Abstract
Diet-induced obesity (DIO) reduces the ability of centrally administered insulin to reduce feeding behavior and also reduces the transport of insulin from the periphery to the central nervous system (CNS). The current study was designed to determine whether reversal of high-fat DIO restores the anorexic efficacy of central insulin and whether this is accompanied by restoration of the compromised insulin transport. Adult male Long-Evans rats were initially maintained on either a low-fat chow diet (LFD) or a high-fat diet (HFD). After 22 weeks, half of the animals on the HFD were changed to the LFD, whereas the other half continued on the HFD for an additional 8 weeks, such that there were 3 groups: 1) a LFD control group (Con; n = 18), 2) a HFD-fed, DIO group (n = 17), and 3) a HFD to LFD, DIO-reversal group (DIO-rev; n = 18). The DIO reversal resulted in a significant reduction of body weight and epididymal fat weight relative to the DIO group. Acute central insulin administration (8 mU) reduced food intake and caused weight loss in Con and DIO-rev but not DIO rats. Fasting cerebrospinal fluid insulin was higher in DIO than Con animals. However, after a peripheral bolus injection of insulin, cerebrospinal fluid insulin increased in Con and DIO-rev rats but not in the DIO group. These data provide support for previous reports that DIO inhibits both the central effects of insulin and insulin's transport to the CNS. Importantly, DIO-rev restored sensitivity to the effects of central insulin on food intake and insulin transport into the CNS.
One of the principal actions of insulin in the brain is the promotion of a net catabolic state. Chronic infusion of small amounts of insulin into the lateral cerebral ventricle was initially demonstrated to reduce food intake and body weight in baboons; and these actions were reversed when the infusion was ceased (1). Since that report, central insulin-induced hypophagia and weight loss have been observed in rats (2–6), mice (7, 8), chicks (9), and sheep (10), as well as in humans after intranasal insulin administration (11, 12). Importantly, the response to central insulin infusion is reduced when animals are genetically obese (2) or else maintained on a high-fat diet (HFD) (13–15), implying that these animals have insulin resistance within the central nervous system (CNS) as well as in the periphery.
Endogenous insulin is thought to enter the brain from the blood via a saturable, insulin receptor-mediated process in brain capillary endothelial cells (16, 17). Because insulin is secreted from the pancreas in direct proportion to body fat (18–21), insulin has been suggested to be an important negative feedback signal to the brain that helps to regulate/maintain body weight (22, 23). Insulin transport into the brain is compromised in obesity (24), such that the normal negative feedback action of insulin to suppress food intake and body weight is deficient. What is not known is whether the compromised insulin transport through the blood brain barrier and/or reduced catabolic action within the brain in animals on a HFD can be reversed by changing them to a low-fat chow diet (LFD). The current study was therefore designed to determine whether reversal of high-fat diet-induced obesity (DIO) will restore the anorexic efficacy of central insulin and whether this is accompanied by restoration of the compromised insulin transport.
Materials and Methods
Animals, housing, and diets
Adult male Long-Evans rats (250–300 g, 12–14 wk of age; Harlan, Indianapolis, Indiana) were housed in individual tub cages and maintained on a 12-hour light, 12-hour dark cycle in the Association for Assessment and Accreditation of Laboratory Animal Care-accredited animal facilities of the Metabolic Disease Institute of the University of Cincinnati. Except where specified, animals had ad libitum access to water and pelleted diet. Animals were maintained on either a LFD (n = 20 [Harlan Teklad Rat Chow, Madison, Wisconsin]; 3.1 kcal/g, percentage of energy from fat: 17%, carbohydrate: 58%, and protein: 25%) or a semipurified HFD (n = 40 [Open Source Diets, New Brunswick, New Jersey]; 4.54 kcal/g, percentage of energy from fat: 40%, carbohydrate: 45%, and protein: 15%) as we have described previously (25). After 22 weeks, half of the animals on the HFD were changed to the LFD, whereas the other half continued on the HFD. Animals were continued on these diets for an additional 8 weeks. Therefore, there were 3 groups in total: 1) a LFD control (Con) group, 2) a HFD-fed, DIO group, and 3) a HFD to LFD, DIO-reversal (DIO-rev) group. All protocols were approved by the University of Cincinnati Institutional Animal Care and Use Committee.
Third-ventricular cannulation (i3vt)
Animals were anesthetized with ip ketamine (70 mg/kg) and xylazine (6 mg/kg). They were shaved and positioned with the skull horizontal in a stereotaxic instrument (David Kopf Instruments, Tujunga, California), as previously described (26). After a small hole was drilled through the skull at a position 2.2 mm posterior to bregma, on the midline, the sagittal sinus was displaced laterally, and a stainless steel guide cannula (Plastics One, Roanoke, Virginia) was lowered 7.5 mm ventral to the dura. The cannula was fixed with dental acrylic anchored to the scull with 4 screws, and an obturator was inserted that extended 0.5 mm below the cannula. The surgery was performed using sterile techniques. Five days before food intake experiments, correct placement/viability of the cannula was confirmed by behavioral means by injecting 10 ng of angiotensin II (American Peptides, Sunnyvale, California) in 1-μL normal saline through the cannula. Rats consuming at least 5 mL of water within 30 minutes were considered to have a viable cannula (see Ref. 27); in total, 7 rats (2 Con, 2 DIO, and 3 DIO-rev) were excluded based on this criterion.
