Abstract
Objective
To determine the association of circulating cell-free hemoglobin with poor clinical outcomes in patients with sepsis and to characterize the potential protective effects of acetaminophen, an inhibitor of hemoprotein-mediated oxidation.
Design
Retrospective observational study.
Patients
A total of 391 critically ill patients with sepsis in multiple intensive care units in an academic tertiary care hospital.
Interventions
None.
Measurements and Main Results
Nonsurvivors had significantly higher plasma cell-free hemoglobin concentrations (median 20 mg/dl, IQR 10–40) measured on enrollment compared to survivors (10 mg/dl, IQR 10–30, p = 0.002). After controlling for potential confounders, patients with higher cell-free hemoglobin concentrations were significantly more likely to die in the hospital (OR 1.078, 95% CI 1.012–1.149, p = 0.02). In addition, receiving acetaminophen in the setting of increased cell-free hemoglobin was independently associated with a protective effect against death (OR 0.48, 95% CI 0.25–0.91, p = 0.026) and lower plasma concentrations of the lipid peroxidation product F2-isoprostanes (18.5 pg/ml IQR 9–22.2) compared to no acetaminophen (42 pg/ml, IQR 29.7–86, p = 0.009).
Conclusions
In critically ill patients with sepsis, elevated concentrations of circulating cell-free hemoglobin are independently associated with an increased risk of death. Acetaminophen may exert a protective effect by reducing cell-free hemoglobin-induced induced oxidative injury.
Keywords: sepsis, mortality, acetaminophen, cell-free hemoglobin, isoprostanes
Introduction
Cell-free hemoglobin can be measured in the plasma of patients with sickle cell anemia (1), hemodialysis (2), patients who have undergone cardiac bypass for coronary artery bypass grafting or aortic repair (3–5), and after red blood cell transfusion (6). In all of these patient populations, cell-free hemoglobin has been associated with poor outcomes including acute kidney injury (3,5), myocardial infarction, and death (7). Mechanisms of toxicity include: the ability of cell-free hemoglobin to scavenge nitric oxide and induce vasoconstriction in various vascular beds (1,2,6,8,9), neutrophil activation (10), damage to the vascular endothelium (11), and the ability of cell-free hemoglobin to undergo redox cycling leading to oxidation of lipid membranes and release of F2-isoprostanes (3,12).
Sepsis is a common condition with a high associated mortality (13,14). Alterations in the red blood cell membrane during sepsis have been described and may be associated with release of hemoglobin into the circulation due to membranous damage (15–17). In animal models of sepsis, cell-free hemoglobin can be measured in the plasma and is associated with mortality, an effect that is potentiated by deficiency of haptoglobin, hemopexin, or heme oxygenase-1 (18). However, it is currently unknown whether circulating cell-free hemoglobin can be detected in humans with sepsis, or whether the presence of cell-free hemoglobin in sepsis is associated with poor clinical outcomes. Additionally, although evidence of oxidant stress and lipid peroxidation as measured by plasma concentrations of F2-isoprostanes has been described in patients with sepsis and is associated with renal, hepatic, and coagulation failure, and mortality (19), the role of cell-free hemoglobin in driving lipid peroxidation in sepsis is unknown.
Acetaminophen, at normal therapeutic concentrations, is a potent inhibitor of hemoprotein-mediated lipid peroxidation owing to its ability to reduce the ferryl-protoporphyrin radical generated with release of hemoproteins into the circulation. Heme-protein induced oxidation of arachidonic acid in lipid membranes and production of isoprostanes and isofurans is blocked by therapeutic concentrations of acetaminophen (20, 21). In experimental studies in rats, acetaminophen was highly effective in preventing oxidative damage and renal failure in the setting of myoglobinemia due to rhabdomyolysis (21); however, the potential for acetaminophen to inhibit hemoprotein-mediated lipid peroxidation has not been studied in humans.
We conducted a retrospective analysis of a prospectively collected cohort to test the hypothesis that high concentrations of cell-free hemoglobin in patients with sepsis are associated with an increased risk of in-hospital mortality. We also hypothesized that exposure to acetaminophen in this patient population would be associated with a protective effect in the setting of cell-free hemoglobin via a reduction of oxidative stress as measured by plasma F2-isoprostanes.
Materials and Methods
Patients
The study population consisted of 400 consecutive patients who were prospectively enrolled in the Validating Acute Lung Injury Markers for Diagnosis (VALID) study (22) and had the diagnosis of sepsis, as defined by the consensus definition (23), on enrollment. The VALID study includes patients > = 18 years old admitted to the Vanderbilt ICUs for at least two days and has been approved by the Vanderbilt Institutional Review Board. Plasma samples were obtained at enrollment on the morning of ICU day two and again 48 hours later. To minimize hemolysis, blood was preferentially drawn through a central venous catheter before using peripheral intravenous access or peripheral blood draw. All blood samples were immediately cooled, centrifuged at 3000 rpm for ten minutes, and plasma was frozen at −80C.
