control) and efficacy (weak, strong); post-hoc paired t-tests were also carried out. Cream value estimates (i.e. WTP) were tested using a one-way repeated measure ANOVA with 3 factor levels and post-hoc t-tests. Placebo effects were calculated by subtracting the mean placebo VAS rating from the mean pain rating of the respective control. Placebo responses from weak and strong conditions were then correlated with each other using Pearson’s correlation coefficient. Furthermore, placebo effects on pain ratings were correlated with corresponding placebo effects on skin conductance responses (SCR) and subjective value differences between placebo and control cream values (see Supplemental Table S2). Linear regression analysis was used for each subject to predict the average pain rating for each cream by its corresponding WTP estimate. Slopes were then tested against zero using Wilcoxon signed-rank test and explained variance was estimated by R2 values. To test for potential effects of placebo order (i.e. weak placebo on day 1 or on day 2) we conducted a linear regression analyses using the placebo condition (dummy coded), the interval between the two sessions (days), the STAI state score, and the placebo order (strong or weak placebo on day 1, dummy coded) as predictors of the placebo response (VAS control – VAS placebo). In all behavioral analyses the significance level was set to p < 0.05, one-tailed in case of a-priori hypotheses, and p-values of post-hoc comparisons were Bonferroni corrected. Data were analyzed using Matlab (Mathworks, Natick, MA) and SPSS (IBM, Armonk, NY).
Skin conductance recordings of each subject were split into individual trials and detrended within trials. A template for a single SCR was estimated for each subject and trials were subsequently decomposed into separate SCRs using the subject specific template (Feld et al., 2010). The SCR amplitudes within the first 5 s of the pain stimulation were log-transformed, summed within trials and averaged for each condition (Levinson and Edelberg, 1985). SCRs were analyzed using a 2 × 2 repeated measures ANOVA and post-hoc t-tests, similar to the pain ratings.
fMRI data analysis
Statistical parametric mapping (SPM8, Wellcome Trust Centre for Neuroimaging, UCL, London, UK) was used to analyze the fMRI data. The first four functional images of each run were discarded and the remaining images were realigned, then normalized to MNI space, and finally smoothed with a 6 mm (FWHM) isotropic Gaussian kernel.
Previous studies reported temporally distinct BOLD activations during placebo analgesia (Wager et al., 2004; Eippert et al., 2009). We therefore analyzed early (1-10s after stimulus onset) and late pain (10-20s) phases separately. Regressors modeling the anticipation period, early pain, late pain and the rating period of each session were specified using boxcar functions. Additionally, six motion regressors, encoding rotation and translation along each axis and four nuisance regressors modeling the mean signal from cerebrospinal fluid and white matter of each hemisphere, respectively, were also included. Together with a session constant, 15 regressors were included per pain session, totaling in 60 regressors per first level model. Condition contrast images (weak placebo, strong placebo and their respective controls) were then raised to a second level random effects analysis for the anticipation phase, early and late pain, respectively. The following group level contrasts were tested for all time periods: main effect of treatment (pooled placebo > pooled control), interaction effects ((strong placebo > control) > (weak placebo > control) and (control > strong placebo) > (control > weak placebo)), as well as specific condition main effects (strong placebo > control; weak placebo > control). Additionally, we evaluated the following contrasts: pooled control > pooled placebo; control > strong placebo; control > weak placebo.
In order to assess correlations between neuronal and behavioral placebo responses, we added placebo effects on pain ratings (control – placebo) as covariates in an additional second level analysis. Since behavioral placebo effects were uncorrelated (see Results), we tested correlations separately for weak and strong placebos.
Correction for multiple comparisons of the functional imaging data was done according to a family-wise error rate (pFWE < 0.05), using small volume correction (SVC) with spheres of 10 mm radius for cortical regions (DLPFC: 15 mm) and 6 mm radius for subcortical regions. Spheres were centered at peak coordinates (ignoring laterality) from previous studies investigating pain processing and value representation in the following regions: DLPFC (Wager et al., 2011), secondary somatosensory cortex (Bingel et al., 2007), insula (anterior part (Bingel et al., 2007); posterior part (Zubieta et al., 2005)), dorsal anterior cingulate cortex (Büchel et al., 2002), rACC (pregenual part (Eippert et al., 2008); subgenual part (Bingel et al., 2007)), ventral striatum (Yacubian et al., 2007), thalamus (Bingel et al., 2007), and the PAG (anatomical center, x=0, y=−32, z=−10, (Eippert et al., 2009)). For completeness, we also report activations significant at p<0.001, uncorrected, within the above specified regions. Supplemental Figure S1 provides an overview about the regions mentioned above.
Results
Behavioral placebo responses
We first tested whether the placebo treatment lowered pain reports compared to the control condition. Secondly, we investigated differences in analgesic efficacy between the two placebo creams (Figure 2a). A 2 × 2 ANOVA revealed a main effect of treatment (F(1,39)=23.43, p<0.001), a main effect of placebo cream (weak vs. strong; F(1,39)=11.46, p=0.002; with lower pain ratings in the strong placebo and corresponding control condition), and an interaction effect (F(1,39)=5.47, p=0.025). Following up on the significant interaction, a comparison of the placebo effects revealed higher pain relief for the strong than for the weak placebo (pain reductionstrong=21.8%; pain reductionweak=8.2%). Post-hoc paired t-tests (Bonferroni corrected) confirmed the significant difference between the two placebo treatments (t(39)=3.54, p=0.001), whereas pain ratings from the control conditions did not differ (t(39)=1.49, p=0.29). Since the post-hoc tests only revealed differences between the two placebo conditions and not between the two controls, the main effect of placebo cream is driven by the lower pain ratings in the strong placebo condition and not by differences between the control conditions. Retrospective reports regarding the perceived efficacy in pain relief also favored the strong over the weak placebo (t(39)=2.67, p=0.011). Interestingly, placebo responses from the two treatments were not correlated (Pearson’s r=0.11, p=0.49; see Supplemental Table S2). Testing for potential effects of the placebo order (i.e. strong placebo on day 1 or day 2) we included several potential placebo response predictors together with the dummy coded test order in a linear regression model. Placebo order did not affect placebo analgesia; its regression weight was not significantly associated with the placebo response (B=1.26, p=0.73). The only significant predictor was the placebo condition, i.e. strong vs. weak irrespective of the test day (B=8.46, p=0.023).