Food intake
The measurement of food intake was based on a previously established paradigm (27). Briefly, animals had food removed at the end of the light for 4 hours/day for 4 days before receiving i3vt injections. In addition, animals were individually handled for 5 minutes/day over these same 4 days. Animals underwent this protocol twice, 1 week apart, in a randomized crossover manner to receive either 8 mU of insulin (333.3 ng; Humulin 2 μL) or 2 μL of sterile normal saline on the 2 experimental days. Injections were given 1 hour before the onset of dark. Food hoppers were returned at the onset of dark, and intake was assessed at 2, 4, and 24 hours.
Cerebrospinal fluid (CSF) sampling
Fourteen to 16 days after the series of injections was completed, CSF samples were taken from each rat during the light cycle after an overnight fast. Thirty minutes before CSF sampling, rats were injected ip with either 1-U/kg insulin or normal saline. Ten minutes after the injection, rats were placed in the chamber of an isoflurane anesthetic machine (RC2 Rodent Anesthesia System; VetEquip, Pleasanton, California). After induction of anesthesia, the area between the shoulders and below the skull was shaved, and the rat was positioned in the stereotaxic instrument. The rat was positioned securely into the ear bars and an isoflurane nose cone with the incisor bar positioned so the head was ventroflexed. CSF sampling was performed based on our previously described CSF collection procedure (28). Briefly, the blunt end of a 25-gauge (1½-inch) needle was connected to microrenathane tubing and mounted onto an electrode holder adjusted to be perpendicular to the ear bars. A 1.0-mL syringe containing normal saline was attached via a 25-gauge needle to the distal end of the tubing. Tubing was flushed with saline, and negative pressure was applied to create a small air pocket in the tubing. After the incision site was swabbed with ethanol, a midsagittal incision of the skin, approximately 2 cm in length, was made inferior to the occipital crest. Tissue was separated by blunt dissection with forceps exposing the atlantooccipital membrane. The area was cleaned with sterile saline-soaked cotton swabs to remove any blood or interstitial fluid. The sharp tip of the needle was advanced toward the membrane using the sterotaxic controls and the needle then penetrated the dura mater, entering the cisternum magnum. CSF flow was spontaneously initiated with the air bubble moving toward the syringe. Slight negative pressure was applied to the syringe until the air bubble entered the syringe, and CSF was then collected via gravity flow into a 500-μL tube on ice. Approximately 150-μl CSF was collected in 2 minutes. The CSF samples were frozen on dry ice and stored at −80°C before analysis. After CSF collection, rats were immediately filled by decapitation, and blood was collected in chilled EDTA-coated tubes and stored on ice before centrifugation. Separated plasma was later stored at −80°C before analysis.
Insulin ELISA
Insulin levels in CSF and plasma were measured using an Ultra-Sensitive Rat Insulin ELISA kit (Crystal Chem, Downers Grove, Illinois), according to the supplied instructions. Briefly, a sample of CSF, plasma, or insulin standard (5 μL/sample) was added to an antibody-coated microplate. After incubation for 2 hours at 4°C, 100 μL of antiinsulin enzyme conjugate were dispensed to each well and incubated at room temperature for 30 minutes. The plate was washed, and 100 μL of enzyme substrate solution were added to each well and incubated for 40 minutes. The stop solution was then added, and insulin concentrations were determined by subtracting the absorbance at 630 nm from the absorbance at 450 nm.
Statistical analyses
Food intake was analyzed using repeated-measures ANOVA with post hoc Fisher's least significant difference tests. Plasma, CSF, and insulin ratios were analyzed using factorial ANOVA with post hoc Fisher's least significant difference tests (Statistica; StatSoft, Tulsa, Oklahoma). Correlations were calculated using SigmaPlot (Systat Software, San Jose, California). Significance was accepted at P < .05 with data reported as mean ± SEM.
Results
Body and epididymal fat pad weights
At 22 weeks, when the diet reversal was initiated, rats that had been fed HFD weighed significantly more than LFD-fed animals (F2,31 = 27.59 P < .05; see Table 1). There were significant differences among all groups at the conclusion of the experiment with Con having the lowest mean body weight and DIO the greatest (F2,31 = 35.23 P < .05). A similar effect was observed with epididymal fat pad weights, with DIO-rev having an intermediate phenotype between the smaller Con and larger DIO groups (F2,31 = 41.07 P < .05).
Table 1.
Body weights, epididymal fat pad weight, and plasma glucose levels
| Con | DIO | DIO-rev | |
|---|---|---|---|
| Body weight before DIO-rev (g) | 481.9 ± 12.8a | 606.8 ± 14.9b | 601.4 ± 12.5b |
| Final body weight (g) | 512.5 ± 13.4a | 652.18 ± 16.3b | 566.7 ± 14.0c |
| Epididymal fat pad weight (g) | 12.3 ± 0.9a | 22.7 ± 1.4b | 16.4 ± 2.0c |
| Fasting plasma glucose (mg/dL) | 132.8 ± 8.8 | 144.2 ± 10.7 | 125 ± 11.2 |
| Plasma glucose after 1-U/kg insulin (mg/dL) | 73.2 ± 5.3* | 103.3 ± 10.3* | 64.3 ± 16.2* |
Data are presented as mean ± SEM. Differing superscript letters indicate differences between dietary conditions, an asterisk indicates differences from fasted state P < .05.
