Abstract
Three different models of MF1 strain mice were studied to measure the effects of gonadal secretions and sex chromosome type and number on body weight and composition, and on related metabolic variables such as glucose homeostasis, feeding, and activity. The 3 genetic models varied sex chromosome complement in different ways, as follows: 1) “four core genotypes” mice, comprising XX and XY gonadal males, and XX and XY gonadal females; 2) the XY* model comprising groups similar to XO, XX, XY, and XXY; and 3) a novel model comprising 6 groups having XO, XX, and XY chromosomes with either testes or ovaries. In gonadally intact mice, gonadal males were heavier than gonadal females, but sex chromosome complement also influenced weight. The male/female difference was abolished by adult gonadectomy, after which mice with 2 sex chromosomes (XX or XY) had greater body weight and percentage of body fat than mice with 1 X chromosome. A second sex chromosome of either type, X or Y, had similar effects, indicating that the 2 sex chromosomes each possess factors that influence body weight and composition in the MF1 genetic background. Sex chromosome complement also influenced metabolic variables such as food intake and glucose tolerance. The results reveal a role for the Y chromosome in metabolism independent of testes and gonadal hormones and point to a small number of X–Y gene pairs with similar coding sequences as candidates for causing these effects.
Compared to men, women have more fat mass, more subcutaneous fat, but less visceral fat (1–3). Women may be at greater risk for obesity, but the visceral adiposity found in men and postmenopausal women may place them at greater risk for cardiometabolic diseases, relative to young women. Most sex differences in humans are attributed to effects of gonadal hormones (4, 5). We recently reported that the number of X chromosomes has important effects on body weight, adiposity, and susceptibility to metabolic disease in C57BL/6J mice (6). After removing the gonads of adults to unmask the role of sex chromosome complement, mice with 2 X chromosomes weigh 24% more than mice with 1 X chromosome, irrespective of the presence of a Y chromosome. The presence of 2 vs 1 X chromosome also led to accelerated weight gain on a high-fat diet (HFD) and promoted the development of fatty liver and elevated lipid and insulin levels. These studies were conducted using several mouse models on a C57BL/6J background in which the number and type of sex chromosomes were manipulated, often independently of gonadal sex.
In the present study, we investigated sex chromosome and gonadal effects on body weight and adiposity of MF1 strain mice with informative sex chromosome genotypes that are not possible to generate on the C57BL/6J background (6). Earlier studies of MF1 mice indicated that mice with a single X chromosome have a modest (∼5%) postnatal growth deficit in the first 5 weeks after birth, compared with XX or XY mice (7). This sex chromosome effect was attributed to haploinsufficiency of a nonpseudoautosomal X gene in XO relative to XX mice. Because a second sex chromosome of either type, X or Y, increased postnatal growth, the genes of interest are likely to be paralogous genes on the X and Y chromosomes. The X gene partners in these X–Y gene pairs typically escape inactivation and are expressed higher in mice with 2 X chromosomes than in mice with 1 X chromosome (6, 8).
To unmask the effects of X and Y genes on obesity and metabolism, we manipulated the number/type of sex chromosomes in MF1 mice, gonadectomized (GDX) as adults to control the levels of adult gonadal hormones. We found that a second sex chromosome, either X or Y, increased body weight and adiposity, and influenced feeding and glucose homeostasis. The results show an overlap in the roles of X and Y genes and point to important nongonadal functions of Y genes other than Sry (sex determining region of the Y chromosome).
Materials and Methods
Mice
Mice were separated by gonadal sex and housed in groups (except where noted) and maintained at 23°C with lights on from 7 am to 7 pm. Mice were fed a regular chow diet (5% fat, 24% protein, Lab Diet 5001, www.labdiet.com). As noted, some mice were fed HFD (35.5% fat, Bioserve F3282, www.bio-serv.com). All mice were GDX at 60 days after birth to allow comparison of groups under conditions of equal (zero) gonadal hormone levels that reveal sex chromosome effects when comparing XX and XY groups, and long-lasting effects of gonadal hormones in groups differing in gonadal type.
All mice were MF1. Although MF1 were originally random-bred, the X chromosomes of all mice used were identical and derived originally from mating an XY mouse with his XO mother to produce a line of mice with identical X chromosomes bred onto outbred stock (Paul Burgoyne, personal communication). Thus, differences between mice with 1 vs 2 X chromosomes were attributable to the number of X chromosomes, not X polymorphism.
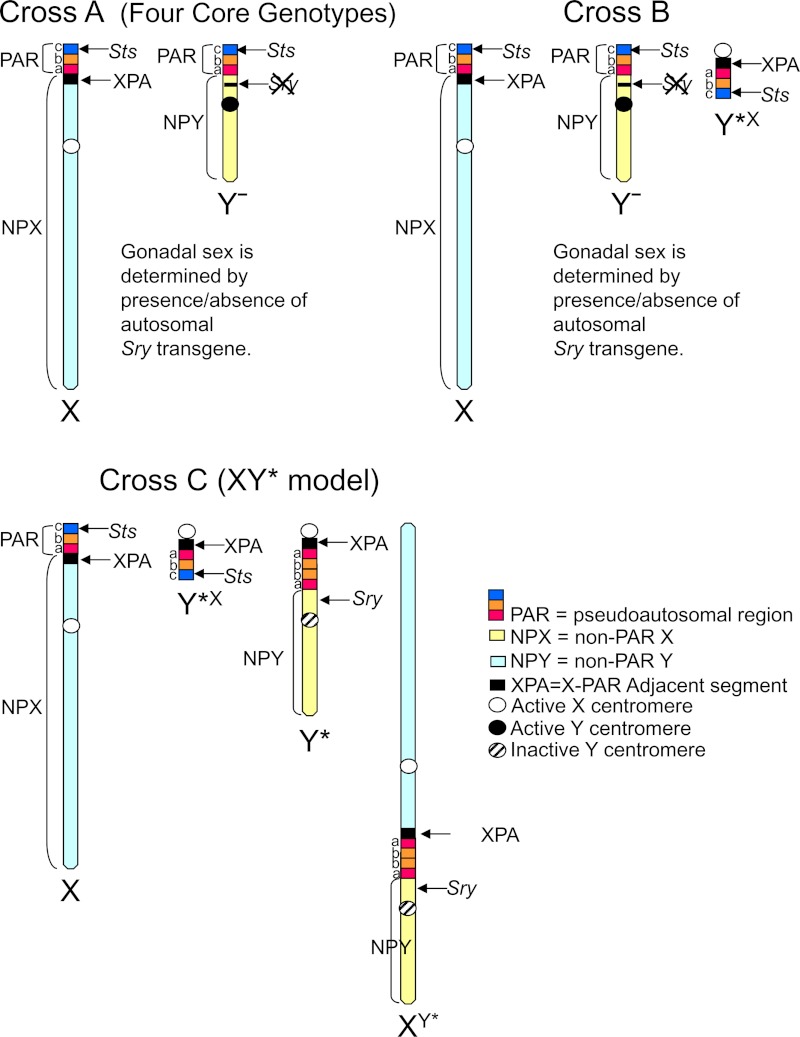
Mice in this study were generated by 3 different crosses. Genotypes and simpler “shorthand” nomenclature are defined in Table 1 and Figure 1.
Table 1.