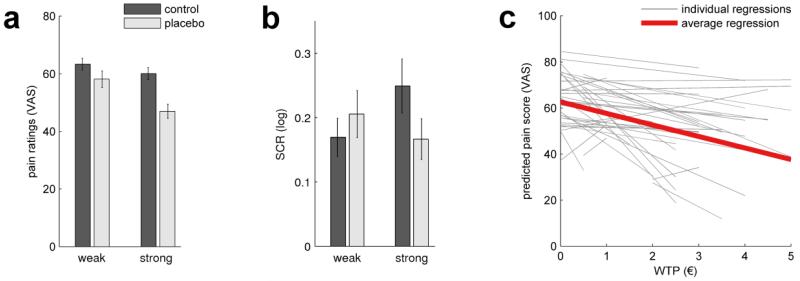
Figure 2.
Behavioral placebo effects. (a) Pain ratings averaged over subjects (± SEM). Temperatures were calibrated to equal a VAS score of 60. Reported pain was significantly reduced by placebo treatment (p<0.001) and the reduction was significantly greater in the strong condition (p=0.025). Pain ratings from the control conditions did not differ significantly. (b) Skin conductance responses (SCR) to heat pain stimuli. The interaction term was significant (p=0.004). Follow-up t-tests revealed a significant reduction of SCRs under strong placebo compared to control (p<0.05). (c) Pain ratings were correlated with value estimates (WTP). The slopes were significantly negative (average slope: −5.01, i.e. the more a subject valued a cream, the lower his corresponding pain rating), thereby relating the group level effect shown in a) to inter-individual differences in treatment valuation.
Skin conductance responses (SCR) to painful stimuli revealed no significant main effect (treatment: F(1,39)=0.9, n.s.; placebo, weak vs. strong: F(1,39)=0.66, n.s.). The interaction term was significant and pointed in the same direction as the VAS ratings (F(1,39)=9.30, p=0.004), indicating stronger placebo analgesic effects for strong vs. weak treatment (Figure 2b). Post-hoc t-tests revealed a significant reduction in SCRs for the strong placebo vs. control (t(39)=2.11, p=0.04). The difference weak placebo vs. control was not significant (t(39)=1.76, n.s.).
We then compared subjective value estimates (willingness-to-pay; WTP) for the three creams. WTP estimates were collected for a set of 21 medical over-the-counter products such as antiseptics or plasters, including the three creams used in the experiment. An ANOVA revealed significant differences between cream values (F(2,78)=77.9, p<0.001). WTP estimates were higher for the strong than for the weak cream (t(39)=4.13, p<0.001; WTPweak: € 2.09; WTPstrong: € 2.86). Notably, the strong placebo received the highest WTP of all 21 goods. As expected, the control cream received the lowest WTP (€ 0.25) and was valued less than both placebo creams (control vs. weak placebo: t(39)=8.16, p<0.001; control vs. strong placebo: t(39)=11.38, p<0.001).
To test for a relationship between pain relief and valuation, we individually predicted pain rating scores by WTP using regression analyses. Value estimates accounted on average for 52% of the pain variance. More importantly, the average slope was negative (mean B=−5.01; z=−3.4; p=0.001), indicating that high subjective treatment value was associated with the magnitude of pain relief (Figure 2c).
Placebo-induced brain responses
After having established graded placebo effects in pain ratings, we investigated brain responses in anticipation of, and during pain stimulation.
Anticipation phase
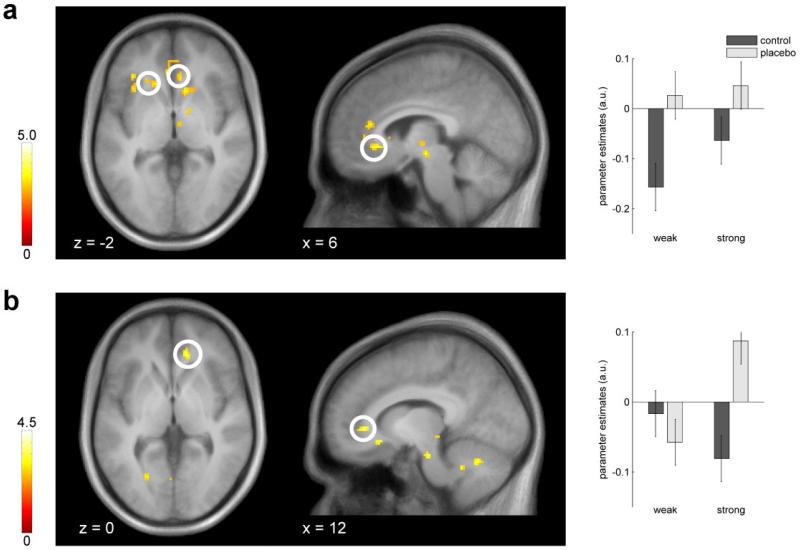
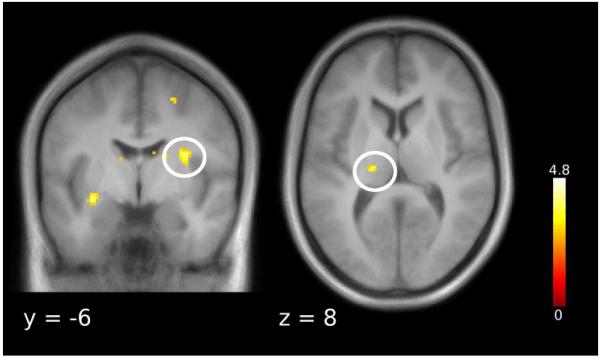
During the anticipation of painful stimuli, the rACC, the ventral striatum and the anterior insula showed placebo related activation (pooled placebo > control; Figure 3a, Table 1). Testing the interaction effect ((strong > control) > (weak > control)) during anticipation yielded enhanced activity for the strong relative to the weak placebo in the rACC (Figure 3b). This interaction term tests for stronger responses to the strong than to the weak placebo. The reverse interaction contrast ((control > strong) > (control > weak)) can be interpreted as stronger deactivations under the strong than under the weak placebo. This pattern was evident in the posterior insula (Table 1). Next, we examined the two placebo anticipation conditions separately: while the strong placebo induced activations in the rACC, the PAG and ventral striatum, the weak placebo induced activity in the rACC and the anterior insula (Table 1). We did not find any effects testing for control > placebo.
