Abstract
Alterations in the X-linked gene MECP2 encoding the methyl-CpG-binding protein 2 (MeCP2) have been linked to autism spectrum disorders (ASD). Most recently, data suggest that overexpression of MECP2 may be related to ASD. To better characterize the relevance of MECP2 overexpression to ASD-related behaviors, we compared the core symptoms of ASD in MECP2 duplication syndrome to nonverbal-mental-age-matched boys with idiopathic ASD. Within the MECP2 duplication group we further delineated aspects of the behavioral phenotype, and also examined how duplication size and gene content corresponded to clinical severity. We compared 10 males with MECP2 duplication syndrome (ages 3–10) to a chronological and mental age-matched sample of 9 nonverbal males with idiopathic ASD. Our results indicate that boys with MECP2 duplication syndrome share the core behavioral features of ASD (e.g. social affect, restricted/repetitive behaviors). Direct comparisons of ASD profiles revealed that a majority of boys with MECP2 duplication syndrome are similar to idiopathic ASD; they have impairments in social affect (albeit to a lesser degree than idiopathic ASD) and similar severity in restricted/repetitive behaviors. Nonverbal mental age did not correlate with severity of social impairment or repetitive behaviors. Within the MECP2 duplication group, breakpoint size does not predict differences in clinical severity. In addition to social withdrawal and stereotyped behaviors, we also found that hyposensitivity to pain/temperature are part of the behavioral phenotype of MECP2 duplication syndrome. Our results illustrate that overexpression/increased dosage of MECP2 is related to core features of ASD.
Keywords: Autism, MECP2, genetics, phenotype, social affect, overexpression
Introduction
Autism spectrum disorder (ASD) is a complex, heterogeneous disorder that likely results from a variety of complex combinations of genetic and environmental factors. ASD is characterized by deficits in social affect, which includes deficits in verbal and nonverbal communication and reciprocal social interaction, accompanied by restricted and repetitive/stereotyped behaviors (RRB). Candidate gene, genome-wide sequencing strategies, and copy number analysis led to the identification of dosage differences in genes linked to ASD (Geschwind, 2011; Sanders et al., 2011; State & Levitt, 2011). Clinical studies of single gene disorders with a high rate of ASD have allowed for better assessment of etiology and gene function because of the significant downstream effects of these mutations. For example, our clinical studies of the X-linked MECP2 duplication syndrome, in which the MECP2 gene encoding the methyl-CpG-binding protein 2 (MeCP2) is overexpressed, informed recent work in mouse models which demonstrated that alterations in MECP2 levels differentially and specifically affect molecular pathways underlying ASD-related social behavior (Samaco et al., 2012). MECP2 mutations that dysregulate MeCP2 protein function are also linked to non-syndromic ASD (Carney et al., 2003; Coutinho et al., 2007; Loat et al., 2008; Shibayama et al., 2004). This is supported by a recent study that further demonstrates the relevance of examining the overlap between idiopathic ASD and MECP2 duplication syndrome. Specifically, individuals with idiopathic ASD and mothers of individuals with ASD were found to overexpress MECP2 in peripheral leukocytes in the absence of MECP2 mutations (Kuwano et al., 2011). In addition, postmortem brain studies show higher MeCP2 protein levels in idiopathic ASD in the anterior cingulate, thalamus, and motor cortex. Taken together, these findings suggest that overexpression of MECP2 may be fundamentally related to some forms of ASD.
Sub-microscopic MECP2 duplications were first identified in a girl with atypical Rett Syndrome (RTT) and preserved speech, a boy with severe intellectual disability (ID) and features of RTT, and in members of three families with X-linked ID (Van Esch et al., 2005) (Ariani, et al., 2004; Meins, et al., 2005). Common core features of MECP2 duplication include: infantile hypotonia, recurrent respiratory infections, severe to profound intellectual disability, limited to absent speech, spasticity, and gastrointestinal motility problems (Friez et al., 2006; Ramocki et al., 2009; Van Esch, 2012; Van Esch, et al., 2005). Dysmorphism and distinct facial features may also be present, as may developmental regression (Van Esch, 2012). Aspects of the behavioral phenotype are still emerging. In our prior studies, we detailed that features of ASD may also be common in the phenotype of MECP2 duplications and include reduced eye gaze and low social interest (Ramocki, et al., 2009). Other associated features include poor to absent speech, severe ID, and epilepsy. Some participants exhibited regression and lost their prior mobility and hand use. Nearly 50% of our cohort was initially diagnosed with ASD prior to receiving their genetic diagnosis (Ramocki, et al., 2009). Within MECP2 duplication syndrome, we have not previously assessed other features that are common in ASD such as irritability, anxiety/social withdrawal, hyperactivity, and sensitivity to pain/temperature. We also have not explicitly made comparisons to a matched group of individuals with non-syndromic ASD. These extensions of our previous research are timely since a recent study in a mouse model of MECP2 duplication syndrome found that doubling MECP2 levels causes heightened anxiety and ASD features and alters the expression of genes that influence anxiety, social behavior, and pain sensitivity/nociception (Samaco, et al., 2012).
MECP2 duplication syndrome is likely underdiagnosed; and because genomic rearrangements including MECP2 copy number gain are typically inherited from a maternal carrier, there is a 50% recurrence risk in future sons in most cases. Due to advantageous skewing of X chromosome inactivation patterns in female carriers, MECP2 duplication syndrome primarily affects males (Ramocki, et al., 2010). More recent data suggest that MECP2 duplication syndrome accounts for approximately 1% of all cases of X-linked intellectual disability (ID), but when males with ID who have other features of MECP2 duplication syndrome are also screened, the likelihood of identifying duplications involving the MECP2 locus approaches 15% (Friez, et al., 2006; Lugtenberg et al., 2009).