Genotypes of the 3 Crosses
| Groups | Genotypes | Shorthand | Gonadal sex | NPX | NPY | PAR (Sts) | Sry | Xp | Xm | XPA |
|---|---|---|---|---|---|---|---|---|---|---|
| Cross A: MF1 XX♀ × ♂XY− (Sry+) (produces the 4 core genotypes) | ||||||||||
| 1♀ | XX | XXF | F | 2 | 0 | 2 | 0 | 1 | 1 | 2 |
| 2♀ | XY− | XYF | F | 1 | 1 | 2 | 0 | 0 | 1 | 1 |
| 3♂ | XX(Sry+) | XXM | M | 2 | 0 | 2 | 1 | 1 | 1 | 2 |
| 4♂ | XY−(Sry+) | XYM | M | 1 | 1 | 2 | 1 | 0 | 1 | 1 |
| Cross B: MF1 XX♀ × XY−Y*X(Sry+) ♂ | ||||||||||
| 1♀♂ | XX | XX | Both | 2 | 0 | 2 | 1♂ or 0♀ | 1 | 1 | 2 |
| 2♀♂ | XY*X | XO + PAR | Both | 1 | 0 | 2 | 1♂ or 0♀ | 0 | 1 | 2 |
| 3♀♂ | XY− | XY | Both | 1 | 1 | 2 | 1♂ or 0♀ | 0 | 1 | 1 |
| 4♀♂ | XO | XO | Both | 1 | 0 | 1 | 1♂ or 0♀ | 0 | 1 | 1 |
| 5♀♂ | XXY*X | 2X + PAR | Both | 2 | 0 | 3 | 1♂ or 0♀ | 1 | 1 | 3 |
| 6♀♂ | XY−Y*X | XY + PAR | Both | 1 | 1 | 3 | 1♂ or 0♀ | 0 | 1 | 2 |
| 7♀♂ | XXY− | XXY | Both | 2 | 1 | 3 | 1♂ or 0♀ | 1 | 1 | 2 |
| Cross C: XX♀ × XY* ♂ (XY* model) | ||||||||||
| 1♀ | XO | XO | F | 1 | 0 | 1 | 0 | 0 | 1 | 1 |
| 2♀ | XY*X | XO + PAR | F | 1 | 0 | 2 | 0 | 0 | 1 | 2 |
| 3♀ | XX | XX | F | 2 | 0 | 2 | 0 | 1 | 1 | 2 |
| 4♂ | XY* | XY | M | 1 | 1 | 1 | 1 | 0 | 1 | 2 |
| 5♂ | XXY* | XXY | M | 2 | 1 | 1 | 1 | 1 | 1 | 2 |
Abbreviations: NPX, nonpseudoautosomal region of the X chromosome; NPY, nonpseudoautosomal region of the Y chromosome; Xp, X chromosome inherited from the father; Xm, X chromosome inherited from the mother; XPA, small PAR-adjacent region of the NPX region containing a small number (∼6–8) coding genes, variably present as an extra copy in some of the progeny in of XY* mice (Figure 1). In the XY* model, 3 regions of PAR are shown in Figure 1, which are variably present on the aberrant chromosomes. This table tallies the numbers of PAR region C shown in Figure 1, which contains steroid sulfatase (Sts), the only known PAR coding gene in mice. The XY* chromosome in group 5 of cross C is the fusion of NPY and NPX with an aberrant intervening PAR. The Y− chromosome is from strain 129 (11). In cross B, genotypes 4 and 5 are rare.
Figure 1.
Schematic diagrams of the component regions of the sex chromosomes of mice used in the present study, expanded from (11). See Table 1 for a comparison of the number of copies of each chromosomal region in the 3 crosses. *P < .05.
Cross A: Four core genotypes (FCG) (Table 1)
The FCG model compares XX and XY mice independent of gonadal sex. The 4 genotypes are as follows: XX and XY mice with testes (XXM and XYM, Sry-positive) and XX and XY mice with ovaries (XXF and XYF, Sry-negative) (6, 9, 10). XX females are mated to XY−(Sry+) males, which have a Y− (“Yminus”) chromosome deleted for the testis-determining gene Sry, plus an Sry autosomal transgene. The Y− chromosome and Sry transgene segregate independently, so that gonadal sex is not determined by sex chromosome complement. The FCG model compares only mice with 2 sex chromosomes, in contrast to the other 2 crosses used here, which vary number and type of sex chromosomes. FCG XY gonadal males are quite similar to wild-type (WT) males (9).
Cross B (Table 1)
This cross, designed by Paul Burgoyne, allows the detection of effects of 2 vs 1 sex chromosome, independent of gonadal sex, keeping the number of pseudoautosomal regions (PAR) equal to 2 as in WT XX and XY mice. XX females were mated with XY−Y*X(Sry+) gonadal males that possess the Y− chromosome from FCG mice (cross A), plus the small Y*X chromosome that is nearly equivalent to a PAR (11), plus the autosomal Sry transgene. The cross produces progeny with 14 different genotypes, 7 different combinations of sex chromosomes each with or without Sry. In this study, we compared only genotypes 1, 2, and 3 (with or without the Sry, 6 genotypes total; Table 1). Cross B allows measuring the effect of a Y chromosome independent of gonadal sex, which cannot be achieved from studies of cross C, where all Y-bearing mice are gonadal males. Once the gonadal independence of a Y effect is established in cross B, as demonstrated here, the much simpler cross C can be used for further studies.
Cross C: Progeny of XY* males (Table 1)
XX gonadal females were mated with XY* gonadal males. The Y* chromosome includes a complex rearrangement of the PAR and produces mice with unusual complements of sex chromosomes genetically similar to XX, XY, XXY, XO, and XO+PAR (11–13). Comparisons include mice with 1 vs 2 copies of most regions of the X chromosome (groups 1 and 2 vs groups 3 and 5; Table 1), or mice with (groups 4 and 5) vs without (groups 1, 2, and 3) most regions of the Y chromosome. Group 5 has 2 non-PAR X regions and 1 non-PAR Y region, with gene dosages closely comparable to those of mice with 3 sex chromosomes. Mice with a Y chromosome have testes.
Four cohorts of mice were studied. One cohort of FCG mice was studied for body weight/composition for 22 weeks after GDX (Figures 2D and 3, A and B), then measured in metabolic cages at 8–9 months after GDX (Figure 3, C–E) and then tested for glucose tolerance (Figure 3, F and G) and euthanized. One cohort of cross B mice was fed chow diet for 20 weeks after GDX (Figures 4 and 5A), then fed an HFD for 7 weeks, and then euthanized (Figure 5, B–G). One cohort of cross C (XY* model) mice were fed regular chow for 19 weeks after GDX (Figure 6A). A second cohort of cross C was fed HFD for 16 weeks beginning 4 weeks after GDX, then given a glucose tolerance test, and euthanized (Figure 6, B and C, and Figure 7, F and G). A subset of this cohort was tested for feeding behavior and body temperature (Figure 7, A–E). In addition, body weights were analyzed from large numbers of cross A (FCG) mice before postnatal day 60 (Figure 1, A–C). Numbers of mice measured are shown in the figures.
Figure 2.
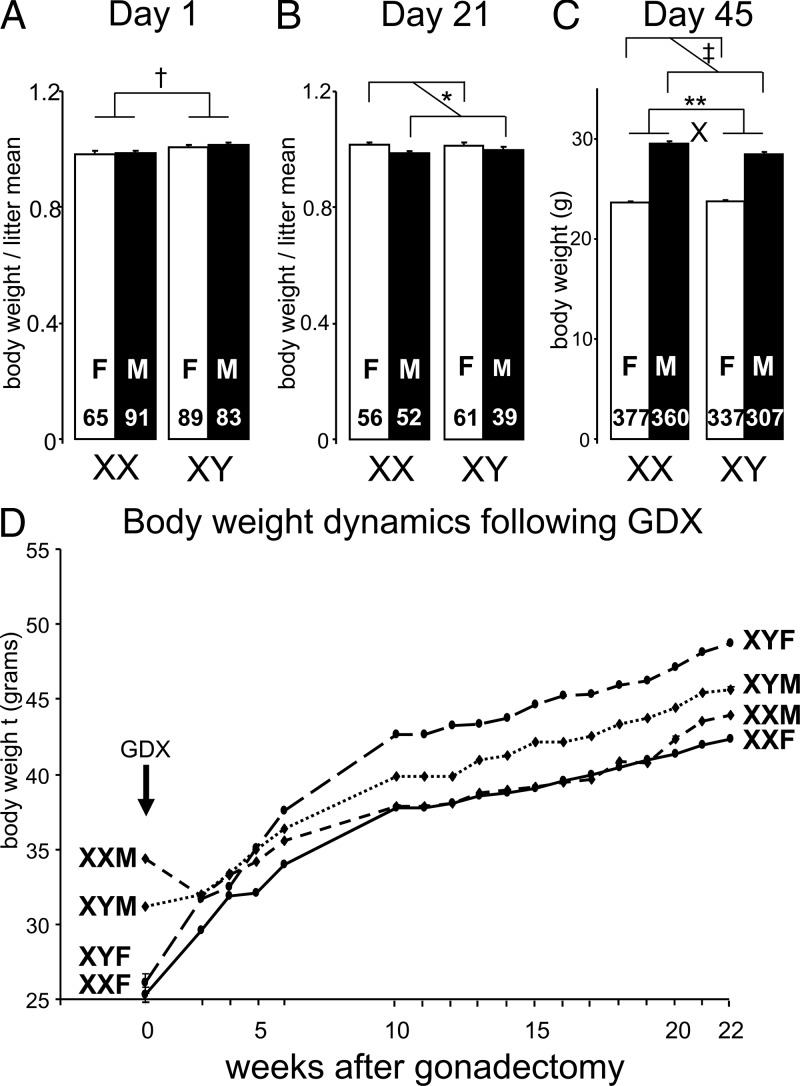
Body weight of FCG mice (cross A). A, Individual body weight, divided by mean litter weight, was greater in XY than XX mice on the day of birth. B, At weaning (day 21), body weight divided by mean litter weight was greater in gonadal females than gonadal males. C, By day 45, gonadal males weighed more than gonadal females, but XX mice weighed more than XY mice in gonadal males, leading to a statistically significant interaction (X, F(1,1377) = 14.35, P < .0002, 2-way ANOVA with factors of sex chromosome complement and gonadal sex). D, After gonadectomy (GDX) at day 60, gonadal males stopped increasing body weight until their weight was not different from that of gonadal females by 4 weeks after GDX. During weeks 10–22 after GDX, XY mice weighed more than XX mice, irrespective of gonadal sex. Group sizes were XXF (9), XXM (10), XYF (10), and XYM (10). *P < .05, **P < .01, †P < .0001, ‡P < .00001. F, female; M, male.
