Abstract
BACKGROUND
Mitochondria are the powerhouse of mammalian cells and the main source of reactive oxygen species (ROS) associated with oxygen consumption. In addition, they also play a strategic role in controlling the fate of cells through regulation of death pathways. Mitochondrial ROS production fulfills a signaling role through regulation of redox pathways, but also contributes to mitochondrial damage in a number of pathological states.
SCOPE OF REVIEW
Mitochondria are exposed to the constant generation of oxidant species, and yet the organelle remains functional due to the existence of an armamentarium of antioxidant defense systems aimed to repair oxidative damage, of which mitochondrial glutathione (mGSH) is of particular relevance. Thus, the aim of the review is to cover the regulation of mGSH and its role in disease.
MAJOR CONCLUSIONS
Cumulating evidence over recent years has demonstrated the essential role for mGSH in mitochondrial physiology and disease. Despite its high concentration in the mitochondrial matrix, mitochondria lack the enzymes to synthesize GSH de novo, so that mGSH originates from cytosolic GSH via transport through specific mitochondrial carriers, which exhibit sensitivity to membrane dynamics. Depletion of mGSH sensitizes cells to stimuli leading to oxidative stress such as TNF, hypoxia or amyloid β-peptide, thereby contributing to disease pathogenesis.
GENERAL SIGNIFICANCE
Understanding the regulation of mGSH may provide novel insights to disease pathogenesis and toxicity and the opportunity to design therapeutic targets of intervention in cell death susceptibility and disease.
INTRODUCTION
Despite its exclusive synthesis in the cytosol, GSH is distributed in intracellular organelles, including endoplasmic reticulum (ER), nucleus and mitochondria. The compartmentalization of GSH in separate redox pools is critical to control compartment-specific needs and functions [1, 2]. In the nucleus, GSH maintains critical protein sulphydryls that are necessary for DNA repair and expression [3] and functions also as a hydrogen donor in ribonucleotide reductase-catalysed reduction of ribonucleotides to deoxyribonucleotides, thus playing a contributory role in DNA synthesis[4]. Intracellularly GSH is predominantly found in its reduced form except in the ER, where it exists mainly as oxidized glutathione (GSSG), GSSG being the main source of oxidizing equivalents to provide the adequate environment necessary for disulphide bond formation and proper folding of nascent proteins [5]. In mitochondria, however, GSH is mainly found in reduced form and represents a minor fraction of the total GSH pool (10–15%). Considering the volume of the mitochondrial matrix, the concentration of mitochondrial GSH (mGSH) is similar to that of cytosol (10–14 mM) [1, 2, 6, 7]. Mitochondria are an excellent example of subcellular organelles whose function is closely linked to maintenance of redox balance. The mitochondria are the primary intracellular site of oxygen consumption and the major source of reactive oxygen species (ROS), most of them originating from the mitochondrial respiratory chain. Associated with this constant flow of ROS generation mitochondria are a target for the damaging effects of oxygen radicals [8–10]. Although normal electron transport in mitochondria involves four-electron reduction of molecular oxygen to water, partial reduction reactions occur even under physiological conditions, causing release of superoxide anion (O·2−) and hydrogen peroxide. In accordance with this, it has been estimated that the steady-state concentration of O·2− in the mitochondrial matrix is five- to tenfold higher than in the cytosol [11].
In addition to the ROS generated under physiological settings, toxic or pathological conditions that lead to an impairment of mitochondrial function can increase the release of ROS. Therefore, although mitochondria are exposed to the constant generation of oxidant species, the organelle remains functional due to the existence of an antioxidant defense system, of which mGSH is a critical component, aimed to prevent or repair oxidative damage generated during normal aerobic metabolism. This review summarizes current knowledge on the physiology and function of mGSH and its role in cell death regulation and pathological states.
1. Mitochondrial Oxidative Stress and defense
The primary function of mitochondria is to transduce oxygen consumption in the electron transport chain (ETC) into energy required for myriad cell functions. Although the process is highly efficient, a small fraction of electrons are transferred directly to molecular oxygen, resulting in the generation of O2·−, which can give rise to other potent ROS as well as reactive nitrogen species (RNS). Therefore, a fine equilibrium between ROS production and removal will determine the physiological vs. pathological function of ROS. Mitochondria contain an arsenal of antioxidant systems with target specificity [1].
Superoxide dismutase
The first line of defense against ROS is guaranteed by the presence of Mn2+-SOD (SOD2) in the mitochondrial matrix, which results in superoxide anion dismutation and the subsequent generation of hydrogen peroxide. The relevance of this strategy is illustrated by the fact that global SOD2 deficiency leads to neonatal death in mice. In turn, control of hydrogen peroxide is achieved by the GSH redox system and other defenses such as glutaredoxins and thioredoxins, as depicted in Figure 1. Hydrogen peroxide can arise through sources other than via superoxide anion dismutation by SOD-2. For instance, p66Shc is a cytoplasmic protein involved in signaling from tyrosine kinases to Ras, which translocates to mitochondria in response to stress contributing to cell death and aging. It has been shown that p66Shc directly stimulates hydrogen peroxide generation, without inhibiting mitochondrial respiration, by transferring electrons to cytochrome c [12].
Figure 1. Mitochondrial antioxidant defense system.
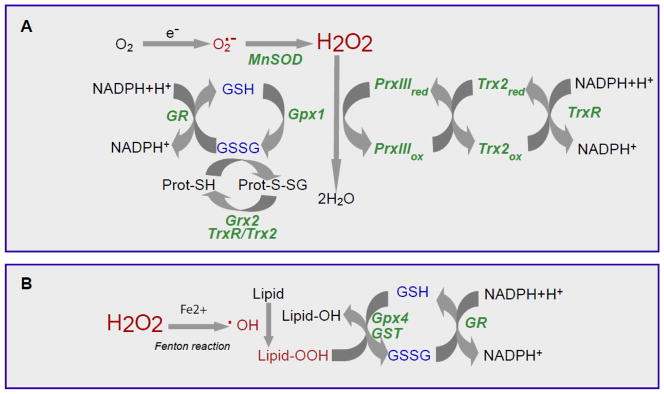
Scheme of the different reactions that take place in the mitochondria to cope with the oxidative stress derived from the presence of superoxide anion, hydrogen peroxide, and hydroxyl radical. A, Removal of H2O2, and B, elimination of hydroxyl radical generated through the Fenton reaction. GSH peroxidase (Gpx), GSSG-reductase (GR); GSSG, glutaredoxin (Grx), Mn-dependent superoxide dismutase (MnSOD), thioredoxin-2 (Trx2), Trx-Reductase (TrxR), peroxiredoxin III (PrxIII).
Glutathione Redox Cycle
Although hydrogen peroxide is not a free radical, it is an oxidant and an intermediate in the chain of reactions that generate reactive free radicals, such as hydroxyl radical, which can oxidize mitochondrial components (proteins, lipids, DNA). Since most mitochondria lack catalase, perhaps with the demonstrated exception of rat heart mitochondria [13], the metabolism of hydrogen peroxide is mainly accomplished by GSH, with the participation of either GSH peroxidase or peroxiredoxins. Associated with this function GSH becomes oxidized to GSSG, which is reduced back to GSH by the NADPH-dependent GSSG reductase (GR), as shown in Figure 1. Among GSH peroxidases (Gpx) that detoxify hydrogen peroxide, Gpx1 is the major isoform localized mainly in the cytosol, with a small fraction also present in the mitochondrial matrix [2, 14]. Some potent electrophiles, especially those generated as a consequence of metabolic processes involving both endogenous compounds and xenobiotics, can be readily removed by GSH via catalysis by glutathione transferases (GSTs). GSTs are distributed in mitochondria (GSTA1), cytosol (alpha, mu, pi, and zeta) and membrane-bound (MGST1) isoforms [15, 16]. Mitochondrial GSTs display both glutathione transferase and peroxidase activities that detoxify harmful byproducts through GSH conjugation or GSH-mediated peroxide reduction [15, 17]. Among human mitochondrial GSTs, the isoforms hGSTA4-4, hGSTA1, hGSTA2, and hGSTP1 showed peroxidase activity, with hGSTA4-4 exhibiting the highest activity [18, 19].
mGSH is also the primary defense against oxidative damage to mitochondrial membranes by insuring the reduction of hydroperoxides present on phospholipids and other lipid peroxides. These modified lipids are detoxified by mGSH through the actions of mitochondrial GSTs with modest Se-independent Gpx activity, as well as specific GSH peroxidases, such as Gpx4 which displays high preference for lipid hydroperoxides (Figure 1). Actually, due to its capacity to reduce hydroperoxide groups on phospholipids, cholesteryl esters and lipoproteins, Gpx4 is considered a critical defense enzyme in protecting membranes against oxidative stress. Gpx4 is synthesized in three forms that arise from the same Gpx4 gene displaying different translation initiation sites. A short form of Gpx4 is present in somatic tissue mitochondria and is essential for survival and protection against apoptosis in mice, whereas a long form has been shown to be important for male fertility [20–22]. Experiments using cell lines overexpressing Gpx4 have shown its critical role in reducing oxidative stress-mediated toxicity [23]. Interestingly, recent reports also suggest that Gpx4 plays a role in the protection against apoptosis and in maintenance of oxidative phosphorylation complexes in gut epithelial cells [24], by a mechanism involving the protein Apoptosis Inducing Factor (AIF). In line with this, TNFα induced ROS formation, phospholipid peroxidation, mitochondrial damage, and apoptotic death in Jurkat cells, was prevented upon ectopic GPx4 expression [25]. Conversely, GPx4 siRNA knockdown enhanced phospholipid peroxidation, increasing TNFα-dependent apoptosis[25]. Moreover, due to its inner membrane location in mitochondria and its ability to repair cardiolipin peroxidation, Gpx4 differentially regulates apoptogenic protein from mitochondria [20]. The importance of Gpx4 is highlighted by the fact that while Gpx1 knockout mice are phenotypically normal and exhibit an expected lifespan, Gpx4 knockout mice die during early embryonic development [26] and Gpx4+/− cell lines are markedly sensitive to inducers of oxidative stress, including gamma-irradiation, tert-butyl-hydroperoxide, and hydrogen peroxide, as compared to cell lines derived from wild-type control littermates [14]. Additionally, Gpx4 has also been suggested to play an important role in life expectancy in mice [26, 27].
For both regulatory and antioxidant roles, it is important for protein thiol redox states to respond rapidly to changes in the GSH/GSSG ratio. Thiol-disulfide exchange between GSSG and a protein thiol or between a glutathionylated protein and GSH is often relatively slow [28]. A potential catalyst for thiol-disulfide exchange is the small, soluble protein glutaredoxin (Grx) [28, 29]. Grx catalyzes the deglutathionylation of protein-glutathione mixed disulfides far more effectively than thioredoxin (Trx) or protein-disulfide isomerase [30]. Two known Grx isoforms are found in mammals, Grx1 and Grx2. Grx1 is of cytosolic expression, and Grx2 localizes to both the mitochondrial matrix and nucleus [31]. The mitochondrial isoform Grx2, discovered in early 2000, is a mature protein of about 15 kDa [29, 31, 32] and displays an interesting characteristic of being reactivated directly by thioredoxin reductase (TrxR) as well as by GSH [33, 34] and is able to fully operate in more oxidatively stressed mitochondrial environment. Therefore, Grx2 is a candidate for catalyzing the interplay of the mitochondrial GSH pool with protein thiols during both antioxidant defense and redox signaling [29].
Thioredoxins
Regarding the thioredoxin (Trx) system in mammals, both Trx and TrxR are expressed as isoforms for both predominantly cytosolic (Trx1 and TrxR1) or mitochondrial (Trx2 and TrxR2) localization [34]. There are direct links between the Trx system and protein glutathionylation [35]; and the direct reduction of mitochondrial Grx2 by TrxR is also of important physiological relevance [33, 34]. However, the reduction of the intermolecular disulfide is specific to Trx and can not be achieved by GSH or glutaredoxin. Mitochondria-specific Trx and TrxR, can interact also with the peroxiredoxins (Prxs), which constitute a novel family of thiol-specific peroxidases that rely on Trx as the hydrogen donor for the reduction of hydrogen peroxides and lipid-hydroperoxides [36].
Peroxiredoxins
One Prx isoform, Prx3, is exclusively detected in mitochondria, suggesting that Prx3 together with Trx2 and TrxR2 provide a primary line of defense against H2O2 produced by the mitochondrial respiratory chain. Prx3 homodimer has a redox-sensitive cysteine that upon reaction with hydrogen peroxide is oxidized to Cys-SOH, which then reacts with neighboring Cys-SH of the other subunit to form an intermolecular disulfide that can be readily reduced by Trx2, but not by Grx or GSH [10, 37]. The fact that the oxidation state of the active site cysteine of Prx can be transferred to other proteins that are less intrinsically susceptible to H2O2 also allows Prx to function as an H2O2 sensor. Similarly, Prx5 the last identified member of the six mammalian peroxiredoxins [38] is widely expressed in tissues but differs by its subcellular distribution. In human cells, it has been shown that Prx5 can be targeted to mitochondria, peroxisomes, the cytosol, and the nucleus. The targeting of Prx5 to mitochondria is highly conserved among species [39], and it has been associated with the protection of mtDNA from oxidative attacks [40]. Prx5 is a peroxidase that can use cytosolic or mitochondrial thioredoxins to reduce alkyl hydroperoxides or peroxynitrite with high rate constants, whereas its reaction with hydrogen peroxide is modest [38]. So far, Prx5 has been viewed mainly as a cytoprotective antioxidant enzyme rather than as a redox sensor. Thus, more than 10 years after its molecular cloning, mammalian Prx5 appears to be a unique peroxiredoxin exhibiting specific functional and structural features [38, 41].