Prior studies have demonstrated considerable phenotypic variability among patients with MECP2 duplications for reasons that are not completely understood (Ramocki, et al., 2010). It is possible that increases in MECP2 copy-number from 2 (duplications) to 3 (triplications) may account for some patients with a very severe phenotype (Carvalho et al., 2011; del Gaudio et al., 2006). Despite the fact that Xq28 duplications spanning MECP2 are non-recurrent rearrangements, the observed phenotypic variability is not explained either by rearrangement size alone or gene content since the smallest region of genomic overlap spans 149 kb, contains the IRAK1 and MECP2 genes, and is sufficient to reproduce the core syndrome phenotypes. Specific genotype-phenotype correlation studies of duplication size and scores on standardized instruments used to assess clinical severity have never been reported.
In this study, we extend our previous work to further characterize the behavioral phenotype in MECP2 duplication syndrome with careful attention to how the triad of core features of autism differs between boys with MECP2 duplication syndrome and nonverbal-mental-age-matched boys with idiopathic ASD. We have focused on behavioral comparisons related to social affect (inclusive of communication and reciprocal social interaction) and repetitive behaviors. Within the MECP2 duplication group, we examined associated features including pain tolerance, and aberrant behavior, and also examined how duplication size and gene content corresponded to severity of the behavioral phenotype. By probing certain ASD phenotypes (e.g. social affect, RRB) and associated behaviors (e.g. social withdrawal, pain sensitivity), and the degree of behavioral overlap with non-syndromic ASD, this approach may lead to further insights into MeCP2 protein function, novel therapeutic strategies, testing in pre-clinical mouse models, and potential human clinical trials (Cobb, Guy, & Bird, 2010; Dolen, Carpenter, Ocain, & Bear, 2010; Samaco, et al., 2012; Veenstra-VanderWeele & Blakely, 2012). Finally, clearly defining how MECP2 duplication syndrome is distinct from idiopathic ASD will enhance early identification and treatment, as well as genetic counseling and testing of family members.
Materials and Methods
Participants
The participants were all male and ranged between the ages of 3–10 years. Inclusion criteria for boys with MECP2 duplication syndrome were: a confirmed genetic diagnosis (see below), independent mobility, and hand use. All participants with an established diagnosis who met our inclusion criteria were offered enrollment and were ascertained as part of their attendance at the first ever worldwide conference on MECP2 duplication syndrome. We excluded boys who did not have sufficient hand use or mobility to complete the Autism Diagnostic Observation Schedule (ADOS). Ten boys with MECP2 duplication syndrome were enrolled in this new study, and were not part of our original cohort group (Ramocki, et al., 2009). Nine boys with ASD were selected through the Vanderbilt local clinical research database and formally participated in either the Simons Simplex collection (SSC) (n=4) or the Autism Treatment Network (ATN) (n=5) research registry. All participants were evaluated by a physician to exclude any underlying genetic conditions (also see Genetic Testing section below). Additional inclusion criteria for boys with idiopathic ASD included matching for chronological age, nonverbal mental age, and language level (i.e. nonverbal) to our sample of boys with MECP2 duplication syndrome. As detailed in other publications, the enrollment criteria for these respective studies is stringent (Lord et al., 2011) (Coury, Jones, Klatka, Winklosky, & Perrin, 2009) and included detailed phenotypic testing to establish the diagnosis of ASD as well as genetic testing (detailed below).
Genetic Testing
All boys with MECP2 duplication syndrome had an established clinical diagnosis. To independently confirm the diagnosis and to determine the size, genomic extent and gene content for each rearrangement, we designed a tiling-path oligonucleotide microarray spanning 4.6 Mb across the MECP2 region on Xq28. The custom 4x44k Agilent Technologies (Santa Clara, CA) microarray was designed using the Agilent e-array website (http://earray.chem.agilent.com/earray/). We selected 22,000 probes covering Chromosome X: 150,000,000–154,600,000 (NCBI build 36), including the MECP2 gene, which represents an average distribution of 1 probe per 250 bp. Probe labeling and hybridization were performed as described (Carvalho, et al., 2011). To estimate duplication size, we calculated the distance between breakpoints/coordinates (i.e. where probe signals traversed from no gain to gain signaling increased dosage of the genomic interval complementary to the interrogating oligonucleotide probe). In boys who had partial triplications, we used weighted correlations to account for the extra copies.
Blood samples were collected from all participants with ASD as per the respective research protocols of the ATN and the SSC. Chromosome microarray analysis (CMA) was performed, as well as DNA testing for Fragile X, and individuals with co-morbid genetic syndromes (e.g. Fragile X, MECP2 duplication syndrome, RTT, Angelman, etc.) were excluded (also by evaluation from a clinician through the SSC and/or ATN).
Instruments
To assess Social Affect (inclusive of communication and reciprocal social interaction) and Restricted/Repetitive behaviours, the Autism Diagnostic Observation Schedule (ADOS), Module 1 was completed on all participants. ADOS scores are reported according to the research algorithm (No Words) for the domains of Social Affect and Restricted/Repetitive Behaviors (RRB). The Communication and Reciprocal Social Interaction domains are combined as part of the Social Affect domain using this research algorithm (Gotham, Risi, Pickles, & Lord, 2007), which results in improved predictive validity (Gotham et al., 2008). All participants included in this study had sufficient mobility and hand use to complete the ADOS. All psychologists who administered the ADOS attained research reliability.
