Abstract
Background
HIV-1-infected individuals with plasma RNA <50 copies/mL on antiretroviral therapy (ART) may have residual, low-level viremia detectable by PCR assays which can detect a single copy of viral RNA (single-copy assay, SCA). The clinical predictors of residual viremia in patients on long-term suppressive ART are incompletely understood.
Methods
We evaluated factors associated with residual viremia in patients on suppressive ART who underwent screening for a raltegravir intensification trial (ACTG A5244). The screened population was HIV-1-infected adults receiving ART for ≥12 months with pre-ART HIV-1 RNA >100,000 copies/mL and on-therapy RNA levels below detection limits of commercial assays for ≥6 months.
Results
Of 103 patients eligible for analysis, the median age was 46 years and the median duration of viral suppression was 4.8 years. Sixty-two percent had detectable viremia (>0.2 copies/mL) by SCA (median 0.2 copies/mL; quartile [Q] 1, Q3 [<0.2, 1.8]). Younger patients had lower HIV-1 RNA levels than older individuals (r=0.27, p=0.005). Patients with virologic suppression on ART for 2 years or less had higher residual viremia than those with suppression for more than 2 years (median 2.3 vs. 0.2 copies/mL, p=0.016).
Conclusions
Among HIV-1-infected patients with pre-ART HIV-1 RNA >100,000 copies/mL, residual viremia was detectable in the majority (62%) despite many years of suppressive ART. Higher level viremia was associated with older age and less than 2 years of virologic suppression on ART. These findings should help in selection of candidates for clinical trials of interventions designed to eliminate residual viremia.
Keywords: HIV-1, Single-copy assay, residual viremia
Introduction
Antiretroviral therapy (ART) prevents HIV-1-related complications by blocking viral replication [1]. Current antiretroviral regimens are highly efficacious in suppressing viral replication and reducing morbidity and mortality [2–3]. However, cure of HIV-1 infection is not currently achievable because of long-lived viral reservoirs that persist despite ART. To design effective strategies to eradicate HIV-1, a more detailed understanding of persistent viral reservoirs is needed. Using PCR assays that can detect a single copy of HIV-1 RNA (single-copy assay, SCA), low-level viremia is detectable in many patients whose plasma HIV-1 RNA is suppressed on ART to below the detection limits of standard commercial assays [4–5]. This low-level viremia may represent ongoing replication or release of virus from long-lived cellular reservoirs, such as resting memory CD4 cells [6–7], and likely other, as yet undefined, sources [8]. Identifying clinical characteristics associated with the level of residual viremia may provide further insight into the host and virologic mechanisms involved in HIV-1 persistence.
AIDS Clinical Trial Group (ACTG) study A5244 was a randomized controlled trial of raltegravir intensification in patients receiving suppressive ART. The primary outcome of this trial, as previously reported [9], is that raltegravir intensification did not reduce residual viremia (plasma HIV-1 RNA) measured by SCA. Using data from patients who screened for enrollment into A5244, we now report the findings of analyses to identify predictors of residual viremia in HIV-1-infected individuals on suppressive ART.
Methods
Study Population
The A5244 trial design and study population have been previously described (NCT#00515827) [9]. The main inclusion criteria to enter A5244 were: 1) HIV-1 infected adults receiving ART for ≥12 months with ≥2 nucleoside reverse transcriptase inhibitors (NRTI) and a ritonavir-boosted protease inhibitor (PI) or a non-nucleoside reverse transcriptase inhibitor (NNRTI); 2) plasma HIV-1 RNA levels below detection limits for ≥6 months using commercial assays; 3) CD4 cell count ≥ 200/mm3; 4) pre-ART HIV-1 RNA >100,000 copies/mL; and 5) detectable HIV-1 RNA by SCA. All screened patients who had SCA results, whether or not viremia was detected, and who met eligibility criteria 1–4 above, were included in the current analyses. Data were cross-sectional and collected at the patients’ screening visit. All patients provided written informed consent for the collection of samples and subsequent analyses.
