Abstract
IGF-I is a key regulator of muscle development and growth. The pre-pro-peptide produced by the Igf1gene undergoes several posttranslational processing steps to result in a secreted mature protein, which is thought to be the obligate ligand for the IGF-I receptor (IGF-IR). The goals of this study were to determine what forms of IGF-I exist in skeletal muscle, and whether the mature IGF-I protein was the only form able to activate the IGF-IR. We measured the proportion of IGF-I species in murine skeletal muscle and found that the predominant forms were nonglycosylated pro-IGF-I and glycosylated pro-IGF-I, which retained the C-terminal E peptide extension, instead of mature IGF-I. These forms were validated using samples subjected to viral expression of IGF-I combined with furin and glycosidase digestion. To determine whether the larger molecular weight IGF-I forms were also ligands for the IGF-IR, we generated each specific form through transient transfection of 3T3 cells and used the enriched media to perform kinase receptor activation assays. Compared with mature IGF-I, nonglycosylated pro-IGF-I had similar ability to activate the IGF-IR, whereas glycosylation of pro-IGF-I significantly reduced receptor activation. Thus, it is important to understand not only the quantity, but also the proportion of IGF-I forms produced, to evaluate the true biological activity of this growth factor.
IGF-I is critical for the growth and development of many tissues. For skeletal muscle, IGF-I coordinates with additional growth factors to promote myoblast proliferation, differentiation, and fiber formation during normal growth as well as during regeneration after injury. Thus, IGF-I is a central therapeutic target for enhancing muscle function in aging and disease. Several strategies have been employed to boost IGF-I levels in muscle, including tissue-specific transgenic expression (1–3), viral-mediated gene transfer (4–6), and directed recombinant IGF-I delivery (7, 8). Increasing IGF-I levels can result in functional hypertrophy in young adult animals, maintenance of mass and regenerative capacity in senescent animals, and enhancement of muscle recovery to counter acute and chronic damage.
IGF-I is produced from the Igf1 gene, which is more than 90% conserved across species, and can undergo alternative splicing at both the 5′- and 3′-ends to generate multiple isoforms (Figure 1A) (reviewed in Ref. 9). Regardless of the isoform transcribed, a pre-pro-peptide is translated, which consists of a signal peptide directing secretion, the mature IGF-I peptide, and a C-terminal extension called the E-peptide. After cleavage of the signal peptide, the pro-IGF-I (mature IGF-I plus an E-peptide) can be subjected to additional processing before secretion. This includes cleavage of the E-peptide by intracellular proteases to release mature IGF-I for secretion (10), maintenance of pro-IGF-I to be secreted without cleavage (11–13), or N-glycosylation in the E-peptide of the predominant IGF-I isoform (IGF-IA) (14), and then secretion (termed Gly-Pro-IGF-I). Hence, three forms of IGF-I protein could exist in the extracellular milieu: mature IGF-I, nonglycosylated pro-IGF-I, and glycosylated-pro-IGF-I. (For this study, we will refer to nonglycosylated pro-IGF-IA as pro-IGF-I.)
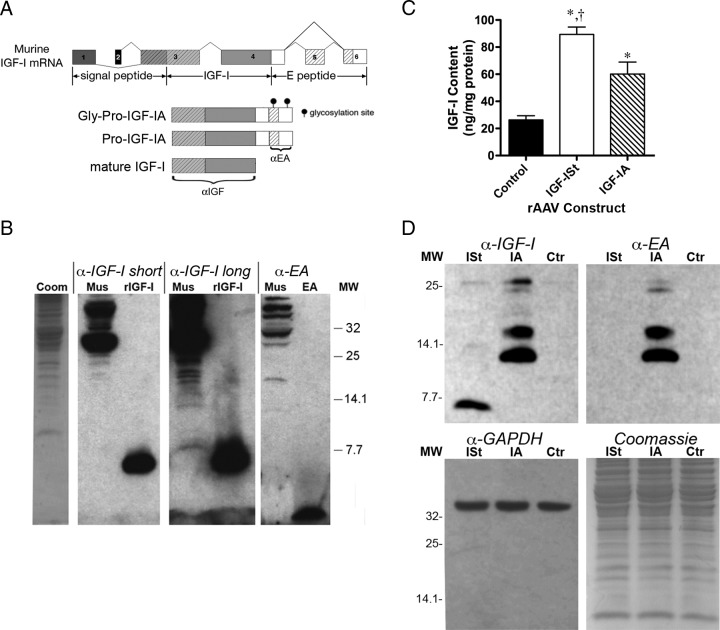
Figure 1.