Visual reception/nonverbal cognitive abilities and fine motor skills (nonverbal cognitive abilities), and expressive/receptive language abilities which comprised “verbal” cognitive abilities were assessed either using the Mullen Scales of Early Learning (MSEL) or the Bayley Scales of Infant and Toddler Development-III (BSID-III). These instruments have been utilized extensively within these populations and in large scale ASD research studies beyond the normative age ranges. In fact, a recent study demonstrated support for the practice of using MSEL age-equivalents to generate nonverbal IQ and verbal IQ scores in a group of individuals with idiopathic ASD beyond the typical age range of the test (Bishop, Guthrie, Coffing, & Lord, 2011). As per standard convention, age equivalents scores from the visual reception/cognitive and fine motor skills subtests were averaged to create the “nonverbal mental age” (NVMA) score, and scores from expressive and receptive language subtests were combined to create the “verbal mental age” (VMA) score (Bishop, et al., 2011; Lord, et al., 2011).The Vineland Adaptive Behavior Scales – II (VABS-II) was completed as a semi-structured interview with parents to assess overall adaptive behavior.
All parents of boys with MECP2 duplication syndrome completed the Aberrant Behavior Checklist (ABC). The ABC is a questionnaire that evaluates the presence of specific maladaptive behaviors in 5 categories (irritability, lethargy/withdrawal, inappropriate speech, hyperactivity, and stereotypic behavior). The ABC has been previously utilized in individuals with a variety of genetic syndromes, and has been used extensively in studies of non-syndromic ASD. ABC raw scores for each of the categories were analyzed, except for the “Inappropriate Speech” category because children in our study had limited speech. Parents of boys with MECP2 duplication syndrome also completed the Sensory Profile Questionnaire. The results of this entire questionnaire are not reported here, but results of whether or not participants had decreased awareness of pain/temperature are reported.
Statistics
Because of the broad age range of our participants, we initially examined correlations between chronological age and the ADOS Social Affect and RRB scales to determine whether or not performance on the ADOS was related to chronological age. Since these correlations were not significant (p=.52, p=.936), chronological age is not used as a covariate. Because of the small sample size, non-parametric statistics (Mann-Whitney U test) were used to compare differences between boys with MECP2 duplication syndrome, and boys with idiopathic ASD on our clinical outcome measures. To determine the relationship between duplication size and clinical severity in boys with MECP2 duplication syndrome, we used bivariate correlations. Since chronological age was not correlated with any outcome variables, partial correlations (controlling for age) were not utilized.
Results
ASD vs. MECP2 duplication syndrome
As expected, our groups were evenly matched for chronological age (See Table 1). All participants were given the MSEL or the BSID-III, and the VABS-II. Our matching procedures were again confirmed since there were no significant differences in NVMA (p=.55) or VMA (p=.27) between individuals with MECP2 duplication syndrome, and those with idiopathic ASD (See Table 1). In addition, individuals who were given the Bayley scales scored comparably to those who were given the Mullen Scales of Early Learning. Similarly, there were no significant differences between boys with MECP2 duplication syndrome and boys with idiopathic ASD on the composite scores of the VABS-II (p=1.00), or any VABS-II subscales (p=.17 to p=.62) (See Table 1).
Table 1.
Demographics
| Chronological Age (M, SD) |
Nonverbal Mental Age (M, SD) |
Verbal Mental Age (M, SD) |
Vineland Adaptive Behavior Composite Standard Score (M, SD) |
|
|---|---|---|---|---|
| Idiopathic ASD | 5.30 years, 1.96 years | 18.67 months, 6.17 months | 10.89 months, 3.90 months | 55.00, 9.15 |
| MECP2 duplication syndrome | 5.64 years, 2.63 years | 17.68 months, 6.56 months | 13.35 months, 5.28 months | 55.00, 4.30 |
Our data indicate that four of 10 boys with MECP2 duplication syndrome had a diagnosis of ASD prior to having a genetic etiological diagnosis. Upon direct evaluation, seven of 10 boys (70%) exceeded total cutoff scores for ASD on the ADOS. Figure 2 shows the ADOS social affect scores of individuals with idiopathic ASD and boys with MECP2 duplication syndrome. Specifically, although the majority of boys with MECP2 duplications exceed threshold cutoff scores, and thus are quite similar to boys with ASD, they do not have as many impairments in social affect as compared to boys with ASD [z=2.34; p=.01]. No differences in RRB were noted between the groups (z=.50, p=ns). It is important to note that there were no significant correlations between NVMA and ADOS social affect or RRB profiles (p=.52; p=.94).
Figure 2.