Figure 3.
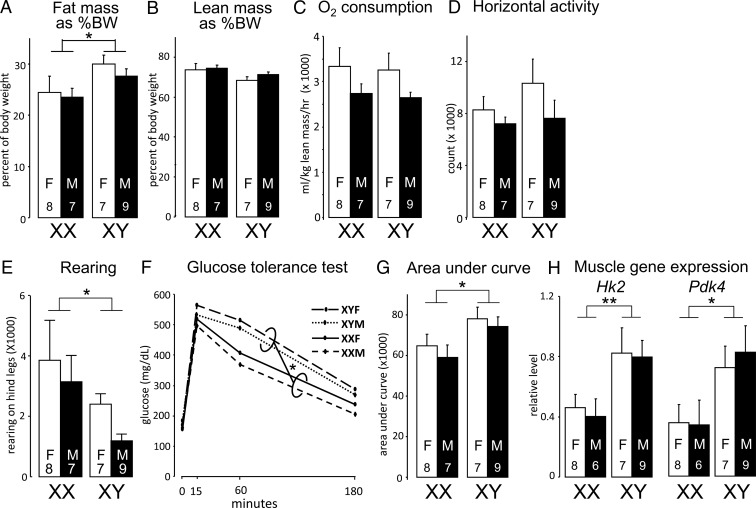
Body composition of FCG (cross A) mice eating a chow diet 9 months after GDX. A, XY mice had more body fat relative to body weight than XX mice (F(1,27) = 5.21, P < .03, 2-way ANOVA). B, Groups did not differ in lean mass as percentage of body weight (BW). C, O2 consumption did not differ among groups. D, Groups did not differ in activity in the horizontal plane. E, XX mice had greater rearing activity, relative to XY mice, as detected by breaking a laser beam in the vertical axis of the cage. F and G, Compared to XX mice, XY mice showed similar increase in blood glucose levels in response to injection of glucose, but impaired glucose clearance. H, Area under the curve in G indicates slower clearance of glucose in XY relative to XX mice. I, XY mice had higher expression levels of hexokinase 2 (Hk2) and pyruvate dehydrogenase kinase 4 (Pdk4) mRNA in skeletal muscle. F, female; M, male. *P < .05, **P < .01.
Figure 4.
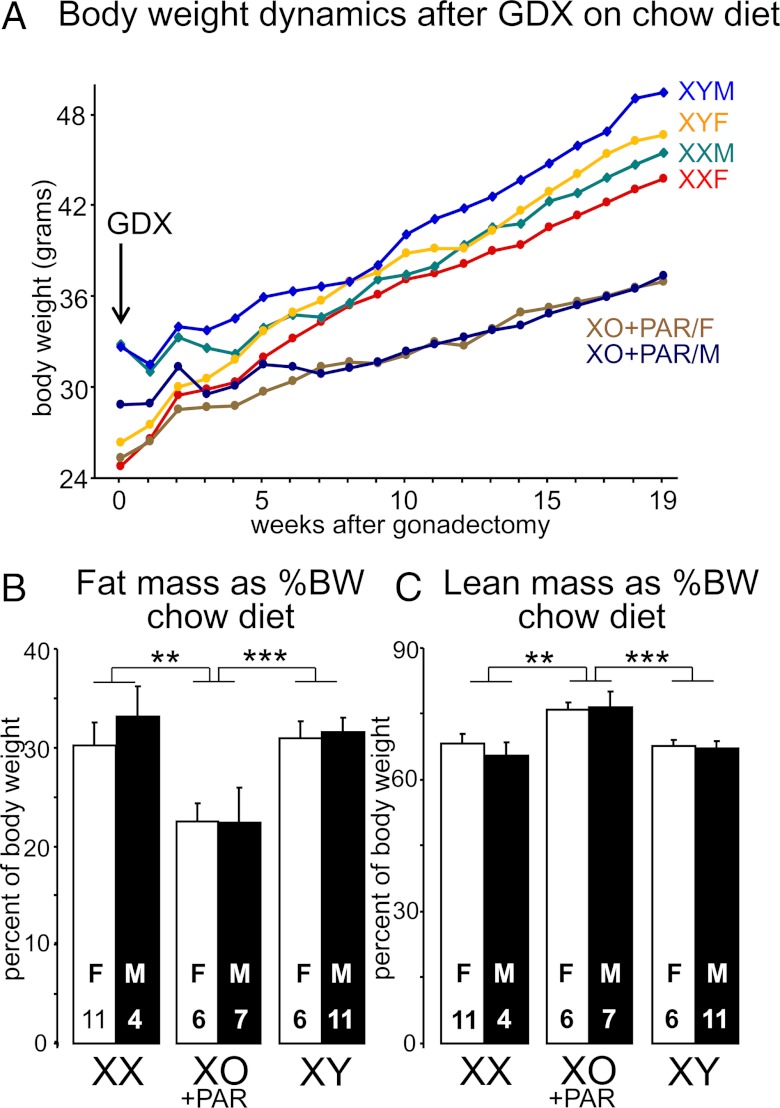
Body weight and composition in cross B eating a chow diet. A, Before gonadectomy (GDX) at day 60, the 3 gonadal male groups weighed 24% more than the 3 gonadal female groups (F(1,42) = 62.8, P < .000001, 2-way ANOVA with factors of genotype and gonadal sex). Nineteen weeks after GDX, genotype influenced body weight (F(2,33) = 10, P < .0005, 2-way ANOVA with factors of gonadal sex and genotype), but there was no effect of gonadal sex. XX mice were 20% heavier than XO+PAR (F(1,22) = 8.64, P < .008), independent of gonadal sex. XY mice were 30% heavier than XO+PAR (F(1,19) = 25.8, P < .00007), independent of gonadal sex. B, Fat mass relative to body weight was higher in XX and XY compared to mice with 1 X chromosome (XO+PAR). C, Lean mass relative to body weight was lower in XX and XY mice than in mice with 1 X chromosome (XO+PAR). **P < .01, ***P < .001.
Figure 5.