IGF-I Forms in Muscle. A, Schematic presentation of alternative splicing of the Igf1 gene and the forms of IGF-I produced by the viral constructs, with the recognition sites for the antibodies indicated. B, Immunoblotting shows the endogenous IGF-I forms in muscle (Mus). IGF-I forms are evident upon long exposure for anti-IGF-I, with a mature IGF-I band at approximately 7 kDa, and multiple bands between 13 and 25 kDa. Blotting with anti-EA-peptide (EA) show that two bands (13 kDa, pro-IGF-I, and 20 kDa, gly-pro-IGF-I) are detectable with antibodies for EA and IGF-I. A total of 100 μg muscle protein per lane, 0.5 ng recombinant murine IGF-I (rIGF-I), and 20 ng recombinant EA-peptide were used as positive controls. Lefthand lane (Coom) shows Coomassie-stained lane of gel containing muscle lysate. C, ELISA measurements demonstrate a 3- to 4-fold increase in IGF-I content after viral delivery to murine skeletal muscle compared with uninjected muscles (Control). Results are shown as mean ± SEM for N = 4 muscles per condition. *P < .05 injected vs control samples, †P < .05, comparisons of injected samples. D, Immunoblotting for mature IGF-I and EA-peptide after viral expression of IGF-IA (IA) and IGF-ISt (ISt) shows that IGF-IA production results in two pro-IGF-I forms, with no detectable mature IGF-I. Only mature IGF-I is produced after viral delivery of IGF-ISt, and IGF-I levels are below the level of detection in control uninjected muscle lysates. Total protein was 20 μg per lane. GAPDH, glyceraldehyde 3-phosphate dehydrogenase; MW, molecular weight.
In a previous study, we compared the response of muscle not only to the IGF-I isoforms (IGF-IA and IGF-IB), but also to expression of mature IGF-I, generated by the insertion of a premature stop codon before the E-peptide sequence (15). Surprisingly, the overexpression of mature IGF-I lacking any E-peptide did not promote muscle hypertrophy in young mice, suggesting that the pro-IGF-I forms are required for this effect. IGF-I acts predominantly via the IGF-I receptor (IGF-IR). Through the inherent tyrosine kinase activity of these receptors, ligand binding mediates the signaling pathways necessary for cell survival and growth. The general consensus is that the mature IGF-I is the obligate ligand for these receptors, and that the pro-IGF forms are precursors that must have the C terminus removed before efficient receptor binding can occur. The important residues for receptor binding are distributed throughout the mature molecule (16, 17), and none have been identified in the E-peptide, suggesting that it is dispensable for receptor binding. However, the recent development of polyethylene glycol (PEG)ylated mature IGF-I protein (18) demonstrates that high molecular weight modifications near the C terminus of mature IGF-I do not inhibit IGF-IR activation and also increase the half-life of the growth factor. By extension, E-peptides found in pro-IGF-I may not inhibit ligand-receptor interactions and, in part, may act in a similar fashion to the PEG group. Consistent with this model, we demonstrated that pro-IGF-I produced by myoblasts has more efficient cell entry into neighboring myoblasts compared with mature IGF-I (19). More recently we have shown that the E-peptides enhance IGF-I-mediated receptor signaling in vitro (20), suggesting that even when the E-peptide is free, it may modulate IGF-I activity. Therefore, the goals of this study were to determine 1) the proportion of mature, pro-, and glycosylated pro-IGF-I found endogenously, 2), the IGF-I forms produced after expression of Igf1 in vitro and in vivo, and 3), the apparent IGF-IR activity induced by these forms.