Effect of exogenous central insulin on food intake and body weight
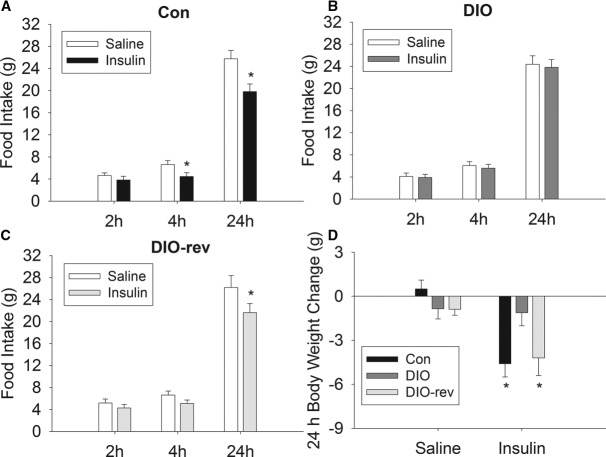
Food intake was significantly reduced by i3vt insulin injection after 4 and 24 hours (F2,68 = 12.657, P < .05; see Figure 1A) in animals maintained on the LFD for the duration of the experiment. In contrast, DIO rats did not change their food intake relative to receiving vehicle in response to i3vt insulin at any time point measured (Figure 1B). The DIO-rev group had a significant decrease in food intake after i3vt insulin at 24 hours (F2,64 = 10.385, P < .05), and there was a trend for a reduction at 4 hours (P = .08; see Figure 1C). There was a significant interaction effect between dietary treatment and insulin injection on body weight change (F2,50 = 9.1205, P < .05). After insulin administration both Con (P < .05) and DIO-rev (P < .05) groups, rats had significant reductions in body weight after 24 hours, relative to changes after saline, and this did not occur in the DIO group (Figure 1D).
Figure 1.
The effect of i3vt insulin on food intake and body weight. i3vt insulin reduced food intake in Con (A) but not DIO (B) rats, and a similar reduction of food intake was observed in DIO-rev (C) rats. There was weight loss 24 hours after i3vt insulin in Con and DIO-rev animals but not in the DIO group (D). Data are presented as mean ± SEM; *P < .05 vs saline.
Effect of DIO and DIO-rev on fasting insulin levels and after ip insulin injection
Plasma
There was a significant interaction effect between dietary treatment and insulin injection on plasma insulin levels (F2,28 = 4.11, P < .05). DIO rats had significantly increased fasting plasma insulin relative to Con levels (P < .05), and plasma insulin in DIO-rev animals was not different from that in animals fed LFD for the duration of the experiment. Administration of 1-U/kg insulin produced a 9- to 10-fold increase in plasma insulin in each of the groups. Therefore, DIO animals continued to have elevated plasma insulin in response to bolus insulin injection compared with the level in the Con group (P < .05), and the DIO-rev group had incremented plasma insulin comparable with that of Con animals (see Figure 2A). There was a significant main effect of insulin treatment on plasma glucose (F1,28 = 22.64, P < .05; see Table 1), no significant effect of dietary treatment was observed on glucose levels.
Figure 2.

The effect of bolus insulin injection on plasma and CSF insulin concentrations. DIO rats had both increased fasting and postinjection plasma insulin relative to the Con group (A). CSF insulin was higher in DIO than Con animals, and CSF insulin in the DIO-rev group was not elevated relative to Con. After bolus peripheral insulin injection, CSF insulin increased in Con and DIO-rev rats but not in the DIO group (B). The ratio of CSF to plasma insulin was not different in the fasted condition among groups. Peripheral insulin reduced the ratio in each of the groups, and this reduction was accentuated in DIO animals relative to the Con and DIO-rev groups (C). Data are presented as mean ± SEM; differences at the P < .05 level are represented by different superscript letters; ie, groups that do not display the same letter are significantly different from one another.
Cerebrospinal fluid
A significant interaction was also observed for CSF insulin levels (F2,28 = 5.43, P < .05). Consistent with the plasma levels, fasting CSF insulin was higher in DIO animals than in the Con group (P < .05), whereas CSF insulin in the DIO-rev group was not elevated relative to that in Con animals. Thirty minutes after exogenous insulin injection, CSF insulin was significantly increased in Con rats (P < .05), whereas no increase was observed in DIO rats. Insulin increased in the CSF of the DIO-rev group similarly to that in the Con group, and this was significantly greater than what occurred in DIO animals (P < .05; see Figure 2B).