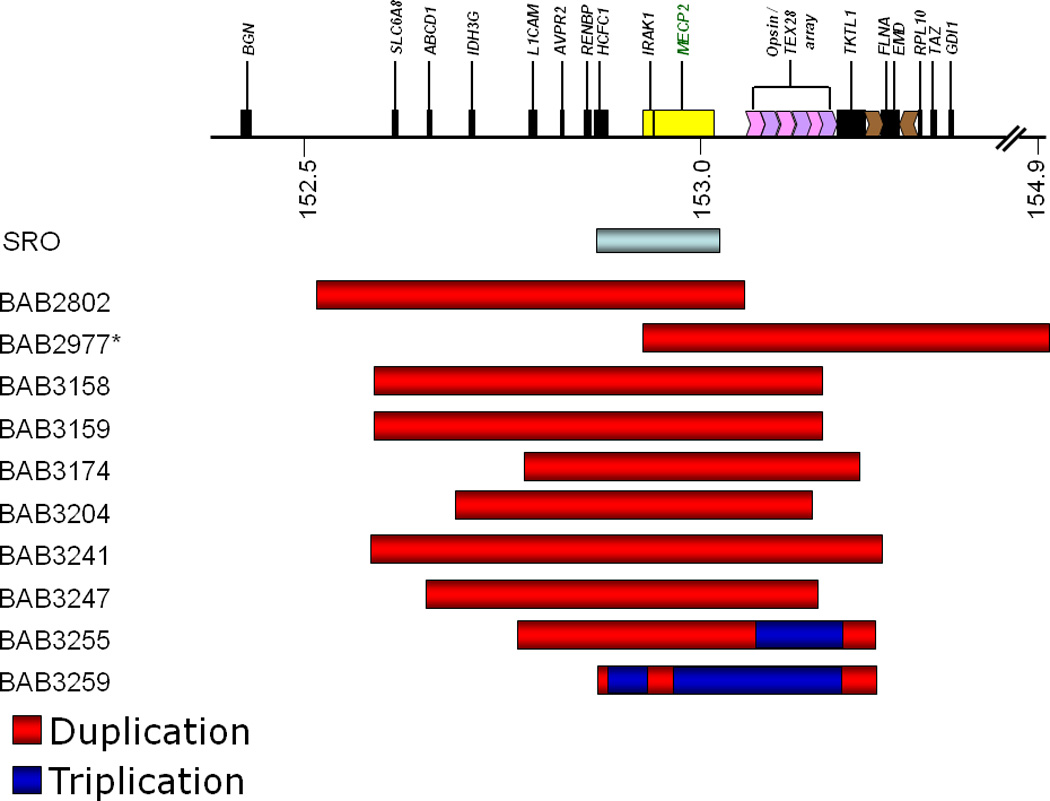
SRO represents the smallest region of overlap (in yellow) that extends from IRAK1 past MECP2. Duplications in this region alone are sufficient to cause MECP2 duplication syndrome.
Within group descriptions for MECP2 duplication syndrome
Table 3 shows the general clinical characteristics of boys with MECP2 duplication syndrome. As is consistent with previous studies, a majority of boys in this cohort have a history of hypotonia and recurrent respiratory infections. Most boys in our sample also have constipation and histories of gastroesophageal reflux. The age of genetic diagnosis of MECP2 duplication syndrome was very variable, ranging between 16 months to 7 years of age. Children who were older in terms of chronological age received later genetic diagnoses (r=.97; p<.001). We also inquired about sensitivity to pain and temperature. Data from the Sensory Profile Questionnaire and clinical interviews revealed that parents of nine of ten participants in our study reported that their boys either “frequently” or “always” (as opposed to occasionally, seldom, or never) had a decreased awareness of pain and temperature. Data from the ABC revealed that eight of ten boys with MECP2 duplication syndrome had clinically elevated scores for the Lethargy/Social Withdrawal subscale, and seven of ten boys had elevated scores for the Stereotyped behaviors subscale. No boys with MECP2 duplication syndrome had clinical elevations for the Irritability subscale, and only two of ten had elevations for the Hyperactivity subscale of the ABC.
Table 3.
Clinical Findings in boys with MECP2 duplication syndrome
| Patient Number |
Age at MECP2 genetic Diagnosis |
Constipation | GER | Infantile Hypotonia |
Recurrent Infections |
Pain Insensitivity |
|---|---|---|---|---|---|---|
| BAB2802 | 4 yo | X | X | X | X | X |
| BAB2977 | 33 months | X | X | X | ||
| BAB 3158 | 7 yo | X | X | X | X | X |
| BAB 3159 | 3 yo | X | X | X | X | X |
| BAB 3174 | 7 yo | X | X | X | X | |
| BAB 3204 | 7 yo | X | X | X | X | |
| BAB 3241 | 19 months | X | X | X | X | X |
| BAB 3247 | 17 months | X | X | X | X | X |
| BAB 3255 | 18 months | X | X | X | X | X |
| BAB 3259 | 16 months | X | X | X | X | X |
Figure 2 shows the mapping of duplications/triplications and the breakpoints of our participants with MECP2 duplication syndrome. As noted in the figure, duplications within the smallest region of overlap (SRO) are sufficient to cause MECP2 duplication syndrome. We examined the correlations between breakpoint size and our clinical outcome measures and did not find any statistically significant correlations for ADOS social affect scores (p=.55), ADOS RRB scores (p=.17), NVMA (p=.45), VMA (p=.48), or the VABS-II adaptive behavior composite (p=.35). There were also no significant correlations with any of the subscales of the ABC (p range=.59 to p=.88).
Discussion
In this study, we extended our previous work by comparing boys with MECP2 duplication syndrome to a chronological and nonverbal mental age matched group of boys with idiopathic ASD with an emphasis on comparing core ASD features and adaptive behavior. Within the MECP2 duplication group, we further delineated aspects of the behavioral phenotype, and examined whether duplication size corresponded to clinical severity. Our results provide further support that core behavioral aspects/features of ASD (e.g. social affect, RRB) are related to MECP2 duplication syndrome/overexpression of MECP2. In addition, our results suggest that within the MECP2 duplication group, breakpoint size alone does not predict differences in clinical severity.