Materials and Methods
Viral and plasmid constructs
Recombinant adeno-associated virus serotype 2/8 (AAV2/8), produced at the University of Pennsylvania Vector Core and harboring the Igf1 cDNA of murine class I IGF-IA and mature IGF-I sequences, was used to express these IGF-I forms in skeletal muscle, as described previously (15). Briefly, IGF-IA included the sequence to encode the class I signal peptide, IGF-I, and the EA-peptide, respectively. IGF-ISt lacked E-peptide sequence, and a stop codon was inserted at the end of the mature IGF-I (A70X).
Plasmid DNA constructs containing the same Igf1 cDNAs used for the viral constructs were also used for transfections of 3T3 cells, in order to produce media containing high levels of IGF-I. Additional mutants were generated by site-directed mutagenesis (QuikChange II; Stratagene, La Jolla, California) to inhibit cleavage between mature IGF-I and the E-peptide and/or N-glycosylation of the EA-peptide. Only the predominant isoform of IGF-I (IGF-IA) was mutagenized, because it represents approximately 90% of the IGF-I produced by the muscle and liver and harbors potential N-glycosylation sites (9). IGF-IA cleavage inhibition required mutagenesis of three sites: K68G, R71A, R77A (IGF-IKRR). Prevention of N-glycosylation in the EA-peptide was achieved through the site-directed mutagenesis of N92D (IGF-IN1), N100D (IGF-IN2), or both residues (IGF-INN). Finally, mutant constructs blocking both cleavage and N-glycosylation sites were generated to produce only pro-IGF-I (IGF-IKRRNN). All cDNA constructs were inserted into the NheI and XhoI restriction sites of pCMV.IRES.eGFP vector (CLONTECH Laboratories, Inc, Mountain View, California) for transient transfection as previously described (19).
Muscle AAV injections
All experiments were approved by the university animal care committee. Viral injections of 1 × 1011 particles diluted in 75–100 μL of PBS were performed into the anterior compartment of one lower hind limb of anesthetized C57Bl/6 mice targeting the tibialis anterior (TA) muscle. The contralateral limb received an equal volume of PBS in the same manner as a control for the injection procedure. After injection, mice were housed in the animal facility until time of analysis. Mice (n = 4 for each construct) were 2–3 weeks of age at the time of injection. They were killed 4 weeks after injection and exsanguinated, and the TA muscle and other tissues were dissected and rapidly frozen in liquid nitrogen for biochemical analysis. Serum was separated from whole blood by centrifugation and stored at –80°C for subsequent analysis.
Cell culture transfection
3T3 cells (1.2 × 106) were grown on six-well plates (Falcon, BD Bioscienses, Sparks, Maryland) in DMEM containing 10% fetal bovine serum and supplemented with 100 U/mL ampicillin and 100 U/mL streptomycin. Transient transfection was performed using Lipofectamine 2000 (Invitrogen, Carlsbad, California). For each transfection, cells were mixed with 0.5 mL of Opti-MEM (Invitrogen) containing 6 μg of plasmid DNA plus 8 μL of Lipofectamine, and then 1.5 mL DMEM plus 10% fetal bovine serum was added, and the cells were incubated for a total of 4 hours. Cells were switched into minimal media (DMEM supplemented with 100 U/mL ampicillin and 100 U/mL streptomycin) for 24 hours after transfection. Controls included transfection of empty vector (green fluorescent protein, GFP) and no transfection (control). The next day, media from transfected 3T3 cells was utilized for ELISA and immunoblotting measurements of IGF-I, and for kinase receptor activation (KIRA) assays (described below). 3T3 cell pellets were also retained for determining transfection efficiency.
Immunoblotting analysis
Tissues were removed from liquid nitrogen storage and homogenized in 10 volumes/muscle wet weight of modified radioimmune precipitation assay lysis buffer (50 mM Tris·HCl [pH 7.4], 1% [wt/vol] Triton X-100, 0.25% sodium deoxycholate, 150 mM NaCl, protease, and phosphatase inhibitor cocktails [P8340 and P5726, Sigma Chemical Co, St Louis, Missouri]). Extracts from transfected cells were obtained by cell lysis in the same RIPA buffer. Tissue homogenates and cell lysates were centrifuged to pellet debris, and the total protein was measured in the supernatant using the Bradford procedure (Bio-Rad protein assay; Bio-Rad Laboratories, Hercules, California). Media from transfected cells were concentrated 10-to 20-fold using microcentrifugal filters (Microcon; Millipore, Billerica, Massachusetts) and with the addition of protease inhibitors (P8340, Sigma), and then subjected to immunoblotting. In addition, media samples were subjected to serial dilution with DMEM and then subjected to immunoblotting.