An interesting issue centers on the relative importance of the mitochondrial GSH redox (mGSH/Gpx) vs PrxIII/Trx2 system on hydrogen peroxide defense. The relevance of GSH peroxidases, in particular the mitochondrial targeted Gpx4, in oxidative stress defense and hydrogen peroxide elimination is illustrated by the fact that Gpx4 knockout mice is embryonic lethal [26]. However, recent studies identified PrxIII as an essential line of defense against mitochondrial hydrogen peroxide [37], with the estimation by kinetic competition analysis that a large amount of hydrogen peroxide generated in mitochondria targets PrxIII [42]. In addition, selective depletion of mGSH has been shown to sensitize hepatocytes to oxidant cell death [2, 6, 8]. While differences in Km for hydrogen peroxide, catalytic efficiency and Kcat may account for the relative importance of mGSH/Gpx and PrxIII/Trx2 against hydrogen peroxide, differences in abundance and post-translational regulation of these antioxidant components in different type of cells may also play a role. For instance, studies addressing the role of PrxIII in oxidative stress defense have been performed in HeLa cells [37], while reporting the role of mGSH in oxidant cell death have been typically done in primary hepatocytes [2, 6, 8]. Although further specific work addressing these features is needed, current evidence has shown that both antioxidant systems (mGSH and PrxIII) are clearly interconnected. For instance, it has been described that depletion of mGSH results in Trx2 oxidation [43]. Similarly, in a recent study using hypercholesterolemic pigs they observed a selective depletion of mGSH in heart mitochondria, with the spare of cytosol GSH that was followed by profound decreases in the levels of the mitochondria-specific antioxidant enzymes such as MnSOD, Trx2, and PrxIII [44]. However, the influence of PrxIII on the GSH redox cycle status has not been addressed. Together, these data provide evidence for an essential role of the mitochondrial GSH redox cycle in maintaining a healthy antioxidant system, and consequently, on hydrogen peroxide homeostasis.
NADPH sources
Since the regeneration of GSSG or oxidized Trx is dependent on reducing equivalents, hence pathways that modulate NADPH may indirectly affect antioxidant efficiency. Several sources contribute to the regeneration of NADPH in mitochondria, including the NADP+-dependent isocitrate dehydrogenase (IDH2), malic enzyme, and the nicotinamide nucleotide transhydrogenase (Nnt) [45]. The redox level of mitochondrial NADP is normally more than 95% reduced, about half of which is uncoupler sensitive [46]. Thus, the uncoupler-sensitive part is consistent with Nnt-generated NADPH, even though contributions by other sources cannot be excluded. Moreover, the relevance of mitochondrial NADPH sources is highlighted by the observation that the diabetic characteristics of C57BL/6J mice, such as glucose intolerance and reduced insulin secretion, are primarily due to an impaired transhydrogenase activity [47]. In this context, it should be pointed out that transhydrogenase is strongly inhibited by fatty acyl CoA, especially long chain acyl CoA, which may further suppress Nnt activity in the presence of a high cellular content of fatty acids, typical of a pre-diabetic condition [46, 48]. On the other hand, IDH2 catalyzes the oxidative decarboxylation of isocitrate into 2-oxoglutarate generating NADPH or NADH from NADP+ or NAD+, respectively. IDH2 can be inactivated by peroxynitrite, as shown recently by alcohol feeding resulting in decreased NADPH equivalents and subsequent GSH defense [49]. In line with these observations, it has been reported that IDH2 silencing sensitizes tumor cells to apoptosis in response to various stimuli, including brefeldin A, anticancer drugs, tumor necrosis factor-α, doxorubicin, actinomycin D and etoposide [50, 51]. Whether this outcome is related directly to perturbation of NADPH equivalents or to the generation of oncometabolites such (R)-2-hydroxyglutarate and subsequent alterations in hypoxia signaling and DNA methylation remains to be established [52].
2. mGSH Transport
Despite the high GSH concentration existing in mitochondrial matrix, GSH is not synthesized de novo as mitochondria lack the enzymes required for the synthesis of GSH from its constituent aminoacids. Therefore, mitochondrial GSH arises from the cytosol GSH by the activity of specific carriers [7]. Furthermore, GSH has an overall negative charge at physiological pH and mitochondria exhibit a large negative membrane potential; consequently, although GSH can cross easily the mitochondrial outer membrane (MOM) through porin channels its transport into mitochondrial matrix cannot be explained by simple diffusion. Indeed, recent findings using dynamic oxidant recovery assays together with GSH-specific fluorescent reporters, determined the free communication of GSH pools between cytosol and the intermitochondrial space (IMS). In contrast, no appreciable communication was observed between the GSH pools of the IMS and matrix [53].
mGSH transport in kidney
Previously, two mitochondrial inner membrane (MIM) anion carriers were identified in kidney and liver as GSH transporters, the 2-oxoglutarate carrier (OGC; Slc25a11) and the dicarboxylate carrier (DIC; Slc25a10) [54–57]. These antiport carriers, OGC and DIC, import cytosolic GSH into mitochondria in exchange for 2-oxoglutarate (2-OG) and inorganic phosphate (Pi2−), respectively [2]. In reference to the presence of mGSH carriers in kidney, Lash et al. [58] showed that overexpression of the rat kidney DIC carrier in a cell line derived from rat kidney proximal tubules (NRK-52E) increased the mGSH pool size 2- to 10-fold with respect to wild-type NRK-52E cells, thus demonstrating a role for this carrier in the mitochondrial transport of GSH in exchange with inorganic phosphate. Moreover, evidence for a role of the OGC carrier as a GSH transporter was based on substrate specificity and pattern of inhibition in rabbit kidney mitochondria and in a partially purified preparation of mitochondrial transporters from kidney mitoplasts reconstituted in proteoliposomes [54, 55].
mGSH transport in liver
Additionally, the functional expression of the hepatic OGC in Xenopus laevis, demonstrated transport of reduced GSH in a phenylsuccinate-sensitive manner [56], further confirming the suggestion that the OGC contributes to the transport of GSH in liver mitochondria. In contrast to OGC, no clear evidence for DIC in the transport of mitochondrial GSH was found in rat liver [56]. For instance, the functional expression in oocytes microinjected with the DIC cRNA from rat liver did not result in significant GSH transport activity [56]. A key insight from this study was the fact that the activity of OGC was sensitive to altered membrane physical properties, in line with what has been reported for the mitochondrial transport of GSH [2]. The initial uptake rate of 2-oxoglutarate was reduced in mitochondria from alcohol-fed rat livers, an effect that was not accompanied by an alcohol-induced decrease in the OGC messenger RNA levels but rather by changes in mitochondrial membrane dynamics induced by alcohol. The fluidization of mitochondria by the fatty acid derivative A2C, a fluidizing molecule, restored the initial transport rate of both GSH and 2-oxoglutarate. Two components have been described for mGSH transport in rat liver mitochondria, at external GSH levels of less than 1 mM, GSH is transported into the mitochondrial matrix by a high-affinity low-capacity component (Km, approximately 60 microM; Vmax, approximately 0.5 nmol/min per mg of protein), which is saturated at levels of 1–2 mM and stimulated by ATP, and a low-affinity high capacity (Km, approximately 5.4 mM; Vmax, approximately 5.9 nmol/min per mg of protein) and is stimulated by ATP and inhibited by glutamate and protonophores that collapse the protonmotive force [59]. Consistent with these findings in liver mitochondria, the transport of GSH into rat kidney mitochondria required ATP production, as oligomycin in the presence of ATP inhibited GSH uptake [54], indicating the dependence of the mitochondrial transport of GSH on the protonmotive force. However, ATP did not stimulate the transport of GSH in kidney mitochondria from fed rats [54]. This discrepancy with respect to previous observations in rat liver mitochondria may relate to the energized state of mitochondria (fasted vs. fed).
Kinetic analyses of 2-oxoglutarate transport in rat liver mitochondria indicated the presence of a single Michaelis-Menten component with kinetic parameters in the range of those reported previously for kidney mitochondria [54–56]. This feature is in contrast to the 2 components for mitochondrial GSH transport [2, 7, 59]. The kinetic parameter of the low-affinity component of GSH transport and that of 2-oxoglutarate are similar, both showing a Michaelis constant in the mmol/L range and a similar maximum velocity [56]. These findings suggest that the OGC accounts for the low-affinity high capacity of GSH transport in liver mitochondria, and imply that the nature of the high affinity GSH transporter remains to be identified. However, whether these carriers also participate in the efflux of mGSH in exchange for cytosolic GSH is unknown. In line with this, intriguing findings indicated that inhibition of OGC or DIC by phenylsuccinate or butylmalonate in kidney HRK523 cells resulted in increased rather than decreased mGSH levels, suggesting the possibility for OGC/DIC to mediate GSH efflux from mitochondria [60]. Moreover, as opposed to release of GSSG from cells, recent studies argue that GSSG does not efflux out of mitochondria, implying that under oxidant conditions, the accumulation of GSSG contributes to mitochondrial protein glutathionylation and subsequent impaired function [61]. In addition, since regeneration of GSH in mitochondria is dependent on reduced environment and NADPH availability, mGSH is highly dependent on the respiratory substrates used to produce NADPH [61], and on the availability of the reduced nucleotide cofactor.
mGSH transport in brain
However little is known about mGSH transport in organs different from liver and kidney. In brain, some studies have examined the mechanisms responsible for mitochondrial GSH transport. In one report, the properties of GSH transport in isolated rat brain mitochondria seemed to be greatly different from those reported previously for mitochondria from kidney, and influenced most by inhibitors of the tricarboxylate carrier rather than OGC or DIC [62]. However, in mouse brain mitochondria another study showed that OGC and DIC are both expressed in cortical neurons and astrocytes [63]. In addition, butylmalonate, an inhibitor of DIC, significantly decreased mGSH, suggesting DIC as the major GSH transporter in mouse cerebral cortical mitochondria [63]. Moreover, a role for UCP2 in the transport of mitochondrial GSH has been described in neurons, suggesting that the transport of protons back into the matrix by UCP2 may favor the movement of GSH [64]. These studies suggest that, in the brain, multiple MIM anion transporters might be involved in mGSH transport, however, essentially nothing is known about how the function of these carriers is regulated. An interesting recent study, suggested the participation of Bcl-2 as a binding partner for mGSH, consequently enhancing its affinity for the OGC [46]. In this context, the binding of recombinant Bcl-2 to GSH in vitro, was antagonized by the Bcl-2 homology-3 domain (BH3) mimetics HA14-1 and compound 6, as well as the BH3-only protein, Bim [65]. Bcl-2 and OGC appear to act in a coordinated manner to increase the mGSH pool and to enhance the resistance of neurons to mitochondrial oxidative stress. Therefore, the antioxidant-like function attributed to Bcl-2 could in part depend on its potential to regulate the cellular glutathione status [57].
Unlike 2-OG and DIC carriers, the evidence for these alternative carriers and regulators in the transport of mGSH in hepatic liver mitochondria remains to be established. A key feature of the transport of GSH through OGC is its strict dependence on appropriate membrane dynamics, which is determined by fatty acid composition and the cholesterol/phospholipid molar ratio [56, 66]. Cholesterol is a critical component of membranes with key structural and functional roles [67]. Compared to plasma membrane, mitochondria are much less enriched in cholesterol with estimates of about 3–5% of the total cholesterol cell load [68]. This restricted pool of cholesterol in mitochondria, however, plays a fundamental physiological role in the synthesis of bile acids in hepatocytes or steroidogenic hormones in specialized tissues [69]. Given the role of cholesterol in the regulation of membrane dynamics and physical properties, the synthesis and supply of membrane cholesterol is highly regulated under physiological conditions. Cholesterol is known to regulate membrane organization [55]. In this regard, it has been shown that cholesterol loading in mitochondria results in increased membrane order parameter which negatively impacts specific membrane carriers, such as the GSH transport system without effect in others, including the S-adenosyl-L-methionine transport system [70]. Functional expression studies in Xenopus laevis oocytes provided clear evidence that the OGC is highly sensitive to mitochondrial membrane fluidity loss induced by cholesterol loading [56]. Thus, one of the functional consequences of mitochondrial cholesterol enrichment is the impairment of mGSH transport which results in depletion of GSH in the mitochondrial matrix. This selective pool of GSH has been shown to play a key role in controlling cell susceptibility to oxidative stress, Ca2+ overload, TNF/Fas, and hypoxia [71–76], and its depletion has been shown to contribute to a number of pathologies, some of which will be covered below [2, 8, 77–80].