Inclusion/Exclusion Criteria
All 400 patients with sepsis on enrollment were considered for inclusion in the cell-free hemoglobin analysis if they had plasma available from the enrollment time point unless gross hemolysis was detected in their plasma sample. For the acetaminophen analysis, all 400 patients were considered for inclusion if they had a non-hemolyzed enrollment blood draw and had data from the medication administration record available as to timing and doses of acetaminophen administered during the 96 hours of the VALID study period. For the F2-isoprostane analysis, a subgroup of patients were consecutively included if they had a plasma cell-free hemoglobin concentration of 10–70 mg/dl on enrollment, had received either the highest quartile cumulative dose of acetaminophen or no acetaminophen in the 48 hours after enrollment, and had a plasma sample available at 48 hours after enrollment. This cell-free hemoglobin range was chosen as the risk factor (detectable cell-free hemoglobin) would be present and the median and IQR of the cell-free hemoglobin concentrations would be similar to the primary analysis.
Measurements
Measurements were made in plasma obtained on the morning of ICU day two (cell-free hemoglobin) at the time of enrollment into the VALID study and 48 hours later on ICU day four (F2-isoprostanes). Cell-free hemoglobin was measured using a HemoCue® Plasma Low/Hb system that has a lower limit of detection of 10 mg/dl. Plasma samples with undetectable cell-free hemoglobin were considered to have concentrations of 0 mg/dl in the statistical analyses. F2-isoprostane measurements were made using stable isotope dilution negative ion chemical ionization gas chromatography mass spectrometry (24).
Statistical Analysis
The primary analysis for this cohort study was hospital mortality in relation to cell-free hemoglobin concentration. The secondary analyses were in-hospital mortality in the setting of cell-free hemoglobin and variable acetaminophen exposure during the entire VALID enrollment period, along with plasma F2-isoprostane concentrations in the setting of cell-free hemoglobin and variable acetaminophen exposure in the 48 hours prior to the blood draw used to measure F2-isoprostanes.
As the majority of the data are not normally distributed, data are expressed as median values with interquartile range for continuous variables and frequencies for categorical variables. Univariate analyses were conducted using Wilcoxon’s rank sum test for continuous variables, Fisher’s exact test for categorical variables of two groups and Pearson’s chi-square test for a trend for categorical variables of greater than two groups, and Spearman’s rank correlation coefficient to assess correlation between two continuous variables. Known risk factors for both poor outcomes and elevated cell-free hemoglobin, and significant variables from univariable analyses that could influence cell-free hemoglobin levels were included in the multivariable logistic regression models used to analyze risks of in-hospital mortality. IBM® SPSS® Statistics (version 19.0) was used for statistical analysis; a two-sided significance level of 0.05 was used for statistical inference.
Results
Clinical Characteristics
Of the 400 patients with sepsis, nine were excluded from the primary analysis due to overt signs of hemolysis during processing. The remaining 391 patients defined the primary study population. Patients were predominantly in the medical ICU (72.1%), followed by the surgical (21.5%), trauma (3.3%), and cardiovascular ICUs (3.1%). Table 1 compares baseline characteristics between hospital survivors and non-survivors. Survivors were younger, had a lower severity of illness by APACHE II score, and were less likely to have chronic liver disease. The median plasma cell-free hemoglobin in all patients measured at enrollment was 20 mg/dl, IQR 10–30. Cell-free hemoglobin was undetectable in 75 (19.2%) of patients. Compared to patients with no detectable cell-free hemoglobin (< 10 mg/dl), patients with any detectable cell-free hemoglobin were similar in regards to age, gender, APACHE II score, chronic liver disease, and percentage with severe sepsis or septic shock; however 6 (8.1%) of patients with undetectable cell-free hemoglobin died compared to 79 (24.9%) of the patients with any amount of detectable cell-free hemoglobin (p = <0.001).
Table 1.
Baseline Characteristics
| Characteristic | Overall n = 391 |
Survivors n = 306 (78.3%) |
Nonsurvivors n = 85 (21.7%) |
p-value |
|---|---|---|---|---|
| Age (years) | 57 (48, 68) | 57 (47, 67) | 61 (54, 70) | 0.011 |
| Men (n, %) | 218 (55.8%) | 165 (53.9%) | 53 (62.4%) | 0.176 |
| APACHE II on enrollment | 28 (22, 33) | 26 (21, 32) | 31 (26, 36) | <0.001 |
| On dialysis at enrollment (n, %) | 14 (3.6%) | 13 (4.2%) | 1 (1.2%) | 0.319 |
| PRBC tranfusion (n, %) | 91 (23.3%) | 66 (21.6%) | 25 (29.4%) | 0.147 |
| Chronic Liver Disease (n, %) | 30 (7.7%) | 16 (5.3%) | 14 (16.5%) | 0.002 |
| Severe Sepsis (n, %) | 377 (96.4%) | 293 (95.8%) | 84 (98.8%) | 0.319 |
| Septic Shock (n, %) | 276 (70.6%) | 209 (68.3%) | 67 (78.8%) | 0.061 |
| ALI/ARDS (n, %) | 165 (42.2%) | 118 (38.6%) | 47 (55.3%) | 0.006 |
| Free Hemoglobin (mg/dl) | 20 (10, 30) | 10 (10, 30) | 20 (10, 40) | 0.002 |
Data given as median (25th percentile, 75th percentile) or number (percentage) of patients. PRBC = packed red blood cells, ALI/ARDS = acute lung injury/acute respiratory distress syndrome.