CSF: plasma ratio and correlations
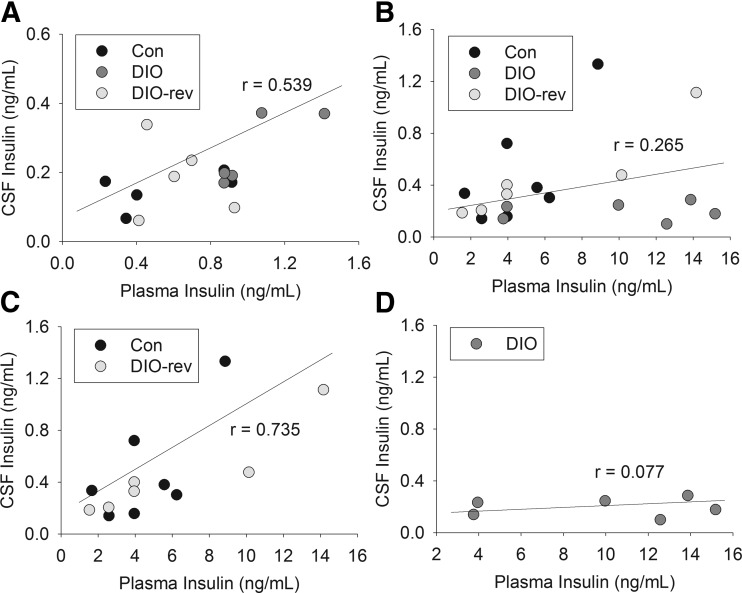
The ratio of CSF to plasma insulin was not different among groups in the fasted condition. When insulin was injected peripherally and CSF sampled soon thereafter, there was a marked reduction in the ratio in each of the groups (F1,28 = 24.16, P < .05), presumably due to the lag in getting insulin into the brain and CSF. Nonetheless, this reduction in the ratio was accentuated in DIO animals relative to the Con (P < .05) and DIO-rev (P < .05) groups (see Figure 2C). There was a strong positive correlation between fasting plasma and CSF insulin levels across the groups (r = 0.539, P < .05; see Figure 3A). However, after insulin injection, plasma and CSF insulin were not significantly correlated (r = 0.265; see Figure 3B). Interestingly, the strong positive correlation between plasma and CSF insulin remained when analyses were confined to the Con and DIO-rev groups (r = 0.735, P < .05; see Figure 3C), with no significant correlation in DIO animals (r = 0.077; see Figure 3D).
Figure 3.
Correlations between plasma and CSF insulin in Con, DIO, and DIO-rev rats. A strong positive correlation between fasting plasma and CSF insulin levels occurred across the groups (A). After insulin injection, plasma and CSF insulin were not significantly correlated when all 3 groups were considered together (B). However, the strong positive correlation remained in Con and DIO-rev groups (C), with no significant correlation in DIO animals (D).
Discussion
Insulin reduces food intake and body weight when it is administered i3vt, chronically or acutely, in male rats consuming LFDs (2–6, 8, 29, 30). Although occasional reports have failed to observe a reduction in food intake after i3vt insulin (eg, Ref. 31), such inconsistencies relating to infusion of insulin as well as other compounds are not uncommon and have recently been reviewed in detail (32). The important point is that most published reports have documented a significant reduction of food intake and body weight after i3vt insulin. When maintained on a HFD, rats become obese, and these DIO rats become resistant to i3vt insulin (15). The current findings confirm that DIO leads to both a decrease in the hypophagic effects of insulin as well as impaired transport of insulin from the periphery to the CNS. Importantly, the reversal of DIO by feeding a chow diet for 8 weeks resulted in recovery of both the hypophagia produced by i3vt and the transport of peripherally injected insulin into the CSF. In addition, there were strong correlations between CSF and plasma insulin levels in fasted animals across groups, and this relationship was retained in the Con and DIO-rev groups but not the DIO group after peripheral insulin injection.
Insulin acts peripherally to reduce circulating glucose by increasing the cellular uptake of glucose, which is then used as fuel or stored as glycogen (33). The important consequence is that the systemic administration of insulin results in a decrease of blood glucose. In hyperglycemic persons with diabetes, exogenous insulin reduces blood glucose toward normal; in individuals with euglycemia, insulin administration causes hypoglycemia. Insulin is secreted in response to elevated or increasing glucose, and the magnitude of the response is directly proportional to body fat; ie, insulin can be considered as an adiposity signal in that leaner individuals secrete less insulin in response to a given level of glucose than individuals with greater adiposity (18–20). The insulin receptor is widely distributed throughout the body, including the brain, reflecting the numerous important functions insulin performs. Within the CNS insulin alters food intake (1, 34) and energy expenditure (30, 35), systemic glucose (36), cognitive function (37–39), and sympathetic output (40).
Given that insulin is a large peptide hormone, it was traditionally assumed that it could not easily penetrate the blood brain barrier (41). However, changes of blood insulin were subsequently found to be paralleled in the CSF, implying that circulating insulin can enter and influence the brain, perhaps providing a hormonal indicator of adiposity (42). It was initially suggested that insulin enters the CSF directly from plasma via the choroid plexus, a possibility supported by evidence of dense insulin binding sites and high receptor concentrations in the choroid plexus, close to the wall of the third ventricle in the ventral hypothalamus (43, 44). This hypothesis is consistent with observations that insulin given into CSF in the third ventricle has catabolic actions (1, 3, 29). Subsequent research suggested rather that insulin is transported into the brain via an insulin receptor-mediated, saturable pathway in brain capillary endothelial cells (16). This work established the 3-compartment model (plasma-brain interstitial fluid-CSF) (45). However, it should be noted that this route of insulin movement through brain parenchyma has not been empirically tested. We therefore assume that insulin measured in the CSF after its peripheral administration has passed through brain interstitial fluid and had an opportunity to act upon insulin receptors on brain cells, a conclusion also reached by Banks (46). The residual insulin that can be assessed in the CSF is subsequently removed by insulin receptors on the choroid plexus. This is because insulin receptors are predominantly located on the CSF side of the choroid plexus (44) and, consequently, better positioned to remove insulin from CSF than to transport it into the CSF from the blood. In actuality, although the route taken by insulin molecules in the blood to get into the CSF is of importance, it is irrelevant to the present findings and conclusions. Insulin levels in the CSF are highly correlated with plasma insulin in the steady state, with peripheral infusions of insulin (or glucose, which elevates pancreatic insulin secretion) resulting in proportionally increased CSF insulin levels (47). The important point is that the appearance of insulin in the CSF after its peripheral administration is reduced in obesity (24); ie, there is insulin resistance at the level of transport. Because the transport relies upon insulin receptors, whether on brain endothelial cells or elsewhere, this is consistent with a generalized insulin resistance in obesity.