Single Copy Assay Methods
Plasma samples from patients were tested with a single copy HIV-1 RNA assay which can detect one RNA molecule per RT-PCR reaction [10]. Controls containing 0 copies and plasma from HIV-1 uninfected individuals were consistently negative. Detectable SCA is defined as HIV-1 RNA above the lower limit of quantification. In this assay, the quantification limit is determined by the volume of plasma assayed. For example, if a single copy of HIV-1 RNA is detected in an 8 mL plasma sample, the quantity of HIV-1 RNA is 0.2 copies/ml (note, 54.5% of the plasma sample is assayed for HIV-1 RNA and the remainder is used for controls). The volume of plasma assayed ranged from 4–10 mL; as previously described [9], 97% of patients had ≥7 mL of plasma assayed and 75% had ≥10 mL of plasma assayed.
Statistical Methods
Associations between screening SCA results and specific variables from screening visits were assessed using rank-based (Spearman) correlations. Continuous variables were dichotomized at the median (age and pre-treatment HIV-1 RNA), a commonly used threshold (screening CD4 cell count ≤ vs. > 500 cells/mm3), or a threshold based on prior findings (years of virologic suppression ≤ vs. > 2 years was chosen because the HIV-1 RNA decay rate slows after 2–3 years of therapy [5])--and were compared using Wilcoxon rank-sum test. Analysis of a particular factor after adjustment for other factors was done using stratified Wilcoxon rank-sum tests. Binary outcomes were compared using Fisher’s exact test. Undetectable screening SCA measurements were assigned an HIV-1 RNA value of ½ the detection limit when analyzed as a continuous variable. All statistical tests were two-sided, exploratory without adjusting for multiple comparisons, and at nominal 0.05 level of significance.
Results
One hundred and twenty-five patients screened for the A5244 study and had a SCA result; 22 of these patients were excluded from this analysis because they failed to meet A5244 entry criteria. The reasons for exclusion were no documented pre-ART HIV-1 RNA measurement or pre-ART HIV-1 RNA ≤100,000 copies/mL (n=12), missing documentation of ≥6 months virologic suppression (n=4), CD4 cell count <200/mm3 or no CD4 cell count (n=4), and ineligible ART regimen (n=2). We examined predictors of screening plasma HIV-1 RNA level in the remaining 103 patients.
The median age of the 103 individuals was 46 years and 89% were male. Sixty percent were white non-Hispanic, 25% were black non-Hispanic, and 13% were Hispanic. Sixty-five percent of the patients were on an NNRTI-containing regimen and 35% were on a PI-containing regimen. The median (quartile [Q] 1, Q3) screening CD4 cell count was 582 (474, 795)/mm3and the median duration on suppressive ART (time since the first HIV-1 RNA below detection limit by commercial assay) was 4.8 (3.1, 6.4) years (N=99, 4 patients did not provide this information). Forty-two (41%) patients had a pre-ART HIV-1 RNA between 100,000–249,999 copies/mL, 23 (22%) were between 250,000 and 499,999 copies/mL, 13 (13%) were between 500,000 – 749,999 copies/mL, 16 (16%) were ≥750,000 copies/mL, and 9 (9%) were greater than the upper limits of quantification by assays with different maximum cutoffs (4 with >500,000 copies/mL and 5 with >100,000 copies/mL). The median pre-ART HIV-1 RNA among the 94 patients with quantified HIV-1 RNA was 5.5 (5.2, 5.8) log10 copies/mL.