Figure 3.
Brain responses during the anticipation period. (a) Activations for placebo > control were observed in the rACC and the ventral striatum. Parameter estimates are plotted for the rACC peak voxel. (b) The rACC showed enhanced placebo induced responses for the strong vs. weak placebo ((strong placebo > control) > (weak placebo > control)). Parameter estimates are plotted for the rACC peak voxel. Error bars indicate 90% confidence interval. Statistical t-maps are overlaid on an average structural image and the significance threshold is set to p < 0.005 (uncorrected) for visualization purposes only.
Table 1. Brain responses during the anticipation of painful heat.
| Region | x | y | z | t value | p value |
|---|---|---|---|---|---|
| placebo > control | |||||
| rACC, pregenual | 8 | 36 | 14 | 3.59 | p < 0.001 * |
| anterior insula | −36 | 20 | 0 | 3.59 | p < 0.001 * |
| rACC, subgenual | 6 | 30 | −4 | 3.48 | p < 0.001 * |
| ventral striatum | −6 | 8 | −6 | 3.35 | p < 0.001 * |
| rACC, subgenual | −4 | 34 | −2 | 3.30 | p < 0.001 |
| control > placebo | |||||
| - | - | - | - | - | - |
| strong placebo > control | |||||
| rACC, pregenual | 12 | 36 | 0 | 4.00 | p < 0.001 * |
| ventral striatum | 6 | 10 | −6 | 3.62 | p < 0.001 * |
| PAG | 4 | −32 | −6 | 3.48 | p < 0.001 * |
| ventral striatum | −6 | 10 | −6 | 3.33 | p < 0.001 * |
| rACC, subgenual | −4 | 34 | −4 | 3.24 | p < 0.001 |
| weak placebo > control | |||||
| anterior insula | −32 | 12 | 10 | 3.83 | p < 0.001 * |
| rACC, pregenual | 8 | 36 | 14 | 3.56 | p < 0.001 * |
| posterior insula | −36 | −16 | 2 | 3.37 | p < 0.001 |
| anterior insula | −36 | 20 | 2 | 3.34 | p < 0.001 |
| anterior insula | −36 | 22 | 10 | 3.25 | p < 0.001 |
| control > strong placebo | |||||
| - | - | - | - | - | - |
| control > weak placebo | |||||
| - - - | - | - | - | ||
| (strong placebo > control) > (weak placebo > control) | |||||
| rACC, pregenual | 12 | 36 | 0 | 3.51 | p < 0.001 * |
| 16 | 32 | 6 | 3.32 | p < 0.001 | |
| (control > strong placebo) > (control > weak placebo) | |||||
| posterior insula | −36 | −16 | 10 | 3.5 | p < 0.001 * |
| anterior insula | −34 | 12 | 8 | 3.46 | p < 0.001 |
| anterior insula | −36 | 22 | 10 | 3.32 | p < 0.001 |
| anterior insula | 40 | 18 | 10 | 3.28 | p < 0.001 |
All coordinates [x,y,z in mm] are in MNI space. Second level contrasts are in italic type face.
rACC – rostral anterior cingulate cortex; PAG – periaqueductal gray.
All activations are from our volumes of interest, significant at p < 0.001, uncorrected;
p < 0.05, corrected;
Pain stimulation period
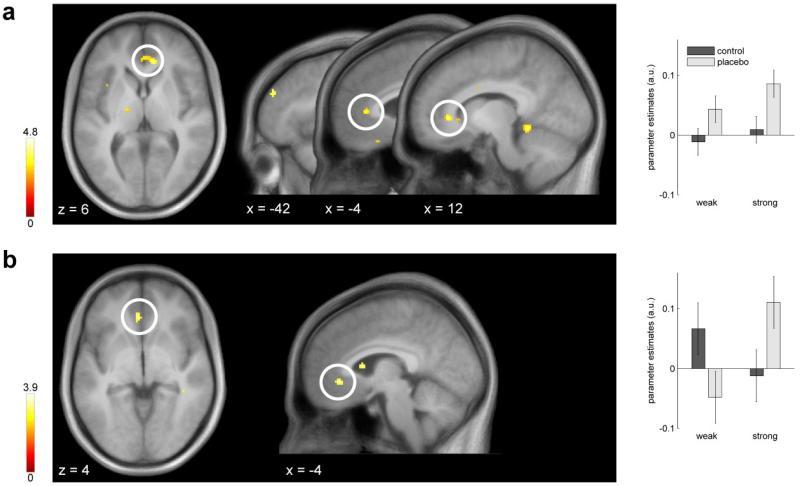
Comparing the pooled placebo conditions to the control conditions during the first half of the painful stimulation, we observed rACC activation (Figure 4a, Table 2). Furthermore, the interaction contrast (strong placebo > control) > (weak placebo > control) revealed enhanced placebo-induced activation within the rACC (Figure 4b, Table 2). When comparing the two placebo conditions separately against their respective controls, the rACC activation was only evident in the strong, but not in the weak placebo condition (Table 2).
Figure 4.
Placebo-induced BOLD responses during pain stimulation. (a) The pooled placebo > control contrast revealed a bilateral cluster in the pregenual rACC during early pain. Parameter estimates are plotted for the left peak. Error bars indicate 90% confidence interval. (b) Comparison of the placebo effects during early pain. The stronger placebo induced increased activity in the rACC, as indicated by the interaction effect (strong> control) > (weak > control). Parameter estimates are plotted for the rACC peak voxels. Statistical t-maps are overlaid on an average structural image and the significance threshold is set to p < 0.005 (uncorrected) for visualization purposes only.