3. mGSH and Cell Death
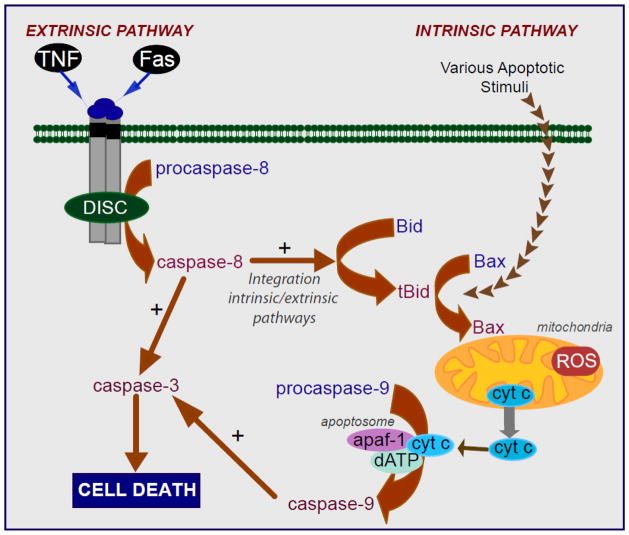
Mitochondria play a central role in various forms of cell death, which are characterized by differential biochemical features, with predominant forms including apoptosis (caspase-dependent and independent), or necrosis. Besides amplifying and mediating extrinsic apoptotic pathways, mitochondria also play a central role in the integration and propagation of death signals originating from inside the cell such as DNA damage, oxidative stress, starvation, as well as those induced by radiation or chemotherapy [81–85]. Apoptosis, also known as programmed cell death, describes a particular mode of cell death that is characterized by a series of biochemical events that lead to a variety of morphological changes, including cell shrinkage, membrane blebbing or budding, nuclear fragmentation, chromatin condensation, and chromosomal DNA fragmentation. Ultimately, the cell is fragmented into compact membrane-enclosed structures, called “apoptotic bodies” which contain cytosol, condensed chromatin, and organelles. Apoptotic bodies are ingested by neighboring cells and phagocytes, preventing inflammation and tissue damage that might ensue upon cell lysis. Apoptosis is induced via two main routes involving either the mitochondria (the intrinsic pathway) or the activation of death receptors (the extrinsic pathway) (Figure 2). Both pathways converge to induce the activation of caspases, the final executioners of cell death [86–88]. In the extrinsic pathway, or “death receptor” pathway, ligand binding results in the formation of a multiprotein complex named death-inducing signaling complex (DISC) and the activation of procaspase-8 [86, 87]. Active caspase-8 then processes downstream effector caspases, such as caspase-3, which subsequently cleave specific substrates resulting in cell death. Cells harboring the capacity to induce such direct and mainly caspase-dependent apoptosis pathways are called type I cells. However, in type II cells, the signal generated from the activated receptor does not results in strong caspase activation to execute cell death. In this case, the signal needs to be amplified via mitochondria-dependent apoptotic pathways. The link between the caspase signaling cascade and the mitochondria is provided by the Bcl-2 family member Bid. Bid is cleaved by caspase-8 and in its truncated form (tBID) translocates to the mitochondria where it induces the translocation, oligomerization, and insertion of other Bcl2-family members such as Bax and/or Bak into the MOM [89], resulting in the integration of the extrinsic and intrinsic pathways, the latter also known as the mitochondrial pathway (Figure 2). This is followed by mitochondrial outer membrane permeabilization (MOMP) and release of several proteins from the mitochondrial intermembrane space, including cytochrome c. Once cytochrome c is released it binds to monomeric Apaf-1 which then, in a dATP-dependent conformational change, oligomerizes to assemble the apoptosome, a complex that triggers the activation of the initiator procaspase-9. Activated caspase-9 subsequently initiates a caspase cascade involving downstream effector caspases, such as caspase-3, ultimately resulting in the execution of apoptosis [90]. Similarly, during the execution of the intrinsic or mitochondrial pathway death signals originating from inside the cell, such as DNA damage, oxidative stress, starvation, as well as those induced by chemotherapeutic drugs, converge in the mitochondria at the level of translocation of Bax/Bak into the outer mitochondrial membrane, triggering MOMP and the consequent release of cytochrome c to the cytosol committing the cell to apoptosis (Figure 2) [82, 83]. In addition to cytochrome c, other proteins from IMS are released to assist or potentiate apoptosis. For instance, Smac/Diablo antagonizes inhibitors of caspases, thereby enhancing caspase activation and apoptosis. Through mitochondria to nuclei translocation, AIF insures caspase-independent mitochondria-mediated apoptosis inducing chromatin condensation and DNA fragmentation [91].
Figure 2. Intrinsic and extrinsic apoptotic pathways.
Intrinsic, or mitochondrial pathways, and extrinsic, or death receptor pathway, converge to a common pathway involving the activation of caspase-3 by the apoptosome culminating in cell death execution. In type II cells, the extrinsic and intrinsic pathways are integrated through the cleavage of Bid by caspase-8 consequently linking mitochondria to the control of cell death.
As opposed to apoptosis, necrosis is a morphologically distinct form of cell death responsible for irreversible tissue destruction due to bioenergetic failure and oxidative damage. The fundamental difference to apoptosis is the rapid loss of cellular membrane potentials due to energy depletion and ion pump/channel failures, leading to swelling, rupture and cytolysis. Rather than being a passive event, necrosis has emerged as a controlled cell death that induces an inflammatory response to stimulate tissue repair by releasing specific factors from dying cells such as HMGB1 and HDGF [92]. Necrosis is dependent on the mitochondrial permeability transition pore (mPT), a channel complex that spans outer and inner mitochondrial membranes at points of contact [93]. mPT is a regulated nonselective water and soluble-passing protein complex whose molecular identity is still debated. It can encompass voltage-dependent anion channel (VDAC) across the MOM and adenine nucleotide translocase (ANT) across the MIM [91, 94]. In contrast to VDAC and ANT, the prolyl isomerase cyclophilin D in the mitochondrial matrix is an essential regulator of mPT and the only genetically proven indispensable mPT component [95–98]. Upon oxidative stress, sudden mPT causes massive ion influx that dissipates mitochondrial membrane potential and shuts down oxidative phosphorylation, ATP production, and ROS overgeneration. Concomitantly, water influx causes matrix swelling, rupture of the rigid MOM and release of apoptogenic proteins sequestered in IMS. Although cytochrome c is released, apoptotic cell death under mPT is inhibited due to energetic failure and ATP exhaustion and oxidative stress-mediated caspase inactivation. Thus, although mitochondria are is the strategic center in the control of cell death, the outcome of MOMP leading to apoptosis or necrosis is determined by the nature and intensity of stimuli and whether mPT is engage [99]. Thus, the mPT induces cell death, and depending on the level of cellular ATP, as ATP is required for the efficient assembly of the apoptosome and/or the extent of ROS generation, apoptosis and/or necrosis will ensue [83, 91, 100–102]. Consequently, understanding the mechanisms leading to MOMP is essential for designing strategies to control cell death.
In this regard, although the field has mainly focused on the role of Bcl-2 family members in the regulation of MOMP and cell death [91, 100, 103], an increasing body of evidence points to a key role of lipids in the regulation of MOMP, cytochrome c release and cell death susceptibility [104]. Recent studies, reported a key role for sphingolipids, particularly sphingosine 1-phosphate and hexedecenal in the regulation of mitochondrial pathway of apoptosis induced by Bax/Bak [105]. Moreover, cardiolipin, an anionic phospholipid of the mitochondrial inner membrane, has emerged as a regulator of apoptosis by playing an important role in cytochrome c release from mitochondria. At physiological pH, cytochrome c is electrostatically bound with cardiolipin. Additionally, this binding is enhanced by the insertion of one chain of cardiolipin into a hydrophobic channel of cytochrome c, while the other cardiolipin acyl chains remain in the membrane, therefore anchoring cytochrome c to the mitochondrial membrane [68, 104, 106]. Hence, after apoptotic stimulus, ROS in mitochondria can oxidize cardiolipin, and oxidized cardiolipin has lower affinity for cytochrome c, resulting in cytochrome c detachment from the mitochondrial inner membrane (Figure 3) [68, 76, 106, 107]. In addition, it has been described that oxidized cardiolipin modulates the biophysical properties of MOMP to allow oligomerized Bax to insert and permeabilize the mitochondrial outer membrane [68, 76, 108]. Altogether, these results support the hypothesis that cardiolipin is a key upstream target in mitochondria-dependent apoptosis. Despite only a small fraction of cytochrome c being bound to cardiolipin (about 15%), the oxidation of this lipid is thought to impact the mobilization of cytochrome c from the mitochondria, although the relevance of this step in the release of cytochrome c to the cytosol is controversial. Since mitochondrial ROS contribute to cardiolipin oxidation and are controlled by antioxidants such as GSH [2, 76], mGSH arises as an important modulator of apoptotic cell death by indirectly controlling the redox state of cardiolipin [68, 76]. Accordingly, mGSH depletion due to alcohol-mediated alteration in mitochondrial membrane dynamics underlies the susceptibility of hepatocytes from alcohol-fed models to tumor necrosis factor (TNF) [109], and in nutritional and genetic models of hepatic steatosis [75, 110], mGSH depletion occurs due to the enrichment of mitochondria in free cholesterol, resulting in decreased mitochondrial membrane fluidity [75]. Of importance, this effect was reproduced after selectively depleting mGSH in “healthy” hepatocytes by means of using 3-hydroxy-4-pentenoate (HP) which undergoes mitochondrial biotransformation into a Michael electrophile followed by its conjugation with GSH in the mitochondrial matrix [111], thereby sensitizing them to the inflammatory cytokine TNF [6, 76, 112, 113]. Moreover, the susceptibility to TNF after mGSH depletion, and subsequent cardiolipin oxidation was observed despite unimpaired GPx, Trx2, and Prx3, thus highlighting the relevance of mGSH in cell protection under oxidative stress [76]. In addition, similar sensitization by mGSH depletion was observed in neurons and glia cell lines challenged with amyloid-beta mediated cell death [80]. In addition, in cerebellar granule neurons, while direct depletion of mitochondrial and cytoplasmic GSH resulted in increased generation of ROS, decrease of the mPT, rapid loss of mitochondrial function, and cell death without apoptotic features, these effects were not observed if mGSH was relatively maintained [114]. Additionally, in astrocytes, a selective loss of mGSH, as a result of treatment with ethacrynic acid, increased cell vulnerability to peroxynitrite and hydrogen peroxide whereas protection was afforded by prior elevation of the mGSH pool by GSH ethyl ester, thus providing new direct evidence for the importance of mGSH in protection against necrotic cell death induced by reactive oxygen and nitrogen species [115]. Overall, these studies in brain cells indicate that the role of mGSH in regulating cell death susceptibility is not tissue specific. mGSH not only regulates cell death susceptibility but the outcome of cell death, that is necrosis or apoptosis. In principle, thiol redox status regulate mPT and enhanced ROS generation can target critical cysteine residues in cyclophilin D, implying that mGSH depletion would favor mPT via redox pathways targeting mPT components. In addition, through modulation of cardiolipin redox state, mGSH can also regulate MOMP and hence the release of apoptogenic proteins.
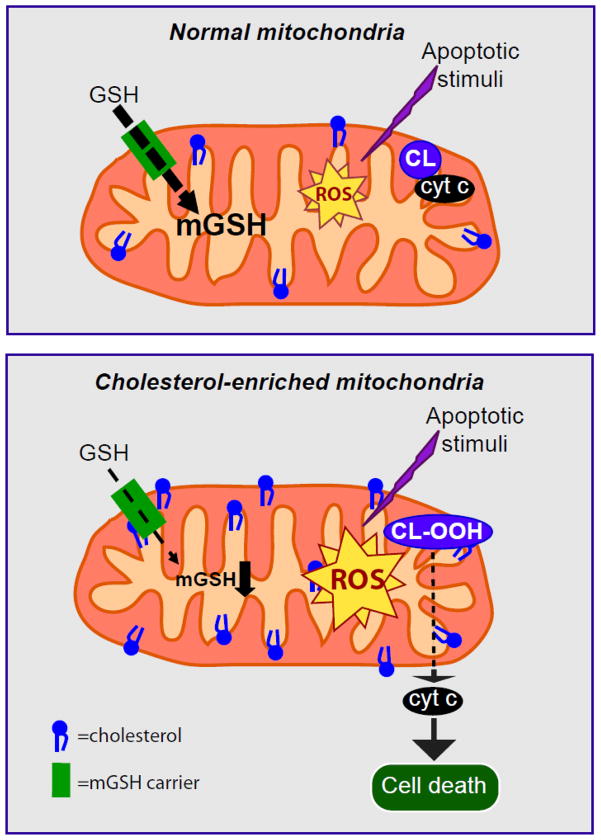
Figure 3. Effect of mitochondrial cholesterol deposition on mitochondrial control of cell death.