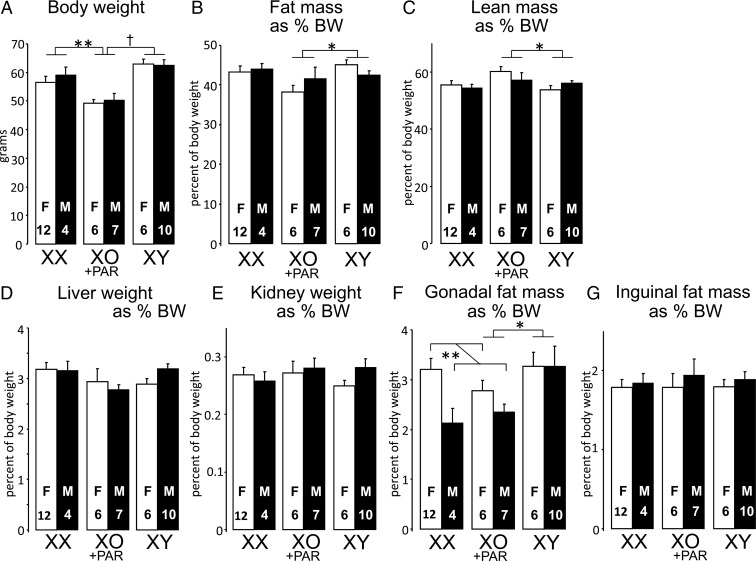
Body weight and composition in cross B mice fed after consuming an HFD for 7 weeks beginning 5 months after GDX. A, XX and XY mice were heavier than mice with 1 X chromosome (XO+PAR). B, XY mice had greater relative fat mass than mice with 1 X chromosome. C, XY mice had less relative lean mass than single-X mice. D and E, Relative liver and kidney weights did not differ among groups. F, Relative gonadal fat weight was higher in mice that previously had ovaries (gonadal female mice) in the XX vs XO+PAR comparison, and higher in XY than XO mice. G, Relative inguinal fat mass did not differ among groups. *P < .05, **P < .01, †P < .0001.
Figure 6.
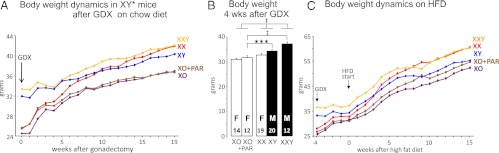
Body weight in cross C (XY* model) mice before and after gonadectomy (GDX) at day 60. A, Before GDX, the 2 groups with testes were heavier than the 3 groups with ovaries. Nineteen weeks after GDX, mice with more than 1 sex chromosome (XX, XY, XXY) were heavier than mice with 1 X chromosome (XO or XO+PAR) (F(1,50) = 7.19, P < .01). Groups sizes: XO (6), XX (11), XO+PAR (11), XY (12), and XXY (12). B, In a second cohort of XY* model mice, 4 weeks after GDX, body weight was greater in mice with more than 1 sex chromosome than in mice with 1 X chromosome, and greater in pairwise comparisons of XXY or XY mice relative to mice with 1 X chromosome (XO+PAR). C, The cohort of mice shown in B was fed HFD for 16 weeks beginning 4 weeks after GDX. All groups increased body weight, but XX mice increased more and became as heavy as XXY mice, significantly heavier than the 2 groups of mice with 1 X chromosome. *P < .05, ***P < .001, ‡P < .00001.
Figure 7.
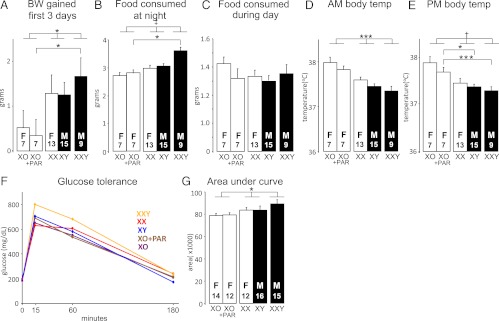
Body weight change, food consumption, body temperature, and glucose tolerance of mice in cross C (XY* model) after eating an HFD for 16 weeks. A, In the first 3 days and nights of the HFD, the amount of weight gain correlated with the number of sex chromosomes (1-way ANOVA, 1 vs 2 vs 3 non-PAR regions of sex chromosomes, F(2,48) = 3.64, P < .033). XXY mice gained more weight than XO+PAR mice. B, Similarly in the first 3 nights during the active period, the amount of food eaten correlated with the number of sex chromosomes (1-way ANOVA, 1 vs 2 vs 3 non-PAR regions of sex chromosomes, F(2,48) = 14.2, P < .00002). XXY mice ate more than XO+PAR mice. C, The amount of food consumed during the first 3 days did not differ among groups. D and E, am and pm body temperature at the end of the 16-week HFD period was higher in mice with fewer sex chromosomes (1-way ANOVA, 1 vs 2 vs 3 sex chromosomes, F(2,48) = 11.1, P < .002). In the pm, pairwise comparisons showed that XXY and XY mice had lower body temperature than XO+PAR mice (F(1,14) = 21, P = .0004, XXY vs XO+PAR; F(1,20) = 7.1, P = .015, XY vs XO+PAR). F, Blood glucose levels after injection of glucose. G, Area under the curve in F. Glucose clearance was slightly more efficient for mice having 1 X chromosome than the other 3 groups (1-way ANOVA on AUC, comparing mice with 1 vs 2 vs 3 non-PAR regions of sex chromosomes [F(2,66) = 4.11, P = .02]). *P < .05, ***P < .001, †P < .0001, ‡P < .00001.
Genotyping
Groups were discriminated by PCR on tail DNA based on the presence/absence of Sry and of Y chromosome gene Ssty, or based on karyotype from metaphase fibroblasts.
Body composition
Body composition (lean and fat mass) was determined by nuclear magnetic resonance spectroscopy using a Mouse Minispec (Bruker Woodlands, Texas) with Echo Medical Systems (Houston, Texas) software (14). Nuclear magnetic resonance spectroscopy was performed on cross A mice at 9 months after GDX, on cross B mice at 5 months after GDX, and then after a further 7 weeks on HFD.
Energy balance
Eight calibrated Oxymax metabolic cages (Columbus Instruments, Columbus, Ohio) were used to study energy balance in cross A (FCG) mice 9 months after GDX (Figure 3, C–E). Mice were housed individually for 4 days and 3 nights to measure parameters at 12-minute intervals: horizontal physical activity, vertical physical activity, and oxygen consumption.
Glucose homeostasis
Baseline glucose levels were determined in mice fasted 5 hours (8 am to 1 am) with a OneTouch Glucose Meter (LifeScan, Milpitas, California), using blood obtained by retro-orbital puncture under isoflurane anesthesia. A glucose tolerance test involved injecting fasted mice intraperitoneally with glucose (2 mg/g body weight) and determining glucose levels at 15, 30, 60, and 180 minutes after injection (Figures 3, G and H, and 7, F and G).
Body temperature
Body temperature was measured as described in cross C (Figure 7, D and E) with a BAT-10 digital thermometer with a Ret-3 rectal probe (PhysiTemp Instruments, Clifton, New Jersey).
HFD and food intake
A cohort of cross C (XY*) mice was fed HFD beginning at 4 weeks after GDX. Mice were habituated to individual housing for 5 days; then HFD was begun and continued for 4 days while mice were individually housed. Food intake, body weight, and temperature were measured at 8:30 am and 5 pm. Then, mice were group-housed in the same social groups as before single housing. At 8 and 16 weeks after onset of HFD, the mice were also individually housed again for 4-day periods for food intake measurements at 8:30 am and 5 pm, each time rehousing mice in the same groups after the 4-day period. During each of the three 4-day periods, the amount of food eaten per day and per night was determined by weighing food remaining from a known starting quantity, at 8:30 am and 5 pm. Care was taken to account for food crumbs that were present on the cage floor.
Quantitative RT-PCR
Tissues (eg, skeletal muscle) were dissected and immediately frozen in liquid nitrogen and then stored at −80°C. RNA was isolated using Trizol (Invitrogen, Carlsbad, California) and quantitative RT-PCR was performed as described (6). Expression of target genes was expressed relative to expression of at least 2 control genes. Primer sequences are provided in Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org.
Statistical analyses
For cross A (FCG), we used 2-way ANOVAs to measure effects of sex chromosome complement (XX vs XY) and gonadal sex (male vs female), or a 3-way ANOVA with the repeated factor of time. For cross B, we used an overall 2-way ANOVA (3 levels of genotype, 2 levels of gonadal sex), and planned 2-way ANOVAs (factors of genotype and gonadal sex) to assess differences between XX vs XO+PAR, XY vs XO+PAR, and XX vs XY. For cross C, we ran 2-way ANOVAs with factors of genotype and repeated factor of time for data of Figure 6, A and C, 1-way ANOVAs to compare gonadal male vs females or mice with 1 vs more than 1 sex chromosomes, 2-way ANOVAs (factors of gonadal sex and number of non-PAR X chromosome regions), and/or 1-way ANOVAs comparing mice with the 1 vs 2 vs 3 non-PAR regions of sex chromosomes. We also directly compared XO+PAR mice pairwise to XX, XY, or XXY mice using 1-way ANOVAs.