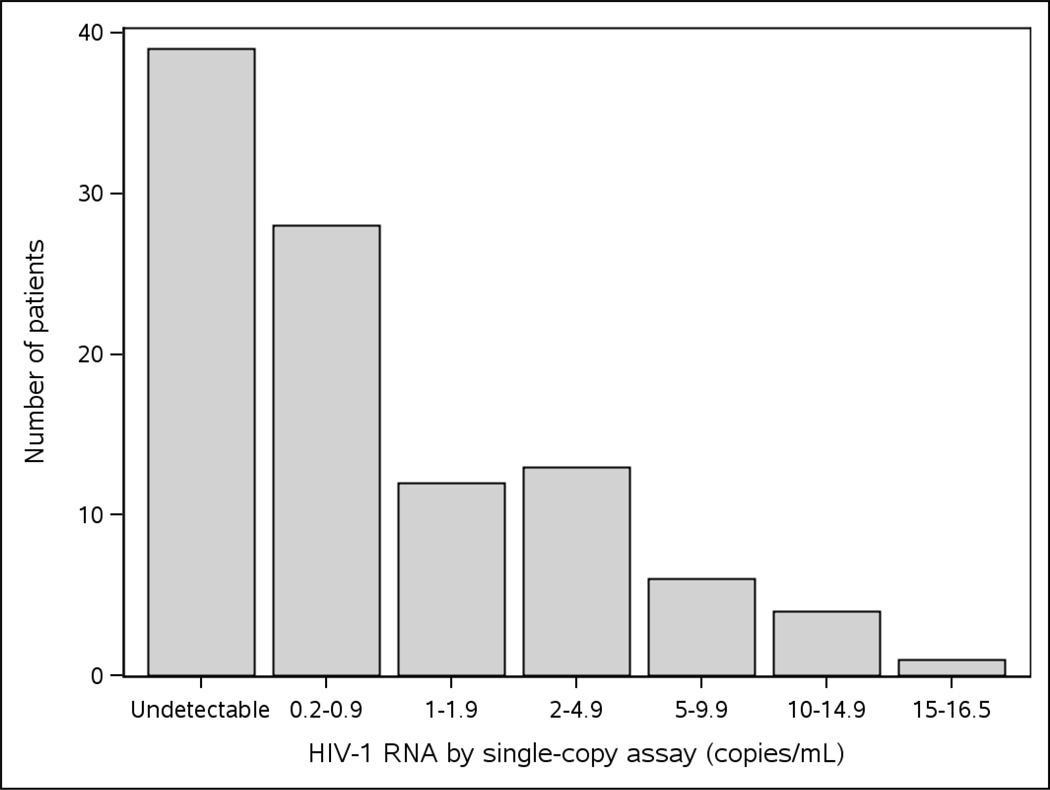
Sixty-four (62%) of the 103 patients had a detectable HIV-1 RNA level by SCA (>0.2 copies/mL), and 39 (38%) patients had an undetectable level. The median HIV-1 RNA by SCA was 0.2 (<0.2, 1.8) copies/mL. Among the 64 patients with a detectable HIV-1 RNA, 28 (44%) had <1 copy/mL and 36 (56%) had ≥1 copy/mL ranging up to 16.4 copies/mL [Figure 1]. Among the 39 patients with an undetectable SCA, 38 patients had ≥8 mL of plasma tested and, therefore, had < 0.2 copies/mL; 1 patient had 4 mL of plasma tested and, therefore, had < 0.4 copies/mL.
Figure 1.
Frequency of screening HIV-1 RNA by single-copy assay
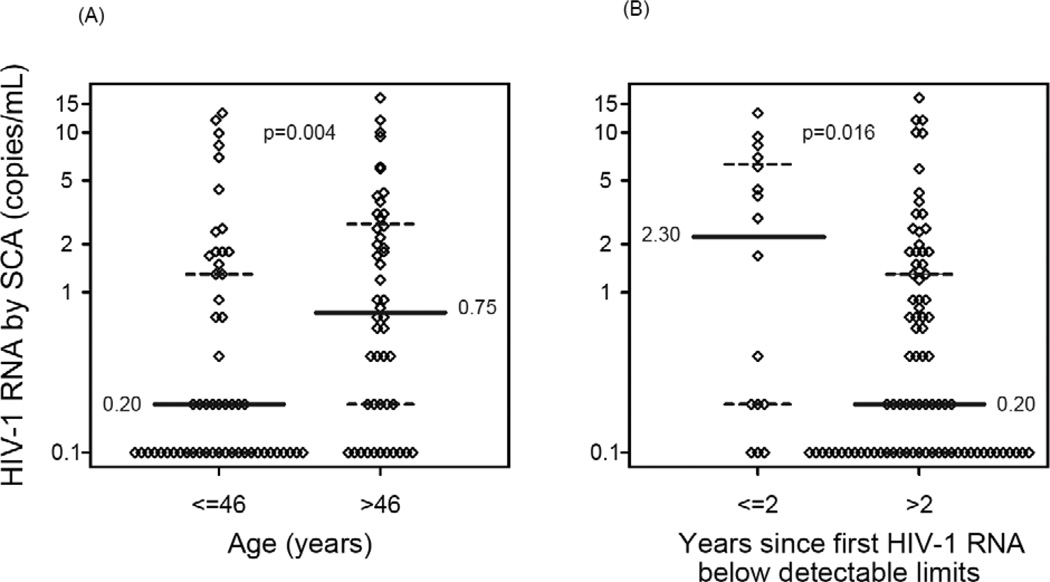
Younger patients had lower HIV-1 RNA values than older individuals (r =0.27, p=0.005, Spearman rank correlation). A smaller proportion of patients ≤46 years of age had a detectable HIV-1 RNA than those >46 years of age (27/55 [49%] vs. 37/48 [77%], p=0.004). The median HIV-1 RNA in patients ≤46 years of age was 0.20 copies/mL whereas the median in those >46 years was 0.75 copies/mL (p=0.004) (Figure 2A). The association between age and HIV-1 RNA level remained significant (p=0.033) after adjustment for screening CD4 cell count, duration of virologic suppression and pre-ART HIV-1 RNA level.