The compromised ability of insulin to reduce food intake and body weight in rats on a HFD has been previously reported (15), and the effect was determined to be independent of the animal's weight or adiposity by the use of a pair-feeding paradigm (27). Additionally, the decreased net catabolic effect of central insulin in animals fed a HFD has been reported to be mediated, at least in part, by the saturated fatty acid, palmitic acid, and its activation of protein kinase C-θ in the arcuate nucleus of the hypothalamus (48). The current study establishes that transferring animals from a HFD to a LFD can reverse the compromised response to central insulin that occurs in HFD-fed animals, as demonstrated by restoration of the hypophagic response to i3vt insulin infusion. Therefore, diet-induced central insulin resistance is reversible when animals are no longer fed the HFD. Based on our findings, this process occurs within 8 weeks in male rats, although the precise time course of this change remains undefined.
Feeding the HFD led to increased fasting insulin relative to LFD-fed control animals in both plasma and CSF. There are numerous reports of increased fasting plasma insulin (19, 49, 50); similar observations have been made in CSF of animal models of obesity (51, 52). Circulating levels of insulin were relatively low in all groups, presumably due to a combination of the isoflurane-mediated suppression of insulin release (53) and the fasted state of the animals. Thirty minutes after peripheral injection of insulin, increases in circulating insulin were greater in DIO animals than in control animals, and those in the DIO-rev group were not different compared with the other dietary conditions. Overall, the plasma insulin levels suggest that the DIO rats had peripheral insulin resistance relative to control and DIO-rev animals.
Consistent with our data on obese rats, insulin levels in the CSF have previously been reported to be elevated in obese rats (52), sheep (54), and humans (55). Although this appears inconsistent with a recent report on humans (56), that group reported only ratios of plasma to CSF insulin without presenting the actual levels of insulin in the CSF. To our knowledge, this is the first report that the compromised blood-CNS transport of insulin after DIO is reversible. Whether the reduced insulin transport relates to consumption of a HFD per se and/or to the consequent adiposity is not known. The DIO state leads to a reduction in central nervous insulin sensitivity in animal models (27, 54) and in humans (57), and many factors, including triglycerides, free-fatty acids, basal insulin, leptin and others, are altered. For this reason, it is difficult to ascertain whether changes in insulin transport and/or insulin-induced hypophagia are mediated by any specific one or combinations of these factors, by individual dietary nutrients, or alternatively by obesity-induced insulin resistance that occurs throughout the body.
There are some limitations to the current study that should be noted. First, we examined central insulin sensitivity by injecting pharmacological doses of insulin into the CSF. Such large doses are used because the administered insulin must move against the normal flow of fluid out of the brain parenchyma. Baskin et al (44) found that insulin administered directly into the CSF penetrates into the hypothalamic parenchyma sufficiently far to reach the arcuate nucleus. Additionally, despite disparate body weights among groups in the present experiment, animals were injected with a fixed dose of insulin. Hence, it could be suggested that a higher dose may have been required to produce an effect in DIO rats. However, given the high pharmacological dose of insulin administered (8 mU), and that doses of 1 mU reduce food intake in lean rats (58), it seems unlikely that a weight-based dose would have altered the outcomes significantly.
The current studies demonstrate the multifaceted effects of DIO on insulin activity within, and transport into, the CNS. Importantly, both the sensitivity to the effects central insulin and insulin transport are restored by DIO-rev. Central insulin resistance, and its association with leptin resistance, may be important factors in understanding the development of obesity. Greater understanding of insulin penetration into the brain will allow studies to incorporate this process as a novel strategy for the prevention and treatment of obesity and type 2 diabetes mellitus.
Acknowledgments
This work was supported by National Institutes of Health (NIH) Grants DK 017844 and DK 095440 (to S.C.W.). D.P.B. was supported by the National Health and Medical Research Council Early Career Fellowship 1013264.
Disclosure Summary: D.P.B., J.D.M., M.L., B.M.R., and S.C.W. have nothing to disclose. D.A.D. consults for Amylin, Lilly, Novo Nordisk, and Zealand and has research support from Ethicon Endo-Surgery and MannKind. R.J.S. has consultancies, research support, or is a paid speaker with the following companies: Ethicon Endo-Surgery/Johnson & Johnson, Novo Nordisk, Merck, Novartis, Angiochem, Zafgen, Takeda, Ablaris, Pfizer, Eli Lilly, and Zealand Pharma.
