Abstract
Background
Obesity increases asthma risk, and may alter asthma severity. In adults, sex appears to modify the effect of obesity on asthma. Among children, the effect of sex on the relationship between obesity and asthma severity remains less clear, particularly when considering race.
Objective
To determine how obesity affects disease characteristics in a diverse cohort of children with poorly controlled asthma, and if obesity effects are altered by sex.
Design
We analyzed 306 children between 6–17 years of age with poorly controlled asthma enrolled in a 6-month trial assessing lansoprazole for asthma control. In this secondary analysis, we determined associations between obesity and symptom severity, spirometry, exacerbation risk, airway biomarkers, bronchial reactivity and airflow perception. We used both a multivariate linear regression and longitudinal mixed-effect model to determine if obesity interacted with sex to affect asthma severity.
Results
Regardless of sex, BMI>95th percentile did not affect asthma control, exacerbation risk or airway biomarkers. Sex changed the effect of obesity on lung function (sex*obesity FEV1%, interaction p-value <.01, sex*obesity FEV1/FVC, interaction p-value=.03). Obese males had significantly worse airflow obstruction compared to non-obese males, while in females there was no obesity effect on airflow obstruction. In females, obesity was associated with significantly greater FEV1 and FVC, and a trend toward reduced airway reactivity.
Conclusions
Obesity did not affect asthma control, airway markers or disease stability; however obesity did affect lung function in a sex-dependent manner. In males, obesity associated with reduced FEV1/FVC, and in females, obesity associated with substantially improved lung function.
Keywords: Asthma, Obesity, Children, Sex, Body Mass Index, Spirometry
INTRODUCTION
Asthma is a complex disease that includes variant forms, or endotypes, driven by differing etiologic mechanisms1. Obesity increases the risk for new asthma in children and adults2, and may alter asthma characteristics2–4. Females who become obese during school-age are significantly more likely to develop early menses, asthma symptoms, and bronchodilator responsiveness5; while women who gain weight during adulthood disproportionately develop late-onset, non-atopic asthma6. Studies mainly involving adults have suggested that the deleterious effects of obesity on asthma severity are greatest in women7,8. Women on average have greater fat stores as a percent of body mass compared to men. The risk of asthma among the obese is most consistent among adult women. One of the largest genetic studies of obesity and asthma found a link only among females9. Obesity may worsen asthma symptoms more in adolescent10 and adult4 females versus males, however these finding require further confirmation. Other factors such as age4 and race11 may be important effect modifiers on the relationship between obesity and asthma. Whether obesity alters the basic mechanisms of asthma in a sex-dependent manner among children remains poorly defined 12–14. Therefore, we sought to determine if obesity has differing effects on asthma based on sex among children utilizing a highly characterized longitudinal cohort. Because this pediatric cohort is predominantly African-American, we also sought to evaluate the impact of the interaction between obesity and race (obesity*race) on asthma phenotype. We hypothesize that obesity worsens asthma symptoms, lung function and disease stability, particularly in females. We compared several baseline and longitudinal outcomes from 306 children, age 6 to 17 years, with poorly controlled asthma enrolled in a randomized clinic trial from 18 American Lung Association – Asthma Clinical Research Centers (ALA-ACRC).
MATERIALS AND METHODS
Details of the main study design have been published15. All participants signed written informed consents. The parent Study of Acid-Reflux in Childhood Asthma (SARCA) study was approved by the Nemours Florida IRB (82404-29) and by all other ALA-ACRC IRBs, and registered at ClinicalTrials.gov (NCT00604851). We included data from over 1900 study visit encounters involving 306 participants age 6–17 years with inadequately controlled persistent asthma. For inclusion, participants required physician diagnosis of asthma, prescription of an asthma controller medication, and either ≥12% post-bronchodilator FEV1 improvement or methacholine PC20 < 16mg/ml. Inadequate control was defined by poor ACQ score, frequent bronchodilator use or exacerbations. Additional inclusion details exist in the online supplementary materials. 2453 participants were screened in order to randomize 306 participants into the 24-week multicenter clinical trial assessing the efficacy of daily oral lansoprazole versus placebo for patients with inadequately controlled asthma.
Data Collected
We collected demographics, daily peak flows, exhaled breath condensate, exhaled nitric oxide, spirometry, methacholine PC20, and Juniper Asthma Control Questionnaires (ACQ) at baseline and periodically during the 24-week follow-up period. Prior to analyzing the data (a priori), we proposed determining if obesity was associated with asthma severity, and if sex or sex*race mediated the obesity effect. We grouped participants as underweight, normal weight, overweight or obese by CDC classification16 based on age and sex-adjusted BMI-percentile. We also measured waist and neck circumferences. Since we were primarily interested in the effect of obesity, we dropped the 6 underweight participants and grouped participants as either obese or non-obese for the multivariate statistical analyses. We reassessed weight and BMI at the completion of the trial to adjust for any significant changes. Asthma symptoms were assessed using the ACQ17 and modified ACQ64. Detailed descriptions exist in the online supplementary materials.
Spirometry, fractional exhaled nitric oxide (FENO) and methacholine challenge testing were performed using ATS/ERS standard procedures. Exhaled breath condensate was collected. We assessed exacerbation risk longitudinally using two methods: incidence of episodes of poor asthma control (EPAC), and peak flow variability (PFvar). Detailed methodology on these measures can be found in the online supplement. Briefly, EPACs were determined from patient diary cards and from interval asthma history forms evaluating peak flow drops, unscheduled health care visits and rescue medication use.
PFvar was calculated for each 14-day block immediately preceding the randomization visit and visits 4–9 using patient home diary cards. We determined the relationship between a participant’s 14-day PFvar and the ACQ6 score for the same period4, to measure subjective symptom reporting normalized for an objective measure of lung function and temporal airway stability.
Data Analysis
Baseline data were taken at randomization and summarized by BMI group. We used Chi-square, Wilcoxon or Kruskal-Wallis tests as appropriate for comparing variables between groups. In the main analyses, we compared asthma characteristics at the randomization visit by BMI category. We used multivariate linear regression adjusting for sleep apnea, snoring, allergic rhinitis, age group, race and pH-probe status to assess the effect of obesity status on lung function and symptom scores. We looked for statistical interaction between BMI group and sex on all asthma-related outcomes. To determine whether obesity influenced the course of asthma, we evaluated longitudinal data using a linear mixed effect model with random intercept and an unstructured within subject correlation structure for repeated measurements. We assessed the effect of obesity and its joint effects with sex on asthma symptom reporting after controlling for effects of race and treatment response on lung function and asthma symptoms. Age of onset, sex, race, atopy, sleep apnea, treatment and low birthweight were accounted for in the models. We are presenting the data analyses from the baseline visit. This is justified because the baseline data were consistent with the results from the longitudinal models. The exception to this is the longitudinal EPAC incidence data.