In-hospital Mortality Related to Cell-free hemoglobin Concentrations
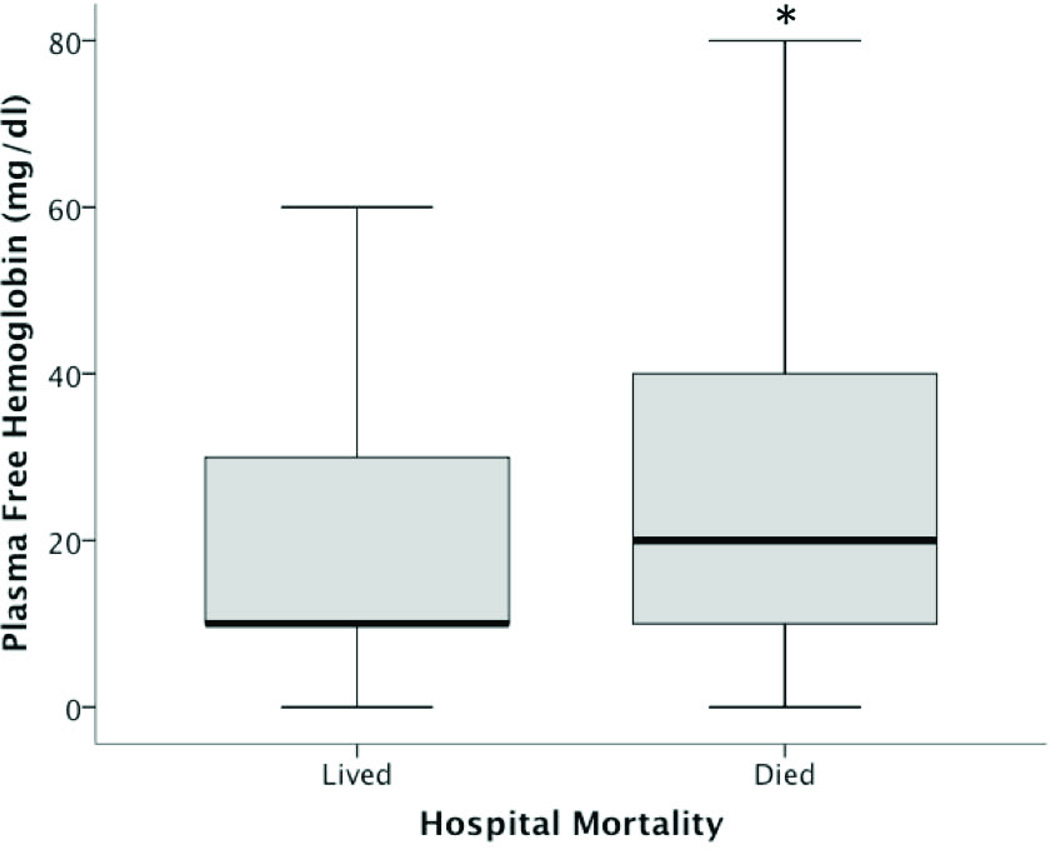
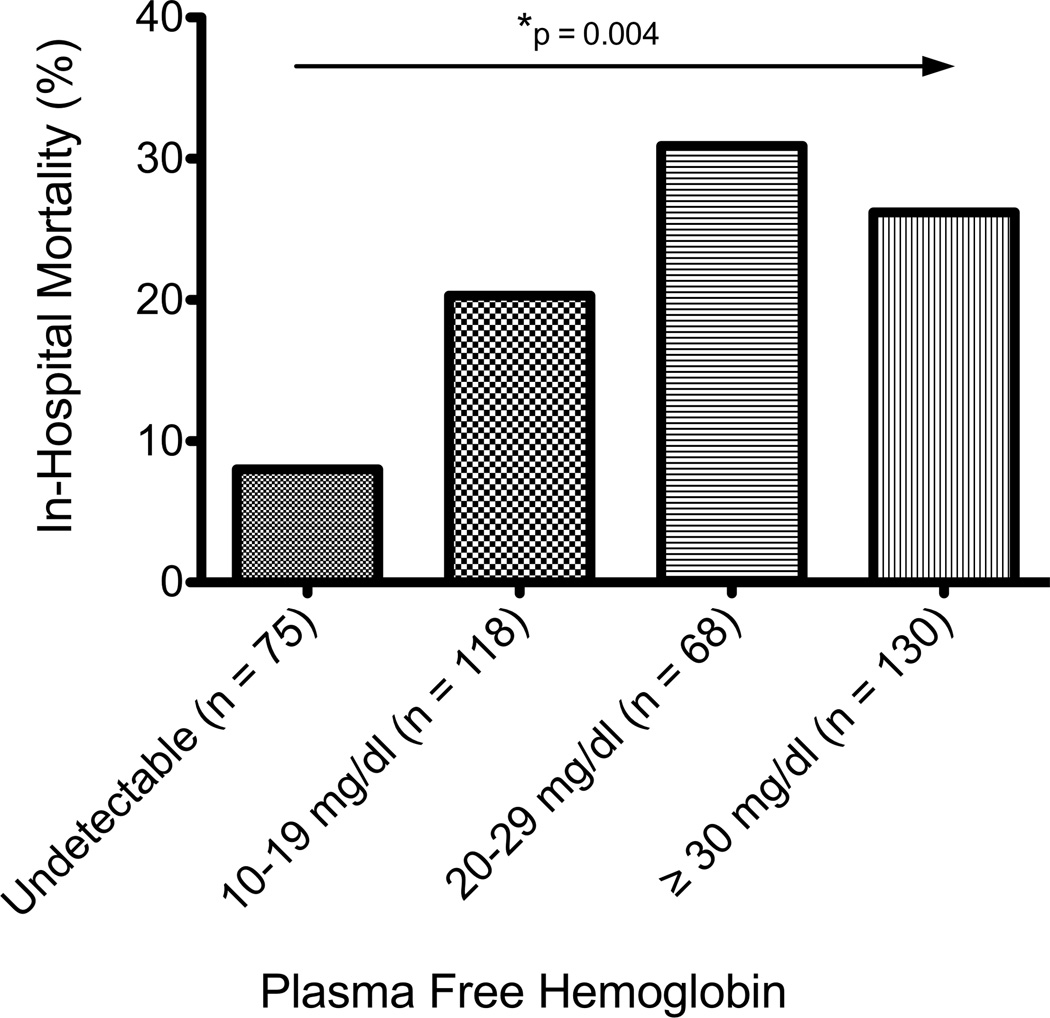
Of the 391 patients in the final analysis, 85 (21.7%) died during hospitalization. Non-survivors had significantly higher free hemoglobin concentrations (median 20 mg/dl, IQR 10–40)(Figure 1) compared to survivors (10 mg/dl, IQR 10–30, p = 0.002). In an unadjusted analysis, higher concentrations of free hemoglobin were associated with an increasing risk of in-hospital mortality (p = 0.004)(Figure 2).