Footnotes
- CNS
- central nervous system
- Con
- LFD control
- CSF
- cerebrospinal fluid
- DIO
- diet-induced obesity
- DIO-rev
- DIO-reversal
- HFD
- high-fat diet
- i3vt
- third-ventricular cannulation
- LFD
- low-fat chow diet.
References
- 1. Woods SC, Lotter EC, McKay LD, Porte D., Jr Chronic intracerebroventricular infusion of insulin reduces food intake and body weight of baboons. Nature. 1979;282:503–505 [DOI] [PubMed] [Google Scholar]
- 2. Ikeda H, West DB, Pustek JJ, Figlewicz DP, Greenwood MR, Porte D, Jr, Woods SC. Intraventricular insulin reduces food intake and body weight of lean but not obese Zucker rats. Appetite. 1986;7:381–386 [DOI] [PubMed] [Google Scholar]
- 3. Air EL, Benoit SC, Clegg DJ, Seeley RJ, Woods SC. Insulin and leptin combine additively to reduce food intake and body weight in rats. Endocrinology. 2002;143:2449–2452 [DOI] [PubMed] [Google Scholar]
- 4. Clegg DJ, Riedy CA, Smith KA, Benoit SC, Woods SC. Differential sensitivity to central leptin and insulin in male and female rats. Diabetes. 2003;52:682–687 [DOI] [PubMed] [Google Scholar]
- 5. Plata-Salaman CR, Oomura Y. Effect of intra-third ventricular administration of insulin on food intake after food deprivation. Physiol Behav. 1986;37:735–739 [DOI] [PubMed] [Google Scholar]
- 6. Brief DJ, Davis JD. Reduction of food intake and body weight by chronic intraventricular insulin infusion. Brain Res Bull. 1984;12:571–575 [DOI] [PubMed] [Google Scholar]
- 7. Brown LM, Clegg DJ, Benoit SC, Woods SC. Intraventricular insulin and leptin reduce food intake and body weight in C57BL/6J mice. Physiol Behav. 2006;30:687–691 [DOI] [PubMed] [Google Scholar]
- 8. Jaillard T, Roger M, Galinier A, Guillou P, Benani A, Leloup C, Casteilla L, Penicaud L, Lorsignol A. Hypothalamic reactive oxygen species are required for insulin-induced food intake inhibition: an NADPH oxidase-dependent mechanism. Diabetes. 2009;58:1544–1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shiraishi J, Yanagita K, Fukumori R, Sugino T, Fujita M, Kawakami S, McMurtry JP, Bungo T. Comparisons of insulin related parameters in commercial-type chicks: evidence for insulin resistance in broiler chicks. Physiol Behav. 2011;103:233–239 [DOI] [PubMed] [Google Scholar]
- 10. Foster LA, Ames NK, Emery RS. Food intake and serum insulin responses to intraventricular infusions of insulin and IGF-I. Physiol Behav. 1991;50(4):745–749 [DOI] [PubMed] [Google Scholar]
- 11. Benedict C, Kern W, Schultes B, Born J, Hallschmid M. Differential sensitivity of men and women to anorexigenic and memory-improving effects of intranasal insulin. J Clin Endocrinol Metab. 2008;93:1339–1344 [DOI] [PubMed] [Google Scholar]
- 12. Hallschmid M, Benedict C, Schultes B, Fehm HL, Born J, Kern W. Intranasal insulin reduces body fat in men but not in women. Diabetes. 2004;53:3024–3029 [DOI] [PubMed] [Google Scholar]
- 13. Arase K, Fisler JS, Shargill NS, York DA, Bray GA. Intracerebroventricular infusions of 3-OHB and insulin in a rat model of dietary obesity. Am J Physiol. 1988;255:R974–R981 [DOI] [PubMed] [Google Scholar]
- 14. Chavez M, Riedy CA, van Dijk G, Woods SC. Central insulin and macronutrient intake in the rat. Am J Physiol. 1996;271:R727–R731 [DOI] [PubMed] [Google Scholar]
- 15. Clegg DJ, Benoit SC, Reed JA, Woods SC, Dunn-Meynell A, Levin BE. Reduced anorexic effects of insulin in obesity-prone rats fed a moderate-fat diet. Am J Physiol Regul Integr Comp Physiol. 2005;288:R981–R986 [DOI] [PubMed] [Google Scholar]
- 16. Baura G, Foster D, Porte D, Jr, Kahn SE, Bergman RN, Cobelli C, Schwartz MW. Saturable transport of insulin from plasma into the central nervous system of dogs in vivo: a mechanism for regulated insulin delivery to the brain. J Clin Invest. 1993;92:1824–1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Calhau C, Martel F, Pinheiro-Silva S, Pinheiro H, Soares-da-Silva P, Hipolito-Reis C, Azevedo I. Modulation of insulin transport in rat brain microvessel endothelial cells by an ecto-phosphatase activity. J Cell Biochem. 2002;84:389–400 [DOI] [PubMed] [Google Scholar]
- 18. Bagdade JD. Basal insulin and obesity. Lancet. 1968;2:630–631 [DOI] [PubMed] [Google Scholar]