In healthy mitochondria, mGSH levels can cope with the ROS generated after an apoptotic stimulus. However, in cholesterol enriched mitochondria, the mGSH transport is concomitantly decreased resulting in mGSH depletion. mGSH levels below a certain threshold will compromise ROS detoxification leading to its accumulation resulting ultimately in cardiolipin oxidation and cell death.
mGSH depletion has also been associated to apoptosis or autophagy induced by chemotherapeutic drugs. For instance, the novel triterpenoid methyl-2-cyano-3,12-dioxooleana-1,9-diene-28-oate (CDDO-Me) potently induced cytotoxicity in imatinib-resistant myeloid leukemia cells. The low-dose effects of CDDO-Me were mainly associated with inhibition of mitochondrial oxygen consumption, whereas the cytotoxic effects appear to be mediated by a rapid and selective depletion of mGSH that accompanied the increased generation of ROS and mitochondrial dysfunction. Interestingly, the toxic effects of CDDO-Me were followed by rapid autophagocytosis of intracellular organelles or the externalization of phosphatidylserine in different cell types [116, 117], indicating that alterations in mitochondrial function by CDDO-Me can result in autophagy or apoptosis of chronic myeloid leukemia. These observations have also been extended to ovarian cancer cells [118]. Similarly, β-phenethyl isothiocyanate (PEITC), a natural product with potent anticancer activity against human leukemia cells, caused a rapid depletion of mitochondrial GSH and a significant elevation of ROS and nitric oxide, induced a disruption of the mitochondrial electron transport complex I, and a significant suppression of mitochondrial respiration that resulted in cytotoxicity in leukemia cells [119]. Taken collectively, these findings provide a basis for developing new therapeutic strategies to effectively cancer cells by using drugs that selectively deplete mGSH stores.
4. mGSH in pathology
Examples for the contribution of mGSH to different diseases have increased over the years. In many pathological settings mGSH depletion is both the consequence of disease progression and the cause of organ failure, and in most cases related to cholesterol-mediated changes in membrane dynamics. Indeed, mitochondrial cholesterol has emerged as an important modulator of MOMP in response to apoptotic stimuli underlying the importance of this lipid in disease pathogenesis, including alcoholic and nonalcoholic steatohepatitis, Alzheimer’s disease or hepatocellular carcinoma [68, 69, 75, 120] by regulating mGSH transport, mGSH homeostasis and subsequent protection of cardiolipin from peroxidation (Figure 3).
In the following sections we will briefly cover the best characterized examples of the role of mGSH depletion in disease pathogenesis.
Neurological disorders
The brain is particularly vulnerable to oxidative damage because of its high oxygen utilization, content of oxidizable polyunsaturated fatty acids, and presence of redox-active metals (Cu, Fe). Oxidative stress increases with age and therefore it can be considered as an important causative factor in several neurodegenerative disorders, such as Alzheimer disease (AD), Parkinson disease (PD) or Huntington disease (HD). The pathogenesis of AD is not clear and two main theories have been put forward, including the accumulation of toxic amyloid plaques and the aberrant phosphorylation of tau proteins. Although evidence has been gathered in favor of both, a recent study provides strong support for the amyloidogenic theory of AD [121]. A rare genetic variant identified in Scandinavians resulting in lower amyloidogenic processing of amyloid β-precursor protein (APP) defended elderly population against AD [121]. The brains of Alzheimer disease patients show a significant extent of oxidative damage associated with marked accumulation of amyloid-β-peptide (Aβ), the main constituent of senile plaques in brain [122, 123]. Epidemiological, genetic and biochemical studies have identified cholesterol, Aβ and mitochondria as key factors in AD pathogenesis. However, the putative participation of cholesterol in AD has been limited to the amyloidogenic processing of APP at the plasma membrane, particularly in lipid rafts where APP is processed by β-secretase 1 to generate Aβ. Of interest, current evidence indicates that targeting of Aβ to intracellular sites, most notably mitochondria, causes oxidative stress, mitochondrial dysfunction and cell death [124, 125]. Not only Aβ may cause mitochondrial ROS production, but also it is becoming clear that oxidative damage occurs early in the AD brain, even before the onset of significant plaque pathology [126]. Recent findings tested the hypothesis that mitochondrial cholesterol modulated Aβ neurotoxicity and AD pathology [68]. Using genetic models of cholesterol loading, it was found that mitochondrial cholesterol sensitized neurons to Aβ in vitro, while intracerebroventricular Aβ infusion resulted in neuroinflammation and neurotoxicity. Isolated mitochondria from brain or cortical neurons of transgenic mice overexpressing SREBP-2 (sterol regulatory element binding protein 2) or NPC1 (Niemann-Pick type C1) knock-out mice exhibited mitochondrial cholesterol accumulation, mGSH depletion and increased susceptibility to Aβ-induced oxidative stress and release of apoptogenic proteins. Similar findings were observed in pharmacologically GSH-restricted rat brain mitochondria, while selective mGSH depletion sensitized human neuronal and glial cell lines to Aβ-mediated cell death [80]. Two lines of evidence indicate that the mGSH depletion specifically accounted for the increased susceptibility to Aβ observed in these genetic models. First, the sensitivity of rat brain mitochondria to Aβ-mediated oxidative stress and release of intermembrane apoptotic proteins was dependent on the levels of mGSH. Second, selective pharmacological depletion of mGSH with the sparing of cytosol GSH in human neuroblastoma and glial cells sensitized to Aβ-mediated cell death. Moreover, in vivo intracerebroventricular human Aβ delivery colocalized with mitochondria resulting in oxidative stress, neuroinflammation and neuronal damage that were enhanced in Tg-SREBP-2 mice and prevented upon mGSH recovery by GSH ethyl ester coinfusion [80]. Hence these findings expand the role of cholesterol and mGSH depletion in Aβ neurotoxicity.
In addition, significant biological changes, related to oxidative stress, have been found in brain tissue of individuals affected by PD. In particular, data from postmortem studies of brains from patients with PD suggest that oxidative stress plays a role in neural degeneration of the pigmented dopaminergic neurons in the substantia nigra. Moreover, the normal metabolism of dopamine can generate free radicals and other ROS. Furthermore, in the human SNpc the autooxidation of dopamine leads to neuromelanin and can generate quinone and semiquinone species and ROS [127, 128]. According to postmortem studies, GSH levels in the SNpc of PD patients are remarkably lower than those of healthy subjects (60% compared to control subjects) while oxidized glutathione (GSSG) levels are slightly increased. GSH loss in PD is also accompanied by a reduction in mitochondrial complex I activity, which is regionally selective for the substantia nigra in PD and does not occur in related basal ganglia degenerative disorders. Consistent with these findings, we have shown that selective mGSH depletion mice sensitized to in vivo injection of 3-nitropropionic acid (3-NP), a mitochondrial toxin used extensively as a model of HD [129]. 3-NP delivery into the striatum of caveolin-1 knockout mice resulted in extensive areas of degeneration and cell death compared to wild type mice. Moreover, following occlusion of the middle cerebral artery in rat brain, early and selective loss of mGSH has been reported [130]. The mGSH depletion showed an apparent association with the tissue damage that developed during subsequent reperfusion, suggesting that it could be an important determinant of susceptibility to cell loss. In fact, brain infusion of GSH-monoethyl ester (GSH-EE) increased cellular GSH and was particularly effective in replenishing the mitochondrial pool, and reduced infarct size from 46% of the total ischaemic hemisphere in saline-treated animals to 16% following GSH-EE treatment, thus providing neuroprotection following transient focal cerebral ischaemia [131]. Thus, these findings illustrate the relevance in searching for optimal means to deliver GSH precursors and prodrugs to the central nervous system as a strategy for neurodegeneration [132]. However, an additional challenge in this aim is to satisfactorily reach mitochondria to increase the mGSH pool despite impaired transport due to loss of membrane physical properties.
Liver diseases
The implication of oxidative stress in liver diseases has been well characterized, playing a role in diseases such as viral hepatitis, metabolic liver diseases, ischemia/reperfusion injury and drug toxicity. Steatohepatitis, however, is one of the best characterized examples where mGSH depletion plays an important role in disease pathogenesis. Steatohepatitis represents an advanced stage in the spectrum of fatty liver disease that encompasses alcoholic (ASH) and non-alcoholic steatohepatitis (NASH), two of the most common forms of liver disease worldwide [112, 133]. Although the primary etiology of ASH and NASH is different, the pathology of ASH is remarkably similar to that of NASH, suggesting some common pathogenic mechanism. These two diseases have almost identical histology characterized by steatosis (macrovesicular > microvesicular), mixed lobular inflammation with scattered polymorphonuclear leukocytes as well as mononuclear cells and hepatocellular cell death due to sensitivity to oxidative stress [112, 133–136]. The pathogenesis of ASH and NASH is currently unknown. A prevalent view according to the two hit hypothesis is that fat infiltration in hepatocytes sensitizes to secondary hits, promoting hepatocellular death, inflammation and fibrosis reflecting disease progression. However, we postulated that the type rather than the amount of fat plays a critical role in the transition from steatosis to ASH/NASH. In line with this hypothesis, previous studies have shown that chronic alcohol feeding in various models results in the depletion of mGSH due to cholesterol loading in mitochondria [6, 109, 110, 112, 137, 138] and that strategies aimed to correct the loss of mitochondrial membrane fluidity restore the mitochondrial transport of GSH and replenish the mGSH pool in alcohol-fed models. This is of relevance in TNF susceptibility as mGSH depletion primes mitochondria to TNF-mediated mitochondrial outer membrane permeabilization by Bax through cardiolipin peroxidation [76]. The mechanisms of alcohol-induced lipogenesis involved endoplasmic reticulum (ER) stress, which triggers the activation of transcription factors SREBP-1c and SREBP-2, resulting in de novo synthesis of fatty acids and cholesterol [133]. Altered alcohol-induced ER stress, in turn, involved perturbed methionine metabolism and subsequent hyperhomocysteinemia [139]. Indeed, supplementing alcohol-fed mice with betaine prevented hyperhomocysteinemia and subsequent ER stress [140]. In line with these findings, we observed that tauroursodeoxycholic acid, a chemical chaperone shown to prevent ER stress in ob/ob mice [141], restored mGSH pool in alcohol fed rats [137] and blocked alcohol-induced ER stress (Fernandez et al, unpublished data). Alcohol-induced mitochondrial cholesterol trafficking is mediated by alcohol-induced upregulation of StAR, the founding member of StAR family of a mitochondrial transporting cholesterol proteins, whose expression is regulated by ER stress (Fernandez et al, unpublished data).
Similarly, using nutritional and genetic models of hepatic steatosis, we have described that free cholesterol loading, but not free fatty acids or triglycerides, sensitizes to TNF- and Fas-induced steatohepatitis [75]. The mechanism involved cholesterol-mediated mGSH depletion as mGSH replenishment by S-adenosyl-L-methionine or GSH ethyl ester protected mice fed high cholesterol diet against LPS-induced liver injury. Although mGSH depletion has been observed in models and patients with NASH [142], other mitochondrial antioxidant defenses are also affected, including the activity of SOD2. This outcome contributes to increased superoxide anion and subsequent peroxynitrite levels that target mitochondrial proteins. Therefore, strategies such as the use of SOD mimetics aimed to improve SOD-2 activity may be of relevance in steatohepatitis. However, the use of SOD mimetics in the face of coexisting mGSH depletion leads to increased hydrogen peroxide, contributing to hepatocellular injury and inflammation in models of nutritional steatohepatitis [143]. These findings widen the concept of oxidative stress implying the importance of a delicate equilibrium between antioxidants to prevent accumulation of reactive species. In addition, the concept that oxidative stress does not necessarily reflect the unbalance between ROS and antioxidants helps explain the failure of antioxidant treatment so far in human liver diseases. Data and models described here could offer an explanation for the important discrepancy between the overwhelming evidence for an important role of ROS and the common clinical failure of trials.
Other pathological settings
In humans, caveolin-1 (CAV1) mutations result in lipodystrophy, cell transformation, and cancer. Moreover, CAV1 gene-disrupted mice exhibit cardiovascular diseases, diabetes, cancer, atherosclerosis, and pulmonary fibrosis. The mechanisms underlying these disparate effects are unknown, but there are a variety of mild phenotypes that link the loss of CAV function with unrelated diseases [144–146], which are likely explained by metabolic failure and oxidative stress [147]. Caveolae are distinctive invaginations of the plasma membrane with reduced fluidity, reflecting cholesterol accumulation, and are present in most mammalian cells [148, 149]. Caveolins (CAVs), integrated plasma membrane proteins, are essential components of caveolae, and complex signaling regulators with numerous partners and whose activity is highly dependent on cellular context [150, 151]. CAV-1 proteins bind cholesterol with high affinity [152], and CAV’s ability to move between these compartments might contribute to regulation of cholesterol fluxes and distributions within cells [148, 152, 153].
In a recent study, it has been uncovered that loss of CAV1 alters mitochondrial function, which may provide a single underlying mechanism for the myriad of pathologies related to CAV1 mutations [129]. Without CAV1, free cholesterol accumulates in mitochondrial membranes, increasing membrane viscosity and reducing efficiency of the respiratory chain and depleting mGSH [121]. Indeed, this mitochondrial dysfunction predisposes CAV1-deficient animals to mitochondrial-related diseases such as steatohepatitis and neurodegeneration. CAV1 deficiency impairs mitochondria by promoting an increased influx and accumulation of free cholesterol in mitochondrial membranes. Although in this particular study only the effect of the mitochondrial failure caused by CAV1 deficiency in liver, brain, and fibroblasts was addressed, naturally occurring CAV1 deficiencies in humans cause disease in other tissues as well [150]. In the lung, for instance, CAV1 is highly expressed and a recent study has been reported that CAV1 deletion results in major functional aberrations, suggesting that caveolin-1 may be crucial to lung homeostasis and development [154]. Furthermore, generation of mutant mice that under-express CAV1 results in severe functional distortion with phenotypes covering the entire spectrum of known lung diseases, including pulmonary hypertension, fibrosis, increased endothelial permeability, and immune defects [155]. The contribution, however, of mitochondrial cholesterol or mGSH in the lung pathogenesis in CAV1 deficient animals remains to be elucidated.