Equal amounts of protein from tissue and cell lysate, or equal volumes of media, were subjected to SDS-PAGE using 16.5% Tris/Tricine or 12.5% Tris/Glycine gels and transferred to polyvinylidene fluoride membranes (Immobilon-P; Millipore Corp). Serial dilutions of recombinant murine IGF-I (R&D Systems, Minneapolis, Minnesota) were loaded onto each blot (0.1–10 ng per lane) in order to establish a standard curve for estimation of the IGF-I content in the samples. The recombinant IGF-I immunoblot and ELISA measurements were consistent with the manufacturers' specifications, with slopes of the comparisons equal to 1 and Y-intercept values less than 0.02. These standard curves were utilized to estimate the IGF-I content in each sample lane. The following primary antibodies were used for the immunodetection of the IGF-I forms (Figure 1A): a rabbit polyclonal anti-IGF-1Ea (20) (1:30 000 dilution), and a goat polyclonal antimature IGF-I antibody (1:500 dilution) (AF791; R&D Systems). Tissue lysates were also probed for glyceraldehyde-3-phosphate dehydrogenase (1:5000) (antimouse glyceraldehyde-3-phosphate dehydrogenase, sc-32233, Santa Cruz Biotechnology, Santa Cruz, California) to ensure equal protein loading. Concentrated media were also probed for IGF-binding protein (IGFBP)-3 (1:1000) (R&D Systems, MAB775), to determine whether the transfected constructs modulated the level of this protein. Immunoblotting of cell pellets for transfection efficiency utilized antibodies for GFP (1:1000) (catalog no. 2955, Cell Signaling Technology, Beverly, Massachusetts), and α-tubulin (1:4000) (T5168, Sigma). Immunoblotting of cell lysates for IGF-IR phosphorylation used antibodies for P-IGF-IR (1:1000) (catalog no. 407707, Calbiochem, San Diego, California) and IGF-IR (1:1000) (sc-713, Santa Cruz Biotechnology). After washes and exposure to secondary antibodies, specific bands were visualized by x-ray film and by Image Quant LAS 4000 (GE Healthcare Biosciences, Pittsburg, Pennsylvania), after incubation with an enhanced chemiluminescent (ECL) substrate (Western lightning-ECL, PerkinElmer, Waltham, Massachusetts). Analysis of band intensity was performed by use of the associated Image Quant software. Membranes were stained with Coomassie brilliant blue R-250 after immunoblotting, and gels were stained with colloidal Coomassie to confirm protein loading.
ELISA assays
Total IGF-I content in muscle protein extracts and in conditioned media was determined by a standard sandwich ELISA protocol using commercially available kit (MG100, R&D Systems) according to manufacturer's recommendations and as previously described (4, 19). This kit detects total rodent IGF-I and can also detect endogenous IGF-I production by C2C12 cells, and there is no cross-reactivity or interference with IGF-II or IGFBPs. The assay can detect IGF-I at 30–2000 pg/mL, with an intraassay precision of 4.3% and an interassay precision of 5.9%. Data were acquired in duplicate using a SpectraMax M5 plate reader (Molecular Devices, Sunnyvale, California) at 450 nm, and the results were averaged.
Furin cleavage
To identify the potential processing products of each IGF-I isoform, total protein lysates from AAV-injected TA muscles from C57BL/6 mice were incubated with recombinant protease furin (Recombinant Human Furin, 1503-SE-010, R&D Systems) according to manufacturer's recommendations. Furin processes proproteins by cleaving at specific motifs (ie, K-X-X-K-X-X-R-X-X-R-X-X-R) usually residing at the end of their pro regions. Equal amounts (30 μg) of total protein from each AAV-injected IGF-IA and IGF-ISt muscle lysate were incubated with furin (20 U of furin per 30 μg of each muscle lysate) overnight at 37°C. The corresponding volume of furin assay buffer (25 mM Tris, 1 mM CaCl2, 0.5% [wt/vol] Brij-35]pH 9.0]) for each muscle lysate was used as control. After overnight incubation with furin or furin assay buffer, muscle total protein extracts were subjected to immunoblotting and to ELISA for IGF-I detection.