Since past asthma studies in children have suggested that BMI-related impairment becomes greatest > 95th percentile, we chose to compare obese (> 95th% BMI) versus normal/overweight (5–94.9th% BMI) children. Central obesity in children is correlated with waist circumference18 and is a risk factor for obesity-related sequelae. We performed a second obesity analysis on asthma outcomes, but instead stratified obesity status using sex and age-adjusted waist-circumference (above or below 90th percentile)18,19.
The statistical packages SAS 9.2 (SAS Institute Inc, Cary NC, USA) and STATA 11 (College Station, TX: StataCorp, 2005) were used. All tests were two-tailed at a level of significance of 0.05.
RESULTS
Baseline Characteristics
The baseline characteristics of 306 children and adolescents are shown by four BMI-percentile groups (table 1). Age, sex, race/ethnicity, age of asthma onset, previous controller treatment and study intervention assignment were similar across BMI groups. Study intervention (lansoprazole) did not affect any of the main outcomes in the current study15. African-Americans made up the largest racial group in each BMI-percentile group except among underweight participants. Before starting the study, more than half of the patients were on some form of step-up therapy (inhaled steroids plus either long-acting beta-agonists or leukotriene modifiers) to treat persistent asthma. Despite the intensive treatment, more than 70% still required urgent care for asthma in the 12 months prior to study enrollment, and required two or more rescue treatments per week on average for breakthrough symptoms.
Table I.
Baseline Demographic Characteristics by BMI-percentile Groups
| Underweight (<5th) |
Normal (5–85th) |
Overweight (85–95th) |
Obese (>95th) |
Total Patients |
P- Value |
|
|---|---|---|---|---|---|---|
| N (%) | 6(2.0) | 150(49.0) | 55(18.0) | 95(31.1) | 306 | |
| Sex, n (%) | 0.75F | |||||
| Male Female |
4(66.7) 2(33.3) |
95(63.3) 55(36.7) |
35(63.6) 20(36.4) |
54(56.8) 41(43.2) |
188 118 |
|
| Race, n (%) | 0.11F | |||||
| Caucasian African-American Other |
3(50.0) 1(16.7) 2(33.3) |
67(44.7) 70(46.7) 13(8.7) |
17(30.9) 31(55.4) 7(12.7) |
31(32.6) 51(53.7) 13(13.7) |
118 152 35 |
|
| Age (years) | 11.8(3.4) | 11.6(3.1) | 10.9(2.7) | 11.5(2.9) | 306 | 0.48 |
| Age of asthma onset | 2.8(3.1) | 3.4(3.4) | 2.9(2.8) | 3.7(2.9) | 306 | 0.23 |
| Weight (Kg) | 33.8(11.4) | 42.9(13.9) | 50.3(16.5) | 73.2(23.2) | 306 | <.0001 |
| BMI (kg/m2) | 14.8(1.7) | 18.5(2.4) | 22.1(2.7 | 29.7(5.2) | 306 | <.0001 |
| Treatment, n (%) | ||||||
| Treatment assignment Lansoprazole Placebo |
4(66.7) 2(33.3) |
73(48.7) 77(51.3) |
23(41.8) 32(58.2) |
49(51.6) 46(48.4) |
149 157 |
0.56F |
| Leukotriene inhibitor | 5(83.3) | 83(55.3) | 36(65.5) | 47(49.4) | 306 | 0.15F |
| ICS-LABA | 3(50) | 88(58.7) | 30(54.5) | 55(57.9) | 306 | 0.89F |
Continuous variable are means (SD), F – value from Fisher’s exact test, BMI – body mass index , Kg – kilograms, cm – centimeters, ICS – inhaled corticosteroid, LABA – long acting beta-agonist.
Lung function differed according to BMI group (table 2). Underweight participants had significantly lower mean FVC and FEV1 before and after bronchodilator, compared to normal weight asthmatics (p<.05). The obese group had the greatest pre- and post-bronchodilator mean FVC percent predicted and smallest mean post-bronchodilator FEV1/FVC compared to all other weight groups. BMI grouping affected the PC20 (p=.02), with underweight participants displaying the greatest airway reactivity and obese participants having the least. BMI did not appear to affect mean post-bronchodilator FEV1 change, asthma symptoms, FENO and EBC measures (pH, nitrogen oxides). BMI group also was not associated with prevalence of bronchodilator responsiveness (defined as having an FEV1 improvement > 12%) or the prevalence of atopy. Age of asthma onset, current age, atopy-status and treatment assignment (data not shown) were not significant modifiers of the effect of obesity on asthma outcomes. Pubertal status (analyzed by age < 12 years vs. age ≥ 12 years) did not affect the relationship between high BMI-percentile and any asthma outcome (data not shown).
Table 2.
Baseline Participant Characteristics by BMI-percentile Groups
| Underweight (<5th) |
Normal (5–85th) |
Overweight (85–95th) |
Obese (>95th) |
Total Patients |
P- Value |
|
|---|---|---|---|---|---|---|
| N (%) | 6(2.0) | 150(49.0) | 55(18.0) | 95(31.1) | 306 | |
| Pulmonary functions, mean (SD) | ||||||
| FEV1% predicted (post) | 85.6(9.9) | 99.4(15.8) | 99.8(13.9) | 100.0(14.7) | 296 | 0.09 |
| FEV1% predicted (pre) | 78.3(9.7) | 91.7(16.3) | 89.9(15.4) | 93.4(16.5) | 306 | 0.07 |
| FVC% predicted (post) | 90.3(12.7) | 101.4(15.5) | 102.0(14.8) | 106.0(12.2) | 296 | <0.01 |
| FVC% predicted (pre) | 87.4(13.9) | 99.5(15.5) | 99.0(12.9) | 103.8(13.4) | 306 | 0.01 |
| FEV1/FVC (post) | 83.8(8.3) | 85.4(8.3) | 85.7(7.0) | 81.9(9.2) | 296 | 0.01 |
| FEV1/FVC (pre) | 79.7(11.4) | 80.3(9.7) | 79.2(9.4) | 77.8(9.6) | 306 | 0.23 |
| FEV1% BD change | 9.7(8.5) | 9.6(10.9) | 11.6(13.0) | 8.6(10.6) | 296 | 0.68 |
| PF variability | 0.3(0.2) | 0.3(0.2) | 0.3(0.2) | 0.3(0.2) | 306 | 0.59 |
| PC20 (mg/mL) | 0.2(0.1) | 2.7(3.7) | 2.8(4.0) | 3.6(4.3) | 214 | 0.02 |
| Asthma symptom scores, mean (SD) | ||||||
| ASUI | 0.75(0.18) | 0.83(0.14) | 0.82(0.14) | 0.82(0.15) | 306 | 0.55 |
| ACQ | 1.7(0.5) | 1.1(0.7) | 1.2(0.7) | 1.2(0.8) | 305 | 0.21 |
| Breath measurements, mean (SD) | ||||||
| FENO (ppb) | 50.4(33.0) | 45.3(54.6) | 37.2(31.1) | 36.1(32.2) | 146 | 0.65 |
| EBC pH | 5.7(0.7) | 6.1(1.1) | 6.1(1.0) | 6.3(0.7) | 241 | 0.41 |
| EBC Nitric oxide (micromol/L) | 2.1(1.6) | 3.2(3.4) | 3.3(3.8) | 3.1(4.3) | 238 | 0.84 |
| Other characteristics, n (%) | ||||||
| Atopy | 6(100) | 117(78.0) | 41(74.6) | 69(72.6) | 306 | 0.48F |
| Sleep apnea | 0(0.0) | 3(2.0) | 1(1.8) | 7(7.4) | 306 | 0.17F |
| ETS exposure | 1(16.7) | 25(16.7) | 9(16.4) | 19(20.0) | 306 | 0.91F |
| Snoring | 3(50.0) | 68(45.3) | 26(47.3) | 56(59.0) | 306 | 0.20F |
| Allergic Rhinitis | 5(83.3) | 93(62.0) | 32(58.2) | 51(53.7) | 306 | 0.40F |
Continuous variable are means (SD), F – value from Fisher’s exact test, BMI – body mass index , FEV1% – forced expiratory volume in 1 second (percent predicted), FVC% - forced vital capacity (percent predicted), pre – before bronchodilator, post – following bronchodilator, FEV1% BD – percent change in FEV1 following bronchodilator, PF – peak flow, PC20 – provocation concentration required to cause 20% FEV1 drop, ASUI – asthma symptom utility index, ACQ – asthma control questionnaire, FENO – fractional exhaled nitric oxide, EBC – exhaled breath condensate, ETS – environmental tobacco smoke exposure determined by questionnaire. Analysis includes 6 underweight participants. All subsequent analyses evaluating obese versus non-obese participants did not include the underweight participants.