As described above, mitochondrial oxidative stress is at the heart of many pathological settings. Since lipophilic cations accumulate in mitochondria, the covalent attachment of a neutral bioactive compound to a lipophilic cation should lead to its selective delivery to mitochondria. In this regard, alkyl-triphenylphosphonium (TPP) cations are an excellent tool for the delivery of compounds to mitochondria as they preferentially accumulate (about hundredfold) within mitochondria in cells making it possible to deliver a wide range of mitochondria-targeted lipophilic TPP cations [156, 157]. Murphy and coworkers have extended this concept to develop a series of cationic antioxidants that include derivatives of the endogenous antioxidants ubiquinol (MitoQ), alpha-tocopherol (MitoVit E), and of the synthetic spin trap PBN (MitoPBN) [156]. These compounds have been found to block oxidative damage in isolated mitochondria and cells far more effectively than untargeted antioxidant analogs due to their concentration within mitochondria, and its oral administration leads to their accumulation in the brain, heart, muscle and liver [143, 156]. In fact, MitoQ has been used in a range of in vivo studies, in rats and mice, and in two phase II human trials demonstrating that it can be safely delivered to patients with promising results, lending further support that mitochondria-targeted antioxidants may be applicable to a wide range of human pathologies that involve mitochondrial oxidative damage [158].
5. Final Remarks
Mitochondria play an essential role in maintaining cells alive by providing the energy needed for multiple signaling cascades and functions. The consumption of molecular oxygen in the respiratory chain is not only the driving force for the ATP synthesis required for cell viability, but also the source of ROS that target mitochondrial and extramitochondrial processes. Mitochondrial GSH plays a critical role to control the damaging effects of mitochondrial generated ROS, and hence its regulation modulates cell death susceptibility and disease progression. In particular, understanding the regulation of mGSH transport may provide novel opportunities for treatment of liver diseases and neurodegeneration. In this regard, evidence has shown that mGSH depletion contributes to susceptibility of hepatocytes or neurons to stimuli that generate oxidative stress including inflammatory cytokines, hypoxia or amyloid β-peptide. Since a key feature of mGSH is that its import from cytosol depends on appropriate mitochondrial membrane physical properties, strategies aimed to increase mGSH by boosting cytosol GSH (e.g. NAC) may not be an optimal approach for steatohepatitis or Alzheimer disease, as it would result in mainly increasing cytosol GSH without replenishing mGSH levels. In this regard, targeting mitochondrial cholesterol trafficking or using membrane permeable GSH prodrugs may constitute an efficient approach to boost mGSH. Thus, these data illustrate the importance of GSH in mitochondria in cell death regulation and in disease progression, indicating that a better understanding of mGSH transport and regulation may have important implications in disease pathogenesis.
HIGHLIGHTS.
Role of mitochondrial GSH in oxidative stress and mitochondrial physiology.
Description and features of mitochondrial GSH transport carriers.
Role and mechanisms of mitochondrial GSH in cell death pathways.
Contribution of mitochondrial GSH in neurodegeneration and liver diseases.
Acknowledgments
The work was supported by grants: SAF2009-11417, SAF2010-15760, and SAF2011-23031 (Plan Nacional de I+D), Proyectos de Investigación en Salud PI10/02114 and PS09/00056 (Instituto de Salud Carlos III), P50-AA-11999 (Research Center for Liver and Pancreatic Diseases, US National Institute on Alcohol Abuse and Alcoholism) and by CIBEREHD from the Instituto de Salud Carlos III. We want to thank the valuable contributions of Drs. Anna Fernandez, Joan Montero, Francisco Caballero and Gorka Basañez to advance our knowledge on the regulation of mitochondrial GSH and role in disease.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mari M, Colell A, Morales A, von Montfort C, Garcia-Ruiz C, Fernandez-Checa JC. Redox control of liver function in health and disease. Antioxidants & redox signaling. 2010;12:1295–1331. doi: 10.1089/ars.2009.2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mari M, Morales A, Colell A, Garcia-Ruiz C, Fernandez-Checa JC. Mitochondrial glutathione, a key survival antioxidant. Antioxidants & redox signaling. 2009;11:2685–2700. doi: 10.1089/ars.2009.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. The international journal of biochemistry & cell biology. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Holmgren A. The function of thioredoxin and glutathione in deoxyribonucleic acid synthesis. Biochemical Society transactions. 1977;5:611–612. doi: 10.1042/bst0050611. [DOI] [PubMed] [Google Scholar]
- 5.Hwang C, Sinskey AJ, Lodish HF. Oxidized redox state of glutathione in the endoplasmic reticulum. Science. 1992;257:1496–1502. doi: 10.1126/science.1523409. [DOI] [PubMed] [Google Scholar]
- 6.Garcia-Ruiz C, Morales A, Ballesta A, Rodes J, Kaplowitz N, Fernandez-Checa JC. Effect of chronic ethanol feeding on glutathione and functional integrity of mitochondria in periportal and perivenous rat hepatocytes. The Journal of clinical investigation. 1994;94:193–201. doi: 10.1172/JCI117306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Griffith OW, Meister A. Origin and turnover of mitochondrial glutathione. Proceedings of the National Academy of Sciences of the United States of America. 1985;82:4668–4672. doi: 10.1073/pnas.82.14.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fernandez-Checa JC, Kaplowitz N. Hepatic mitochondrial glutathione: transport and role in disease and toxicity. Toxicology and applied pharmacology. 2005;204:263–273. doi: 10.1016/j.taap.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 9.Kaelin WG., Jr ROS: really involved in oxygen sensing. Cell metabolism. 2005;1:357–358. doi: 10.1016/j.cmet.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 10.Orrenius S, Gogvadze V, Zhivotovsky B. Mitochondrial oxidative stress: implications for cell death. Annual review of pharmacology and toxicology. 2007;47:143–183. doi: 10.1146/annurev.pharmtox.47.120505.105122. [DOI] [PubMed] [Google Scholar]
- 11.Cadenas E, Davies KJ. Mitochondrial free radical generation, oxidative stress, and aging. Free radical biology & medicine. 2000;29:222–230. doi: 10.1016/s0891-5849(00)00317-8. [DOI] [PubMed] [Google Scholar]
- 12.Giorgio M, Migliaccio E, Orsini F, Paolucci D, Moroni M, Contursi C, Pelliccia G, Luzi L, Minucci S, Marcaccio M, Pinton P, Rizzuto R, Bernardi P, Paolucci F, Pelicci PG. Electron transfer between cytochrome c and p66Shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell. 2005;122:221–233. doi: 10.1016/j.cell.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 13.Radi R, Turrens JF, Chang LY, Bush KM, Crapo JD, Freeman BA. Detection of catalase in rat heart mitochondria. The Journal of biological chemistry. 1991;266:22028–22034. [PubMed] [Google Scholar]
- 14.Legault J, Carrier C, Petrov P, Renard P, Remacle J, Mirault ME. Mitochondrial GPx1 decreases induced but not basal oxidative damage to mtDNA in T47D cells. Biochemical and biophysical research communications. 2000;272:416–422. doi: 10.1006/bbrc.2000.2800. [DOI] [PubMed] [Google Scholar]
- 15.Aniya Y, Imaizumi N. Mitochondrial glutathione transferases involving a new function for membrane permeability transition pore regulation. Drug metabolism reviews. 2011;43:292–299. doi: 10.3109/03602532.2011.552913. [DOI] [PubMed] [Google Scholar]
- 16.Li W, James MO, McKenzie SC, Calcutt NA, Liu C, Stacpoole PW. Mitochondrion as a novel site of dichloroacetate biotransformation by glutathione transferase zeta 1. The Journal of pharmacology and experimental therapeutics. 2011;336:87–94. doi: 10.1124/jpet.110.173195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hayes JD, Flanagan JU, Jowsey IR. Glutathione transferases. Annual review of pharmacology and toxicology. 2005;45:51–88. doi: 10.1146/annurev.pharmtox.45.120403.095857. [DOI] [PubMed] [Google Scholar]
- 18.Gardner JL, Gallagher EP. Development of a peptide antibody specific to human glutathione S-transferase alpha 4-4 (hGSTA4-4) reveals preferential localization in human liver mitochondria. Archives of biochemistry and biophysics. 2001;390:19–27. doi: 10.1006/abbi.2001.2352. [DOI] [PubMed] [Google Scholar]
- 19.Gallagher EP, Gardner JL, Barber DS. Several glutathione S-transferase isozymes that protect against oxidative injury are expressed in human liver mitochondria. Biochemical pharmacology. 2006;71:1619–1628. doi: 10.1016/j.bcp.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 20.Liang H, Ran Q, Jang YC, Holstein D, Lechleiter J, McDonald-Marsh T, Musatov A, Song W, Van Remmen H, Richardson A. Glutathione peroxidase 4 differentially regulates the release of apoptogenic proteins from mitochondria. Free radical biology & medicine. 2009;47:312–320. doi: 10.1016/j.freeradbiomed.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Imai H, Nakagawa Y. Biological significance of phospholipid hydroperoxide glutathione peroxidase (PHGPx, GPx4) in mammalian cells. Free radical biology & medicine. 2003;34:145–169. doi: 10.1016/s0891-5849(02)01197-8. [DOI] [PubMed] [Google Scholar]
- 22.Savaskan NE, Ufer C, Kuhn H, Borchert A. Molecular biology of glutathione peroxidase 4: from genomic structure to developmental expression and neural function. Biological chemistry. 2007;388:1007–1017. doi: 10.1515/BC.2007.126. [DOI] [PubMed] [Google Scholar]
- 23.Yagi K, Komura S, Kojima H, Sun Q, Nagata N, Ohishi N, Nishikimi M. Expression of human phospholipid hydroperoxide glutathione peroxidase gene for protection of host cells from lipid hydroperoxide-mediated injury. Biochemical and biophysical research communications. 1996;219:486–491. doi: 10.1006/bbrc.1996.0260. [DOI] [PubMed] [Google Scholar]
- 24.Cole-Ezea P, Swan D, Shanley D, Hesketh J. Glutathione peroxidase 4 has a major role in protecting mitochondria from oxidative damage and maintaining oxidative phosphorylation complexes in gut epithelial cells. Free radical biology & medicine. 2012 doi: 10.1016/j.freeradbiomed.2012.05.029. [DOI] [PubMed] [Google Scholar]
- 25.Latchoumycandane C, Marathe GK, Zhang R, McIntyre TM. Oxidatively Truncated Phospholipids Are Required Agents of Tumor Necrosis Factor alpha (TNFalpha)-induced Apoptosis. The Journal of biological chemistry. 2012;287:17693–17705. doi: 10.1074/jbc.M111.300012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muller FL, Lustgarten MS, Jang Y, Richardson A, Van Remmen H. Trends in oxidative aging theories. Free radical biology & medicine. 2007;43:477–503. doi: 10.1016/j.freeradbiomed.2007.03.034. [DOI] [PubMed] [Google Scholar]
- 27.Ran Q, Liang H, Ikeno Y, Qi W, Prolla TA, Roberts LJ, 2nd, Wolf N, Van Remmen H, Richardson A. Reduction in glutathione peroxidase 4 increases life span through increased sensitivity to apoptosis. The journals of gerontology. Series A, Biological sciences and medical sciences. 2007;62:932–942. doi: 10.1093/gerona/62.9.932. [DOI] [PubMed] [Google Scholar]
- 28.Beer SM, Taylor ER, Brown SE, Dahm CC, Costa NJ, Runswick MJ, Murphy MP. Glutaredoxin 2 catalyzes the reversible oxidation and glutathionylation of mitochondrial membrane thiol proteins: implications for mitochondrial redox regulation and antioxidant DEFENSE. The Journal of biological chemistry. 2004;279:47939–47951. doi: 10.1074/jbc.M408011200. [DOI] [PubMed] [Google Scholar]
- 29.Murphy MP. Mitochondrial thiols in antioxidant protection and redox signaling: distinct roles for glutathionylation and other thiol modifications. Antioxidants & redox signaling. 2012;16:476–495. doi: 10.1089/ars.2011.4289. [DOI] [PubMed] [Google Scholar]