Figure 2. Baseline predictors of HIV-1 RNA by single-copy assay (SCA).
(A) Age (≤ 46, > 46 years) versus HIV-1 RNA by SCA (median age of the study population for this analysis was 46 years). (B) Years (≤ 2 vs. >2) since first HIV-1 RNA below detectable limits by commercial assays versus HIV-1 RNA by SCA. When the SCA measurement was below the detection limit, a value of 1/2 of the detection limit was imputed. The solid horizontal line indicates the median SCA value; the dashed lines indicate 25th and 75th percentile. (The 25th percentile was 0.1 copies/mL for the group with age ≤46 years old and the group with >2 years since first HIV-1 RNA below detectable limits by commercial assays).
In addition, among patients who had ≤2 years of virologic suppression, 75% (12/16) had a detectable screening SCA HIV-1 RNA, whereas among those with more than two years of virologic suppression, 58% (48/83) had a detectable HIV-1 RNA (p=0.27). Patients with ≤ 2 years of virologic suppression had significantly higher HIV-1 RNA levels than those who had suppressed for more than two years (median 2.3 vs. 0.2 copies/mL, p=0.016) (Figure 2B); this association remained significant after adjusting for age (p=0.015), screening CD4 cell count (p=0.021), and pre-ART HIV-1 RNA level (p=0.016). When duration of virologic suppression was considered as a continuous covariate, no association was apparent (r=−0.04, p=0.70).
We found no association between HIV-1 RNA level by SCA and the following factors: sex, race, background regimen (PI vs. NNRTI), or screening CD4 cell count (all p≥0.20). Pre-ART HIV-1 RNA was not associated with HIV-1 RNA by SCA (p=0.58), but all patients in the analysis had a pre-ART HIV-1 RNA >100,000 copies/mL.
A sensitivity analysis in which data from all patients for whom screening SCA results were available regardless of inclusion/exclusion criteria (n=125) gave similar results. Moreover, the precision of the SCA HIV-1 RNA values below 1 copy/mL did not substantially affect our conclusions: if HIV-1 RNA values below 1 copy/mL were all assigned a value of 0.5 copies/mL, our conclusions did not change.
Discussion
In this study of 103 HIV-1-infected patients with a median duration of virologic suppression of almost 5 years, 62% had detectable HIV-1 RNA in plasma by the single-copy assay. However, a substantial minority of patients had levels below the limit of detection and the overall median HIV-1 RNA level was quite low (0.2 copies/mL) despite all patients having pre-ART plasma HIV-1 RNA >100,000 copies/mL. On average, plasma HIV-1 RNA was reduced by 500,000-fold from pre-ART levels. This finding underscores the effectiveness of current ART regimens in suppressing viral replication. Whether similar viral suppression is achieved in other anatomical sites is unresolved [11–13]. In addition, a critical unanswered question is whether the level of residual viremia is related to the size of latent or productive HIV-1 reservoirs.