Obesity and Asthma Outcomes by Sex
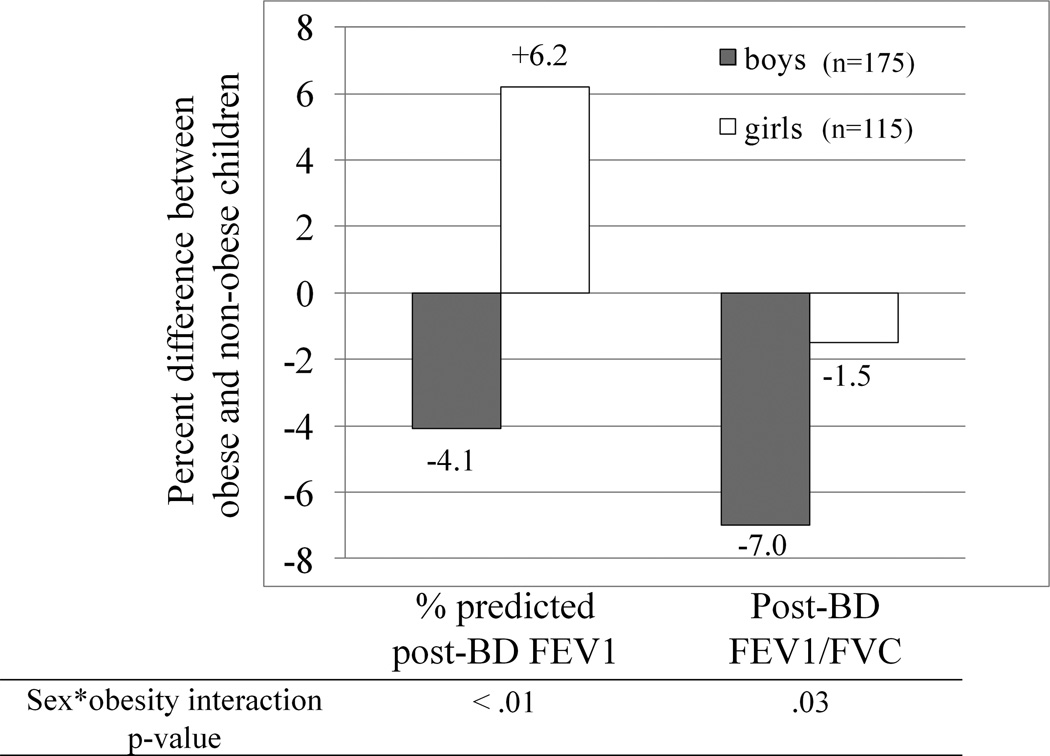
For both sexes, BMI>95th percentile did not affect asthma control, exacerbation risk, bronchodilator response or airway biomarkers. However, the effect of obesity on post-BD FEV1% (sex interaction pvalue <0.01) and FEV1/FVC (sex interaction pvalue =.03) was different among males and females (figure 1). Among males, obesity was associated with a significantly reduced post-BD FEV1/FVC (78.6% vs. 84.5%, p<0.001) and there was a trend among obese males to have lower post-BD FEV1 (95.9% vs. 100.0%, table 3a). Obese females had significantly greater mean FEV1% (pre-BD 97.4% vs. 91.0% and post-BD 104.9% vs. 98.7%) and mean FVC% (pre- 103.9% vs. 98.3% and post-BD 107.3% vs. 100.7%) compared with non-obese females, while there was no association of obesity with FEV1/FVC (Table 3b).
Figure 1.
Differential Obesity-Effect by Sex. FEV1 percent predicted point difference and FEV1/FVC percentage difference when comparing obese to non-obese children by sex. Boys (n=175), Girls (n=115). Row below graph shows the interaction p-value for the effect of sex on the relationship between obesity status and asthma outcomes. FEV1 – forced expiratory volume in 1 second, FVC – forced vital capacity.
Table 3.