Figure 1. Plasma concentrations of cell-free hemoglobin and in-hospital mortality.
Survivors had significantly lower cell-free hemoglobin (10 mg/dl) than non-survivors (20 mg/dl)(*p = 0.002). Values are medians (middle bold horizontal line), interquartile ranges (IQR: box borders), and 10th to 90th percentiles (whiskers).
Figure 2. In-Hospital Mortality based on increasing concentrations of Plasma Cell-free hemoglobin.
There was an increasing mortality trend amongst patients with higher plasma cell-free hemoglobin concentrations (Pearson Χ2 for a trend, p = 0.004).
A multivariable logistic regression model was used to examine the association between plasma free hemoglobin concentration and mortality. After controlling for age, APACHE II score, requirement of hemodialysis, and chronic liver disease, there was a significant increase in risk of in-hospital mortality in patients with higher concentrations of cell-free hemoglobin (OR 1.078 per 10 mg/dl increase in plasma cell-free hemoglobin concentration, 95% CI 1.012–1.149, p = 0.02)(Table 2).
Table 2.
Logistic Regression Model for In-Hospital Mortality
| Characteristic | Odds Ratio | 95% Confidence Interval |
p-value |
|---|---|---|---|
| Age (years) | 1.020 | 1.002–1.038 | 0.028 |
| APACHE II Score | 1.075 | 1.04–1.111 | <0.001 |
| On Hemodialysis | 0.215 | 0.025–1.838 | 0.16 |
| Chronic Liver Disease | 3.824 | 1.703–8.587 | 0.001 |
| Free Hemoglobin Level (mg/dl) | 1.078 | 1.012–1.149 | 0.020 |
The odds ratio for APACHE II score is for every one point above a score of seven. The odds ratio for Cell-free hemoglobin Concentration is for an increment of every 10 mg/dl above 0 mg/dl. The Hosmer and Lemeshow goodness-of-fit statistic for this model is 0.605.
Clinical Characteristics of Patients with Acetaminophen Exposure
Of the 391 patients included in the primary analysis, accurate records regarding presence or absence of acetaminophen exposure and dose during the VALID enrollment period were available on 292 patients from the electronic medication administration record. Patients included in the acetaminophen analysis were similar to patients in the primary analysis (Table 3). During the 96 hours of VALID enrollment, 146 (50%) of these 292 patients received any dose of acetaminophen and the median cumulative acetaminophen dose among all patients was 325mg, IQR 0–1500 (Table 3).
Table 3.
Baseline Characteristics for Patients Included in the Acetaminophen Analysis
| Characteristic | Overall n = 292 | Survivors n = 235 (80.4%) |
Nonsurvivors n = 57 (19.6%) |
p-value |
|---|---|---|---|---|
| Age (years) | 57.5 (49, 68) | 57 (47, 68) | 63 (55, 71) | 0.011 |
| Men (n, %) | 155 (53.1%) | 120 (51.1%) | 35 (61.4%) | 0.184 |
| APACHE II Score | 28 (21–33) | 27 (21, 32) | 31 (27, 36) | <0.001 |
| On Hemodialysis | 13 (4.5%) | 12 (5.1%) | 1 (1.8%) | 0.475 |
| PRBC transfusion (n, %) | 77 (26.4%) | 55 (23.4%) | 22 (38.6%) | 0.147 |
| Chronic Liver Disease (n, %) | 20 (6.8%) | 11 (4.7%) | 9 (15.8%) | 0.006 |
| Free Hemoglobin (mg/dl) | 20 (10–30) | 10 (10, 30) | 20 (10, 40) | 0.002 |
| Received Acetaminophen (n, %) | 146 (50%) | 127 (54%) | 19 (33.3%) | 0.008 |
| Cumulative Acetaminophen dose (mg) | 325 (0, 1500) | 650 (0, 1950) | 0 (0, 650) | 0.002 |
Data given as median (25th percentile, 75th percentile) or number (percentage) of patients. PRBC transfusion = packed red blood cell transfusion in the 48 hours prior to enrollment.