Our direct comparisons of ASD profiles revealed many similarities between boys with MECP2 duplication syndrome, and the matched group of boys with ASD. A majority of boys with MECP2 duplication syndrome have impairments in social affect, albeit to a lesser degree than a matched sample of kids with idiopathic ASD. There were no differences in RRB between boys with idiopathic ASD and boys with MECP2 duplication syndrome. Of importance is that the ASD-related behaviors across groups cannot be attributable to low cognition alone since nonverbal mental age did not correlate with severity of ADOS social affect or RRB scores in our study. Further support for the spectrum of ASD-related behaviors being associated with overexpression of MECP2/MECP2 duplications is found in recent reports of girls with MECP2 duplications who have a milder cognitive phenotype but still exhibit ASD features (Grasshoff et al., 2011). Beyond ASD, alterations in MECP2 are connected to a broad range of social impairments, even within the context of “normal” cognitive functioning (Piton et al., 2011) (Ramocki, et al., 2009), further suggesting that low cognition alone cannot account for ASD-related phenotypes, and that common pathophysiological mechanisms may be involved across a broader range of disorders. When taken together with the findings that individuals with idiopathic ASD and mothers of individuals with idiopathic ASD were found to overexpress MECP2 in peripheral leukocytes in the absence of MECP2 mutations (Kuwano, et al., 2011), the aggregate data suggest that overexpression of MECP2 may be fundamentally related to the core social features of ASD. Our data also suggest that the MeCP2 protein may have an important role in “normal” social development.
In further delineating the core behavioral phenotype in MECP2 duplication syndrome, our data from the ABC suggested that stereotyped behaviors and lethargy/social withdrawal were common. In contrast, levels of irritability were not in a clinically significant range for any boys with MECP2 duplication syndrome and aggression does not appear to be a core aspect of the behavioral phenotype. Also, while two of ten boys with MECP2 duplication syndrome exceeded clinically significant levels for hyperactivity, hyperactivity also does not appear to be a core aspect of the behavioral phenotype in MECP2 duplication syndrome.
There is increasing evidence that altered levels of MeCP2 may modulate pain sensitivity (Geranton, Fratto, Tochiki, & Hunt, 2008; Samaco et al., 2008). For this reason, we had parents of boys with MECP2 duplication syndrome complete the Sensory Profile Questionnaire. Parents of nine out of ten boys in our study reported decreased sensitivity to pain and temperature, indicating that sensory hypo-responsiveness may be a core aspect of the phenotype in MECP2 duplication syndrome. These findings mirror those from a recent study of girls with RTT, where parents reported decreased sensitivity to pain, and decreased/delayed responses in situations likely to cause pain (e.g. falls, burns, injections, etc.) (Downs et al., 2010). Hypo-responsiveness to sensory stimuli has also been reported in a subset of individuals with idiopathic ASD; with a recent study demonstrating that hypo-responsiveness correlates strongly with ASD-related Social Affect profiles, but less so with repetitive behaviors (Foss-Feig, Heacock, & Cascio, 2012). Since the majority of our boys with MECP2 duplication syndrome “frequently” or “always” had decreased responses to pain and temperature, we did not calculate how severity of impairments in Social Affect corresponded to pain/temperature sensitivity within this study (because of decreased variability in scores), but this would be of importance to examine in future work.
There was variability in the age of genetic diagnosis in our boys with MECP2 duplications. Of note, however, is that older boys received later diagnoses, suggesting that in recent years, there may be increased awareness of the features of MECP2 duplication syndrome leading to formal genetic testing and diagnosis. Still, 40% of our cohort had an original diagnosis of an autism spectrum disorder. Our cohort group for this study consisted of boys with mobility and hand use, introducing a selection bias as compared to those boys who exhibited significant regression and were hospitalized. Although most boys in our sample also had hypotonia and recurrent respiratory infections, it may have been more challenging to sort out the distinct aspects of the phenotype of MECP2 duplication syndrome in these participants. Careful inquiry regarding family history may lead to an increased diagnostic yield in that often more than one family member was affected across generations; sometimes diagnoses of severe ASD were all that had been made.
Another goal of this study was to examine whether or not duplication size in boys with MECP2 duplication syndrome corresponded to clinical severity (e.g. NVMA, VMA, ADOS scores, adaptive behavior). As is consistent with previous studies (Lugtenberg, et al., 2009; Van Esch, et al., 2005), we did not find any significant correlations with duplication size and any of our clinical outcome measures. While this lack of significance may be attributable, in part, to our small sample size, another plausible explanation is that varying levels of MeCP2 protein or MECP2 transcript may account for the differences in clinical severity amongst our patients. For example, two brothers BAB3158 and BAB3159, have identical rearrangements, but differing levels of clinical severity, particularly with regard to ASD-related Social Affect (communication and reciprocal social interaction) and lethargy/social withdrawal. We previously reported a series of 30 male patients with MECP2 duplications (Carvalho, et al., 2009), in which the duplication sizes were between 250 kb and 2.6 MB. The smallest region of overlap (SRO) in our series combined with our unpublished results surmised from 44 patients is approximately 140 kb encompassing HCFC1, IRAK1, MECP2 as well as all known MECP2 cis-regulatory regions (Liu & Francke, 2006). By comparison, case BAB2977, despite carrying the largest duplication rearrangement, does not include the entire pre-defined SRO which may help explain why his clinical parameters are not as severe as all other patients who have the entire SRO duplicated (Figure 2).
While this study clearly extends our prior work, it does have limitations. First, we acknowledge that our sample size was small and this may limit the generalizability of our findings. As noted above, we included boys with MECP2 duplication syndrome who were mobile and had hand use, and thus were less severely affected than the broader population of individuals with MECP2 duplications. In addition, ASD is a very heterogeneous disorder, and although this study focused on the identification of common behaviors, we did not investigate common biomarkers (e.g. MECP2 expression levels) across idiopathic ASD and MECP2 duplication syndrome. Given recent research demonstrating altered MECP2 expression levels in idiopathic ASD, this approach has relevance in identifying possible sensitive diagnostic markers and treatment targets/outcomes and provides for a better examination of the potential contribution of individual genes such as MECP2 to specific behaviors such as ASD-related social impairment. Future studies should also examine the physiological correlates of ASD-related social impairment and features such as anxiety in MECP2 duplication syndrome and idiopathic ASD, given that CRH, which encodes corticotrophin releasing hormone, is a target gene of MeCP2 (Samaco, et al., 2012).