| a. Effect of Obesity Status on Asthma Characteristics in Males | ||||
|---|---|---|---|---|
| Variables | Non-obese | Obese | n | P |
| N ( %) | 130(71%) | 54(29%) | 184 | |
| BMI, raw | 19.3(2.8) | 29.5(5.0) | 184 | <.0001 |
| Neck circumference > 90th%, n (%) | 26 (21) | 39 (75) | 177 | <.0001 |
| Waist circumference > 90th%, n (%) | 2 (2) | 21 (40) | 177 | <.0001 |
| Race, n (%) | ||||
| Caucasian African-American Other |
52(40%) 64(49%) 14(11%) |
19(35%) 26(48%) 9(17%) |
71 90 23 |
0.52 |
| Allergic rhinitis, n (%) | 73(56%) | 30(56%) | 184 | 0.94 |
| Any Atopy, n (%) | 97(75%) | 41(76%) | 184 | 0.85 |
| Pulmonary Function, mean (SD) | ||||
| FEV1 % predicted-post | 100.0(14.7) | 95.9(15.4) | 175 | 0.10 |
| FEV1 % predicted-pre | 91.4(15.5) | 90.3(17.0) | 184 | 0.52 |
| FVC % predicted-post | 102.0(14.9) | 105.0(13.6) | 175 | 0.15 |
| FVC % predicted-pre | 99.9(14.3) | 103.7(14.5) | 184 | 0.12 |
| FEV1/FVC-post | 84.5(7.2) | 78.6(9.9) | 175 | <0.001 |
| FEV1/FVC-pre | 78.7(9.1) | 74.6(9.5) | 184 | <0.01 |
| FEV1 % change | 10.5(11.9) | 8.4(9.8) | 175 | 0.25 |
| PF variability | 0.3(0.2) | 0.3(0.2) | 184 | 0.69 |
| PC20 (mg/ml) | 2.7(4.1) | 3.1(4.2) | 129 | 0.35 |
| Asthma symptom scores, mean (SD) | ||||
| ACQ | 1.1(0.6) | 1.2(0.8) | 183 | 0.43 |
| ASUI | 0.84(0.12) | 0.85(0.13) | 184 | 0.60 |
| Other | ||||
| airflow perception score | 4.6(4.5) | 4.8(4.2) | 183 | 0.65 |
| b. Effect of Obesity Status on Asthma Characteristics in Females | ||||
|---|---|---|---|---|
| Variables | Non-obese | Obese | n | P-value |
| N (%) | 75(65%) | 41(35%) | 116 | |
| BMI, raw | 19.8(3.2) | 30.0(5.5) | 116 | <.0001 |
| Neck circumference > 90th%, n (%) | 22 (30) | 38 (93) | 115 | <.0001 |
| Waist circumference > 90th%, n (%) | 0 (0) | 25 (63) | 114 | <.0001 |
| Race, n (%) | ||||
| Caucasian African-American Other |
32(43%) 37(49%) 6(8%) |
12(29%) 25(61%) 4(10%) |
44 25 10 |
0.36F |
| Allergic Rhinitis, n (%) | 52(69%) | 21(51%) | 116 | 0.05 |
| Any Atopy | 61(81%) | 28(68%) | 116 | 0.11 |
| Pulmonary Function, mean (SD) | ||||
| FEV1 % predicted-post | 98.7(16.3) | 104.9(12.4) | 115 | 0.03 |
| FEV1 % predicted-pre | 91.0(17.1) | 97.4(15.1) | 116 | 0.03 |
| FVC % predicted-post | 100.7(15.9) | 107.3(10.3) | 115 | 0.02 |
| FVC % predicted-pre | 98.3(15.8) | 103.9(11.8) | 116 | 0.03 |
| FEV1/FVC-post | 87.2(8.8) | 85.9(6.4) | 115 | 0.06 |
| FEV1/FVC-pre | 82.2(10.2) | 82.1(7.9) | 116 | 0.45 |
| FEV1 % change | 9.5(10.8) | 8.9(11.6) | 115 | 0.62 |
| PF variability | 0.3(0.3) | 0.3(0.2) | 116 | 0.48 |
| PC20 | 2.8(3.1) | 4.2(4.3) | 81 | 0.08 |
| Asthma Symptom Scores, mean (SD) | ||||
| ACQ | 1.3(0.8) | 1.2(0.8) | 116 | 0.26 |
| ASUI | 0.80(0.16) | 0.79(0.18) | 116 | 0.87 |
| Other | ||||
| airflow perception score | 6.5(7.2) | 4.6(3.9) | 116 | 0.33 |
F – value from Fisher’s exact test, BMI – body mass index, FEV1% – forced expiratory volume in 1 second (percent predicted), FVC% - forced vital capacity (percent predicted), pre – before bronchodilator, post – following bronchodilator, FEV1% BD – percent change in FEV1 following bronchodilator, PF – peak flow, BDR – bronchodilator reversibility, PC20 – provocation concentration required to cause 20% FEV1 drop, ACQ – asthma control questionnaire, ASUI – asthma symptom utility index.
Obese males had a significantly higher prevalence of sleep disordered breathing compared to non-obese males (p<0.01), while obese females had a significantly lower prevalence of allergic rhinitis (p=.05) compared with non-obese females (data not shown). We saw a trend toward reduced airway reactivity in obese females (compared to non-obese females, 2.8 vs. 4.2 mg/ml p=.08), while obese and non-obese males had similar PC20 (2.7 vs. 3.1 mg/ml, p=.35, supplementary figure).
There was weak evidence that the relationship between post-BD FEV1% predicted and obesity in males was different in white and black children (p=0.02 for interaction between obesity and race). White males who were obese had lower percent predicted FEV1 compared with non-obese white males (87% vs. 97%, p=0.02; figure 2). White males who were obese also displayed greater evidence of airflow obstruction by FEV1/FVC (74% vs. 82%, p<0.01), worse ACQ scores (1.4 vs. 0.8, p<0.01), and greater PFvar (p=.02) compared with non-obese white males. A significantly higher percentage of obese white males had bronchodilator responsiveness (39% vs. 16%, p=.04) compared with similar non-obese participants. In black males, obesity status was not associated with these decrements.
Figure 2.
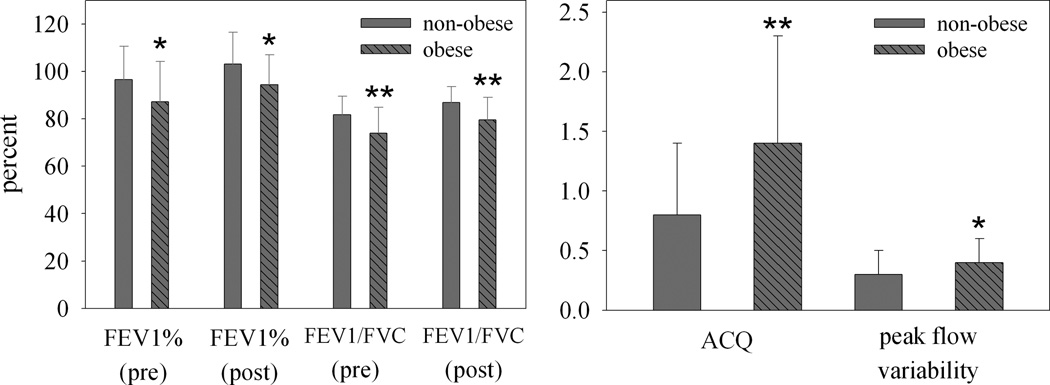
Obesity and Asthma Severity in White Male Children. Pre-bronchodilator [non-obese (n=52) and obese (n=19)] and post-bronchodilator [non-obese (n=50) and obese (n=19)] spirometry (left panel). Disease severity based on Asthma Control Questionnaire and Peak Flow Variability prior to randomization [non-obese (n=52), obese (n=19) (right panel); FEV1-forced expiratory volume in 1 second (percent predicted), FVC – forced vital capacity, PFvar – peak flow variability, ACQ – asthma control questionnaire, * - p ≤ .05, ** - p < .01.