In-hospital Mortality and Acetaminophen Exposure
Of the 292 patients in this analysis, 57 (19.6%) died during hospitalization. Survivors were more likely to have received some amount of acetaminophen (p = 0.008) along with being exposed to higher cumulative doses (median 650mg, IQR 0–1950) compared to nonsurvivors (median 0 mg, IQR 0–650, p = 0.002). After controlling for age, the presence of chronic liver disease, APACHE II score, and cell-free hemoglobin concentration, receiving any dose of acetaminophen had a protective association with in-hospital mortality (OR 0.481, 95% CI 0.252–0.916, p = 0.026)(Table 4). In addition, when analyzing only the subset of patients with no detectable plasma cell-free hemoglobin (n = 34), the associated protective effect between acetaminophen and mortality was no longer apparent (OR 1.328, 95% CI 0.103–17.164, p = 0.852).
Table 4.
Logistic Regression Model for In-Hospital Mortality in Relation to Acetaminophen Exposure
| Characteristic | Odds Ratio | 95% Confidence Interval |
p-value |
|---|---|---|---|
| Age (years) | 1.023 | 1.001–1.046 | 0.039 |
| APACHE II Score | 1.081 | 1.036–1.128 | <0.001 |
| Chronic Liver Disease | 4.458 | 1.6–12.416 | 0.004 |
| Free Hemoglobin Level (mg/dl) | 1.082 | 1.006–1.164 | 0.035 |
| Acetaminophen | 0.481 | 0.252–0.916 | 0.026 |
The odds ratio for Cell-free hemoglobin Concentration is for an increment of every 10 mg/dl above 0 mg/dl. Acetaminophen refers to a patient receiving any amount of acetaminophen over the 96-hour study period compared to none. The Hosmer and Lemeshow goodness-of-fit statistic for this model is 0.518.
F2-Isoprostane Concentrations in the Setting of Variable Acetaminophen Exposure
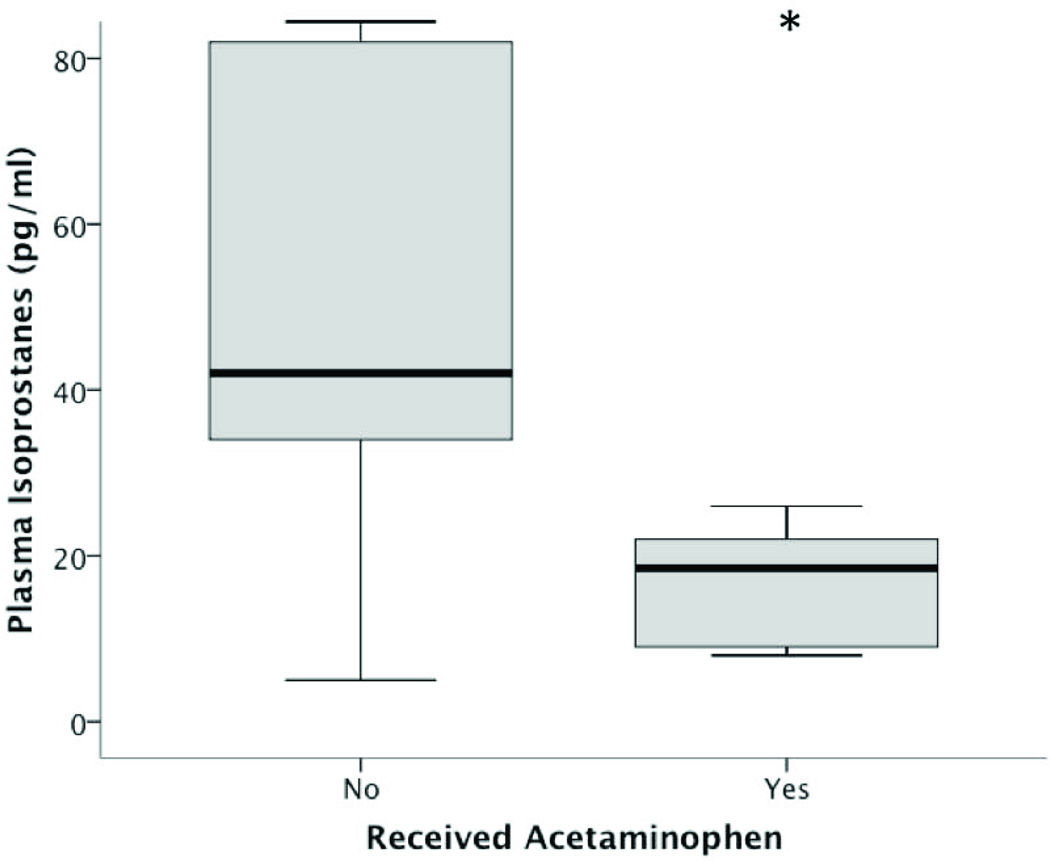
We next determined whether administration of acetaminophen was associated with a reduction in lipid peroxidation in the setting of circulating cell-free hemoglobin in a nested case control study. We studied 20 consecutively selected patients from the larger cohort of 391 sepsis patients who had plasma cell-free hemoglobin concentrations of 10–70 mg/dl measured on enrollment. Half of the patients (n = 10) had received no acetaminophen and half of the patients had received a cumulative acetaminophen dose in the highest quartile prior to a second blood draw 48 hours after enrollment for measurement of F2-isoprostanes. There was no difference in cell-free hemoglobin concentrations between the acetaminophen and no acetaminophen groups (20mg/dl compared to 30mg/dl, respectively, p = 0.409). Patients who had received acetaminophen in the 48 hours prior to the second blood draw had significantly lower concentrations of F2-isoprostanes (18.5 pg/ml, IQR 9–22.2) compared to those who received no acetaminophen (42 pg/ml, IQR 29.7–86, p = 0.009)(Figure 3). In addition, the cumulative dose of acetaminophen received was inversely correlated with F2-isoprostane concentrations measured 48 hours after enrollment (rs = −0.669, p = 0.001).