In spite of these limitations, the present study illustrates that overexpression/increased dosage of MECP2 is related to core features of ASD. In fact, both increases and decreases in MECP2 gene dosage and expression are related to ASD features (e.g. regression, stereotyped behaviors, social withdrawal) across mouse and human studies (Ramocki & Zoghbi, 2008; Samaco et al., 2009) (Coutinho, et al., 2007; Samaco, et al., 2008), demonstrating the importance of tight regulation of MECP2. Together, these studies should provide a potential framework for investigating treatments which could have broader impact for individuals with idiopathic ASD and those with MECP2 duplications.
Figure 1.
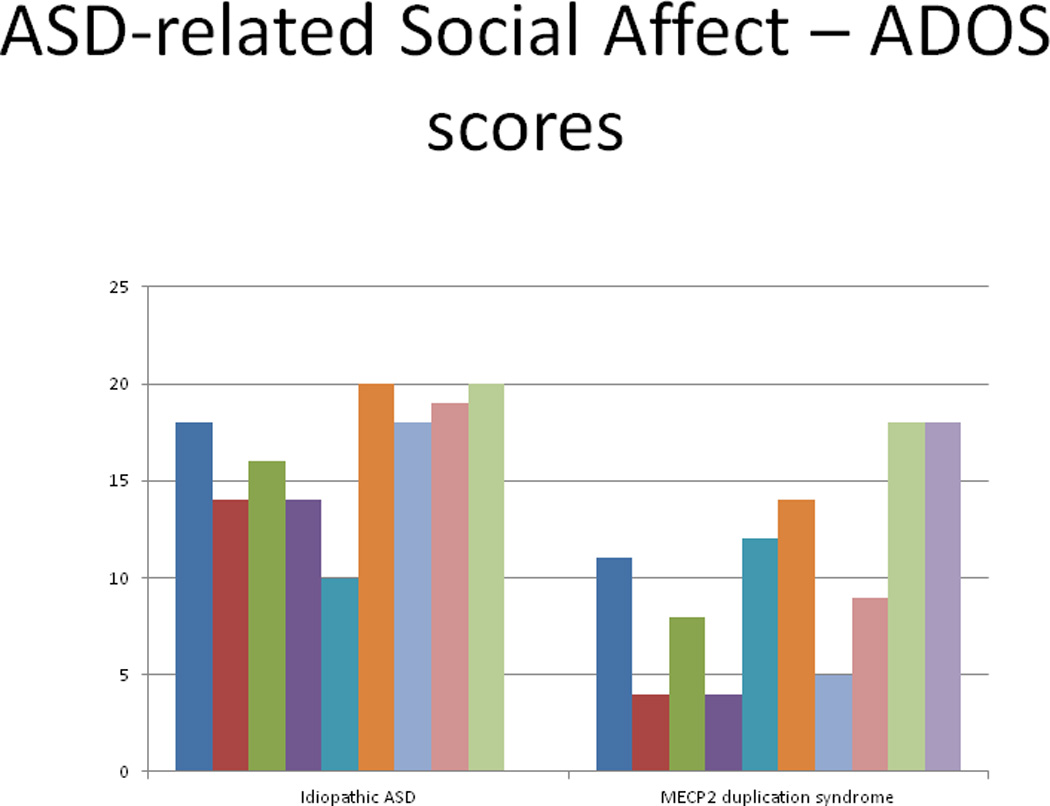
ADOS social affect scores for idiopathic ASD and MECP2 duplication syndrome
Table 2.
Aberrant Behavior Checklist subscale raw scores
| Irritability (M, SD) |
Lethargy/Social Withdrawal (M, SD) |
Stereotypy (M, SD) |
Hyperactivity (M, SD) |
|
|---|---|---|---|---|
| Idiopathic ASD (n=4) | 18.75, 12.09 | 11.50, 4.93 | 11.50, 2.89 | 31.25, 5.56 |
| MECP2 duplication syndrome (n=10) | 3.10, 2.77 | 9.70, 6.98 | 8.80, 7.98 | 8.30, 5.16 |
Acknowledgements
Funding for this project has been provided by a Vanderbilt Kennedy Center Hobbs Discovery Grant (to SUP), by 5P30HD015052-30 (to Elisabeth Dykens PI), and by NINDS grant 5K08NS062711 (to M.B.R.). We also wish to thank the individuals and families who so graciously participated in this study.