Effect of Obesity on Disease Stability and Risk of Exacerbation
We recorded 288 episodes of poor asthma control (EPAC) during the 24-week intervention period and found that obesity did not affect the overall risk of poor asthma control (p=.68). Sex did not modify the impact of obesity on exacerbation risk (p>0.1 for sex*obesity interaction). Also, we saw no effect of obesity on PFvar at any point during the 24-week trial.
Effect of Obesity on Airflow Perception
There was a significant relationship between PFvar and ACQ6 for both obese (beta=1.17, p<.0001) and non-obese (beta=0.83, p<.0001) males, and for non-obese females (beta=1.17, p<.0001). The weakest correlation between PFvar and symptom reporting was with obese females (beta=0.67, p=.05). Among both sexes, obesity did not significantly affect airflow perception (p=0.21, for the difference in slopes between obese vs. non-obese females; p=0.22, for the difference in slopes between obese vs. non-obese males).
Waist-Circumference and Asthma Outcomes
When we defined obesity-status as an age- and sex-adjusted waist circumference > 90th percentile, we found similar degrees of improved lung function in obese females that we saw in the BMI-percentile-based analysis (Supplementary table B). However, among males, the results changed using waist measures. We did not see significant obesity-related airflow obstruction, however males with a waist-circumference >90th percentile had significantly worse asthma control by ACQ (1.1 vs. 1.4, p=.03, Supplementary table A). In both conventions for determining obesity, obesity was associated with improved lung function in females and worse asthma measures in males.
DISCUSSION
Contrary to our hypothesis, our data showed that obesity, defined as BMI>95th percentile, had little effect on symptom control, exacerbation risk or airway biomarkers among children with inadequately controlled asthma. However, obesity’s effect on lung function depended in part on sex and to a lesser degree on race. Obesity in males was associated with significantly greater airflow obstruction (reduced FEV1/FVC), while among females, obesity was associated with improved lung function and a trend toward reduced airway reactivity to methacholine. When using waist-circumference to define obesity, we still saw improved lung function among obese females compared to lean females.
Several studies have evaluated the association of obesity with asthma severity in children. Outcome measures in past studies have varied, and conclusions regarding the effect of obesity on asthma severity have ranged widely 20,21. These discrepancies may be related to specific inclusion criteria (e.g. asthma severity, ethnicity) or outcome measures analyzed (subjective symptoms versus objective measures). Our cohort was unique being predominantly African-American, with poorly controlled asthma and with a significant portion being obese. Of 306 children, 49% and 31% were overweight/obese and obese, respectively. Furthermore, we evaluated both subjective symptom reporting and objective measures of airway biomarkers and lung function testing.
Underweight status was highly associated with reduced lung function and increased symptom severity in our study. Though it is difficult to make conclusions from such a small sample of underweights (n=6), these results validate our recent work in a larger sample22 that showed that underweight asthmatics have worse lung function and symptom severity.
Obesity status defined as BMI > 95th percentile did not affect airway pH, FENO, day-to-day peak flow variability, or the risk of asthma exacerbation in males or females. BMI>95th percentile did affect lung function and this effect depended on sex. BMI>95th percentile in males was associated with significant worsening of airflow obstruction, a central component of asthma. Greater airflow obstruction in obese asthmatic males (versus obese asthmatic females) has been described previously23, though not consistently24,25. Males compared to females are known to have reduced airway caliber relative to lung size early in life26, and likely contributes to male infants having greater early wheezing and airway responsiveness. A possible explanation for the findings of enhanced airflow obstruction in obese males relative to obese females may be related to lung growth in early life. Since the timing and acceleration of somatic growth differs by sex 27,28, obesity-related alterations of lung growth may partially explain the sex-differences we are witnessing in the current study. Sex differences in obese-asthma among children may not be driven primarily by altered asthma mechanisms, but rather sex-specific differences in growth patterns and fat deposition that may alter growth and development of the lung. It is possible that greater truncal and intrathoracic adipose among males leads to greater impairment of lung function and sex-specific dysanapsis. More data is needed regarding pediatric obesity-asthma sex dimorphism. . Central obesity, which is generally more common in males, can restrict lung function 29,30. Among non-asthmatic children, obesity’s impact on lung function from previous research does not appear to be modified by gender, particularly when defining obesity by total body fat31,32. Specifically, there is little consistent evidence that obesity increases lung function in non-asthmatic girls. Therefore, improved lung function in obese girls seen in our study may not be solely attributable to greater lung growth but may result from changes in asthma.
Interestingly, when we used waist-circumference instead of BMI-percentile to define obesity, we found that the sex disparity remained qualitatively the same. Males with a waist-circumference >90th% reported worse asthma control compared to similar females; and females with a waist-circumference >90th% still had significantly better lung function.
It is important to note that among this predominantly African-American cohort, the association between obesity and airflow obstruction was driven primarily by participants who were White. Due to the weak interaction effect (obesity*race, p=.02), this finding could be due in part to random sampling error. However, Clerisme-Beaty previously reported no association between obesity and asthma severity among African-American adults 11. Our data and those of Clerisme-Beaty and colleagues point to the possibility of a weaker obesity-asthma association among African-Americans. Furthermore, our data further suggest that obesity is unlikely to be a significant epidemiologic cause of the excess asthma morbidity seen among African-Americans.
Previous reports have suggested that obesity leads to a greater risk for asthma exacerbation requiring systemic steroids or hospitalization 21,33. Carroll reported that obese children with asthma-related symptoms were more likely to be hospitalized33. However, in the same study there was no obesity effect on objective markers of asthma severity. Similar acute-care studies failed to find an obesity-related increase in objective asthma severity34. In the current study, BMI>95th percentile did not affect either the risk of incident episodes of poor asthma control or day-to-day PFvar. We included several possible criteria for meeting an ‘episode of poor asthma control’ (EPAC), including worsening symptoms or home peak flow values, greater bronchodilator use, new systemic steroids or controller therapy or an unscheduled healthcare visit for asthma. The EPAC is likely to be a very sensitive detector of exacerbation risk. In addition, PFvar has been shown to correlate with exacerbation risk and PFvar was also not affected by BMI>95th percentile status. In total, we conclude that obesity defined by BMI>95th percentile does not to contribute to increased disease instability or exacerbation risk in children with generally high symptom burden.
Next, by assessing the relationship between individual level PFvar and symptom reporting, we assessed whether obesity factors not specific to asthma (such as exercise intolerance or exertional dyspnea) might obscure the obese asthma phenotype. Obese asthmatics have reported greater asthma symptoms compared to lean counterparts, but it remains unclear if these findings are asthma-specific. Among adults, it does not appear that greater symptoms in obese patients are explained solely by enhanced perception. We previously showed that obesity was associated with less, not greater, airflow perception4, suggesting that obesity itself was unlikely to be causing over-reporting of symptoms. In children from the same study, obesity was associated with greater airflow perception, and in mild asthmatics airflow perception may partially explain why some obese asthmatics report greater symptoms. Enhanced perception seen in milder asthma was not seen in the current study which would not support the hypothesis that obesity heightens symptom perception in children with more symptomatic asthma.