For analysis of oxygen consumption of FCG mice in Oyxmax cages (Figure 3C), we performed multiple regression analysis of the volume of oxygen consumed, analyzed for day and night separately, with covariates of gonadal sex (male vs female), sex chromosome complement (XX vs XY), fat mass, and lean mass. Fat and lean mass were analyzed as either absolute or percentage of body mass and considered as either individual covariates separately or 2 factors.
Results
Gonadal sex and sex chromosome complement influence prepubertal and postpubertal body weight
In FCG mice, the 4 genotypes had similar body weights at postnatal day 1 and at weaning (day 21) (not shown). Because body weight in neonatal mice varies inversely with litter size, we divided body weight of each mouse by mean body weight of its litter and found that newborn XY mice were 4% heavier than XX, regardless of gonadal sex (F(1,285) = 11.1, P < .001; Figure 2A) (15). By 21 days, the sex chromosome effect was no longer evident (Figure 2B). By day 45, gonadal males weighed 22% more than females (F(1,1377) = 1157, P < .000001; Figure 2C). In gonadal male but not female groups, XX mice were 4% heavier than XY mice (interaction of sex and sex chromosome complement, F(1,1377) =14.4, P < .0002). Similar patterns were found at day 60 (0 week, XX > XY 9%, male [M] > female [F] 28%; Figure 2D). Thus, sex chromosome complement influences body weight during the first 45 days after birth, but gonadal hormones cause greater body weight in males by day 45.
Effects of sex chromosome complement on adiposity of GDX adult FCG mice
To assess the effects of sex chromosome complement better, FCG MF1 mice were GDX at 60 days of age to remove acute effects of gonadal hormones (Figure 2D). Sex chromosome complement affected the response to GDX (F(17,647) = 5.39, P < .000001, interaction of sex chromosome complement and time, repeated measures ANOVA with factors of sex chromosome complement, gonadal sex, and time). By 5 weeks after GDX, the difference between gonadal males and females had disappeared, indicating that it was caused by acute differences in levels of gonadal secretions prior to GDX, and not by nongonadal effects of Sry. Beginning at 5 weeks after GDX, a new pattern for weight differences emerged, with XY mice weighing progressively more than XX mice. During 10–22 weeks after GDX, XY mice weighed more than XX (F(1,466) = 5.96, P < .02). There were no significant effects of gonadal sex, or significant interactions between gonadal sex, sex chromosome complement, and age (repeated measures 3-way ANOVA).
Chromosome complement also affected body composition. At 9 months after GDX, XY mice (vs XX) had ∼20% higher percentage body fat (F(1,27) = 5.21, P < .03; Figure 3A), or nearly one-third higher absolute fat mass (F(1,27) = 5.6, P < .03; Supplemental Figure 1A). The XY mice had a trend to corresponding reduced percentage lean body mass (F(1,27) =4.17, P = .051; Figure 3B and Supplemental Figure 1B). To identify factors that could contribute to greater adiposity in XY mice, we investigated energy expenditure and physical activity in FCG mice at 9 months after GDX. No significant differences were detected among the 4 genotypes in oxygen consumption (analyzed by multiple regression; see Materials and Methods) (Figure 3C). However, XX mice exhibited greater rearing activity, as detected by breaking a laser beam in the vertical axis of the cage (F(1,27) =4.23, P = .049; Figure 3E), with no differences detected in activity along the horizontal axis (Figure 3D). Overall, these findings raise the possibility that increased activity contributes to the lower adiposity in XX compared to XY mice.
Sex chromosome complement influences glucose homeostasis in FCG mice
Obesity is associated with impaired glucose metabolism. To determine whether the increased adiposity in XY mice was associated with metabolic dysregulation, we assessed glucose tolerance in fasted FCG mice. XY and XX mice showed a similar increase in blood glucose levels in response to an acute glucose challenge, but XY showed impaired glucose clearance relative to XX (area under the curve [AUC], F(1,27) = 6.33, P < .02; Figure 3, F and G). Gonadal males and females were not different. XY mice exhibited higher expression levels of hexokinase 2 (Hk2) and pyruvate dehydrogenase kinase 4 (Pdk4) in skeletal muscle (F(1,26) =13.8, P < .001 for Pdk4, F(1,26) =6.85, P < .02 for Hk2; Figure 2H). This suggests that XY mice do not have impaired early steps in glycolysis, such as phosphorylation of glucose by hexokinase, but may have altered gluconeogenesis and/or glucose oxidation, both of which are regulated by Pdk4. Together our results indicate that GDX XY mice exhibit increased adiposity and impaired glucose disposal. It is unclear whether an inherent deficit in glucose tolerance in XY mice is a cause or consequence of excess adiposity.
The presence of 2 sex chromosomes, compared to 1 sex chromosome, increases body weight and adiposity
The study of FCG mice led to the initial conclusion that sex chromosome complement (XX vs XY) influences body weight, adiposity, and glucose homeostasis in MF1 mice lacking gonadal hormones. The genetic differences that could explain this XX vs XY difference include the presence/absence of the Y chromosome, and the number or parental imprint of the X chromosome. To discriminate between X-linkage and Y-linkage of the relevant sex determinant(s), we measured body weight and composition in cross B, which varies the number of X chromosomes and the presence/absence of the Y chromosome, independently of each other. The 6 groups are XX, XY, and XO+PAR gonadal males, and XX, XY, and XO+PAR gonadal females. In cross B, the XO+PAR mice also carry 2 PARs, as do WT XX and XY.
Just before GDX on day 60 (Figure 4A), the 3 gonadal male groups weighed 24% more than the 3 gonadal female groups (F(1,42) = 62.8, P < .000001, 2-way ANOVA with factors of genotype and gonadal sex). In addition, the 2 groups with a Y chromosome were 3% to 9% heavier than those without a Y chromosome (F(1,42) = 6.46, P < .02, 2-way ANOVA with factors of Y chromosome and sex), independent of the gonadal sex of the mice. Genotype affected the change in body weight after GDX (interaction of genotype and time, F(38,760) = 10.5, P < .000001, repeated measures 3-way ANOVA with factors of genotype, sex, and time; Figure 4A). Nineteen weeks after GDX, genotype influenced body weight (F(2,33) = 10, P < .0004, 2-way ANOVA with factors of gonadal sex and genotype), but there was no effect of gonadal sex. Two-X mice were 20% heavier than 1-X mice (F(1,22) = 8.64, P < .008), independent of gonadal sex. XY mice were 30% heavier than 1-X mice (F(1,19) = 25.8, P < .00007), independent of gonadal sex.
These results support the conclusion that a second sex chromosome, either an X or a Y, increases body weight, relative to mice with 1 X chromosome. This conclusion leads to a reinterpretation of results from FCG mice (Figure 2D), where the main contrast was between mice with XX vs XY sex chromosomes. In light of the results from cross B, the greater body weight and adiposity of XY FCG mice relative to XX suggests that, under some conditions, the effect of the second sex chromosome to increase body weight is greater if that chromosome is Y rather than X. The stronger effect of the Y chromosome is reflected in some, but not all, measurements of cross B (see Discussion).
At 20 weeks after GDX (Figure 4A), XX and XY mice had about 40% greater relative body fat than XO+PAR mice, regardless of gonadal sex (XX vs XO+PAR, F(1,24) = 9.48, P < .005; XY vs XO+PAR, F(1,23) = 15.1, P < .0002, 2-way ANOVAs with factors of genotype and sex; Figure 4B and Supplemental Figure 2). XO+PAR mice had approximately 11% greater percentage of lean body mass than either XX or XY mice (Figure 4C; XX<XO+PAR, F(1,24) = 9.64, P < .005; XY<XO+PAR, F(1,23) = 14.3, P < .001). The type of gonads originally present had no effect, as gonadal males and females of each genotype were indistinguishable in both fat and lean mass. Body composition was indistinguishable between XX and XY mice, indicating that a second sex chromosome—whether X or Y—increases the proportion of body mass in the adipose compartment, relative to that found in single-X mice.