Figure 3. F2-isoprostanes and acetaminophen exposure.
Patients exposed to acetaminophen in the 48 hours prior to their blood draw had significantly lower plasma F2-isoprostane concentrations (18.5 pg/ml) compared to those who received no acetaminophen (42 pg/ml, *p = 0.009). Values are medians (middle bold horizontal line), interquartile ranges (IQR: box borders), and 10th to 90th percentiles (whiskers).
Discussion
In this large study of critically ill patients with sepsis, there was a significant association between plasma cell-free hemoglobin concentrations and in-hospital mortality even when controlling for factors that might predispose to higher circulating cell-free hemoglobin concentrations such as hemodialysis and chronic liver disease. In addition, sepsis patients who received acetaminophen, a potent inhibitor of hemoprotein-mediated lipid peroxidation, early in their hospital course had significantly lower hospital mortality and a decrease in oxidative stress as measured by circulating F2-isoprostanes. To our knowledge, this study is the first description of an association of cell-free hemoglobin concentrations with mortality in a large population of patients with sepsis. In addition, a potential protective effect of acetaminophen in sepsis has not previously been reported.
In experimental studies, circulating cell-free hemoglobin has been observed in rodents with sepsis and higher concentrations were associated with mortality (18). There are several potential mechanisms by which cell-free hemoglobin could be injurious in the setting of sepsis. Cell-free hemoglobin can scavenge nitric oxide leading to vasoconstriction (1, 2, 6, 8, 9). In addition, when hemoglobin is released into the extracellular compartment, ferrous heme is oxidized to ferryl heme protoporphyrin radical species which are potent oxidants that cause lipid peroxidation (21, 25). Both vasoconstriction and lipid peroxidation have been associated with poor outcomes in other patient populations (1–3, 6, 8,9, 12, 17).
By virtue of its reducing properties, acetaminophen can inhibit the production of radicals in the setting of free hemoproteins such as hemoglobin or myoglobin (20, 21, 26, 27). In animal models, acetaminophen is protective against acute kidney injury caused by rhabdomyolysis (21). Since cell-free hemoglobin was detectable in the majority of severe sepsis patients and was associated with poor clinical outcomes, we hypothesized that exposure to acetaminophen would be protective by limiting hemoglobin-mediated lipid peroxidation. In fact, not only did we find an independent association between treatment with acetaminophen and reduced mortality in the setting of an oxidant stimulus (cell-free hemoglobin), we also found that patients who received the highest cumulative amount of acetaminophen had a reduction in lipid peroxidation as measured by F2-isoprostanes compared to patients who received no acetaminophen.
Plasma cell-free hemoglobin concentrations measured in our patient population are similar to concentrations reported in other disease states such as sickle cell anemia and those undergoing dialysis or cardiac bypass (1–5). However, the current study suggests a different interaction between acetaminophen and sepsis compared to a recent observational study of over 700 critically ill patients that reported an association between acetaminophen administration and increased mortality in the subgroup with sepsis (28). Unlike that study, which only analyzed acetaminophen given for fever and thus may have been subject to confounding by indication, our study analyzed any form of acetaminophen given for any indication, including as a pain reliever in combination with opiates. Furthermore, acetaminophen may only be protective in a subset of sepsis patients who have detectable concentrations of plasma cell-free hemoglobin as there was no associated protective effect in our study when analyzing only patients with undetectable cell-free hemoglobin.
This study has several limitations. First, it is not possible to determine causation from this observational study. Although we adjusted for multiple potential confounders in our multivariable analysis, it is possible that elevated plasma cell-free hemoglobin is a marker of more serious illness rather than a mediator. Second, although the sample size for the primary analysis was large, the sample sizes for secondary analyses were smaller due to data on acetaminophen exposure being available only in a subset of patients as well as there being relatively few patients who met the inclusion criteria for the analysis of F2-isoprostanes. Third, although we controlled for severity of illness and conditions that may preclude treatment with acetaminophen, i.e. chronic liver disease or a high severity of illness, when analyzing the effect of acetaminophen on mortality, it is possible that the association between acetaminophen dose and improved clinical outcomes is still confounded. Data describing the reasons for acetaminophen administration and route were not available, which further limits our ability to control for potential confounders. In addition, the association between higher F2-isoprostane concentrations in patients having received no acetaminophen compared to patients receiving the highest quartile of acetaminophen may be a marker of severity of illness rather than a pharmacologic effect of acetaminophen. The potential for confounding is mitigated to some extent by the strong, inverse correlation between cumulative acetaminophen dose and isoprostane concentrations. The lower limit of detection for our cell-free hemoglobin assay being 10 mg/dl along with the inability to determine which patients in the VALID study had disseminated intravascular coagulation reduces the granularity of the data we have presented. Finally, we cannot account for patients who died during the acetaminophen exposure window, which would decrease their chance to receive acetaminophen, although these patients accounted for less than 5% (n = 14) of patients included in the acetaminophen analysis. There is no survivor bias in the F2-isoprostane analysis as none of the patients died during the potential acetaminophen exposure window.