Deglycosylation
To identify the putative glycosylated (and nonglycosylated) forms of IGF-I, total protein isolated from AAVIA-injected TA muscles was incubated with N-Glycosidase F (PNGase F), which cleaves between the innermost GlcNAc and asparagine residues of high mannose and complex oligosaccharides from N-linked glycoproteins. Aliquots containing 80 μg of AAV IGF-IA muscle protein extract were incubated with 2500 U of PNGase F (catalog no. P0705S; New England Biolabs, Ipswich, Massachusetts) for 3 hours at 37°C, according to manufacturer's recommendations. The corresponding volume of PNGase assay reaction buffer (G7 reaction buffer [10X], glycoprotein denaturing buffer [10X], Nonidet P-40 [10%]) lacking the enzyme PNGase F was used as control. After a 3-hour incubation with PNGase F or reaction buffer, muscle protein samples were purified using detergent removal spin columns (catalog no. 87777, Pierce Chemical Co, Rockford, Illinois), and then incubated with furin, or furin assay buffer (control) overnight at 37°C as described in the previous section. The final reactions were then subjected to immunoblotting for IGF-I detection. The deglycosylation buffer conditions were not compatible with ELISA measurements, and therefore they were not pursued.
IGF-IR activation assay
To compare the potency of IGF-IR activation by the IGF-I forms secreted from 3T3 cells after transfection, a KIRA assay was performed as previously described (21) with a few alterations. Briefly, 2.5 × 104 P6 cells, which overexpress IGF-IR (kind gift from Dr Renato Baserga, Thomas Jefferson University, Philadelphia, Pennsylvania) were seeded into 96-well plates. They were maintained in growth media supplemented with 200 μg/mL G418. The cells were serum starved for 6 hours, and then treated for 15 min with fresh media harvested from the transfected 3T3 cells. Controls included P6 cells treated with media alone, or with recombinant IGF-I (0.5–600 nM). For each KIRA experiment replicates, one well of 3T3 cells was transfected with a single IGF-I construct, giving rise to conditioned media containing the IGF-I form produced by the construct. Each media sample was tested in duplicate or triplicate on P6 cells. The P6 cells were lysed and IGF-IR was captured onto an ELISA plate coated with an antibody to IGF-IR (MAB1120, Millipore Corp.). A horseradish peroxidase-conjugated antibody to phosphorylated tyrosines (16–454, Millipore Corp.) and TMB substrate (N301, Thermo Scientific, Rockford, Illinois) were used for colorimetric quantification. Absorbance was read at 450 nm via the SpectraMax M5 plate reader (Molecular Devices, Sunnyvale, California), and values were averaged within each replicate. As validated previously (20) and in our hands, the assay had an intra-assay precision of 4% for standards and 9% for test samples, and an interassay precision of 12% for both standards and samples. Calculation of relative activity for each replicate was performed as described in Supplemental Appendix 1 published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org.
Statistical analysis
All statistical comparisons were performed in Prism (version 5.0c for Mac OS X, GraphPad Software, Inc, San Diego, California). One-way ANOVA was employed to evaluate changes in IGF-I content in both muscle lysates and cell-conditioned media in all viral infection and transfection conditions, as well as the KIRA assays, and was used to compare the apparent activity of IGF-I. Where significant F ratios were found for main effects (P < .05), means were compared using Tukey's post hoc tests. Unpaired t-tests were used for comparisons of results from furin experiments. KIRA dose-response curves were compared by an extra sum-of-squares F test. All data are presented as mean ± SEM. The level of statistical significance was set at P < .05.