Effect of 2 vs 1 sex chromosome on response to HFD
The mice of Figure 3A were fed a high-fat, simple carbohydrate diet for 7 weeks, beginning at 20 weeks after GDX. During this period, all genotypes gained weight (Figure 5A and Supplemental Figure 3). At the end of this period, XX mice weighed 16% more than XO+PAR mice (F(1,25) = 10.4, P < .006), and XY mice weighed 26% more than XO+PAR mice (F(1,25) = 39, P = .00003). XY mice were not significantly heavier than XX mice (F(1,28) = 4.01, P < .06). There was no effect of gonadal sex.
Body composition on HFD showed similar patterns as body weight. XY mice had higher percentage body fat than XO+PAR (Figure 5B; F(1,23) = 5.01, P < .04), and lower percentage lean mass (Figure 5C; F(1,23) = 5.21, P < .04). XX mice were similar to XY but not significantly different from XO+PAR (Figure 5, B and C). At the end of the HFD period (at 9 months of age), liver and kidney weight were proportionally similar in all genotypes (Figure 5, D and E), and their absolute weight paralleled body weight (Supplemental Figure 3). However, genotypes differed in the distribution of body fat. Gonadal fat weighed more in XY mice relative to XX (F(1,26) = 4.42, P < .05) or XO+PAR (F(1,24) = 9.82, P < .005; Figure 5F). In addition, gonadal fat pads were larger in gonadal females than males in XX and XO+PAR mice (F(1,24) = 9.48, P < .005; Figure 5F). In contrast to gonadal fat depots, inguinal subcutaneous fat pad was proportionally similar in all genotypes (Figure 5G). Thus, the larger adipose tissue depots in mice with 2 (vs 1) sex chromosomes were primarily evident in the gonadal fat depot.
Confirmation of the X and Y chromosome effects on body weight in an independent genetic model
The experiments with cross B demonstrated that the metabolic effects of a second sex chromosome in mice on a chow diet were independent of gonadal type (Figure 4). To evaluate the effect of having the 3 non-PAR regions of sex chromosomes compared to 2 or 1, we produced mice using the XY* model, with the genotypes comparable to the following: XX, XY, XXY, XO, and XO+PAR (cross C; Figure 1 and Table 1). This model is unique in allowing a determination of the Y chromosome effect in the presence of 1 or 2 non-PAR regions of the X chromosome, and the ability to compare XO with and without a second PAR.
Just before GDX at 60 days, the gonadal males (XY, XXY) were 29% heavier than gonadal females (XO, XX, and XO+PAR) (F(1,50) = 164, P < .000001; Figure 6A, 1-way ANOVA). In addition, mice with 2 X chromosomes were 13% heavier than mice with 1 X chromosome (F(1,48) = 4.46, P < .04). After GDX, the body weights changed over time (interaction of genotype and time, F(72,924) = 3.39, P < .000001, 2-way repeated measures ANOVA with factors of genotype and time; Figure 6A). Body weight of gonadal males and females converged and were no longer significantly different by 11 weeks after GDX. By contrast, 2 X mice became significantly heavier than 1 X mice by 6–10 weeks after GDX. At 19 weeks after GDX, body weight of 1 X mice (XO and XO+PAR) was 12% to 14% less than in those with 2 or 3 non-PAR regions of sex chromosomes (XX, XY, or XXY, F(1,50) = 7.19, P < .01, 1-way ANOVA). The results from crosses B and C are consistent in demonstrating that single-X chromosome mice have lower body weight than mice with more than 1 sex chromosome.
X chromosome number affects body weight, feeding, and body temperature in mice on an HFD
A second cohort of cross C mice received the HFD for 16 weeks, beginning 4 weeks after GDX at day 60. Genotype affected the change in body weight (interaction of genotype and time, F(72,1322) = 4.14, P < 000001; Figure 6C, 2-way repeated measures ANOVA with factors of genotype and time). At 4 weeks after GDX, gonadally intact mice with more than 1 sex chromosome weighed more than 1 X mice (XXY > XY > XX > XO+PAR > XO, F(1,73) = 19.7, P(XX vs X) = 0.0003, 2-way ANOVA with factors of gonadal sex and number of non-PAR regions of the X chromosomes; Figure 6B). In addition, gonadal males weighed more than gonadal females (F(1,73) = 54.4, P [Y vs no Y] = .000001). The relative order of body weights was maintained over the 16 weeks of HFD, except that the XX group increased body weight more than XY (Figure 6C). At the end of the study, the body weight of 2 X mice were the same, and all 1 X groups, regardless of the presence of a Y chromosome or second PAR, had similarly lower body weights (Figure 6C). In a 2-way ANOVA with factors of Y (present vs absent) and X chromosome number (1 vs 2 non-PAR X), both factors were significant at the beginning of the HFD (X: F(1,73) = 11.5, P < .0003; Y: F(1,73) = 54.4, P < .00001), but only X chromosome number was significant at the end (F(1,73) = 11.5, P < .001).
We monitored body weight and food intake for the first few days of HFD feeding and found that XO mice gained less weight than mice with more sex chromosomes (Figure 7A; 1-way ANOVA: 1 vs 2 vs 3 non-PAR regions of sex chromosomes, F(2,48) = 3.64, P < .033). This was associated with increased food intake by mice with more than 1 sex chromosome compared to XO mice specifically during the active period (night; Figure 7B); food intake was similar among all genotypes during the daytime (Figure 7C). Food consumption was measured again at 8 and 16 weeks after onset of the HFD, at which point the amount of food consumed was significantly lower than at the outset and was not different among groups (data not shown).
After 16 weeks of HFD, body temperature at either 8:30 am or 5 pm, over 3 consecutive days, was inversely related to the number of sex chromosomes (F(2,48) = 11.1, P < .002, 1-way ANOVA, 1 vs 2 vs 3 non-PAR regions of sex chromosomes; Figure 7, D and E). Thus, in general, the mice gaining the least weight had the highest body temperatures. Additionally, mice with a Y chromosome had significantly lower body temperature than mice with no Y chromosome (Figure 7, D and E). Thus, some of the sex chromosome effect on body temperature could potentially be explained by long-lasting effects of gonadal hormones acting before GDX.
At the end of 16 weeks of HFD feeding, glucose clearance after an acute glucose challenge was slightly more efficient for mice having fewer sex chromosomes, as assessed for 3 hours following glucose administration (Figure 7, F and G; 1-way ANOVA on AUC, 1 vs 2 vs 3 non-PAR regions of sex chromosomes, F(2,67) = 4.11, P = .02). The reduced glucose tolerance parallels the increased body weight and adiposity in mice with 2 vs 1 sex chromosome.
Discussion
The present results show that a second sex chromosome, either Y or X, causes similar increases in body weight, adiposity, and lean mass of GDX MF1 mice, relative to mice with a single X chromosome. Importantly, the effects are independent of the gonadal sex of the mouse. We also demonstrated effects of X and Y chromosome complement on feeding behavior and body temperature in mice fed an HFD, which may contribute to the observed differences in weight and adiposity. In addition, there was evidence for metabolic dysregulation in the form of impaired glucose tolerance in mice with more than 1 sex chromosomes compared to those with a single X chromosome. The results provide new evidence for the role of non-Sry Y genes in controlling nongonadal traits. The similar effects of a second sex chromosome—either X or Y—suggest that the 2 sex chromosomes possess similar determinant(s) affecting body weight and metabolic regulation. One similarity of the X and Y chromosomes, despite their dramatic difference in size and gene content and the lack of recombination with each other in non-PAR regions, is that they harbor pairs of X–Y paralogous genes with very similar protein coding sequences. The X partners of the paired X–Y genes usually escape inactivation and are expressed at higher levels in XX than in XY or XO mice, in numerous tissues including metabolic tissues such as fat and liver (6, 8, 16, 17). Thus, the present results support the hypothesis that 1 or more of these X–Y gene pairs has a significant impact on metabolism, growth, and adiposity.