- 19. Bagdade JD, Bierman EL, Porte D., Jr The significance of basal insulin levels in the evaluation of the insulin response to glucose in diabetic and nondiabetic subjects. J Clin Invest. 1967;46:1549–1557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stephan F, Reville P, Thierry R, Schlienger JL. Correlations between plasma insulin and body weight in obesity, anorexia nervosa and diabetes mellitus. Diabetologia. 1972;8:196–201 [DOI] [PubMed] [Google Scholar]
- 21. Woods SC, Decke E, Vasselli JR. Metabolic hormones and regulation of body weight. Psychol Rev. 1974;81:26–43 [DOI] [PubMed] [Google Scholar]
- 22. Woods SC, Seeley RJ, Porte D, Jr, Schwartz MW. Signals that regulate food intake and energy homeostasis. Science. 1998;280:1378–1383 [DOI] [PubMed] [Google Scholar]
- 23. Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–671 [DOI] [PubMed] [Google Scholar]
- 24. Kaiyala K, Prigeon RL, Kahn SE, Woods SC, Schwartz MW. Obesity induced by a high-fat diet is associated with reduced brain insulin transport in dogs. Diabetes. 2000;49:1525–1533 [DOI] [PubMed] [Google Scholar]
- 25. Woods SC, Seeley RJ, Rushing PA, D'Alessio DA, Tso P. A controlled high-fat diet induces an obese syndrome in rats. J Nutr. 2003;133:1081–1087 [DOI] [PubMed] [Google Scholar]
- 26. Begg DP, Kent S, McKinley MJ, Mathai ML. Suppression of endotoxin-induced fever in near-term pregnant rats is mediated by brain nitric oxide. Am J Physiol Regul Integr Comp Physiol. 2007;292:R2174–R2178 [DOI] [PubMed] [Google Scholar]
- 27. Clegg DJ, Gotoh K, Kemp C, Wortman MD, Benoit SC, Brown LM, D'Alessio D, Tso P, Seeley RJ, Woods SC. Consumption of a high-fat diet induces central insulin resistance independent of adiposity. Physiol Behav. 2011;103:10–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu M, Shen L, Begg DP, D'alessio DA, Woods SC. Insulin increases central apolipoprotein E levels as revealed by an improved technique for collection of cerebrospinal fluid from rats. J Neurosci Methods. 2012;209(1):106–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Air EL, Benoit SC, Blake Smith KA, Clegg DJ, Woods SC. Acute third ventricular administration of insulin decreases food intake in two paradigms. Pharmacol Biochem Behav. 2002;72:423–429 [DOI] [PubMed] [Google Scholar]
- 30. Benoit SC, Air EL, Coolen LM, Strauss R, Jackman A, Clegg DJ, Seeley RJ, Woods SC. The catabolic action of insulin in the brain is mediated by melanocortins. J Neurosci. 2002;22:9048–9052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jessen L, Clegg DJ, Bouman SD. Evaluation of the lack of anorectic effect of intracerebroventricular insulin in rats. Am J Physiol Regul Integr Comp Physiol. 2010;298:R43–R50 [DOI] [PubMed] [Google Scholar]
- 32. Woods SC, Langhans W. Inconsistencies in the assessment of food intake. Am J Physiol Endocrinol Metab. 2012;303(12):E1408–E1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cooney GJ, Caterson ID, Newsholme EA. The effect of insulin and noradrenaline on the uptake of 2-[1–14C]deoxyglucose in vivo by brown adipose tissue and other glucose-utilising tissues of the mouse. FEBS Lett. 1985;188:257–261 [DOI] [PubMed] [Google Scholar]
- 34. Bruning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, Klein R, Krone W, Muller-Wieland D, Kahn CR. Role of brain insulin receptor in control of body weight and reproduction. Science. 2000;289:2122–2125 [DOI] [PubMed] [Google Scholar]
- 35. Muller C, Voirol MJ, Stefanoni N, Surmely JF, Jequier E, Gaillard RC, Tappy L. Effect of chronic intracerebroventricular infusion of insulin on brown adipose tissue activity in fed and fasted rats. Int J Obes Relat Metab Disord. 1997;21:562–566 [DOI] [PubMed] [Google Scholar]
- 36. Fisher SJ, Bruning JC, Lannon S, Kahn CR. Insulin signaling in the central nervous system is critical for the normal sympathoadrenal response to hypoglycemia. Diabetes. 2005;54:1447–1451 [DOI] [PubMed] [Google Scholar]
- 37. Stockhorst U, de Fries D, Steingrueber HJ, Scherbaum WA. Insulin and the CNS: effects on food intake, memory, and endocrine parameters and the role of intranasal insulin administration in humans. Physiol Behav. 2004;83:47–54 [DOI] [PubMed] [Google Scholar]
- 38. Stockhorst U, Huenig A, Ziegler D, Scherbaum W. Unconditioned and conditioned effects of intravenous insulin and glucose on heart rate variability in healthy men. Physiol Behav. 2011;103:31–38 [DOI] [PubMed] [Google Scholar]