Table 2. Brain responses during early and late pain.
| Region | x | y | z | t value | p value |
|---|---|---|---|---|---|
| Early pain | |||||
| placebo > control | |||||
| rACC, pregenual | 12 | 34 | 2 | 4.21 | p < 0.001 * |
| rACC, pregenual | −6 | 34 | 8 | 3.76 | p < 0.001 * |
| DLPFC | −42 | 44 | 26 | 3.61 | p < 0.001 |
| anterior insula | −40 | 24 | 6 | 3.19 | p < 0.001 |
| control > placebo | |||||
| DLPFC | −44 | 18 | 30 | 3.39 | p < 0.001 |
| strong placebo > control | |||||
| rACC, pregenual | −8 | 32 | 8 | 3.47 | p < 0.001 * |
| anterior insula | −40 | 24 | 6 | 3.32 | p < 0.001 |
| weak placebo > control | |||||
| rACC, pregenual | 12 | 34 | 2 | 3.33 | p < 0.001 |
| DLPFC | 38 | 32 | 36 | 3.15 | p < 0.001 |
| control > strong placebo | |||||
| - | - | - | - | - | - |
| control > weak placebo | |||||
| - | - | - | - | - | - |
| (strong placebo > control) > (weak placebo > control) | |||||
| rACC, subgenual | −4 | 34 | −4 | 3.45 | p < 0.001 * |
| rACC, pregenual | −10 | 30 | 10 | 3.22 | p < 0.001 |
| (control > strong placebo) > (control > weak placebo) | |||||
| - | - | - | - | - | - |
| Late pain | |||||
| placebo > control | |||||
| - | - | - | - | - | - |
| control > placebo | |||||
| SII | 32 | −8 | 20 | 3.77 | p < 0.001 * |
| Thalamus | −16 | −22 | 6 | 3.45 | p < 0.001 * |
| strong placebo > control | |||||
| - | - | - | - | - | - |
| weak placebo > control | |||||
| Thalamus | −12 | −14 | 2 | 3.42 | p < 0.001 * |
| control > strong placebo | |||||
| dACC | 10 | 4 | 40 | 3.26 | p < 0.001 |
| dACC | −6 | 4 | 38 | 3.24 | p < 0.001 |
| control > weak placebo | |||||
| DLPFC | 36 | 34 | 20 | 4.28 | p < 0.001 * |
| (strong placebo > control) > (weak placebo > control) | |||||
| DLPFC | 36 | 34 | 20 | 3.98 | p < 0.001 * |
| DLPFC | 48 | 30 | 28 | 3.17 | p < 0.001 |
| (control > strong placebo) > (control > weak placebo) | |||||
| rACC, subgenual | −12 | 30 | −16 | 3.65 | p < 0.001 * |
| thalamus | −12 | −16 | 2 | 3.64 | p < 0.001 * |
| posterior insula | −36 | −16 | 6 | 3.48 | p < 0.001 * |
| thalamus | 8 | −22 | 4 | 3.36 | p < 0.001 * |
| thalamus | −8 | −20 | 6 | 3.25 | p < 0.001 * |
All coordinates [x,y,z in mm] are in MNI space. Second level contrasts are in italic type face.
rACC – rostral anterior cingulate cortex; DLPFC – dorsolateral prefrontal cortex; SII – secondary somatosensory cortex; dACC – dorsal anterior cingulate cortex.
All activations p < 0.001, uncorrected, are from our volumes of interest;
p < 0.05 corrected.
Within the second half of the painful stimulation, no regions showed a main effect of placebo versus control. Interestingly, activity in the thalamus and secondary somatosensory cortex (SII) was reduced under pooled placebo compared to control (Figure 5). Contrasting the two placebo effects (i.e. using the interaction (strong > control) > (weak > control)) revealed enhanced activation for the strong placebo in the dorsolateral prefrontal cortex (DLPFC, Table 2). Testing the second interaction term ((control > strong) > (control > weak)) revealed effects in the rACC, the thalamus and, as during anticipation, the posterior insula. The rACC activation was located ventrally and did not overlap with any other rACC activation in this study. Parameter estimates at rACC peak show that both placebos induced a positive effect, but the difference to the respective control was bigger for the weak placebo. Investigating the two placebo conditions separately, revealed a deactivation of dorsal ACC (dACC) in the strong placebo condition, at p<0.001, uncorrected. Under weak placebo, the thalamus was activated and this activation led to the interaction effect reported above for the second interaction.
Figure 5.
Reductions in brain responses to placebo. SII (left) and thalamus (right) showed reduced activity under pooled placebo vs. control. Statistical t-maps are overlaid on an average structural image and the significance threshold is set to p < 0.005 (uncorrected) for visualization purposes only.
Correlations between neural and behavioral placebo responses
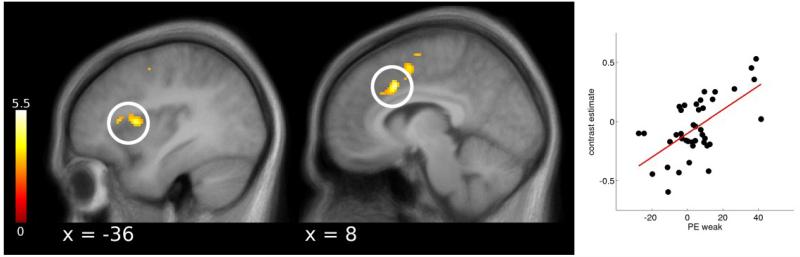
As the behavioral placebo responses were uncorrelated (see Behavioral placebo responses), we computed brain-behavior correlations separately for the weak and strong placebo. Behavioral responses to the strong placebo correlated positively with placebo induced activity in the DLPFC during early pain and with the rACC during late pain (Supplemental Table S3). Furthermore, weak placebo effects correlated with deactivations in the thalamus, anterior insula and dACC during early pain. During late pain, this correlation was evident in the dACC and the anterior insula (Figure 6). Larger reductions in pain reports hence predicted lower activation in pain processing areas such as the dACC and the anterior insula.