Results
The first goal of this study was to examine the forms of IGF-I produced in skeletal muscle. We measured endogenous forms of IGF-I in muscle by immunoblotting high amounts of protein (100 μg) extracted from tissues. Muscle had high molecular weight bands in addition to mature IGF-I (Figure 1B). Two bands of approximately 13 and 20 kDa were also detected by the antibody for EA, supporting that these bands were glycosylated pro and pro-IGF-I. We do not know the identity of the higher molecular weight bands (those greater than 25 kDa). Therefore, a significant proportion of IGF-I found in naïve muscle is pro-IGF-I.
To improve IGF-I detection, we utilized muscle samples subjected to viral mediated delivery of IGF-IA and mature IGF-I (IGF-ISt). The levels of IGF-I in the AAV-injected and control muscles were quantified by ELISA. Although all injections resulted in significantly higher IGF-I content than in uninjected controls, the production after viral delivery of IGF-ISt exceeded the levels produced by IGF-IA by more than 40% (Figure 1C). To determine the forms of IGF-I produced after viral delivery to murine skeletal muscle, immunoblotting of muscle lysates (20 μg total protein) was performed with antibodies specific to IGF-I as well as to the EA-peptide (Figure 1D). In contrast to the ELISA measurements, the bands detected after AAV injection of IGF-IA were of higher intensity than the bands found in samples injected by AAV-IGF-ISt. Further, the predominant bands from IGF-IA samples migrated at a higher molecular weight (Figure 1D). IGF-ISt produced one band approximately 7 kDa in size, which was the predicted size for mature IGF-I, yet IGF-IA expression generated 13- and 17-kDa bands. Immunoblotting with an antibody recognizing the EA-peptide was used (21) and also detected the same bands (Figure 1C). Thus, most IGF-I species retained in muscle after viral delivery of the full-length Igf1a open reading frame are glycosylated pro- and pro-IGF-I forms, not mature.
To clarify the identity of IGF-I forms produced by viral expression of IGF-IA, muscle lysates were incubated with furin and/or glycosidase to cleave mature IGF-I from the E-peptide, and to separate the glycosylated side chains from asparagine residues, respectively. This experiment required levels of IGF-I produced by AAV, because endogenous IGF-I levels were insufficient for immunoblot detection after enzyme digestion. As shown in Figure 2A, furin treatment increased the presence of mature IGF-I in IGF-IA muscle lysates, and glycosidase collapsed the gly-pro-IGF-IA forms to a single pro-IGF-I band. Furin cleavage efficiency was not affected by glycosylation, because mature IGF-I was evident after furin treatment regardless of whether the samples had been treated previously with glycosidase. Importantly, it appeared that ELISA measurements were most sensitive to mature IGF-I, not the proforms (Figure 1), suggesting that the presence of the E-peptide impaired the ability for the IGF-I antibody in the ELISA to recognize the protein. To determine whether the retention of the E-peptide impaired ELISA detection, muscle lysates were incubated with furin, and then subjected to IGF-I ELISA quantification. Comparison of the same sample before and after furin treatment showed that IGF-I levels measured by ELISA increased after furin treatment in IGF-IA muscles, even though immunoblotting did not show an increase in total IGF-I (Figure 2B). In contrast, IGF-ISt samples did not exhibit the discordance between the ELISA measurements before and after furin treatment. Thus, it appeared that the E-peptide in pro-IGF-I impaired the ability to accurately measure IGF-I content under nondenaturing conditions.
Figure 2.