Our study has several limitations. It is possible that we are underpowered to appreciate important effects of obesity on asthma outcomes, particularly if they are modified by other co-variates such as race, sex and age. We may have been able to appreciate the effects of sex and BMI>95th percentile on lung function because these participants had more severe asthma, or because obesity in early life may affect boys differently than girls in terms of lung growth. Another limitation is the fact that these are secondary data analyses. However, it is important to note that our hypothesis and statistical approach was declared a priori (prior to data analysis). Because of the modest sample size and possible alpha error, our finding of improved lung function among obese females needs further replication and mechanistic analysis. Lastly, we chose the most common convention for pediatric obesity (BMI>95th percentile). However, high BMI percentile is not specific to adiposity and can become elevated, particularly in shorter or muscular children. Better markers of adiposity may be needed to decipher the true associations between obesity and asthma characteristics. Since this analysis did not follow BMI-percentile over several years and did not involve a non-asthmatic comparison group, we are only able to assess phenotype (lung function, asthma symptoms) associations with obesity among asthmatics. This study cannot delineate whether the obesity-related findings stem from alterations in lung growth or asthma itself. Future studies will be helpful that follow young children for several years and measure both adiposity and respiratory mechanics (including residual and expiratory reserve volumes) among children with and without asthma.
Overall, obesity was very prevalent in this cohort of children with poorly controlled asthma, while underweight status was relatively rare. Underweight status associated with profoundly impaired lung growth and lung function, and requires further evaluation. The relationship between obesity and pediatric asthma is complex and appears to depend on sex, race and method of defining obesity. BMI>95th percentile in asthmatic children does not affect asthma control, airway biomarkers or exacerbation risk. However, BMI>95th percentile appears to affect lung function among children with difficult-to-control asthma in a manner that is sex-dependent. Obesity was associated with improved lung function and reduced airway reactivity in females, and heightened airflow obstruction in males.
Supplementary Material
Airways reactivity represented by PC20 from Methacholine Challenge Testing for non-obese (n=90) and obese (n=39) boys, and non-obese (n=51) and obese (n=30) girls. Error bars represent standard deviations. PC20 – provocation concentration to elicit a 20% reduction in the forced expiratory volume in 1 sec (FEV1), * - p=0.08 compared to non-obese females.
ACKNOWLEDGEMENTS
This research was performed by the American Lung Association Asthma Clinical Research Centers (ALA-ACRC).
FUNDING
Support: Supported by grants from the National Heart Lung and Blood Institute and American Lung Association. Support provided by Takeda Pharmaceuticals North America, Inc (Lansoprazole and Placebo) and by GlaxoSmithKline (Albuterol HFA).
Appendix
The members of the ALA-ACRC research group for the trial were as follows:
American Lung Association Asthma Clinical Research Centers
Baylor College of Medicine, Houston: N. A. Hanania (principal investigator), M. Sockrider (co-principal investigator), L. Bertrand (principal clinic coordinator), M. Atik, L. Giraldo, B, Flores (coordinators);
Columbia University–New York University Consortium, New York: J. Reibman (principal investigator), E. DiMango, L. Rogers (co-principal investigators), C. Cammarata and K. Carapetyan (clinic coordinators at New York University), J. Sormillon and E. Simpson (clinic coordinators at Columbia University);
Duke University Medical Center, Durham, N.C.: L. Williams (principal investigator), J. Sundy (co-principal investigator), G. Dudek (principal clinic coordinator), R. Newton and A. Dugdale (coordinators);
Emory University School of Medicine, Atlanta: W.G. Teague (principal investigator), Anne Fitzpatrick, Sumita Khatri (co-principal investigators), R. Patel (principal clinic coordinator), J. Peabody, E Hunter, D Whitlock (coordinators);
Illinois Consortium, Chicago: L. Smith (principal investigator), J. Moy, E. Naureckas, A. Prestridge (co-principal investigators), J. Hixon (principal clinic coordinator), A. Brees, J. Judge (coordinators);
Indiana University, Asthma Clinical Research Center, Indianapolis: M. Busk (principal investigator), P. Puntenney (principal clinic coordinator), N. Busk, J. Hutchins (coordinators);
University of Pennsylvania, Philadelphia: F. Leone (principal investigator), M. Hayes-Hampton (principal clinic coordinator);
National Jewish Health, Denver: R. Katial (principal investigator), M. Krawiecz (co-principal investigator), H. Currier (principal clinic coordinator);
Nemours Children’s Clinic–University of Florida Consortium, Jacksonville: J. Lima (principal investigator), K. Blake (co-principal investigator), J Lang (co-principal investigator), D Schaeffer (investigator), A Santos (principal coordinator), M McRae (coordinator)
Hofstra University School of Medicine (formerly North Shore–Long Island Jewish Health System), New Hyde Park, N.Y.: J. Karpel (principal investigator), R. Cohen (co-principal investigator), R. Ramdeo (principal clinic coordinator);
Northern New England Consortium (formerly Vermont Lung Center at the University of Vermont), Colchester, Vt.: C.G. Irvin (principal investigator), A.E. Dixon, D.A. Kaminsky (co-principal investigators), R. Colletti (GI consultant), S.M. Burns, L.M. Bourassa, S.E. Lang, L.V. Griffes (coordinators), R. Pratt, K.B. Nakos, K.J. Girard;
The Ohio State University Medical Center/Columbus Children’s Hospital, Columbus: J. Mastronarde (principal investigator), K. McCoy (co-principal investigator), J. Parsons (co-investigator), J. Drake (principal clinic coordinator), R. Compton, L. Raterman, D. Cosmar (coordinators);
Maria Fareri Children’s Hospital at Westchester Medical Center and New York Medical College: A. Dozor (principal investigator), I. Gherson (principal clinic coordinator)
University of Alabama at Birmingham, Birmingham: L.B. Gerald (principal investigator), W.C. Bailey, R. Grad (co-principal investigators), S. Erwin (principal clinic coordinator), A. Kelley, D. Laken (coordinators);
University of Miami, Miami–University of South Florida, Tampa: A. Wanner (principal investigator, Miami), R. Lockey (principal investigator, Tampa), E. Mendes (principal clinic coordinator for University of Miami), S. McCullough (principal clinic coordinator for University of South Florida), M. Grandstaff-Singleton, D. Miller (coordinators);
University of Minnesota, Minneapolis: M.N. Blumenthal (principal investigator), G. Brottman, J. Hagen (co-principal investigators), A. Decker, D. Lascewski, S. Kelleher (principal clinic coordinators), K. Bachman, C. Quintard, C. Sherry (coordinators);
University of Missouri, Kansas City School of Medicine, Kansas City: G. Salzman (principal investigator), C. Dinakar, D. Pyszczynski (co-principal investigators), P. Haney (principal clinic coordinator);
St. Louis Asthma Clinical Research Center: Washington University, St. Louis: M. Castro (principal investigator), L. Bacharier, K. Sumino (co-investigators), J. Tarsi (principal coordinator), B. Patterson (coordinator);
University of California San Diego: S. Wasserman (principal investigator), J. Ramsdell (co-principal investigator), P. Ferguson, K. Kinninger, T. Greene (clinic coordinators);
Chairman’s Office University of Alabama, Birmingham (formerly at Respiratory Hospital, Winnipeg, Man., Canada): W. Bailey and N. Anthonisen (research group chair);
Data Coordinating Center, Johns Hopkins University Center for Clinical Trials, Baltimore: R. Wise (center director), J. Holbrook (deputy director), E. Brown (principal coordinator), D. Amend-Libercci, K. Barry, M. Daniel, A. Lears, G. Leatherman, C. Levine, D. Nowakowski, N. Prusakowski, S. Rayapudi, S. Roettger, A. Thurman, D. Shade, E. Sugar, C. Wei;
Esophageal pH Probe Quality Control Center, Children’s Center for Digestive Healthcare Pediatric Gastroenterology, Hepatology, and Nutrition, (formerly at Emory University School of Medicine): B. Gold (center director);
Data and Safety Monitoring Board: S. Lazarus (chair), W. Calhoun, M. Cloutier, B. McWilliams, A. Rogatko, C. Sorkness;
Project Office, American Lung Association, New York: E. Lancet (project officer), N. Edelman (scientific consultant), S. Rappaport;
Project Office, National Heart Lung and Blood Institute: V. Taggart (project officer), G. Weinmann (DSMB secretary, airway branch chief);
ALA Scientific Advisory Committee: E. N. Schachter (chair), L. A. Baggott (vice-chair) W. C. Bailey, A. L. Brannen II, M. Castro, B. W. Christman, A. Chuang, R. M. Donaldson, C. Holloway, T. A. Mahr, J. A. Neubauer, J. M. Samet, E. R. Swenson, D. J. Upson, D. J. Weiss, R. Wise.