Figure 6.
Correlations between behavioral and cortical placebo responses. During late pain, reductions in pain ratings of the weak placebo correlated with placebo induced reductions in the anterior insula and dACC. Statistical t-maps are overlaid on an average structural image and the significance threshold is set to p < 0.005 (uncorrected) for visualization purposes only.
Discussion
This study examined the relationship between levels of previous pain experience, placebo analgesia and brain responses, particularly in the rACC. We observed stronger placebo analgesic effects for a placebo that was presented to the subjects as more effective and more valuable compared to a second placebo. Placebo-induced BOLD responses during anticipation and heat pain stimulation were observed most prominently in the rACC, a region implicated in endogenous pain control in humans (Petrovic et al., 2002; Wager et al., 2004; Zubieta et al., 2005; Bingel et al., 2006; Bingel and Tracey, 2008; Eippert et al., 2009). Additionally, the rACC activation mirrored the better analgesic efficacy of the strong placebo during anticipation and painful heat stimulation. On the behavioral level, better analgesic efficacy led to higher subjective valuation for the strong placebo.
A stronger temperature reduction in the manipulation phase combined with verbal information about the ostensible cream value induced better analgesic efficacy for the strong placebo. This result replicates findings from previous studies (Price et al., 1999; De Pascalis et al., 2002; Nakamura et al., 2012). It also supports the idea that placebo effects are shaped by previous experience (Voudouris et al., 1989; Colloca et al., 2008, 2010), since subjects experienced different levels of pain during the manipulation phase. With regard to the previously demonstrated value effect on placebo efficacy (Waber et al., 2008), it may have been sufficient to use verbal value information only to induce graded placebo responses. However, Waber and colleagues (Waber et al., 2008) used a between subject design and labeled the price in one condition as “discounted to $0.10 per pill”. Using the term “discounted” might have not only affected the direct valuation of the pill, but also led to additional negative attributions to the pill via Halo effects (Thorndike, 1920). Since we wanted to obtain an estimate of subjective value unbiased by given prices, we labeled the weak placebo as “low priced”. This might be considered a weaker intervention, in particular when subjects can directly compare analgesic efficacy in a repeated measures design.
If prior treatment experience (i.e. the temperature reduction during the manipulation phase) would be the only factor influencing placebo responses, one would expect a correlation between the two placebo effects. Analgesic responses, however, did not correlate between the two placebos. A previous study of placebo and nocebo responses found these to be correlated in one group, but not in a second group, depending on stimulation intensity and number of conditioning trials (Colloca et al., 2010). The lack of a significant correlation can of course be due to insufficient power, but our sample size of N=40 should provide enough power to detect medium sized effects. Another explanation would be a non-linear relationship between the two placebo responses. The close to zero correlation from our sample does not necessarily rule out the importance of personality traits as predictors for placebo responses (De Pascalis et al., 2002; Morton et al., 2009). But it is likely that the perception or subjective valuation of a given treatment interacts with personality factors in generating placebo responses (Whalley et al., 2008; Hyland and Whalley, 2008).
Nakamura et al. (2012) reported dose dependent placebo effects on pain ratings, evoked potentials and skin conductance. They used different shock intensities during the placebo test phase in order to assess placebo effects on the sensitivity, i.e. the slope, of their dependent variables. In the present study, pain ratings and the rACC showed a dose-dependent response. The lack of such a response pattern in the SCR might be due to the use of thermal pain stimuli which do not elicit as distinct SCRs as electric shocks.
The individual subjective values of the two placebo treatments enabled us to elucidate the functional significance of the group-level effect of treatment. High treatment value, as estimated by willingness-to-pay, correlated with reduced pain ratings, suggesting a link between subjective treatment valuation and analgesic efficacy. Even though subjects were aware about the experimental setting, placebo treatment efficacy led them to spend more of their money on the more effective cream even after pain stimulation was completed. This extends the notion that subjects are willing to pay more to avoid a high intensity pain stimulus (Vlaev et al., 2009) and demonstrates the behavioral relevance of placebo effects in real life decision-making situations.
Anticipatory activation of the ventral striatum was identified when comparing the placebo to the control condition. When testing the two placebos separately we found this effect to be driven by the strong placebo. Interestingly, gray matter density in the striatum has been correlated with behavioral placebo analgesia (Schweinhardt et al., 2009). Dopamine release in the nucleus accumbens, as measured with positron emission tomography, also correlated with the magnitude of anticipated placebo analgesia (Scott et al., 2007) and experienced pain relief (Scott et al., 2008). These correlations together with the observed anticipatory striatal activity in the strong placebo condition, point towards an involvement of the ventral striatum in placebo analgesia. The ventral striatum has been shown to encode value predictions and prediction errors about reinforcing stimuli (Schultz et al., 1997; Peters and Büchel, 2009; Jocham et al., 2011). These findings are compatible with the observation that the striatum was activated during anticipation, but not during the actual stimulation phase. Since our paradigm did not allow a direct computation of prediction errors or reward expectations, the observed signal could also be due to other processes than predictive learning or value encoding. Hence, a potential causal relationship between the dopaminergic response in the striatum (Scott et al., 2008) and the placebo response remains an interesting but open question.
Activation of the DLPFC was higher for the strong compared to the weak placebo during late pain stimulation. An observation that is in line with previous studies which associated DLPFC signal changes with placebo analgesia (Wager et al., 2004; Kong et al., 2006; Eippert et al., 2009; Watson et al., 2009) and other mechanisms of pain control (Lorenz et al., 2003). In addition, it has recently been shown that disruption of DLPFC functioning by repetitive transcranial magnetic stimulation (rTMS) abolishes placebo analgesia (Krummenacher et al., 2010) which provides a more direct test of DLPFC function in placebo analgesia.