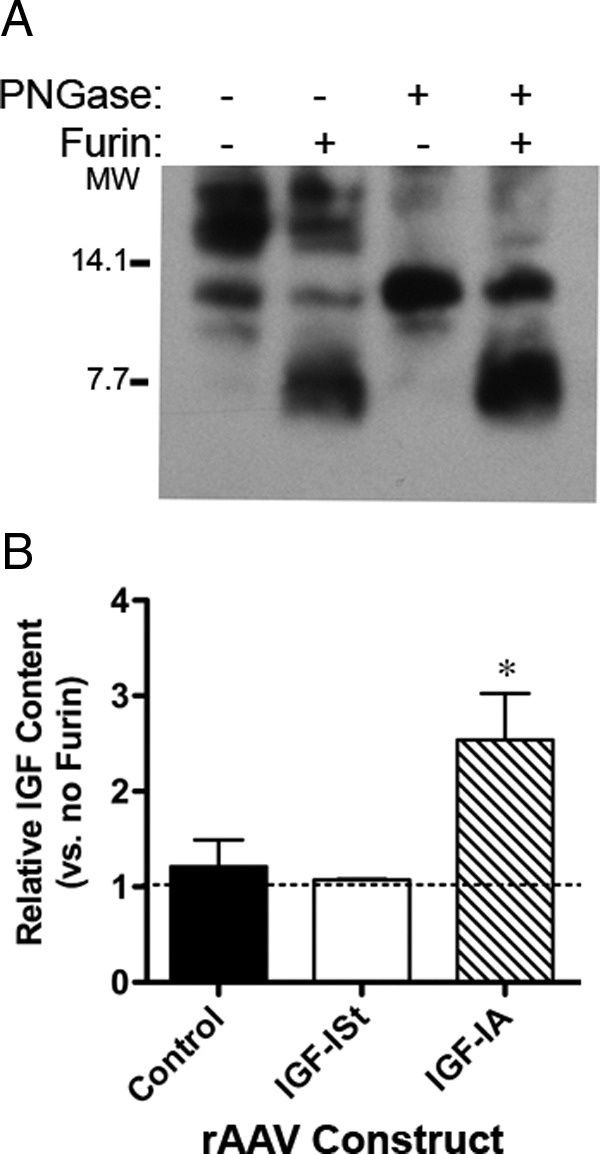
Identification of the Multiple Forms Produced by Viral Delivery of the IGF-I Isoforms. A, Furin digest confirms that the higher molecular weight forms produced by IGF-IA can be cleaved to produce mature IGF-I. Deglycosylation of IGF-IA by PNGase treatment confirms that the highest molecular weight bands produced by this isoform are glycosylated. PNGase followed by furin digest results in increased mature IGF-I in the sample. Blot shows the same muscle lysate sample of 30-μg aliquots subjected to buffer only, furin alone, PNGase alone, or PNGase followed by furin. B, ELISA quantification before and after furin digestion overnight at 37°C shows the cleavage of the C-terminal extension increases the detectable pool of IGF-I after viral delivery of IGF-IA, but does not change the observed IGF-I in control muscle (no viral delivery) nor after delivery of AAVIGF-ISt. *P < .05 for comparisons between apparent IGF-I levels before and after furin digest. MW, molecular weight.
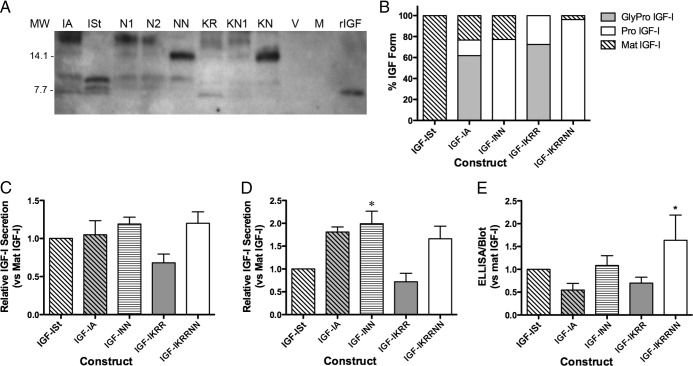
The multiple species of IGF-I produced in muscle raises the question of which form(s) can activate the IGF-IR. We moved to a cell-based system in order to control both the form produced and to enable quantification of receptor activation. Initially, we determined which asparagine residue was utilized for glycosylation by replacing one or both residues with aspartate. Immunoblotting of media from 3T3 cells transfected with IGF-IA revealed a cluster of glycosylated bands ranging from 17–20 kDa (Figure 3A). Transfection of IGF-IN1 or IGF-IN2 resulted in a single 17-kDa band, whereas transfection of IGF-INN, harboring a double mutation of N92 and N100, resulted in a 13-kDa band lacking any glycosylation. Thus, both asparagine residues could be utilized for glycosylation and result in the 20-kDa band.
Figure 3.