- 39. Craft S, Baker LD, Montine TJ, Minoshima S, Watson GS, Claxton A, Arbuckle M, Callaghan M, Tsai E, Plymate SR, Green PS, Leverenz J, Cross D, Gerton B. Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: a pilot clinical trial. Arch Neurol. 2012;69:29–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ward KR, Bardgett JF, Wolfgang L, Stocker SD. Sympathetic response to insulin is mediated by melanocortin 3/4 receptors in the hypothalamic paraventricular nucleus. Hypertension. 2011;57:435–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Havrankova J, Schmechel D, Roth J, Brownstein MJ. Identification of insulin in the rat brain. Proc Natl Acad Sci USA. 1978;75:5737–5741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Woods SC, Porte D., Jr Insulin and the set-point regulation of body weight. In: Hunger: Basic Mechanisms and Clinical Implications. New York, NY: Raven Press; 1976;273–280 [Google Scholar]
- 43. Havrankova J, Roth J, Browstein M. Insulin receptors are widely distributed in the central nervous system of the rat. Nature. 1978;272:827–829 [DOI] [PubMed] [Google Scholar]
- 44. Baskin DG, Porte D, Jr, Guest K, Dorsa DM. Regional concentrations of insulin in the rat brain. Endocrinology. 1983;112:898–903 [DOI] [PubMed] [Google Scholar]
- 45. Schwartz MW, Figlewicz DP, Baskin DG, Woods SC, Porte D., Jr Insulin in the brain: a hormonal regulator of energy balance. Endocr Rev. 1992;13:387–414 [DOI] [PubMed] [Google Scholar]
- 46. Banks WA. The source of cerebral insulin. Eur J Pharmacol. 2004;490:5–12 [DOI] [PubMed] [Google Scholar]
- 47. Woods SC, Porte D., Jr Relationship between plasma and cerebrospinal fluid insulin levels of dogs. Am J Physiol. 1977;233:E331–E334 [DOI] [PubMed] [Google Scholar]
- 48. Benoit SC, Kemp CJ, Elias CF, Abplanalp W, Herman JP, Migrenne S, Lefevre A, Cruciani-Guglielmacci C, Magnan C, Yu F, Niswender K, Irani BG, Holland WL, Clegg DJ. Palmitic acid mediates hypothalamic insulin resistance by altering PKC-Î̧ subcellular localization in rodents. J Clin Invest. 2009;119:2577–2589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Schwartz MW, Boyko EJ, Kahn SE, Ravussin E, Bogardus C. Reduced insulin secretion: an independent predictor of body weight gain. J Clin Endocrinol Metab. 1995;80:1571–1576 [DOI] [PubMed] [Google Scholar]
- 50. Carey DG, Jenkins AB, Campbell LV, Freund J, Chisholm DJ. Abdominal fat and insulin resistance in normal and overweight women: direct measurements reveal a strong relationship in subjects at both low and high risk of NIDDM. Diabetes. 1996;45:633–638 [DOI] [PubMed] [Google Scholar]
- 51. Israel PA, Park CR, Schwartz MW, Green PK, Sipols AJ, Woods SC, Porte D, Jr, Figewicz DP. Effect of diet-induced obesity and experimental hyperinsulinemia on insulin uptake into CSF of the rat. Brain Res Bull. 1993;30:571–575 [DOI] [PubMed] [Google Scholar]
- 52. Stein LJ, Dorsa DM, Baskin DG, Figlewicz DP, Ikeda H, Frankmann SP, Greenwood MR, Porte D, Jr, Woods SC. Immunoreactive insulin levels are elevated in the cerebrospinal fluid of genetically obese Zucker rats. Endocrinology. 1983;113:2299–2301 [DOI] [PubMed] [Google Scholar]
- 53. Kawano T, Tanaka K, Chi H, Eguchi S, Yamazaki F, Kitamura S, Kumagai N, Yokoyama M. Biophysical and pharmacological properties of glucagon-like peptide-1 in rats under isoflurane anesthesia. Anesth Analg. 2012;115:62–69 [DOI] [PubMed] [Google Scholar]
- 54. Adam CL, Findlay PA, Aitken RP, Milne JS, Wallace JM. In vivo changes in central and peripheral insulin sensitivity in a large animal model of obesity. Endocrinology. 2012;153:3147–3157 [DOI] [PubMed] [Google Scholar]
- 55. Owen OE, Reichard GAJ, Boden G, Shuman C. Comparative measurements of glucose, β-hydroxybutyrate, acetoacetate, and insulin in blood and cerebrospinal fluid during starvation. Metabolism. 1974;23:7–14 [DOI] [PubMed] [Google Scholar]
- 56. Kern W, Benedict C, Schultes B, Plohr F, Moser A, Born J, Fehm HL, Hallschmid M. Low cerebrospinal fluid insulin levels in obese humans. Diabetologia. 2006;49:2790–2792 [DOI] [PubMed] [Google Scholar]
- 57. Tschritter O, Preissl H, Hennige AM, Stumvoll M, Porubska K, Frost R, Marx H, Klosel B, Lutzenberger W, Birbaumer N, Haring HU, Fritsche A. The cerebrocortical response to hyperinsulinemia is reduced in overweight humans: a magnetoencephalographic study. Proc Natl Acad Sci USA. 2006;103:12103–12108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Riedy CA, Chavez M, Figlewicz DP, Woods SC. Central insulin enhances sensitivity to cholecystokinin. Physiol Behav. 1995;58:755–760 [DOI] [PubMed] [Google Scholar]