- 30.Jung CH, Thomas JA. S-glutathiolated hepatocyte proteins and insulin disulfides as substrates for reduction by glutaredoxin, thioredoxin, protein disulfide isomerase, and glutathione. Archives of biochemistry and biophysics. 1996;335:61–72. doi: 10.1006/abbi.1996.0482. [DOI] [PubMed] [Google Scholar]
- 31.Lundberg M, Johansson C, Chandra J, Enoksson M, Jacobsson G, Ljung J, Johansson M, Holmgren A. Cloning and expression of a novel human glutaredoxin (Grx2) with mitochondrial and nuclear isoforms. The Journal of biological chemistry. 2001;276:26269–26275. doi: 10.1074/jbc.M011605200. [DOI] [PubMed] [Google Scholar]
- 32.Gladyshev VN, Liu A, Novoselov SV, Krysan K, Sun QA, Kryukov VM, Kryukov GV, Lou MF. Identification and characterization of a new mammalian glutaredoxin (thioltransferase), Grx2. The Journal of biological chemistry. 2001;276:30374–30380. doi: 10.1074/jbc.M100020200. [DOI] [PubMed] [Google Scholar]
- 33.Johansson C, Lillig CH, Holmgren A. Human mitochondrial glutaredoxin reduces S-glutathionylated proteins with high affinity accepting electrons from either glutathione or thioredoxin reductase. The Journal of biological chemistry. 2004;279:7537–7543. doi: 10.1074/jbc.M312719200. [DOI] [PubMed] [Google Scholar]
- 34.Enoksson M, Fernandes AP, Prast S, Lillig CH, Holmgren A, Orrenius S. Overexpression of glutaredoxin 2 attenuates apoptosis by preventing cytochrome c release. Biochemical and biophysical research communications. 2005;327:774–779. doi: 10.1016/j.bbrc.2004.12.067. [DOI] [PubMed] [Google Scholar]
- 35.Casagrande S, Bonetto V, Fratelli M, Gianazza E, Eberini I, Massignan T, Salmona M, Chang G, Holmgren A, Ghezzi P. Glutathionylation of human thioredoxin: a possible crosstalk between the glutathione and thioredoxin systems. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:9745–9749. doi: 10.1073/pnas.152168599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chae HZ, Kang SW, Rhee SG. Isoforms of mammalian peroxiredoxin that reduce peroxides in presence of thioredoxin. Methods in enzymology. 1999;300:219–226. doi: 10.1016/s0076-6879(99)00128-7. [DOI] [PubMed] [Google Scholar]
- 37.Chang TS, Cho CS, Park S, Yu S, Kang SW, Rhee SG. Peroxiredoxin III, a mitochondrion-specific peroxidase, regulates apoptotic signaling by mitochondria. The Journal of biological chemistry. 2004;279:41975–41984. doi: 10.1074/jbc.M407707200. [DOI] [PubMed] [Google Scholar]
- 38.Knoops B, Goemaere J, Van der Eecken V, Declercq JP. Peroxiredoxin 5: structure, mechanism, and function of the mammalian atypical 2-Cys peroxiredoxin. Antioxidants & redox signaling. 2011;15:817–829. doi: 10.1089/ars.2010.3584. [DOI] [PubMed] [Google Scholar]
- 39.Van der Eecken V, Clippe A, Van Veldhoven PP, Knoops B. Mitochondrial targeting of peroxiredoxin 5 is preserved from annelids to mammals but is absent in pig Sus scrofa domesticus. Mitochondrion. 2011;11:973–981. doi: 10.1016/j.mito.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 40.Banmeyer I, Marchand C, Clippe A, Knoops B. Human mitochondrial peroxiredoxin 5 protects from mitochondrial DNA damages induced by hydrogen peroxide. FEBS letters. 2005;579:2327–2333. doi: 10.1016/j.febslet.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 41.Zhu H, Santo A, Li Y. The antioxidant enzyme peroxiredoxin and its protective role in neurological disorders. Exp Biol Med (Maywood) 2012;237:143–149. doi: 10.1258/ebm.2011.011152. [DOI] [PubMed] [Google Scholar]
- 42.Cox AG, Winterbourn CC, Hampton MB. Mitochondrial peroxiredoxin involvement in antioxidant defence and redox signalling. The Biochemical journal. 2010;425:313–325. doi: 10.1042/BJ20091541. [DOI] [PubMed] [Google Scholar]
- 43.Zhang H, Go YM, Jones DP. Mitochondrial thioredoxin-2/peroxiredoxin-3 system functions in parallel with mitochondrial GSH system in protection against oxidative stress. Archives of biochemistry and biophysics. 2007;465:119–126. doi: 10.1016/j.abb.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 44.McCommis KS, McGee AM, Laughlin MH, Bowles DK, Baines CP. Hypercholesterolemia increases mitochondrial oxidative stress and enhances the MPT response in the porcine myocardium: beneficial effects of chronic exercise. American journal of physiology. Regulatory, integrative and comparative physiology. 2011;301:R1250–1258. doi: 10.1152/ajpregu.00841.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pollak N, Dolle C, Ziegler M. The power to reduce: pyridine nucleotides--small molecules with a multitude of functions. The Biochemical journal. 2007;402:205–218. doi: 10.1042/BJ20061638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rydstrom J. Mitochondrial NADPH, transhydrogenase and disease. Biochimica et biophysica acta. 2006;1757:721–726. doi: 10.1016/j.bbabio.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 47.Freeman HC, Hugill A, Dear NT, Ashcroft FM, Cox RD. Deletion of nicotinamide nucleotide transhydrogenase: a new quantitive trait locus accounting for glucose intolerance in C57BL/6J mice. Diabetes. 2006;55:2153–2156. doi: 10.2337/db06-0358. [DOI] [PubMed] [Google Scholar]
- 48.Lowell BB, Shulman GI. Mitochondrial dysfunction and type 2 diabetes. Science. 2005;307:384–387. doi: 10.1126/science.1104343. [DOI] [PubMed] [Google Scholar]
- 49.Lee JH, Yand ES, Park JW. Inactivation of NADP+-dependent isocitrate dehydrogenase by peroxynitrite. Implication in cytotoxicity and alcohol-induced liver injury. The Journal of biological chemistry. 2003;278:51360–51371. doi: 10.1074/jbc.M302332200. [DOI] [PubMed] [Google Scholar]
- 50.Moon JL, Kim SY, Shin SW, Park JW. Regulation of brefeldin A-induced ER stress and apoptosis by mitochondrial NADP(+)-dependent isocitrate dehydrogenase. Biochemical and biophysical research communications. 2012;417:760–764. doi: 10.1016/j.bbrc.2011.12.030. [DOI] [PubMed] [Google Scholar]
- 51.Kil IS, Kim SY, Lee SJ, Park JW. Small interfering RNA-mediated silencing of mitochondrial NADP+-dependent isocitrate dehydrogenase enhances the sensitivity of HeLa cells toward tumor necrosis factor-alpha and anticancer drugs. Free radical biology & medicine. 2007;43:1197–1207. doi: 10.1016/j.freeradbiomed.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 52.Dang L, Jin S, Su SM. IDH mutations in glioma and acute myeloid leukemia. Trends in molecular medicine. 2010;16:387–397. doi: 10.1016/j.molmed.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 53.Kojer K, Bien M, Gangel H, Morgan B, Dick TP, Riemer J. Glutathione redox potential in the mitochondrial intermembrane space is linked to the cytosol and impacts the Mia40 redox state. The EMBO journal. 2012;31:3169–3182. doi: 10.1038/emboj.2012.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen Z, Lash LH. Evidence for mitochondrial uptake of glutathione by dicarboxylate and 2-oxoglutarate carriers. The Journal of pharmacology and experimental therapeutics. 1998;285:608–618. [PubMed] [Google Scholar]
- 55.Chen Z, Putt DA, Lash LH. Enrichment and functional reconstitution of glutathione transport activity from rabbit kidney mitochondria: further evidence for the role of the dicarboxylate and 2-oxoglutarate carriers in mitochondrial glutathione transport. Archives of biochemistry and biophysics. 2000;373:193–202. doi: 10.1006/abbi.1999.1527. [DOI] [PubMed] [Google Scholar]
- 56.Coll O, Colell A, Garcia-Ruiz C, Kaplowitz N, Fernandez-Checa JC. Sensitivity of the 2-oxoglutarate carrier to alcohol intake contributes to mitochondrial glutathione depletion. Hepatology. 2003;38:692–702. doi: 10.1053/jhep.2003.50351. [DOI] [PubMed] [Google Scholar]
- 57.Wilkins HM, Marquardt K, Lash LH, Linseman DA. Bcl-2 is a novel interacting partner for the 2-oxoglutarate carrier and a key regulator of mitochondrial glutathione. Free radical biology & medicine. 2012;52:410–419. doi: 10.1016/j.freeradbiomed.2011.10.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lash LH, Putt DA, Matherly LH. Protection of NRK-52E cells, a rat renal proximal tubular cell line, from chemical-induced apoptosis by overexpression of a mitochondrial glutathione transporter. The Journal of pharmacology and experimental therapeutics. 2002;303:476–486. doi: 10.1124/jpet.102.040220. [DOI] [PubMed] [Google Scholar]
- 59.Martensson J, Lai JC, Meister A. High-affinity transport of glutathione is part of a multicomponent system essential for mitochondrial function. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:7185–7189. doi: 10.1073/pnas.87.18.7185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Circu ML, Rodriguez C, Maloney R, Moyer MP, Aw TY. Contribution of mitochondrial GSH transport to matrix GSH status and colonic epithelial cell apoptosis. Free radical biology & medicine. 2008;44:768–778. doi: 10.1016/j.freeradbiomed.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Garcia J, Han D, Sancheti H, Yap LP, Kaplowitz N, Cadenas E. Regulation of mitochondrial glutathione redox status and protein glutathionylation by respiratory substrates. The Journal of biological chemistry. 2010;285:39646–39654. doi: 10.1074/jbc.M110.164160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wadey AL, Muyderman H, Kwek PT, Sims NR. Mitochondrial glutathione uptake: characterization in isolated brain mitochondria and astrocytes in culture. Journal of neurochemistry. 2009;109(Suppl 1):101–108. doi: 10.1111/j.1471-4159.2009.05936.x. [DOI] [PubMed] [Google Scholar]
- 63.Kamga CK, Zhang SX, Wang Y. Dicarboxylate carrier-mediated glutathione transport is essential for reactive oxygen species homeostasis and normal respiration in rat brain mitochondria. American journal of physiology. Cell physiology. 2010;299:C497–505. doi: 10.1152/ajpcell.00058.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.de Bilbao F, Arsenijevic D, Vallet P, Hjelle OP, Ottersen OP, Bouras C, Raffin Y, Abou K, Langhans W, Collins S, Plamondon J, Alves-Guerra MC, Haguenauer A, Garcia I, Richard D, Ricquier D, Giannakopoulos P. Resistance to cerebral ischemic injury in UCP2 knockout mice: evidence for a role of UCP2 as a regulator of mitochondrial glutathione levels. Journal of neurochemistry. 2004;89:1283–1292. doi: 10.1111/j.1471-4159.2004.02432.x. [DOI] [PubMed] [Google Scholar]
- 65.Zimmermann AK, Loucks FA, Schroeder EK, Bouchard RJ, Tyler KL, Linseman DA. Glutathione binding to the Bcl-2 homology-3 domain groove: a molecular basis for Bcl-2 antioxidant function at mitochondria. The Journal of biological chemistry. 2007;282:29296–29304. doi: 10.1074/jbc.M702853200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lluis JM, Colell A, Garcia-Ruiz C, Kaplowitz N, Fernandez-Checa JC. Acetaldehyde impairs mitochondrial glutathione transport in HepG2 cells through endoplasmic reticulum stress. Gastroenterology. 2003;124:708–724. doi: 10.1053/gast.2003.50089. [DOI] [PubMed] [Google Scholar]
- 67.Ikonen E. Cellular cholesterol trafficking and compartmentalization. Nature reviews. Molecular cell biology. 2008;9:125–138. doi: 10.1038/nrm2336. [DOI] [PubMed] [Google Scholar]
- 68.Montero J, Mari M, Colell A, Morales A, Basanez G, Garcia-Ruiz C, Fernandez-Checa JC. Cholesterol and peroxidized cardiolipin in mitochondrial membrane properties, permeabilization and cell death. Biochimica et biophysica acta. 2010;1797:1217–1224. doi: 10.1016/j.bbabio.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Garcia-Ruiz C, Mari M, Colell A, Morales A, Caballero F, Montero J, Terrones O, Basanez G, Fernandez-Checa JC. Mitochondrial cholesterol in health and disease. Histology and histopathology. 2009;24:117–132. doi: 10.14670/HH-24.117. [DOI] [PubMed] [Google Scholar]
- 70.Fernandez A, Colell A, Caballero F, Matias N, Garcia-Ruiz C, Fernandez-Checa JC. Mitochondrial S-adenosyl-L-methionine transport is insensitive to alcohol-mediated changes in membrane dynamics. Alcoholism, clinical and experimental research. 2009;33:1169–1180. doi: 10.1111/j.1530-0277.2009.00940.x. [DOI] [PubMed] [Google Scholar]