Conclusions
In critically ill patients with sepsis, cell-free hemoglobin can commonly be detected and higher concentrations are associated with a higher risk of death. In addition, acetaminophen exposure during early sepsis and in the setting of increased cell-free hemoglobin is associated with a lower risk death, and evidence of oxidative stress is decreased in patients with detectable cell-free hemoglobin who received acetaminophen. These observations suggest that cell-free hemoglobin could be an important mediator of oxidative injury in clinical sepsis and that acetaminophen might attenuate this injury. Prospective studies are needed to more specifically define the injurious role of cell-free hemoglobin in sepsis and the potential protective effect of acetaminophen in this clinical setting.
Acknowledgements
Supported in part by the Vanderbilt CTSA grant UL1 RR024975-01 from NCRR/NIH, NIH HL103836, NIH HL090785, NIH GM42056, an American Heart Association Clinical Research Award, and an American Heart Association Established Investigator Award. The authors would also like to thank Todd W Rice and Arthur P Wheeler for their advice on the analysis and presentation of this data.
Abbreviations
- APACHE
Acute Physiology and Chronic Health Evaluation
- VALID
Validating Acute Lung Injury markers for Diagnosis
- ICU
intensive care unit
- OR
odds ratio
- CI
confidence interval
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors’ Contributions
DRJ, JAB, and LBW were involved in the study design. DRJ, JFP, GS, NW, AKM, and LJR collected the data. DRJ and LBW performed the statistical analysis. DRJ drafted the manuscript and all authors participated in the revision of this manuscript. All authors read and approved the final manuscript.
Conflict of Interest Statement
The authors declare that they have no conflicts of interest.
References
- 1.Reiter CD, Wang X, Tanus-Santos JE, Hogg N, Cannon RO, Schechter AN, Gladwin MT. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nature Med. 2002;8:1383–1389. doi: 10.1038/nm1202-799. [DOI] [PubMed] [Google Scholar]
- 2.Meyer C, Heiss C, Drexhage C, Kehmeier ES, Balzer J, Muhlfeld A, Merx MW, Lauer T, Kuhl H, Floege J, Kelm M, Rassaf T. Hemodialysis-induced release of hemoglobin limits nitric oxide bioavailability and impairs vascular function. J Am Coll Cardiol. 2010;55:454–459. doi: 10.1016/j.jacc.2009.07.068. [DOI] [PubMed] [Google Scholar]
- 3.Billings FT, Ball SK, Roberts JR, III, Pretorius M. Postoperative acute kidney injury is associated with hemoglobinemia and an enhanced oxidative stress response. Free Rad Biol and Med. 2011;50:1480–1487. doi: 10.1016/j.freeradbiomed.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vermeulen Windsant IC, Hanssen SJ, Buurman WA, Jacobs MJ. Cardiovascular surgery and organ damage: time to reconsider the role of hemolysis. J Thor Card Surg. 2011;142:1–11. doi: 10.1016/j.jtcvs.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 5.Vermeulen Windsant IC, Snoeijs MG, Hanssen SJ, ALtintas S, Heijmans JH, Koeppel TA, Schurink GW, Buurman WA, Jacobs MJ. Hemolysis is associated with acute kidney injury during major aortic surgery. Kidney Int. 2010;77:913–920. doi: 10.1038/ki.2010.24. [DOI] [PubMed] [Google Scholar]
- 6.Gladwin MT, Kim-Shapiro DB. Storage lesion in banked blood due to hemolysis-dependent disruption of nitric oxide homeostasis. Curr Opin Hematol. 2009;16:515–523. doi: 10.1097/MOH.0b013e32833157f4. [DOI] [PubMed] [Google Scholar]
- 7.Natanson C, Kern SJ, Lurie P, Banks SM, Wolfe SM. Cell-free hemoglobin-based blood substitutes and risk of myocardial infarction and death. JAMA. 2008;299:2304–2312. doi: 10.1001/jama.299.19.jrv80007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Minneci PC, Deans KJ, Zhi H, Yuen PST, Star RA, Banks SM, Schechter AN, Natanson C, Gladwin MT, Solomon SB. Hemolysis-associated endothelial dysfunction mediated by accelerated NO inactivation by decompartmentalized oxyhemoglobin. J Clin Invest. 2005;115:3409–3417. doi: 10.1172/JCI25040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donadee C, Raat NJH, Kanias T, Tejero J, Lee JS, Kelley EE, Zhao X, Liu C, Reynolds H, Azarov I, Frizzell S, Meyer EM, Donnenberg AD, Qu L, Triulzi D, Kim-Shapiro DB, Gladwin MT. Nitric oxide scavenging by red blood cell microparticles and cell-free hemoglobin as a mechanism for the red cell storage lesion. Circulation. 2011;124:465–476. doi: 10.1161/CIRCULATIONAHA.110.008698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Graca-Souza AV, Arruda MAB, de Freitas MS, et al. Neutrophil activation by heme: implications for inflammatory processes. Blood. 2002;99:4160–4165. doi: 10.1182/blood.v99.11.4160. [DOI] [PubMed] [Google Scholar]