References
- Ariani F, Mari F, Pescucci C, Longo I, Bruttini M, Meloni I, et al. Real-time quantitative PCR as a routine method for screening large rearrangements in Rett syndrome: Report of one case of MECP2 deletion and one case of MECP2 duplication. Hum Mutat. 2004;24(2):172–177. doi: 10.1002/humu.20065. [DOI] [PubMed] [Google Scholar]
- Bishop SL, Guthrie W, Coffing M, Lord C. Convergent validity of the mullen scales of early learning and the differential ability scales in children with autism spectrum disorders. Am J Intellect Dev Disabil. 2011;116(5):331–343. doi: 10.1352/1944-7558-116.5.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney RM, Wolpert CM, Ravan SA, Shahbazian M, Ashley-Koch A, Cuccaro ML, et al. Identification of MeCP2 mutations in a series of females with autistic disorder. Pediatr Neurol. 2003;28(3):205–211. doi: 10.1016/s0887-8994(02)00624-0. [DOI] [PubMed] [Google Scholar]
- Carvalho CM, Ramocki MB, Pehlivan D, Franco LM, Gonzaga-Jauregui C, Fang P, et al. Inverted genomic segments and complex triplication rearrangements are mediated by inverted repeats in the human genome. Nat Genet. 2011;43(11):1074–1081. doi: 10.1038/ng.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho CM, Zhang F, Liu P, Patel A, Sahoo T, Bacino CA, et al. Complex rearrangements in patients with duplications of MECP2 can occur by fork stalling and template switching. Hum Mol Genet. 2009;18(12):2188–2203. doi: 10.1093/hmg/ddp151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb S, Guy J, Bird A. Reversibility of functional deficits in experimental models of Rett syndrome. Biochem Soc Trans. 2010;38(2):498–506. doi: 10.1042/BST0380498. [DOI] [PubMed] [Google Scholar]
- Coury D, Jones NE, Klatka K, Winklosky B, Perrin JM. Healthcare for children with autism: the Autism Treatment Network. Curr Opin Pediatr. 2009;21(6):828–832. doi: 10.1097/MOP.0b013e328331eaaa. [DOI] [PubMed] [Google Scholar]
- Coutinho AM, Oliveira G, Katz C, Feng J, Yan J, Yang C, et al. MECP2 coding sequence and 3'UTR variation in 172 unrelated autistic patients. Am J Med Genet B Neuropsychiatr Genet. 2007;144B(4):475–483. doi: 10.1002/ajmg.b.30490. [DOI] [PubMed] [Google Scholar]
- del Gaudio D, Fang P, Scaglia F, Ward PA, Craigen WJ, Glaze DG, et al. Increased MECP2 gene copy number as the result of genomic duplication in neurodevelopmentally delayed males. Genet Med. 2006;8(12):784–792. doi: 10.1097/01.gim.0000250502.28516.3c. [DOI] [PubMed] [Google Scholar]
- Dolen G, Carpenter RL, Ocain TD, Bear MF. Mechanism-based approaches to treating fragile X. Pharmacol Ther. 2010;127(1):78–93. doi: 10.1016/j.pharmthera.2010.02.008. [DOI] [PubMed] [Google Scholar]
- Downs J, Geranton SM, Bebbington A, Jacoby P, Bahi-Buisson N, Ravine D, et al. Linking MECP2 and pain sensitivity: the example of Rett syndrome. Am J Med Genet A. 2010;152A(5):1197–1205. doi: 10.1002/ajmg.a.33314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischbach GD, Lord C. The Simons Simplex Collection: a resource for identification of autism genetic risk factors. Neuron. 2010;68(2):192–195. doi: 10.1016/j.neuron.2010.10.006. [DOI] [PubMed] [Google Scholar]
- Foss-Feig JH, Heacock JL, Cascio CJ. TACTILE RESPONSIVENESS PATTERNS AND THEIR ASSOCIATION WITH CORE FEATURES IN AUTISM SPECTRUM DISORDERS. Res Autism Spectr Disord. 2012;6(1):337–344. doi: 10.1016/j.rasd.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friez MJ, Jones JR, Clarkson K, Lubs H, Abuelo D, Bier JA, et al. Recurrent infections, hypotonia, and mental retardation caused by duplication of MECP2 and adjacent region in Xq28. Pediatrics. 2006;118(6):e1687–e1695. doi: 10.1542/peds.2006-0395. [DOI] [PubMed] [Google Scholar]
- Geranton SM, Fratto V, Tochiki KK, Hunt SP. Descending serotonergic controls regulate inflammation-induced mechanical sensitivity and methyl-CpG-binding protein 2 phosphorylation in the rat superficial dorsal horn. Mol Pain. 2008;4:35. doi: 10.1186/1744-8069-4-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind DH. Genetics of autism spectrum disorders. Trends Cogn Sci. 2011;15(9):409–416. doi: 10.1016/j.tics.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotham K, Risi S, Dawson G, Tager-Flusberg H, Joseph R, Carter A, et al. A replication of the Autism Diagnostic Observation Schedule (ADOS) revised algorithms. J Am Acad Child Adolesc Psychiatry. 2008;47(6):642–651. doi: 10.1097/CHI.0b013e31816bffb7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotham K, Risi S, Pickles A, Lord C. The Autism Diagnostic Observation Schedule: revised algorithms for improved diagnostic validity. J Autism Dev Disord. 2007;37(4):613–627. doi: 10.1007/s10803-006-0280-1. [DOI] [PubMed] [Google Scholar]
- Grasshoff U, Bonin M, Goehring I, Ekici A, Dufke A, Cremer K, et al. De novo MECP2 duplication in two females with random X-inactivation and moderate mental retardation. Eur J Hum Genet. 2011;19(5):507–512. doi: 10.1038/ejhg.2010.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwano Y, Kamio Y, Kawai T, Katsuura S, Inada N, Takaki A, et al. Autism-associated gene expression in peripheral leucocytes commonly observed between subjects with autism and healthy women having autistic children. PLoS One. 2011;6(9):e24723. doi: 10.1371/journal.pone.0024723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasalle JM, Yasui DH. Evolving role of MeCP2 in Rett syndrome and autism. Epigenomics. 2009;1(1):119–130. doi: 10.2217/epi.09.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Francke U. Identification of cis-regulatory elements for MECP2 expression. Hum Mol Genet. 2006;15(11):1769–1782. doi: 10.1093/hmg/ddl099. [DOI] [PubMed] [Google Scholar]