- 71.Armstrong JS, Jones DP. Glutathione depletion enforces the mitochondrial permeability transition and causes cell death in Bcl-2 overexpressing HL60 cells. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2002;16:1263–1265. doi: 10.1096/fj.02-0097fje. [DOI] [PubMed] [Google Scholar]
- 72.Lluis JM, Buricchi F, Chiarugi P, Morales A, Fernandez-Checa JC. Dual role of mitochondrial reactive oxygen species in hypoxia signaling: activation of nuclear factor-{kappa}B via c-SRC and oxidant-dependent cell death. Cancer research. 2007;67:7368–7377. doi: 10.1158/0008-5472.CAN-07-0515. [DOI] [PubMed] [Google Scholar]
- 73.Lluis JM, Morales A, Blasco C, Colell A, Mari M, Garcia-Ruiz C, Fernandez-Checa JC. Critical role of mitochondrial glutathione in the survival of hepatocytes during hypoxia. The Journal of biological chemistry. 2005;280:3224–3232. doi: 10.1074/jbc.M408244200. [DOI] [PubMed] [Google Scholar]
- 74.Lu C, Armstrong JS. Role of calcium and cyclophilin D in the regulation of mitochondrial permeabilization induced by glutathione depletion. Biochemical and biophysical research communications. 2007;363:572–577. doi: 10.1016/j.bbrc.2007.08.196. [DOI] [PubMed] [Google Scholar]
- 75.Mari M, Caballero F, Colell A, Morales A, Caballeria J, Fernandez A, Enrich C, Fernandez-Checa JC, Garcia-Ruiz C. Mitochondrial free cholesterol loading sensitizes to TNF- and Fas-mediated steatohepatitis. Cell metabolism. 2006;4:185–198. doi: 10.1016/j.cmet.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 76.Mari M, Colell A, Morales A, Caballero F, Moles A, Fernandez A, Terrones O, Basanez G, Antonsson B, Garcia-Ruiz C, Fernandez-Checa JC. Mechanism of mitochondrial glutathione-dependent hepatocellular susceptibility to TNF despite NF-kappaB activation. Gastroenterology. 2008;134:1507–1520. doi: 10.1053/j.gastro.2008.01.073. [DOI] [PubMed] [Google Scholar]
- 77.Garcia-Ruiz C, Colell A, Morales A, Calvo M, Enrich C, Fernandez-Checa JC. Trafficking of ganglioside GD3 to mitochondria by tumor necrosis factor-alpha. The Journal of biological chemistry. 2002;277:36443–36448. doi: 10.1074/jbc.M206021200. [DOI] [PubMed] [Google Scholar]
- 78.Krahenbuhl S, Talos C, Lauterburg BH, Reichen J. Reduced antioxidative capacity in liver mitochondria from bile duct ligated rats. Hepatology. 1995;22:607–612. doi: 10.1002/hep.1840220234. [DOI] [PubMed] [Google Scholar]
- 79.Fernandez-Checa JC, Fernandez A, Morales A, Mari M, Garcia-Ruiz C, Colell A. Oxidative stress and altered mitochondrial function in neurodegenerative diseases: lessons from mouse models. CNS & neurological disorders drug targets. 2010;9:439–454. doi: 10.2174/187152710791556113. [DOI] [PubMed] [Google Scholar]
- 80.Fernandez A, Llacuna L, Fernandez-Checa JC, Colell A. Mitochondrial cholesterol loading exacerbates amyloid beta peptide-induced inflammation and neurotoxicity. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:6394–6405. doi: 10.1523/JNEUROSCI.4909-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xue X, You S, Zhang Q, Wu Y, Zou GZ, Wang PC, Zhao YL, Xu Y, Jia L, Zhang X, Liang XJ. Mitaplatin increases sensitivity of tumor cells to cisplatin by inducing mitochondrial dysfunction. Molecular pharmaceutics. 2012;9:634–644. doi: 10.1021/mp200571k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kaufmann SH, Earnshaw WC. Induction of apoptosis by cancer chemotherapy. Experimental cell research. 2000;256:42–49. doi: 10.1006/excr.2000.4838. [DOI] [PubMed] [Google Scholar]
- 83.Wang GQ, Gastman BR, Wieckowski E, Goldstein LA, Gambotto A, Kim TH, Fang B, Rabinovitz A, Yin XM, Rabinowich H. A role for mitochondrial Bak in apoptotic response to anticancer drugs. The Journal of biological chemistry. 2001;276:34307–34317. doi: 10.1074/jbc.M103526200. [DOI] [PubMed] [Google Scholar]
- 84.Li H, Zhang X, Song X, Zhu F, Wang Q, Guo C, Liu C, Shi Y, Ma C, Wang X, Zhang L. PDCD5 promotes cisplatin-induced apoptosis of glioma cells via activating mitochondrial apoptotic pathway. Cancer biology & therapy. 2012;13 doi: 10.4161/cbt.20565. [DOI] [PubMed] [Google Scholar]
- 85.Montaigne D, Hurt C, Neviere R. Mitochondria death/survival signaling pathways in cardiotoxicity induced by anthracyclines and anticancer-targeted therapies. Biochemistry research international. 2012;2012:951539. doi: 10.1155/2012/951539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Boldin MP, Goncharov TM, Goltsev YV, Wallach D. Involvement of MACH, a novel MORT1/FADD-interacting protease, in Fas/APO-1- and TNF receptor-induced cell death. Cell. 1996;85:803–815. doi: 10.1016/s0092-8674(00)81265-9. [DOI] [PubMed] [Google Scholar]
- 87.Muzio M, Chinnaiyan AM, Kischkel FC, O’Rourke K, Shevchenko A, Ni J, Scaffidi C, Bretz JD, Zhang M, Gentz R, Mann M, Krammer PH, Peter ME, Dixit VM. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death--inducing signaling complex. Cell. 1996;85:817–827. doi: 10.1016/s0092-8674(00)81266-0. [DOI] [PubMed] [Google Scholar]
- 88.Zhivotovsky B. Caspases: the enzymes of death. Essays in biochemistry. 2003;39:25–40. doi: 10.1042/bse0390025. [DOI] [PubMed] [Google Scholar]
- 89.Schmitz I, Walczak H, Krammer PH, Peter ME. Differences between CD95 type I and II cells detected with the CD95 ligand. Cell death and differentiation. 1999;6:821–822. doi: 10.1038/sj.cdd.4400569. [DOI] [PubMed] [Google Scholar]
- 90.Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 91.Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiological reviews. 2007;87:99–163. doi: 10.1152/physrev.00013.2006. [DOI] [PubMed] [Google Scholar]
- 92.Zong WX, Thompson CB. Necrotic death as a cell fate. Genes & development. 2006;20:1–15. doi: 10.1101/gad.1376506. [DOI] [PubMed] [Google Scholar]
- 93.Halestrap AP. Calcium, mitochondria and reperfusion injury: a pore way to die. Biochemical Society transactions. 2006;34:232–237. doi: 10.1042/BST20060232. [DOI] [PubMed] [Google Scholar]
- 94.Baines CP. The cardiac mitochondrion: nexus of stress. Annual review of physiology. 2010;72:61–80. doi: 10.1146/annurev-physiol-021909-135929. [DOI] [PubMed] [Google Scholar]
- 95.Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, Robbins J, Molkentin JD. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- 96.Basso E, Fante L, Fowlkes J, Petronilli V, Forte MA, Bernardi P. Properties of the permeability transition pore in mitochondria devoid of Cyclophilin D. The Journal of biological chemistry. 2005;280:18558–18561. doi: 10.1074/jbc.C500089200. [DOI] [PubMed] [Google Scholar]
- 97.Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, Yamagata H, Inohara H, Kubo T, Tsujimoto Y. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature. 2005;434:652–658. doi: 10.1038/nature03317. [DOI] [PubMed] [Google Scholar]
- 98.Schinzel AC, Takeuchi O, Huang Z, Fisher JK, Zhou Z, Rubens J, Hetz C, Danial NN, Moskowitz MA, Korsmeyer SJ. Cyclophilin D is a component of mitochondrial permeability transition and mediates neuronal cell death after focal cerebral ischemia. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:12005–12010. doi: 10.1073/pnas.0505294102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hockenbery DM, Giedt CD, O’Neill JW, Manion MK, Banker DE. Mitochondria and apoptosis: new therapeutic targets. Advances in cancer research. 2002;85:203–242. doi: 10.1016/s0065-230x(02)85007-2. [DOI] [PubMed] [Google Scholar]
- 100.Zamzami N, Hirsch T, Dallaporta B, Petit PX, Kroemer G. Mitochondrial implication in accidental and programmed cell death: apoptosis and necrosis. Journal of bioenergetics and biomembranes. 1997;29:185–193. doi: 10.1023/a:1022694131572. [DOI] [PubMed] [Google Scholar]
- 101.Lemasters JJ, Qian T, Bradham CA, Brenner DA, Cascio WE, Trost LC, Nishimura Y, Nieminen AL, Herman B. Mitochondrial dysfunction in the pathogenesis of necrotic and apoptotic cell death. Journal of bioenergetics and biomembranes. 1999;31:305–319. doi: 10.1023/a:1005419617371. [DOI] [PubMed] [Google Scholar]
- 102.Golstein P, Kroemer G. Cell death by necrosis: towards a molecular definition. Trends in biochemical sciences. 2007;32:37–43. doi: 10.1016/j.tibs.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 103.Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nature reviews. Molecular cell biology. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 104.Kalanxhi E, Wallace CJ. Cytochrome c impaled: investigation of the extended lipid anchorage of a soluble protein to mitochondrial membrane models. The Biochemical journal. 2007;407:179–187. doi: 10.1042/BJ20070459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chipuk JE, McStay GP, Bharti A, Kuwana T, Clarke CJ, Siskind LJ, Obeid LM, Green DR. Sphingolipid metabolism cooperates with BAK and BAX to promote the mitochondrial pathway of apoptosis. Cell. 2012;148:988–1000. doi: 10.1016/j.cell.2012.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Schug ZT, Gottlieb E. Cardiolipin acts as a mitochondrial signalling platform to launch apoptosis. Biochimica et biophysica acta. 2009;1788:2022–2031. doi: 10.1016/j.bbamem.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 107.Kriska T, Korytowski W, Girotti AW. Role of mitochondrial cardiolipin peroxidation in apoptotic photokilling of 5-aminolevulinate-treated tumor cells. Archives of biochemistry and biophysics. 2005;433:435–446. doi: 10.1016/j.abb.2004.09.025. [DOI] [PubMed] [Google Scholar]
- 108.Landeta O, Landajuela A, Gil D, Taneva S, Di Primo C, Sot B, Valle M, Frolov VA, Basanez G. Reconstitution of proapoptotic BAK function in liposomes reveals a dual role for mitochondrial lipids in the BAK-driven membrane permeabilization process. The Journal of biological chemistry. 2011;286:8213–8230. doi: 10.1074/jbc.M110.165852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Colell A, Garcia-Ruiz C, Miranda M, Ardite E, Mari M, Morales A, Corrales F, Kaplowitz N, Fernandez-Checa JC. Selective glutathione depletion of mitochondria by ethanol sensitizes hepatocytes to tumor necrosis factor. Gastroenterology. 1998;115:1541–1551. doi: 10.1016/s0016-5085(98)70034-4. [DOI] [PubMed] [Google Scholar]
- 110.Colell A, Garcia-Ruiz C, Morales A, Ballesta A, Ookhtens M, Rodes J, Kaplowitz N, Fernandez-Checa JC. Transport of reduced glutathione in hepatic mitochondria and mitoplasts from ethanol-treated rats: effect of membrane physical properties and S-adenosyl-L-methionine. Hepatology. 1997;26:699–708. doi: 10.1002/hep.510260323. [DOI] [PubMed] [Google Scholar]
- 111.Shan X, Jones DP, Hashmi M, Anders MW. Selective depletion of mitochondrial glutathione concentrations by (R,S)-3-hydroxy-4-pentenoate potentiates oxidative cell death. Chemical research in toxicology. 1993;6:75–81. doi: 10.1021/tx00031a012. [DOI] [PubMed] [Google Scholar]
- 112.Garcia-Ruiz C, Fernandez-Checa JC. Mitochondrial glutathione: hepatocellular survival-death switch. Journal of gastroenterology and hepatology. 2006;21(Suppl 3):S3–6. doi: 10.1111/j.1440-1746.2006.04570.x. [DOI] [PubMed] [Google Scholar]
- 113.Mari M, Colell A, Morales A, Paneda C, Varela-Nieto I, Garcia-Ruiz C, Fernandez-Checa JC. Acidic sphingomyelinase downregulates the liver-specific methionine adenosyltransferase 1A, contributing to tumor necrosis factor-induced lethal hepatitis. The Journal of clinical investigation. 2004;113:895–904. doi: 10.1172/JCI19852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wullner U, Seyfried J, Groscurth P, Beinroth S, Winter S, Gleichmann M, Heneka M, Loschmann P, Schulz JB, Weller M, Klockgether T. Glutathione depletion and neuronal cell death: the role of reactive oxygen intermediates and mitochondrial function. Brain research. 1999;826:53–62. doi: 10.1016/s0006-8993(99)01228-7. [DOI] [PubMed] [Google Scholar]