- 11.Baek JH, D’Agnillo F, Vallelian F, et al. Hemoglobin-driven pathophysiology is an in vivo consequence of the red blood cell storage lesion that can be attenuated in guinea pigs by haptoglobin therapy. J Clin Invest. 2012;122:1444–1458. doi: 10.1172/JCI59770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reeder BL, Svistunenko DA, Cooper CE, Wilson MT. The radical and redox chemistry of myoglobin and hemoglobin: from in vitro studies to human pathology. Antioxid Redox Signaling. 2004;6:954–966. doi: 10.1089/ars.2004.6.954. [DOI] [PubMed] [Google Scholar]
- 13.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 15.Piagnerelli M, Zouaoui Boudjeltia K, Vanhaeverbeek M, Vincent JL. Red blood cell rheology in sepsis. Int Care Med. 2003;29:1052–1061. doi: 10.1007/s00134-003-1783-2. [DOI] [PubMed] [Google Scholar]
- 16.Machiedo GW, Powell RJ, Rush BF, Swislocki NI, Dikdan G. The incidence of decreased red blood cell deformability in sepsis and the association with oxygen free radical damage and multiple-system organ failure. Arch Surg. 1989;124:1386–1389. doi: 10.1001/archsurg.1989.01410120032007. [DOI] [PubMed] [Google Scholar]
- 17.Baskurt OK, Gelmont D, Meiselman HJ. Red blood cell deformability in sepsis. Am J Respir Crit Care Med. 1998;157:421–427. doi: 10.1164/ajrccm.157.2.9611103. [DOI] [PubMed] [Google Scholar]
- 18.Larsen R, Gozzelino R, Jeney V, Tokaji L, Bozza FA, Japiassu AM, Bonaparte D, Cavalcante MM, Chora A, Ferreira A, Marguti I, Cardoso S, Sepulveda N, Smith A, Soares MP. A central role for free heme in the pathogenesis of severe sepsis. Sci Transl Med. 2010;2:1–12. doi: 10.1126/scitranslmed.3001118. [DOI] [PubMed] [Google Scholar]
- 19.Ware LB, Fessel JP, May AK, Roberts II LJ. Plasma biomarkers of oxidant stress and development of organ failure in severe sepsis. Shock. 2011;36:12–17. doi: 10.1097/SHK.0b013e318217025a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ouellet M, Percival MD. Mechanism of acetaminophen inhibition of cyclooxygenase isoforms. Arch Biochem Biophys. 2001;387:273–280. doi: 10.1006/abbi.2000.2232. [DOI] [PubMed] [Google Scholar]
- 21.Boutaud O, Moore KP, Reeder BJ, Harry D, Howie AJ, Wang S, Carney CK, Masterson TS, Amin T, Wright DW, Wilson MT, Oates JA, Roberts LJ., II Acetaminophen inhibits hemoprotein-catalyzed lipid peroxidation and attenuates rhabdomyolysis-induced renal failure. Proc Nat Acad Sci. 2010;107:2699–2704. doi: 10.1073/pnas.0910174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Siew ED, Ikizler TA, Gebretsadik T, et al. Elevated urinary IL-18 levels at the time of ICU admission predict adverse clinical outcomes. Clin J Am Soc Nephrol. 2010;5:1497–1505. doi: 10.2215/CJN.09061209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM consensus conference committee. Chest. 1992;101:1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 24.Milne GL, Yin H, Brooks JD, et al. Quantification of F2-isoprostanes in biological fluids and tissues as a measure of oxidant stress. Methods Enzymol. 2007;433:113–126. doi: 10.1016/S0076-6879(07)33006-1. [DOI] [PubMed] [Google Scholar]
- 25.Hogg N, Rice-Evans C, Darley-Usmar V, et al. The role of lipid hydroperoxides in the myoglobin-dependent oxidation of LDL. Arch Biochem Biophys. 1994;314:39–44. doi: 10.1006/abbi.1994.1409. [DOI] [PubMed] [Google Scholar]
- 26.Grisham MB. Myoglobin-catalyzed hydrogen peroxide dependent arachidonic acid peroxidation. J Free Radic Biol Med. 1985;1:227–232. doi: 10.1016/0748-5514(85)90122-9. [DOI] [PubMed] [Google Scholar]
- 27.Boutard O, Aronoff DM, Richardson JH, et al. Determinants of the cellular specificity of acetaminophen as an inhibitor of prostaglandin H(2) synthases. Proc Natl Acad Sci. 2002;99:7130–7135. doi: 10.1073/pnas.102588199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee BH, Inui D, Suh GY, et al. Association of body temperature and antipyretic treatments with mortality of critically ill patients with and without sepsis: a multi-center prospective observational study. Crit Care. 2012;16:R33. doi: 10.1186/cc11211. [DOI] [PMC free article] [PubMed] [Google Scholar]