Footnotes
COMPETING INTERESTS
Disclosures:
Dr. Lang has no conflicts of interest in the subject matter of this manuscript
Dr. Holbrook has no conflicts of interest in the subject matter of this manuscript
Dr. Wise has no conflicts of interest in the subject matter of this manuscript
Dr. Dixon has no conflicts of interest in the subject matter of this manuscript
Dr. Teague has no conflicts of interest in the subject matter of this manuscript
Ms. Wei has no conflicts of interest in the subject matter of this manuscript
Dr. Irvin has no conflicts of interest in the subject matter of this manuscript
Dr. Shade as no conflicts of interest in the subject matter of this manuscript
Dr. Lima has no conflicts of interest in the subject matter of this manuscript
Contributor Information
Jason E. Lang, Email: jelang@nemours.org.
Janet T. Holbrook, Email: jholbroo@jhsph.edu.
Robert A. Wise, Email: rwise@jhmi.edu.
Anne E. Dixon, Email: anne.dixon@vtmednet.org.
W. Gerald Teague, Email: WGT2P@hscmail.mcc.virginia.edu.
Christine Y. Wei, Email: cwei@jhsph.edu.
Charles Irvin, Email: charles.irvin@uvm.edu.
David Shade, Email: dshade@jhsph.edu.
John J. Lima, Email: jlima@nemours.org.
REFERENCES
- 1.Lotvall J, Akdis CA, Bacharier LB, Bjermer L, Casale TB, Custovic A, Lemanske RF, Jr, Wardlaw AJ, Wenzel SE, Greenberger PA. Asthma endotypes: a new approach to classification of disease entities within the asthma syndrome. J Allergy Clin Immunol. 2011;127(2):355–360. doi: 10.1016/j.jaci.2010.11.037. [DOI] [PubMed] [Google Scholar]
- 2.Beuther DA, Sutherland ER. Overweight, obesity, and incident asthma: a meta-analysis of prospective epidemiologic studies. Am J Respir Crit Care Med. 2007;175(7):661–666. doi: 10.1164/rccm.200611-1717OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sutherland ER, Goleva E, Strand M, Beuther DA, Leung DY. Body mass and glucocorticoid response in asthma. Am J Respir Crit Care Med. 2008;178(7):682–687. doi: 10.1164/rccm.200801-076OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lang JE, Hossain J, Dixon AE, Shade D, Wise RA, Peters SP, Lima JJ. Does age impact the obese asthma phenotype? Longitudinal asthma control, airway function, and airflow perception among mild persistent asthmatics. Chest. 2011;140(6):1524–1533. doi: 10.1378/chest.11-0675. [DOI] [PubMed] [Google Scholar]
- 5.Castro-Rodriguez JA, Holberg CJ, Morgan WJ, Wright AL, Martinez FD. Increased incidence of asthmalike symptoms in girls who become overweight or obese during the school years. Am J Respir Crit Care Med. 2001;163(6):1344–1349. doi: 10.1164/ajrccm.163.6.2006140. [DOI] [PubMed] [Google Scholar]
- 6.Moore WC, Meyers DA, Wenzel SE, Teague WG, Li H, Li X, D'Agostino R, Jr, Castro M, Curran-Everett D, Fitzpatrick AM, et al. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med. 2010;181(4):315–323. doi: 10.1164/rccm.200906-0896OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ford ES. The epidemiology of obesity and asthma. J Allergy Clin Immunol. 2005;115(5):897–909. doi: 10.1016/j.jaci.2004.11.050. quiz 910. [DOI] [PubMed] [Google Scholar]
- 8.Sood A. Sex differences: implications for the obesity-asthma association. Exerc Sport Sci Rev. 2011;39(1):48–56. doi: 10.1097/JES.0b013e318201f0c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomsen SF, Ulrik CS, Kyvik KO, Sorensen TI, Posthuma D, Skadhauge LR, Steffensen I, Backer V. Association between obesity and asthma in a twin cohort. Allergy. 2007;62(10):1199–1204. doi: 10.1111/j.1398-9995.2007.01480.x. [DOI] [PubMed] [Google Scholar]
- 10.Kattan M, Kumar R, Bloomberg GR, Mitchell HE, Calatroni A, Gergen PJ, Kercsmar CM, Visness CM, Matsui EC, Steinbach SF, et al. Asthma control, adiposity, and adipokines among inner-city adolescents. J Allergy Clin Immunol. 125(3):584–592. doi: 10.1016/j.jaci.2010.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clerisme-Beaty EM, Karam S, Rand C, Patino CM, Bilderback A, Riekert KA, Okelo SO, Diette GB. Does higher body mass index contribute to worse asthma control in an urban population? J Allergy Clin Immunol. 2009;124(2):207–212. doi: 10.1016/j.jaci.2009.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Camargo CA, Jr, Boulet LP, Sutherland ER, Busse WW, Yancey SW, Emmett AH, Ortega HG, Ferro TJ. Body mass index and response to asthma therapy: fluticasone propionate/salmeterol versus montelukast. J Asthma. 2010;47(1):76–82. doi: 10.3109/02770900903338494. [DOI] [PubMed] [Google Scholar]
- 13.Peters-Golden M, Swern A, Bird SS, Hustad CM, Grant E, Edelman JM. Influence of body mass index on the response to asthma controller agents. Eur Respir J. 2006;27(3):495–503. doi: 10.1183/09031936.06.00077205. [DOI] [PubMed] [Google Scholar]
- 14.Forno E, Lescher R, Strunk R, Weiss S, Fuhlbrigge A, Celedon JC. Decreased response to inhaled steroids in overweight and obese asthmatic children. J Allergy Clin Immunol. 2011;127(3):741–749. doi: 10.1016/j.jaci.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holbrook JT, Wise RA, Gold BD, Blake K, Brown ED, Castro M, Dozor AJ, Lima JJ, Mastronarde JG, Sockrider MM, et al. Lansoprazole for children with poorly controlled asthma: a randomized controlled trial. JAMA. 2012;307(4):373–381. doi: 10.1001/jama.2011.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barlow SE. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120(Suppl 4):S164–S192. doi: 10.1542/peds.2007-2329C. [DOI] [PubMed] [Google Scholar]