- 115.Muyderman H, Wadey AL, Nilsson M, Sims NR. Mitochondrial glutathione protects against cell death induced by oxidative and nitrative stress in astrocytes. Journal of neurochemistry. 2007;102:1369–1382. doi: 10.1111/j.1471-4159.2007.04641.x. [DOI] [PubMed] [Google Scholar]
- 116.Samudio I, Konopleva M, Hail N, Jr, Shi YX, McQueen T, Hsu T, Evans R, Honda T, Gribble GW, Sporn M, Gilbert HF, Safe S, Andreeff M. 2-Cyano-3,12-dioxooleana-1,9-dien-28-imidazolide (CDDO-Im) directly targets mitochondrial glutathione to induce apoptosis in pancreatic cancer. The Journal of biological chemistry. 2005;280:36273–36282. doi: 10.1074/jbc.M507518200. [DOI] [PubMed] [Google Scholar]
- 117.Samudio I, Kurinna S, Ruvolo P, Korchin B, Kantarjian H, Beran M, Dunner K, Jr, Kondo S, Andreeff M, Konopleva M. Inhibition of mitochondrial metabolism by methyl-2-cyano-3,12-dioxooleana-1,9-diene-28-oate induces apoptotic or autophagic cell death in chronic myeloid leukemia cells. Molecular cancer therapeutics. 2008;7:1130–1139. doi: 10.1158/1535-7163.MCT-07-0553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Petronelli A, Saulle E, Pasquini L, Petrucci E, Mariani G, Biffoni M, Ferretti G, Scambia G, Benedetti-Panici P, Greggi S, Cognetti F, Russo MA, Sporn M, Testa U. High sensitivity of ovarian cancer cells to the synthetic triterpenoid CDDO-Imidazolide. Cancer letters. 2009;282:214–228. doi: 10.1016/j.canlet.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 119.Chen G, Chen Z, Hu Y, Huang P. Inhibition of mitochondrial respiration and rapid depletion of mitochondrial glutathione by beta-phenethyl isothiocyanate: mechanisms for anti-leukemia activity. Antioxidants & redox signaling. 2011;15:2911–2921. doi: 10.1089/ars.2011.4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Colell A, Fernandez A, Fernandez-Checa JC. Mitochondria, cholesterol and amyloid beta peptide: a dangerous trio in Alzheimer disease. Journal of bioenergetics and biomembranes. 2009;41:417–423. doi: 10.1007/s10863-009-9242-6. [DOI] [PubMed] [Google Scholar]
- 121.Jonsson T, Atwal JK, Steinberg S, Snaedal J, Jonsson PV, Bjornsson S, Stefansson H, Sulem P, Gudbjartsson D, Maloney J, Hoyte K, Gustafson A, Liu Y, Lu Y, Bhangale T, Graham RR, Huttenlocher J, Bjornsdottir G, Andreassen OA, Jonsson EG, Palotie A, Behrens TW, Magnusson OT, Kong A, Thorsteinsdottir U, Watts RJ, Stefansson K. A mutation in APP protects against Alzheimer’s disease and age-related cognitive decline. Nature. 2012;488:96–99. doi: 10.1038/nature11283. [DOI] [PubMed] [Google Scholar]
- 122.Tanzi RE, Moir RD, Wagner SL. Clearance of Alzheimer’s Abeta peptide: the many roads to perdition. Neuron. 2004;43:605–608. doi: 10.1016/j.neuron.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 123.Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid beta-peptide. Nature reviews. Molecular cell biology. 2007;8:101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- 124.Lin MT, Beal MF. Alzheimer’s APP mangles mitochondria. Nature medicine. 2006;12:1241–1243. doi: 10.1038/nm1106-1241. [DOI] [PubMed] [Google Scholar]
- 125.Manczak M, Anekonda TS, Henson E, Park BS, Quinn J, Reddy PH. Mitochondria are a direct site of A beta accumulation in Alzheimer’s disease neurons: implications for free radical generation and oxidative damage in disease progression. Human molecular genetics. 2006;15:1437–1449. doi: 10.1093/hmg/ddl066. [DOI] [PubMed] [Google Scholar]
- 126.Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 127.Sian J, Dexter DT, Lees AJ, Daniel S, Agid Y, Javoy-Agid F, Jenner P, Marsden CD. Alterations in glutathione levels in Parkinson’s disease and other neurodegenerative disorders affecting basal ganglia. Annals of neurology. 1994;36:348–355. doi: 10.1002/ana.410360305. [DOI] [PubMed] [Google Scholar]
- 128.Damier P, Hirsch EC, Zhang P, Agid Y, Javoy-Agid F. Glutathione peroxidase, glial cells and Parkinson’s disease. Neuroscience. 1993;52:1–6. doi: 10.1016/0306-4522(93)90175-f. [DOI] [PubMed] [Google Scholar]
- 129.Bosch M, Mari M, Herms A, Fernandez A, Fajardo A, Kassan A, Giralt A, Colell A, Balgoma D, Barbero E, Gonzalez-Moreno E, Matias N, Tebar F, Balsinde J, Camps M, Enrich C, Gross SP, Garcia-Ruiz C, Perez-Navarro E, Fernandez-Checa JC, Pol A. Caveolin-1 deficiency causes cholesterol-dependent mitochondrial dysfunction and apoptotic susceptibility. Current biology : CB. 2011;21:681–686. doi: 10.1016/j.cub.2011.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Anderson MF, Nilsson M, Sims NR. Glutathione monoethylester prevents mitochondrial glutathione depletion during focal cerebral ischemia. Neurochemistry international. 2004;44:153–159. doi: 10.1016/s0197-0186(03)00133-5. [DOI] [PubMed] [Google Scholar]
- 131.Anderson MF, Nilsson M, Eriksson PS, Sims NR. Glutathione monoethyl ester provides neuroprotection in a rat model of stroke. Neuroscience letters. 2004;354:163–165. doi: 10.1016/j.neulet.2003.09.067. [DOI] [PubMed] [Google Scholar]
- 132.Cacciatore I, Baldassarre L, Fornasari E, Mollica A, Pinnen F. Recent advances in the treatment of neurodegenerative diseases based on GSH delivery systems. Oxidative medicine and cellular longevity. 2012;2012:240146. doi: 10.1155/2012/240146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Garcia-Ruiz C, Mari M, Colell A, Morales A, Fernandez-Checa JC. Metabolic therapy: lessons from liver diseases. Current pharmaceutical design. 2011;17:3933–3944. doi: 10.2174/138161211798357700. [DOI] [PubMed] [Google Scholar]
- 134.Angulo P. Nonalcoholic fatty liver disease. The New England journal of medicine. 2002;346:1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 135.Brunt EM. Nonalcoholic steatohepatitis. Seminars in liver disease. 2004;24:3–20. doi: 10.1055/s-2004-823098. [DOI] [PubMed] [Google Scholar]
- 136.Tilg H, Diehl AM. Cytokines in alcoholic and nonalcoholic steatohepatitis. The New England journal of medicine. 2000;343:1467–1476. doi: 10.1056/NEJM200011163432007. [DOI] [PubMed] [Google Scholar]
- 137.Colell A, Coll O, Garcia-Ruiz C, Paris R, Tiribelli C, Kaplowitz N, Fernandez-Checa JC. Tauroursodeoxycholic acid protects hepatocytes from ethanol-fed rats against tumor necrosis factor-induced cell death by replenishing mitochondrial glutathione. Hepatology. 2001;34:964–971. doi: 10.1053/jhep.2001.28510. [DOI] [PubMed] [Google Scholar]
- 138.Zhao P, Kalhorn TF, Slattery JT. Selective mitochondrial glutathione depletion by ethanol enhances acetaminophen toxicity in rat liver. Hepatology. 2002;36:326–335. doi: 10.1053/jhep.2002.34943. [DOI] [PubMed] [Google Scholar]
- 139.Ji C, Kaplowitz N. Hyperhomocysteinemia, endoplasmic reticulum stress, and alcoholic liver injury. World journal of gastroenterology : WJG. 2004;10:1699–1708. doi: 10.3748/wjg.v10.i12.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ji C, Kaplowitz N. Betaine decreases hyperhomocysteinemia, endoplasmic reticulum stress, and liver injury in alcohol-fed mice. Gastroenterology. 2003;124:1488–1499. doi: 10.1016/s0016-5085(03)00276-2. [DOI] [PubMed] [Google Scholar]
- 141.Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, Gorgun CZ, Hotamisligil GS. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137–1140. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Serviddio G, Bellanti F, Tamborra R, Rollo T, Capitanio N, Romano AD, Sastre J, Vendemiale G, Altomare E. Uncoupling protein-2 (UCP2) induces mitochondrial proton leak and increases susceptibility of non-alcoholic steatohepatitis (NASH) liver to ischaemia-reperfusion injury. Gut. 2008;57:957–965. doi: 10.1136/gut.2007.147496. [DOI] [PubMed] [Google Scholar]
- 143.Montfort C, Matias N, Fernandez A, Fucho R, Conde de la Rosa L, Martinez-Chantar ML, Mato JM, Machida K, Tsukamoto H, Murphy MP, Mansouri A, Kaplowitz N, Garcia-Ruiz C, Fernandez-Checa JC. Mitochondrial GSH determines the toxic or therapeutic potential of superoxide scavenging in steatohepatitis. Journal of hepatology. 2012;57:852–859. doi: 10.1016/j.jhep.2012.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Le Lay S, Kurzchalia TV. Getting rid of caveolins: phenotypes of caveolin-deficient animals. Biochimica et biophysica acta. 2005;1746:322–333. doi: 10.1016/j.bbamcr.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 145.Cao H, Alston L, Ruschman J, Hegele RA. Heterozygous CAV1 frameshift mutations (MIM 601047) in patients with atypical partial lipodystrophy and hypertriglyceridemia. Lipids in health and disease. 2008;7:3. doi: 10.1186/1476-511X-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kim CA, Delepine M, Boutet E, El Mourabit H, Le Lay S, Meier M, Nemani M, Bridel E, Leite CC, Bertola DR, Semple RK, O’Rahilly S, Dugail I, Capeau J, Lathrop M, Magre J. Association of a homozygous nonsense caveolin-1 mutation with Berardinelli-Seip congenital lipodystrophy. The Journal of clinical endocrinology and metabolism. 2008;93:1129–1134. doi: 10.1210/jc.2007-1328. [DOI] [PubMed] [Google Scholar]
- 147.Wallace DC, Fan W, Procaccio V. Mitochondrial energetics and therapeutics. Annual review of pathology. 2010;5:297–348. doi: 10.1146/annurev.pathol.4.110807.092314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Parton RG, Simons K. The multiple faces of caveolae. Nature reviews. Molecular cell biology. 2007;8:185–194. doi: 10.1038/nrm2122. [DOI] [PubMed] [Google Scholar]
- 149.Bosch M, Mari M, Gross SP, Fernandez-Checa JC, Pol A. Mitochondrial cholesterol: a connection between caveolin, metabolism, and disease. Traffic. 2011;12:1483–1489. doi: 10.1111/j.1600-0854.2011.01259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Boscher C, Nabi IR. Caveolin-1: role in cell signaling. Advances in experimental medicine and biology. 2012;729:29–50. doi: 10.1007/978-1-4614-1222-9_3. [DOI] [PubMed] [Google Scholar]
- 151.Murata M, Peranen J, Schreiner R, Wieland F, Kurzchalia TV, Simons K. VIP21/caveolin is a cholesterol-binding protein. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:10339–10343. doi: 10.1073/pnas.92.22.10339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Pol A, Martin S, Fernandez MA, Ingelmo-Torres M, Ferguson C, Enrich C, Parton RG. Cholesterol and fatty acids regulate dynamic caveolin trafficking through the Golgi complex and between the cell surface and lipid bodies. Molecular biology of the cell. 2005;16:2091–2105. doi: 10.1091/mbc.E04-08-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Pol A, Luetterforst R, Lindsay M, Heino S, Ikonen E, Parton RG. A caveolin dominant negative mutant associates with lipid bodies and induces intracellular cholesterol imbalance. The Journal of cell biology. 2001;152:1057–1070. doi: 10.1083/jcb.152.5.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Drab M, Verkade P, Elger M, Kasper M, Lohn M, Lauterbach B, Menne J, Lindschau C, Mende F, Luft FC, Schedl A, Haller H, Kurzchalia TV. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science. 2001;293:2449–2452. doi: 10.1126/science.1062688. [DOI] [PubMed] [Google Scholar]
- 155.Maniatis NA, Chernaya O, Shinin V, Minshall RD. Caveolins and lung function. Advances in experimental medicine and biology. 2012;729:157–179. doi: 10.1007/978-1-4614-1222-9_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ross MF, Kelso GF, Blaikie FH, James AM, Cocheme HM, Filipovska A, Da Ros T, Hurd TR, Smith RA, Murphy MP. Lipophilic triphenylphosphonium cations as tools in mitochondrial bioenergetics and free radical biology. Biochemistry. Biokhimiia. 2005;70:222–230. doi: 10.1007/s10541-005-0104-5. [DOI] [PubMed] [Google Scholar]
- 157.Sheu SS, Nauduri D, Anders MW. Targeting antioxidants to mitochondria: a new therapeutic direction. Biochimica et biophysica acta. 2006;1762:256–265. doi: 10.1016/j.bbadis.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 158.Smith RA, Murphy MP. Animal and human studies with the mitochondria-targeted antioxidant MitoQ. Annals of the New York Academy of Sciences. 2010;1201:96–103. doi: 10.1111/j.1749-6632.2010.05627.x. [DOI] [PubMed] [Google Scholar]