The most consistent and prominent placebo related brain activation was observed within the rACC. The rACC demonstrated a greater activity for both placebos compared to control and an interaction effect, indicating enhanced activation for the strong placebo. These results are in line with placebo analgesia fMRI studies reporting increased rACC activity, either during anticipation (Wager et al., 2004; Watson et al., 2009), or during pain stimulation (Bingel et al., 2006; Kong et al., 2006; Eippert et al., 2009). Furthermore, enhanced activation of the rACC for a more effective placebo treatment is in line with its role in endogenous pain control (Bingel and Tracey, 2008) and affect regulation (Petrovic et al., 2005; Etkin et al., 2010). Two studies reported a placebo related deactivation within the rACC (Wager et al., 2004; Eippert et al., 2009). We did not find a deactivation, but during late pain, the subgenual portion of the rACC was activated stronger by the weak than by the strong placebo. This cluster did not overlap with any other cluster identified in the present study.
In a cue-based pain modulation paradigm, Atlas et al. (2010) investigated brain mediators of pain perception. In this study, the rACC mediated cue effects on pain perception. The rACC showed increased activity during pain stimulation preceded by low pain cues, and decreased rACC activity predicted higher pain ratings (Atlas et al., 2010). In the present study, the rACC was more active under placebo during both, anticipation and early pain phases. As we also presented a cue before each pain stimulus, the observed rACC responses probably reflect processes similar to those observed by Atlas et al. (2010). The presence of the graded rACC activation already during the anticipation of painful stimuli could be due to the constant conditions within a block in contrast to a randomized cue presentation (Atlas et al., 2010). Taken together, these findings support the idea of an active preparatory engagement of the rACC in contextual pain modulation (Bingel and Tracey, 2008).
We also informed our subjects about the ostensible placebo value which might have had an impact on rACC activity. Value signals for food items have been reported within the rACC (Hare et al., 2008; Litt et al., 2011) and subjective value for a variety of goods and choice tasks is encoded in nearby ventromedial prefrontal cortex (Chib et al., 2009; Gläscher et al., 2009; Levy and Glimcher, 2011). A recent study examined the integration of prospective monetary rewards and painful electric shocks (Talmi et al., 2009). In their study, the rACC signaled the anticipated reward and this signal was attenuated by pain compared to non-painful stimuli. One could therefore argue that part of the rACC activity observed for the strong and expensive placebo could be driven by the placebo value. However, this is rather unlikely as our subjects did not receive any rewards in contrast to the study by Talmi et al. (2009).
We observed BOLD signal reductions under the pooled placebo within secondary somatosensory cortex and thalamus. In the thalamus and dACC placebo related signal reductions correlated with behavioral placebo effects of the weak placebo. These findings add to the literature on decreased activations in pain processing areas under placebo (Wager et al., 2004; Price et al., 2007; Eippert et al., 2009).
In summary, our study demonstrated graded analgesic efficacy of two placebo treatments based on experience and verbal information. Successful pain relief was reflected in value estimates obtained with a validated economical sciences procedure, underpinning the relevance of placebo analgesia. On the neural level, the most consistent placebo-related responses were observed in the rACC, mirroring the behavioral placebo effects, with increased placebo related responses for the more effective placebo.
Supplementary Material
Acknowledgements
We thank Ulrike Bingel and Sabrina Boll for helpful discussions regarding this study, and Matthias Gamer for providing the SCR decomposition script. This work was supported by the DFG, SFB 936 (A06) and the ERC, ERC-2010-AdG_20100407, Grant Agreement Number 269661.
Footnotes
The authors declare no conflict of interest.
References
- Amanzio M, Benedetti F. Neuropharmacological dissection of placebo analgesia: expectation-activated opioid systems versus conditioning-activated specific subsystems. The Journal of Neuroscience. 1999;19:484–94. doi: 10.1523/JNEUROSCI.19-01-00484.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amanzio M, Pollo A, Maggi G, Benedetti F. Response variability to analgesics: a role for non-specific activation of endogenous opioids. Pain. 2001;90:205–15. doi: 10.1016/S0304-3959(00)00486-3. [DOI] [PubMed] [Google Scholar]
- Atlas LY, Bolger N, Lindquist MA, Wager TD. Brain Mediators of Predictive Cue Effects on Perceived Pain. The Journal of Neuroscience. 2010;30:12964–12977. doi: 10.1523/JNEUROSCI.0057-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker GM, DeGroot MH, Marschak J. Measuring utility by a single-response sequential method. Behavioral science. 1964;9:226–32. doi: 10.1002/bs.3830090304. [DOI] [PubMed] [Google Scholar]
- Becker S, Gandhi W, Schweinhardt P. Cerebral interactions of pain and reward and their relevance for chronic pain. Neuroscience letters. 2012;520:182–7. doi: 10.1016/j.neulet.2012.03.013. [DOI] [PubMed] [Google Scholar]
- Bingel U, Lorenz J, Schoell ED, Weiller C, Büchel C. Mechanisms of placebo analgesia: rACC recruitment of a subcortical antinociceptive network. Pain. 2006;120:8–15. doi: 10.1016/j.pain.2005.08.027. [DOI] [PubMed] [Google Scholar]
- Bingel U, Schoell ED, Herken W, Büchel C, May A. Habituation to painful stimulation involves the antinociceptive system. Pain. 2007;131:21–30. doi: 10.1016/j.pain.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Bingel U, Tracey I. Imaging CNS modulation of pain in humans. Physiology (Bethesda) 2008;23:371–80. doi: 10.1152/physiol.00024.2008. [DOI] [PubMed] [Google Scholar]