In our analysis of predictors of residual viremia, we found that SCA values were higher in those who had 2 years or less of suppressive therapy compared with those who had been suppressed for >2 years. This finding is consistent with known phases of HIV-1 RNA decay after ART initiation: following the rapid first and second phases, there is a slower third phase of decay, which has a half-life of 39 weeks, followed by a fourth phase in which there is essentially no further decrease in HIV-1 RNA level [5]. Studies that seek to assess the effect of an intervention on residual viremia should focus on patients who have had virologic suppression for at least >2 years. Trials that include patients with shorter durations of suppression may observe third phase declines in HIV-1 RNA that are unrelated to the intervention; if there is not a control group, such declines could be misinterpreted.
The association of higher level residual viremia with older age is more difficult to explain and was not observed in one previous study [4]. Older individuals may have higher levels of immune activation [14] that might drive release of HIV-1 from the latent T cell or tissue reservoirs. Although we did not observe an association between age and T cell activation in the randomized portion of the A5244 study [9], the sample size in the randomized study was smaller (n = 53) and may have not been adequate to detect a modest relationship. Alternatively, older age may be associated with a less effective immune response to HIV-1 and, therefore, less efficient suppression or clearance of cells that are producing HIV-1. The relations among age, immune activation and function, and HIV-1 reservoirs should be studied further.
We did not find an association between pre-ART HIV-1 RNA level and residual viremia, although a clear limitation of our study is that all patients in the analysis had a pre-ART HIV-1 RNA >100,000 copies/mL. This inclusion criterion was chosen for A5244 because residual viremia is more likely to be detected in individuals with higher pre-treatment HIV-1 RNA levels [4]. The range of our pre-ART HIV-1 RNA levels was only 10-fold and having a broader range of pre-ART RNA levels and a larger sample size may reveal an association between pre-ART HIV-1 RNA and residual viremia level as was reported in another study [4]. Another limitation of our study is that, even though it is one of the largest populations investigated to date with the single-copy assay, the moderate sample size does not allow us to detect factors that have a small effect on residual viremia. We also do not have information on adherence or drug levels prior to or at the time of screening nor do we have data on HIV-1 RNA levels in non-blood compartments.
In summary, we find that residual viremia persists in most HIV-1 infected patients on long-term suppressive ART and that the level of this residual viremia is higher in older patients and those who have had ≤2 years of virologic suppression. Studies of interventions to eliminate residual viremia should focus on patients who have reached a steady-state level of residual viremia, which requires at least 2 years of suppressive ART. A better understanding of the relations between residual viremia, age, immune activation, immune function, and tissue reservoirs in patients on long-term suppressive therapy is needed to develop effective strategies to reduce or eliminate long-lived HIV-1 reservoirs— a high priority for HIV-1 research.
Acknowledgements
We would like to thank all the members of the ACTG A5244 team, including Randi Leavitt, John Coffin, Sarah Palmer, Carla Pettinelli, Ana Martinez, Lisa M. Demeter, Jeffrey M Jacobson, Richard D’Aquila, Barbara Philpotts, Betty A. Donoval, and Robert Levaro. We would like to express our special appreciation to Ann Wiegand at the National Institutes of Health for performing the single-copy assays, to Beatrice Kallungal for her exceptional work as the clinical trials specialist, to Jennifer Janik for her excellent work as the study data manager, and to Heather Sprenger and Amy Jennings for their outstanding work on coordinating the laboratory data for the study.
Financial Disclosure (sources of funding)
The project was supported by Award Number U01AI068636 from the National Institute of Allergy and Infectious Diseases. The project was also supported by a grant from the National Institute of Allergy and Infectious Diseases to the Statistical and Data Analysis Center (AI 06834). RTG is supported by NIH R01 AI066992-04A1 and NIH G08LM008830-01 and by grants to the AIDS Clinical Trials Group (NIH U01 AI 694722) and the Harvard University Center for AIDS Research (NIH 2P30 AI060354-06).
Footnotes
Disclosure statement
The results of this analysis were previously presented at the XVII International AIDS Conference, Mexico City, Mexico, August 3–8, 2008.
Conflict of interest: JWM is a consultant for Gilead Sciences and RFS Pharma and owns shares in RFS Pharma. JJE is a consultant for Merck, GSK/ViiV, Gilead, Tibotec and Bristol Myers Squibb. He also receives research support from Tobira and GSK/ViiV through the University of North Carolina.