- Loat CS, Curran S, Lewis CM, Duvall J, Geschwind D, Bolton P, et al. Methyl-CpG-binding protein 2 polymorphisms and vulnerability to autism. Genes Brain Behav. 2008;7(7):754–760. doi: 10.1111/j.1601-183X.2008.00414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord CBS. The autism spectrum: definitions, assessment and diagnoses. Br J Hosp Med. 2009 Mar;70(3):132–135. doi: 10.12968/hmed.2009.70.3.40552. [DOI] [PubMed] [Google Scholar]
- Lord C, Petkova E, Hus V, Gan W, Lu F, Martin DM, et al. A Multisite Study of the Clinical Diagnosis of Different Autism Spectrum Disorders. Arch Gen Psychiatry. 2011 doi: 10.1001/archgenpsychiatry.2011.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg D, Kleefstra T, Oudakker AR, Nillesen WM, Yntema HG, Tzschach A, et al. Structural variation in Xq28: MECP2 duplications in 1% of patients with unexplained XLMR and in 2% of male patients with severe encephalopathy. Eur J Hum Genet. 2009;17(4):444–453. doi: 10.1038/ejhg.2008.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meins M, Lehmann J, Gerresheim F, Herchenbach J, Hagedorn M, Hameister K, et al. Submicroscopic duplication in Xq28 causes increased expression of the MECP2 gene in a boy with severe mental retardation and features of Rett syndrome. J Med Genet. 2005;42(2):e12. doi: 10.1136/jmg.2004.023804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagarajan RP, Hogart AR, Gwye Y, Martin MR, LaSalle JM. Reduced MeCP2 expression is frequent in autism frontal cortex and correlates with aberrant MECP2 promoter methylation. Epigenetics. 2006;1(4):e1–e11. doi: 10.4161/epi.1.4.3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piton A, Gauthier J, Hamdan FF, Lafreniere RG, Yang Y, Henrion E, et al. Systematic resequencing of X-chromosome synaptic genes in autism spectrum disorder and schizophrenia. Mol Psychiatry. 2011;16(8):867–880. doi: 10.1038/mp.2010.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramocki MB, Peters SU, Tavyev YJ, Zhang F, Carvalho CM, Schaaf CP, et al. Autism and other neuropsychiatric symptoms are prevalent in individuals with MeCP2 duplication syndrome. Ann Neurol. 2009;66(6):771–782. doi: 10.1002/ana.21715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramocki MB, Tavyev YJ, Peters SU. The MECP2 duplication syndrome. Am J Med Genet A. 2010;152A(5):1079–1088. doi: 10.1002/ajmg.a.33184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramocki MB, Zoghbi HY. Failure of neuronal homeostasis results in common neuropsychiatric phenotypes. Nature. 2008;455(7215):912–918. doi: 10.1038/nature07457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaco RC, Fryer JD, Ren J, Fyffe S, Chao HT, Sun Y, et al. A partial loss of function allele of methyl-CpG-binding protein 2 predicts a human neurodevelopmental syndrome. Hum Mol Genet. 2008;17(12):1718–1727. doi: 10.1093/hmg/ddn062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaco RC, Mandel-Brehm C, Chao HT, Ward CS, Fyffe-Maricich SL, Ren J, et al. Loss of MeCP2 in aminergic neurons causes cell-autonomous defects in neurotransmitter synthesis and specific behavioral abnormalities. Proc Natl Acad Sci U S A. 2009;106(51):21966–21971. doi: 10.1073/pnas.0912257106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaco RC, Mandel-Brehm C, McGraw CM, Shaw CA, McGill BE, Zoghbi HY. Crh and Oprm1 mediate anxiety-related behavior and social approach in a mouse model of MECP2 duplication syndrome. Nat Genet. 2012;44(2):206–211. doi: 10.1038/ng.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders SJ, Ercan-Sencicek AG, Hus V, Luo R, Murtha MT, Moreno-De-Luca D, et al. Multiple recurrent de novo CNVs, including duplications of the 7q11.23 Williams syndrome region, are strongly associated with autism. Neuron. 2011;70(5):863–885. doi: 10.1016/j.neuron.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibayama A, Cook EH, Jr, Feng J, Glanzmann C, Yan J, Craddock N, et al. MECP2 structural and 3'-UTR variants in schizophrenia, autism and other psychiatric diseases: a possible association with autism. Am J Med Genet B Neuropsychiatr Genet. 2004;128B(1):50–53. doi: 10.1002/ajmg.b.30016. [DOI] [PubMed] [Google Scholar]
- State MW, Levitt P. The conundrums of understanding genetic risks for autism spectrum disorders. Nat Neurosci. 2011;14(12):1499–1506. doi: 10.1038/nn.2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanseem I, Anitha A, Nakamura K, Suda S, Iwata K, Matsuzaki H, et al. Elevated transcription factor specificity protein 1 in autistic brains alters the expression of autism candidate genes. Biol Psychiatry. 2012;71(5):410–418. doi: 10.1016/j.biopsych.2011.09.020. [DOI] [PubMed] [Google Scholar]
- Van Esch H. MECP2 Duplication Syndrome. Mol Syndromol. 2012;2(3–5):128–136. doi: 10.1159/000329580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Esch H, Bauters M, Ignatius J, Jansen M, Raynaud M, Hollanders K, et al. Duplication of the MECP2 region is a frequent cause of severe mental retardation and progressive neurological symptoms in males. Am J Hum Genet. 2005;77(3):442–453. doi: 10.1086/444549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenstra-VanderWeele J, Blakely RD. Networking in autism: leveraging genetic, biomarker and model system findings in the search for new treatments. Neuropsychopharmacology. 2012;37(1):196–212. doi: 10.1038/npp.2011.185. [DOI] [PMC free article] [PubMed] [Google Scholar]