- Büchel C, Bornhovd K, Quante M, Glauche V, Bromm B, Weiller C. Dissociable Neural Responses Related to Pain Intensity, Stimulus Intensity, and Stimulus Awareness within the Anterior Cingulate Cortex: A Parametric Single-Trial Laser Functional Magnetic Resonance Imaging Study. The Journal of Neuroscience. 2002;22:970–6. doi: 10.1523/JNEUROSCI.22-03-00970.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chib VS, Rangel A, Shimojo S, O’Doherty JP. Evidence for a common representation of decision values for dissimilar goods in human ventromedial prefrontal cortex. The Journal of Neuroscience. 2009;29:12315–20. doi: 10.1523/JNEUROSCI.2575-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colloca L, Petrovic P, Wager TD, Ingvar M, Benedetti F. How the number of learning trials affects placebo and nocebo responses. Pain. 2010;151:430–9. doi: 10.1016/j.pain.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colloca L, Sigaudo M, Benedetti F. The role of learning in nocebo and placebo effects. Pain. 2008;136:211–8. doi: 10.1016/j.pain.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Deichmann R, Gottfried J, Hutton C, Turner R. Optimized EPI for fMRI studies of the orbitofrontal cortex. NeuroImage. 2003;19:430–441. doi: 10.1016/s1053-8119(03)00073-9. [DOI] [PubMed] [Google Scholar]
- Eippert F, Bingel U, Schoell E, Yacubian J, Büchel C. Blockade of endogenous opioid neurotransmission enhances acquisition of conditioned fear in humans. The Journal of Neuroscience. 2008;28:5465–72. doi: 10.1523/JNEUROSCI.5336-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eippert F, Bingel U, Schoell ED, Yacubian J, Klinger R, Lorenz J, Büchel C. Activation of the opioidergic descending pain control system underlies placebo analgesia. Neuron. 2009;63:533–43. doi: 10.1016/j.neuron.2009.07.014. [DOI] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in cognitive sciences. 2010;15:85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feld GB, Specht M, Gamer M. Differential electrodermal and phasic heart rate responses to personally relevant information: Comparing sleep and wakefulness. Sleep and Biological Rhythms. 2010;8:72–78. [Google Scholar]
- Gläscher J, Hampton AN, O’Doherty JP. Determining a role for ventromedial prefrontal cortex in encoding action-based value signals during reward-related decision making. Cerebral Cortex. 2009;19:483–95. doi: 10.1093/cercor/bhn098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, O’Doherty JP, Camerer CF, Schultz W, Rangel A. Dissociating the role of the orbitofrontal cortex and the striatum in the computation of goal values and prediction errors. The Journal of Neuroscience. 2008;28:5623–30. doi: 10.1523/JNEUROSCI.1309-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyland ME, Whalley B. Motivational concordance: an important mechanism in self-help therapeutic rituals involving inert (placebo) substances. Journal of psychosomatic research. 2008;65:405–13. doi: 10.1016/j.jpsychores.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Jensen KB, Kaptchuk TJ, Kirsch I, Raicek J, Lindstrom KM, Berna C, Gollub RL, Ingvar M, Kong J. Nonconscious activation of placebo and nocebo pain responses. Proceedings of the National Academy of Sciences. 2012;109:15959–64. doi: 10.1073/pnas.1202056109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jocham G, Klein TA, Ullsperger M. Dopamine-Mediated Reinforcement Learning Signals in the Striatum and Ventromedial Prefrontal Cortex Underlie Value-Based Choices. The Journal of Neuroscience. 2011;31:1606–1613. doi: 10.1523/JNEUROSCI.3904-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, Gollub RL, Rosman IS, Webb JM, Vangel MG, Kirsch I, Kaptchuk TJ. Brain activity associated with expectancy-enhanced placebo analgesia as measured by functional magnetic resonance imaging. The Journal of Neuroscience. 2006;26:381–388. doi: 10.1523/JNEUROSCI.3556-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krummenacher P, Candia V, Folkers G, Schedlowski M, Schönbächler G. Prefrontal cortex modulates placebo analgesia. Pain. 2010;148:368–74. doi: 10.1016/j.pain.2009.09.033. [DOI] [PubMed] [Google Scholar]
- la Fuente-Fernández R de, Ruth T, Sossi V, Schulzer M, Calne D, Stoessl A. Expectation and dopamine release: mechanism of the placebo effect in Parkinson’s disease. Science. 2001;293:1164–6. doi: 10.1126/science.1060937. [DOI] [PubMed] [Google Scholar]
- Levine JD, Gordon NC, Fields HL. The mechanism of placebo analgesia. Lancet. 1978;312:654–7. doi: 10.1016/s0140-6736(78)92762-9. [DOI] [PubMed] [Google Scholar]
- Levinson DF, Edelberg R. Scoring criteria for response latency and habituation in electrodermal research: a critique. Psychophysiology. 1985;22:417–26. doi: 10.1111/j.1469-8986.1985.tb01626.x. [DOI] [PubMed] [Google Scholar]
- Levy DJ, Glimcher PW. Comparing Apples and Oranges: Using Reward-Specific and Reward-General Subjective Value Representation in the Brain. Journal of Neuroscience. 2011;31:14693–14707. doi: 10.1523/JNEUROSCI.2218-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litt A, Plassmann H, Shiv B, Rangel A. Dissociating valuation and saliency signals during decision-making. Cerebral cortex. 2011;21:95–102. doi: 10.1093/cercor/bhq065. [DOI] [PubMed] [Google Scholar]
- Lorenz J, Minoshima S, Casey KL. Keeping pain out of mind: the role of the dorsolateral prefrontal cortex in pain modulation. Brain. 2003;126:1079–1091. doi: 10.1093/brain/awg102. [DOI] [PubMed] [Google Scholar]
- Meissner K, Bingel U, Colloca L, Wager TD, Watson A, Flaten MA. The Placebo Effect: Advances from Different Methodological Approaches. The Journal of Neuroscience. 2011;31:16117–16124. doi: 10.1523/JNEUROSCI.4099-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery GH, Kirsch I. Classical conditioning and the placebo effect. Pain. 1997;72:107–13. doi: 10.1016/s0304-3959(97)00016-x. [DOI] [PubMed] [Google Scholar]