References
- 1.Murray JS, Elashoff MR, Iacono-Connors LC, Cvetkovich TA, Struble KA. The use of plasma HIV RNA as a study endpoint in efficacy trials of antiretroviral drugs. AIDS. 1999;13(7):797–804. doi: 10.1097/00002030-199905070-00008. [DOI] [PubMed] [Google Scholar]
- 2.Walensky R, Paltiel A, Losina E, et al. The Survival Benefits of AIDS Treatment in the United States. J Infect Dis. 2006;194(1):11–19. doi: 10.1086/505147. [DOI] [PubMed] [Google Scholar]
- 3.Daar ES, Tierney C, Fischl MA, et al. Atazanavir plus ritonavir or efavirenz as part of a 3-drug regimen for initial treatment of HIV-1. Ann Intern Med. 2011;154(7):445–456. doi: 10.1059/0003-4819-154-7-201104050-00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maldarelli F, Palmer S, King MS, et al. ART suppresses plasma HIV-1 RNA to a stable set point predicted by pretherapy viremia. PLoS Pathog. 2007;3(4):e46. doi: 10.1371/journal.ppat.0030046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palmer S, Maldarelli F, Wiegand A, et al. Low-level viremia persists for at least 7 years in patients on suppressive antiretroviral therapy. Proc Natl Acad Sci USA. 2008;105(10):3879–3884. doi: 10.1073/pnas.0800050105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haggerty CM, Pitt E, Siliciano RF. The latent reservoir for HIV-1 in resting CD4+ T cells and other viral reservoirs during chronic infection: insights from treatment and treatment-interruption trials. Curr Opin HIV AIDS. 2006;1(1):62–68. doi: 10.1097/01.COH.0000191897.78309.70. [DOI] [PubMed] [Google Scholar]
- 7.Anderson JA, Archin NM, Ince W, et al. Clonal sequences recovered from plasma from patients with residual HIV-1 viremia and on intensified antiretroviral therapy are identical to replicating viral RNAs recovered from circulating resting CD4+ T cells. J Virol. 2011;85(10):5220–5223. doi: 10.1128/JVI.00284-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brennan TP, Woods JO, Sedaghat AR, Siliciano JD, Siliciano RF, Wilke CO. Analysis of human immunodeficiency virus type 1 viremia and provirus in resting CD4+ T cells reveals a novel source of residual viremia in patients on antiretroviral therapy. J Virol. 2009;83(17):8470–8481. doi: 10.1128/JVI.02568-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gandhi RT, Zheng L, Bosch RJ, et al. The effect of raltegravir intensification on low-level residual viremia in HIV-infected patients on antiretroviral therapy: a randomized controlled trial. PLoS Med. 2010;7(8) doi: 10.1371/journal.pmed.1000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palmer S, Wiegand AP, Maldarelli F, et al. New real-time reverse transcriptase-initiated PCR assay with single-copy sensitivity for human immunodeficiency virus type 1 RNA in plasma. J Clin Microbiol. 2003;41(10):4531–4536. doi: 10.1128/JCM.41.10.4531-4536.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chun TW, Nickle DC, Justement JS, et al. Persistence of HIV in gut-associated lymphoid tissue despite long-term antiretroviral therapy. J Infect Dis. 2008;197(5):714–720. doi: 10.1086/527324. [DOI] [PubMed] [Google Scholar]
- 12.Yukl SA, Shergill AK, McQuaid K, et al. Effect of raltegravir-containing intensification on HIV burden and T-cell activation in multiple gut sites of HIV-positive adults on suppressive antiretroviral therapy. AIDS. 2010;24:2451–2460. doi: 10.1097/QAD.0b013e32833ef7bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yukl SA, Gianella S, Sinclair E, et al. Differences in HIV burden and immune activation within the gut of HIV-positive patients receiving suppressive antiretroviral therapy. J Infect Dis. 2010;202(10):1553–1561. doi: 10.1086/656722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Appay V, Sauce D. Immune activation and inflammation in HIV-1 infection: causes and consequences. J Pathol. 2008;214:231–241. doi: 10.1002/path.2276. [DOI] [PubMed] [Google Scholar]