The present results provide an interesting comparison to our recent report that the number of X chromosomes contributes to sex differences in body weight and adiposity in mice (6). In FCG mice on a C57BL/6J background, GDX in adulthood, XX mice became much more obese than XY mice, irrespective of their gonadal sex. Moreover, GDX C57BL/6J XX mice fed an HFD showed more metabolic dysregulation relative to XY mice, including hyperinsulinemia and development of fatty liver. Based on analysis of the C57BL/6J XY* cross (analogous to cross C), the XX vs XY difference was attributed to the number of X chromosomes, and the presence or absence of a Y chromosome had no apparent effect. Here, we report that similar effects of X chromosome number are found in random-bred MF1 mice. In MF1 mice, however, we found that the effect of a second X chromosome was mimicked by the effect of a Y chromosome.
The current results suggest several metabolic processes that are influenced by sex chromosome number, which might underlie the enhanced adiposity in MF1 mice with more than 1 sex chromosome. In cross C mice (XY* model), groups of mice with lower body weight and adiposity had higher body temperatures, raising the possibility that differences in sex chromosome type or number affected metabolic processes influencing body temperature that in turn produced differences in body weight and adiposity. The GDX cross C mice with more than 1 sex chromosome also showed significantly higher food intake during the night, relative to single-X mice, when they were first fed the HFD; this effect warrants further investigation regarding the effects of sex chromosome complement on feeding behavior. Interestingly, in C57BL/6J FCG mice, we observed that XX mice ate more during the daytime than XY mice (6); studies are in progress to determine the physiological mechanism involved. Finally, in GDX FCG mice, we found some evidence for more rearing activity in XX than XY mice, but not more activity in the horizontal plane. Because activity was measured over a relatively short period, longer periods of observation are needed to characterize fully the effects of sex chromosome complement on physical activity and the downstream effects on body weight. Other possible mediators of the sex chromosome effects include alterations in the inflammatory response in adipose tissue, liver, or brain (18).
The results from crosses B (Figures 4 and 5) and C (Figures 6 and 7) provide convergent support for the conclusion that a second sex chromosome, either X or Y, increases body weight after adult gonadectomy. In cross B, XY and XX mice, relative to mice with 1 X chromosome, were clearly heavier and had greater relative body fat and lower relative lean mass, independent of gonad type. Because all mice in cross B had 2 PARs, differences between groups are attributable to the number of copies of the non-PAR regions of the X or Y chromosomes. In cross C, XO mice, GDX as adults, weighed less than XX, XY, or XXY mice. At 4 weeks after GDX, the presence of the Y chromosome or second X chromosome increased body weight relative to that of single-X mice. Mice with 2 sex chromosomes (XX, XY, 2 non-PAR regions) did not differ significantly from mice with 3 non-PAR regions (similar to XXY), suggesting a plateau in the effects of more than 2 sex chromosomes.
The analysis of FCG mice (Figures 2 and 3) suggests that sometimes the effect of the second sex chromosome is stronger if that chromosome is Y rather than X. For example, XY mice, relative to XX, were heavier, had greater relative body fat, and showed greater glucose intolerance. However, the differences between XX and XY were present but less consistent (or did not reach statistical significance) in the analysis of Figures 4 and 5 (cross B). For example, at 19 weeks after GDX, XY mice, relative to XX, had greater absolute lean mass but not fat mass (Supplemental Figure 2), and the 2 groups did not differ in body weight or percentage fat or lean mass. After eating an HFD, XX and XY mice did not differ in body weight or body composition (Figure 5). The variable results could stem from insufficient statistical power in some studies, or genetic or environmental variability across experiments. The sometimes greater effect of the Y chromosome remains a question for further research.
The functional similarity of non-PAR regions of the X and Y chromosomes is predicted from theories of evolution of the sex chromosomes. The Y chromosome is thought to be a degenerate version of the X chromosome (19–21). Nevertheless, a few genes have survived the massive degenerative forces on the Y chromosome, including the Y partners of X–Y gene pairs. The explanation for survival of the Y genes is that they and their X partners perform similar essential functions, so that null mutation of the Y gene in XY animals is highly deleterious and results in rapid removal from the population of the Y chromosome carrying such a mutation. In the present example, we find that the non-PAR X and Y have similar effects on metabolism. Thus, although the many Y genes are involved in male sexual development and function (22–24), the Y chromosome does not just have testis-specific functions. Although the Y genes have a role only in males, the present evidence suggests that these Y genes do not make males phenotypically different from females, but makes them phenotypically more similar to females because they compensate (25) for the lack of the second X chromosome.
The candidate X–Y paralogous non-PAR gene pairs, which may cause the similar effects of the X and Y chromosomes reported here, include Ddx3x/Ddx3y, Eif2s3x/Eif2s3y, Kdm5c/Kdm5d, Kdm6a/Uty, Uba1/Ube1y1, and Usp9x/Usp9y. All of the X partners of these pairs show higher expression in XX vs XY or XX vs XO mice in 1 or more metabolic or other tissues in adult mouse (6, 8, 26–28), as expected if the X gene escapes inactivation. Many of these genes are also found on human sex chromosomes and escape inactivation. However, only the first 4 of these 6 X genes have been reported to escape inactivation based on assays performed to date (16, 29–32). These genes are generally widely expressed in many tissues and are positioned in important pathways that could influence metabolic processes, because of their known roles in translation initiation, posttranslational modification, epigenetic programming, cell proliferation and apoptosis, tumor suppression, tissue differentiation, and so on (33–38). The sex differences in expression of these genes occur at all ages tested to date, and therefore, it is not clear whether the sex chromosome effects are exerted early in development (akin to organizational effects of gonadal hormones) or throughout life.
The present results are relevant to a dissection of genetic effects of sex chromosome aneuploidy, including Turner (XO) and Klinefelter syndrome (XXY). Both of these syndromes are associated with greater adiposity and metabolic disease (39, 40). In studies of humans, the direct effects of aneuploidy on metabolic tissues are difficult to dissociate from indirect effects resulting from different levels of gonadal hormones. For example, the greater adiposity of gonadally intact XO women, relative to XX (opposite in direction to the effects reported here), could be based on endocrine differences rather than direct effects of X chromosome genes. In our mouse models, the XO vs XX genotypes can be compared in individuals with testes or ovaries, with or without their gonads. The mouse models therefore offer significant advantages for disentangling the hormonal and genetic components regulating metabolism. The X genes discovered to influence metabolism in mice may later be tested for similar effects in humans.
The present results show the advantages of comparing sex chromosome effects in different mouse strains. Our analysis of MF1 mice reinforces the conclusion, based on study of C57BL/6J mice, that the second X chromosome of females increases body weight and adiposity and has strong influences on metabolic regulation. Part of this effect is the result of increased food intake in XX relative to XY. The present analysis extends the previous conclusions, because we find that in some genetic backgrounds, the Y chromosome shows effects similar to those of the X chromosome. Moreover, we present evidence for the first time that the number of sex chromosomes influences body temperature and alters glucose homeostasis. The results raise questions about which of these effects is primary and which are secondary to the increased adiposity or other effects. The sex chromosome effects that we observe are present in mice with or without their gonads, but are larger in the absence of gonadal hormones in adults (6). Thus, they may be most relevant to humans that are hypogonadal, for example, in postmenopausal women or aging men. Identifying the X and Y genetic determinants could provide a set of novel targets for therapies to treat or prevent obesity.
Supplementary Material
Acknowledgments
Thanks to Paul Burgoyne for many discussions, for founder populations of mice used here, for contributing the design of novel cross B, and for comments on the manuscript. We thank Simon Beaven and Peter Tontonoz for the use of the metabolic cages. Thanks also to Hector Alcala, Jenny Link, Ping Xu, and Lauren Csaki for assistance.
This work was supported by National Institutes of Health Grants DK083561 and NS043196.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AUC
- area under the curve
- FCG
- four core genotypes
- GDX
- gonadectomized
- HFD
- high-fat diet
- PAR
- pseudoautosomal region
- Sry
- sex-determining region of the Y chromosome
- WT
- wild type.