- Morton DL, Watson A, El-Deredy W, Jones AKP. Reproducibility of placebo analgesia: Effect of dispositional optimism. Pain. 2009;146:194–8. doi: 10.1016/j.pain.2009.07.026. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Donaldson GW, Kuhn R, Bradshaw DH, Jacobson RC, Chapman CR. Investigating dose-dependent effects of placebo analgesia: A psychophysiological approach. Pain. 2012;153:227–37. doi: 10.1016/j.pain.2011.10.024. [DOI] [PubMed] [Google Scholar]
- Pascalis V De, Chiaradia C, Carotenuto E. The contribution of suggestibility and expectation to placebo analgesia phenomenon in an experimental setting. Pain. 2002;96:393–402. doi: 10.1016/S0304-3959(01)00485-7. [DOI] [PubMed] [Google Scholar]
- Peters J, Büchel C. Overlapping and distinct neural systems code for subjective value during intertemporal and risky decision making. The Journal of Neuroscience. 2009;29:15727–34. doi: 10.1523/JNEUROSCI.3489-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovic P, Dietrich T, Fransson P, Andersson J, Carlsson K, Ingvar M. Placebo in emotional processing - induced expectations of anxiety relief activate a generalized modulatory network. Neuron. 2005;46:957–69. doi: 10.1016/j.neuron.2005.05.023. [DOI] [PubMed] [Google Scholar]
- Petrovic P, Kalso E, Petersson KM, Ingvar M. Placebo and opioid analgesia - imaging a shared neuronal network. Science. 2002;295:1737–40. doi: 10.1126/science.1067176. [DOI] [PubMed] [Google Scholar]
- Plassmann H, O’Doherty JP, Rangel A. Orbitofrontal cortex encodes willingness to pay in everyday economic transactions. The Journal of Neuroscience. 2007;27:9984–8. doi: 10.1523/JNEUROSCI.2131-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price DD, Craggs J, Verne GN, Perlstein WM, Robinson ME. Placebo analgesia is accompanied by large reductions in pain-related brain activity in irritable bowel syndrome patients. Pain. 2007;127:63–72. doi: 10.1016/j.pain.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Price DD, Milling LS, Kirsch I, Duff A, Montgomery GH, Nicholls SS. An analysis of factors that contribute to the magnitude of placebo analgesia in an experimental paradigm. Pain. 1999;83:147–156. doi: 10.1016/s0304-3959(99)00081-0. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A Neural Substrate of Prediction and Reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Schweinhardt P, Seminowicz DA, Jaeger E, Duncan GH, Bushnell MC. The anatomy of the mesolimbic reward system: a link between personality and the placebo analgesic response. The Journal of Neuroscience. 2009;29:4882–7. doi: 10.1523/JNEUROSCI.5634-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott DJ, Stohler CS, Egnatuk CM, Wang H, Koeppe RA, Zubieta J-K. Individual differences in reward responding explain placebo-induced expectations and effects. Neuron. 2007;55:325–36. doi: 10.1016/j.neuron.2007.06.028. [DOI] [PubMed] [Google Scholar]
- Scott DJ, Stohler CS, Egnatuk CM, Wang H, Koeppe RA, Zubieta J-K. Placebo and nocebo effects are defined by opposite opioid and dopaminergic responses. Archives of General Psychiatry. 2008;65:220–31. doi: 10.1001/archgenpsychiatry.2007.34. [DOI] [PubMed] [Google Scholar]
- Stewart-Williams S, Podd J. The placebo effect: dissolving the expectancy versus conditioning debate. Psychological bulletin. 2004;130:324–40. doi: 10.1037/0033-2909.130.2.324. [DOI] [PubMed] [Google Scholar]
- Talmi D, Dayan P, Kiebel SJ, Frith CD, Dolan RJ. How humans integrate the prospects of pain and reward during choice. The Journal of neuroscience. 2009;29:14617–26. doi: 10.1523/JNEUROSCI.2026-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorndike EL. A constant error in psychological ratings. Journal of Applied Psychology. 1920;4:25–29. [Google Scholar]
- Vlaev I, Seymour B, Dolan RJ, Chater N. The price of pain and the value of suffering. Psychological science. 2009;20:309–17. doi: 10.1111/j.1467-9280.2009.02304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voudouris NJ, Peck CL, Coleman G. Conditioned response models of placebo phenomena: further support. Pain. 1989;38:109–116. doi: 10.1016/0304-3959(89)90080-8. [DOI] [PubMed] [Google Scholar]
- Waber RL, Shiv B, Carmon Z, Ariely D. Commercial features of placebo and therapeutic efficacy. The journal of the American Medical Association (JAMA) 2008;299:1016–7. doi: 10.1001/jama.299.9.1016. [DOI] [PubMed] [Google Scholar]
- Wager TD, Atlas LY, Leotti LA, Rilling JK. Predicting Individual Differences in Placebo Analgesia: Contributions of Brain Activity during Anticipation and Pain Experience. The Journal of Neuroscience. 2011;31:439–452. doi: 10.1523/JNEUROSCI.3420-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, Kosslyn SM, Rose RM, Cohen JD. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science. 2004;303:1162–7. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- Watson A, El-Deredy W, Iannetti GD, Lloyd D, Tracey I, Vogt BA, Nadeau V, Jones AKP. Placebo conditioning and placebo analgesia modulate a common brain network during pain anticipation and perception. Pain. 2009;145:24–30. doi: 10.1016/j.pain.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalley B, Hyland ME, Kirsch I. Consistency of the placebo effect. Journal of psychosomatic research. 2008;64:537–41. doi: 10.1016/j.jpsychores.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Yacubian J, Sommer T, Schroeder K, Gläscher J, Kalisch R, Leuenberger B, Braus DF, Büchel C. Gene-gene interaction associated with neural reward sensitivity. Proceedings of the National Academy of Sciences. 2007;104:8125–30. doi: 10.1073/pnas.0702029104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubieta J-K, Bueller JA, Jackson LR, Scott DJ, Xu Y, Koeppe RA, Nichols TE, Stohler CS. Placebo effects mediated by endogenous opioid activity on mu-opioid receptors. The Journal of Neuroscience. 2005;25:7754–7762. doi: 10.1523/JNEUROSCI.0439-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.