- 17.Juniper EF, O'Byrne PM, Guyatt GH, Ferrie PJ, King DR. Development and validation of a questionnaire to measure asthma control. Eur Respir J. 1999;14(4):902–907. doi: 10.1034/j.1399-3003.1999.14d29.x. [DOI] [PubMed] [Google Scholar]
- 18.Taylor RW, Jones IE, Williams SM, Goulding A. Evaluation of waist circumference, waist-to-hip ratio, and the conicity index as screening tools for high trunk fat mass, as measured by dual-energy X-ray absorptiometry, in children aged 3–19 y. Am J Clin Nutr. 2000;72(2):490–495. doi: 10.1093/ajcn/72.2.490. [DOI] [PubMed] [Google Scholar]
- 19.Zimmet P, Alberti G, Kaufman F, Tajima N, Silink M, Arslanian S, Wong G, Bennett P, Shaw J, Caprio S. The metabolic syndrome in children and adolescents. Lancet. 2007;369(9579):2059–2061. doi: 10.1016/S0140-6736(07)60958-1. [DOI] [PubMed] [Google Scholar]
- 20.Mahut B, Beydon N, Delclaux C. Overweight is not a comorbidity factor during childhood asthma: the GrowthOb study. Eur Respir J. 2012;39(5):1120–1126. doi: 10.1183/09031936.00103311. [DOI] [PubMed] [Google Scholar]
- 21.Quinto KB, Zuraw BL, Poon KY, Chen W, Schatz M, Christiansen SC. The association of obesity and asthma severity and control in children. J Allergy Clin Immunol. 2011;128(5):964–969. doi: 10.1016/j.jaci.2011.06.031. [DOI] [PubMed] [Google Scholar]
- 22.Lang JE, Hossain J, Smith K, Lima JJ. Asthma Severity, Exacerbation Risk, and Controller Treatment Burden in Underweight and Obese Children. J Asthma. 2012 doi: 10.3109/02770903.2012.677895. [DOI] [PubMed] [Google Scholar]
- 23.Tantisira KG, Litonjua AA, Weiss ST, Fuhlbrigge AL. Association of body mass with pulmonary function in the Childhood Asthma Management Program (CAMP) Thorax. 2003;58(12):1036–1041. doi: 10.1136/thorax.58.12.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Gent R, van der Ent CK, Rovers MM, Kimpen JL, van Essen-Zandvliet LE, de Meer G. Excessive body weight is associated with additional loss of quality of life in children with asthma. J Allergy Clin Immunol. 2007;119(3):591–596. doi: 10.1016/j.jaci.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 25.Ross KR, Hart MA, Storfer-Isser A, Kibler AM, Johnson NL, Rosen CL, Kercsmar CM, Redline S. Obesity and obesity related co-morbidities in a referral population of children with asthma. Pediatr Pulmonol. 2009;44(9):877–884. doi: 10.1002/ppul.21065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pagtakhan RD, Bjelland JC, Landau LI, Loughlin G, Kaltenborn W, Seeley G, Taussig LM. Sex differences in growth patterns of the airways and lung parenchyma in children. J Appl Physiol. 1984;56(5):1204–1210. doi: 10.1152/jappl.1984.56.5.1204. [DOI] [PubMed] [Google Scholar]
- 27.Lampl M, Veldhuis JD, Johnson ML. Saltation and stasis: a model of human growth. Science. 1992;258(5083):801–803. doi: 10.1126/science.1439787. [DOI] [PubMed] [Google Scholar]
- 28.Boezen HM, Jansen DF, Postma DS. Sex and gender differences in lung development and their clinical significance. Clin Chest Med. 2004;25(2):237–245. doi: 10.1016/j.ccm.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 29.Salome CM, King GG, Berend N. Physiology of obesity and effects on lung function. J Appl Physiol. 2010;108(1):206–211. doi: 10.1152/japplphysiol.00694.2009. [DOI] [PubMed] [Google Scholar]
- 30.Musaad SM, Patterson T, Ericksen M, Lindsey M, Dietrich K, Succop P, Khurana Hershey GK. Comparison of anthropometric measures of obesity in childhood allergic asthma: central obesity is most relevant. J Allergy Clin Immunol. 2009;123(6):1321–1327. e12. doi: 10.1016/j.jaci.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lazarus R, Colditz G, Berkey CS, Speizer FE. Effects of body fat on ventilatory function in children and adolescents: cross-sectional findings from a random population sample of school children. Pediatr Pulmonol. 1997;24(3):187–194. doi: 10.1002/(sici)1099-0496(199709)24:3<187::aid-ppul4>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 32.Li AM, Chan D, Wong E, Yin J, Nelson EA, Fok TF. The effects of obesity on pulmonary function. Arch Dis Child. 2003;88(4):361–363. doi: 10.1136/adc.88.4.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carroll CL, Stoltz P, Raykov N, Smith SR, Zucker AR. Childhood overweight increases hospital admission rates for asthma. Pediatrics. 2007;120(4):734–740. doi: 10.1542/peds.2007-0409. [DOI] [PubMed] [Google Scholar]
- 34.Ginde AA, Santillan AA, Clark S, Camargo CA., Jr Body mass index and acute asthma severity among children presenting to the emergency department. Pediatr Allergy Immunol. 2009;21(3):480–488. doi: 10.1111/j.1399-3038.2009.00911.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Airways reactivity represented by PC20 from Methacholine Challenge Testing for non-obese (n=90) and obese (n=39) boys, and non-obese (n=51) and obese (n=30) girls. Error bars represent standard deviations. PC20 – provocation concentration to elicit a 20% reduction in the forced expiratory volume in 1 sec (FEV1), * - p=0.08 compared to non-obese females.