References
- 1. Karastergiou K, Smith SR, Greenberg AS, Fried SK. Sex differences in human adipose tissues—the biology of pear shape. Biol Sex Differ. 2012;3:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lovejoy JC, Sainsbury A. Sex differences in obesity and the regulation of energy homeostasis. Obes Rev. 2009;10:154–167 [DOI] [PubMed] [Google Scholar]
- 3. Paeratakul S, Lovejoy JC, Ryan DH, Bray GA. The relation of gender, race and socioeconomic status to obesity and obesity comorbidities in a sample of US adults. Int J Obes Relat Metab Disord. 2002;26:1205–1210 [DOI] [PubMed] [Google Scholar]
- 4. Shi H, Seeley RJ, Clegg DJ. Sexual differences in the control of energy homeostasis. Front Neuroendocrinol. 2009;30:396–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brown LM, Gent L, Davis K, Clegg DJ. Metabolic impact of sex hormones on obesity. Brain Res. 2010;1350:77–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen X, McClusky R, Chen J, et al. The number of X chromosomes causes sex differences in adiposity in mice. PLoS Genet. 2012;8:e1002709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Burgoyne PS, Ojarikre OA, Turner JM. Evidence that postnatal growth retardation in XO mice is due to haploinsufficiency for a non-PAR X gene. Cytogenet Genome Res. 2002;99:252–256 [DOI] [PubMed] [Google Scholar]
- 8. Lopes AM, Burgoyne PS, Ojarikre A, et al. Transcriptional changes in response to X chromosome dosage in the mouse: implications for X inactivation and the molecular basis of Turner Syndrome. BMC Genomics. 2010;11:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. De Vries GJ, Rissman EF, Simerly RB, et al. A model system for study of sex chromosome effects on sexually dimorphic neural and behavioral traits. J Neurosci. 2002;22:9005–9014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Arnold AP, Chen X. What does the “four core genotypes” mouse model tell us about sex differences in the brain and other tissues? Front Neuroendocrinol. 2009;30:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen X, Watkins R, Delot E, et al. Sex difference in neural tube defects in p53-null mice is caused by differences in the complement of X not Y genes. Dev Neurobiol. 2008;68:265–273 [DOI] [PubMed] [Google Scholar]
- 12. Eicher EM, Hale DW, Hunt PA, et al. The mouse Y* chromosome involves a complex rearrangement, including interstitial positioning of the pseudoautosomal region. Cytogenet Cell Genet. 1991;57:221–230 [DOI] [PubMed] [Google Scholar]
- 13. Burgoyne PS, Mahadevaiah SK, Perry J, Palmer SJ, Ashworth A. The Y* rearrangement in mice: new insights into a perplexing PAR. Cytogenet Cell Genet. 1998;80:37–40 [DOI] [PubMed] [Google Scholar]
- 14. Taicher GZ, Tinsley FC, Reiderman A, Heiman ML. Quantitative magnetic resonance (QMR) method for bone and whole-body-composition analysis. Anal Bioanal Chem. 2003;377:990–1002 [DOI] [PubMed] [Google Scholar]
- 15. Burgoyne PS, Thornhill AR, Boudrean SK, Darling SM, Bishop CE, Evans EP. The genetic basis of XX-XY differences present before gonadal sex differentiation in the mouse. Philos Trans R Soc Lond B Biol Sci. 1995;350:253–260 [DOI] [PubMed] [Google Scholar]
- 16. Yang F, Babak T, Shendure J, Disteche CM. Global survey of escape from X inactivation by RNA-sequencing in mouse. Genome Res. 2010;20:614–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Werler S, Poplinski A, Gromoll J, Wistuba J. Expression of selected genes escaping from X inactivation in the 41, XX(Y) * mouse model for Klinefelter's syndrome. Acta Paediatr. 2011;100:885–891 [DOI] [PubMed] [Google Scholar]
- 18. Dulloo AG, Montani JP. Body composition, inflammation and thermogenesis in pathways to obesity and the metabolic syndrome: an overview. Obes Rev. 2012;13(suppl 2):1–5 [DOI] [PubMed] [Google Scholar]
- 19. Charlesworth B. The evolution of sex chromosomes. Science. 1991;251:1030–1033 [DOI] [PubMed] [Google Scholar]
- 20. Charlesworth D, Charlesworth B, Marais G. Steps in the evolution of heteromorphic sex chromosomes. Heredity. 2005;95:118–128 [DOI] [PubMed] [Google Scholar]
- 21. Graves JAM. Sex chromosome specialization and degeneration in mammals. Cell. 2006;124:901–914 [DOI] [PubMed] [Google Scholar]
- 22. Lahn BT, Page DC. Functional coherence of the human Y chromosome. Science. 1997;278:675–680 [DOI] [PubMed] [Google Scholar]
- 23. Sekido R, Lovell-Badge R. Genetic control of testis fevelopment. Sex Dev. 2013;7:21–32 [DOI] [PubMed] [Google Scholar]
- 24. Burgoyne PS, Mitchell MJ. The role of mouse Y chromosome genes in spermatogenesis. In: Lau YFC, Chan WY, eds. Y Chromosome and Male Germ Cell Biology. Hackensack, NJ: World Scientific Publishers; 2007;27–45 [Google Scholar]
- 25. De Vries GJ. Minireview: Sex differences in adult and developing brains: compensation, compensation, compensation. Endocrinology. 2004;145:1063–1068 [DOI] [PubMed] [Google Scholar]
- 26. Xu J, Burgoyne PS, Arnold AP. Sex differences in sex chromosome gene expression in mouse brain. Hum Mol Genet. 2002;11:1409–1419 [DOI] [PubMed] [Google Scholar]
- 27. Xu J, Deng X, Watkins R, Disteche CM. Sex-specific differences in expression of histone demethylases Utx and Uty in mouse brain and neurons. J Neurosci. 2008;28:4521–4527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xu J, Deng X, Disteche CM. Sex-specific expression of the X-linked histone demethylase gene Jarid1c in brain. PLoS One. 2008;3:e2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Agulnik AI, Mitchell MJ, Mattei MG, et al. A novel X gene with a widely transcribed Y-linked homologue escapes X-inactivation in mouse and human. Hum Mol Genet. 1994;3:879–884 [DOI] [PubMed] [Google Scholar]
- 30. Ehrmann IE, Ellis PS, Mazeyrat S, et al. Characterization of genes encoding translation initiation factor eIF-2gamma in mouse and human: sex chromosome localization, escape from X- inactivation and evolution. Hum Mol Genet. 1998;7:1725–1737 [DOI] [PubMed] [Google Scholar]
- 31. Wu J, Salido EC, Yen PH, et al. The murine Xe169 gene escapes X-inactivation like its human homologue. Nat Genet. 1994;7:491–496 [DOI] [PubMed] [Google Scholar]
- 32. Greenfield A, Carrel L, Pennisi D, et al. The UTX gene escapes X inactivation in mice and humans. Hum Mol Genet. 1998;7:737–742 [DOI] [PubMed] [Google Scholar]
- 33. Swigut T, Wysocka J. H3K27 demethylases, at long last. Cell. 2007;131:29–32 [DOI] [PubMed] [Google Scholar]
- 34. Mansour AA, Gafni O, Weinberger L, et al. The H3K27 demethylase Utx regulates somatic and germ cell epigenetic reprogramming. Nature. 2012;488:409–413 [DOI] [PubMed] [Google Scholar]
- 35. Lai MC, Chang WC, Shieh SY, Tarn WY. DDX3 regulates cell growth through translational control of cyclin E1. Mol Cell Biol. 2010;30:5444–5453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Moudry P, Lukas C, Macurek L, et al. Ubiquitin-activating enzyme UBA1 is required for cellular response to DNA damage. Cell Cycle. 2012;11:1573–1582 [DOI] [PubMed] [Google Scholar]
- 37. Vucic D, Dixit VM, Wertz IE. Ubiquitylation in apoptosis: a post-translational modification at the edge of life and death. Nat Rev Mol Cell Biol. 2011;12:439–452 [DOI] [PubMed] [Google Scholar]
- 38. Rosner A, Rinkevich B. The DDX3 subfamily of the DEAD box helicases: divergent roles as unveiled by studying different organisms and in vitro assays. Curr Med Chem. 2007;14:2517–2525 [DOI] [PubMed] [Google Scholar]
- 39. Gravholt CH, Jensen AS, Host C, Bojesen A. Body composition, metabolic syndrome and type 2 diabetes in Klinefelter syndrome. Acta Paediatr. 2011;100:871–877 [DOI] [PubMed] [Google Scholar]
- 40. Rubin KR. Turner syndrome: transition from pediatrics to adulthood. Endocr Pract. 2008;14:775–781 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.