Secretion of IGF-I Forms after Transient Transfection of IGF-I Constructs. A, Immunoblot detection of the IGF-I forms secreted (30 μL media per lane). IA, transfection of wild-type IGF-IA; ISt, IGF-ISt transfection; N1, transfection of IGF-IA with N92A mutation; N2, transfection of IGF-IA with N100A mutation; NN, transfection of IGF-IA with N92A/N100A mutation; KR, blockade of cleavage site between mature IGF-I and the E-peptide; KN1, blockage of cleavage and N92A mutation; KN, blockade of both cleavage and glycosylation; V, vector only; M, mock transfection. B, The proportion of IGF-I forms secreted after transient transfection. C, ELISA measurements of IGF-I secreted after transient transfection. Data are presented as IGF-I levels normalized to that in media from mature IGF-I transfections for four experiments. D, Quantification of total IGF-I secreted after transient transfection based on immunoblot. *P < .05 significantly different compared with mature IGF-I. E, Ratio of ELISA and immunoblot quantification shows that presence of glycosylation causes an underestimation of IGF-I by ELISA. Data are presented as the sum of all IGF-I bands produced by each construct normalized to bands from transfection of mature IGF-I in four experiments. *P < .05 significantly different compared with mature IGF-I. MW, molecular weight.
We expanded our sets of constructs to include those with blockade of cleavage between mature IGF-I and the E-peptide (IGF-IKRR) as well as blockade of cleavage and glycosylation (IGF-IKRRN1, IGF-IKRRNN). As shown in Figure 3A, IGF-ISt-transfected cells secreted only mature IGF-I. Cells transfected with IGF-IA secreted mature, pro and gly-pro forms, and the presence of gly-pro-IGF-I was eliminated by transfection of IGF-INN. Likewise, the mutation of the cleavage sites between mature IGF-I and the E-peptides by IGF-IKRR transfection did not produce mature IGF-I. Glycosylation was preserved in the N1 mutant of pro-IGF-I (IGF-IKRRN1 vs IGF-IKRRNN). Finally, mutation of both cleavage and glycosylation eliminated most mature IGF-I as well as gly-pro-IGF-I in the media. These band patterns were consistent for N = 4 separate series of transfections. The mean proportions of each IGF-I form with respect to the specific construct are displayed in Figure 3B. Immunoblotting of cell lysates for GFP confirmed that the variability in IGF-I secretion was not due to the transfection efficiency (Supplemental Figure 1A).
Comparison of ELISA and immunoblotting for estimation of IGF-I in the enriched media reflected the observations made in vivo. Specifically, ELISA measurements (Figure 3C) underestimated the amount of IGF-I produced by constructs when there was gly-pro-IGF-I present, compared with measurements by immunoblotting (Figure 3, D and E). Therefore, although ELISA is a robust measurement for mature IGF-I, the presence of additional forms of IGF-I, specifically glycosylated pro-IGF-I, impairs the accurate quantification of the total IGF-I pool. This was particularly problematic for IGF-IA and IGF-IKRR constructs.
To determine the potency for IGF-IR activation by each IGF-I form, media from the transfected 3T3 cells were utilized for KIRA assays (Figure 4A). The amount of IGF-I per transfection was measured by immunoblotting taking into account all IGF-I forms in each lane. Immunoblotting was used instead of ELISA so that we did not underestimate the amount of glycosylated pro-IGF-I in any sample. Compared with mature IGF-I, we found that media containing gly-pro-IGF-I had lower apparent IGF-IR activation. Specifically, IGF-IA and IGF-IKRR samples caused 30 ± 8% and 27 ± 7%, respectively, less IGF-IR phosphorylation per IGF-I molecule than mature IGF-I. In contrast, media containing predominantly pro-IGF-I after IGF-INN and IGF-IKRRNN transfections had equivalent IGF-IR phosphorylation to mature IGF-I. To determine whether the difference in IGF-IR phosphorylation was affected by a change in binding proteins, media content of IGFBP3 was evaluated by immunoblotting, because 3T3 cells are known to secret IGFBP3 (22). However, there was no detectable IGFBP3 in the concentrated media (Supplemental Figure 1B). Thus, it appeared that gly-pro-IGF-I was less able to activate the IGF-IR independent of changes in binding protein level.
Figure 4.
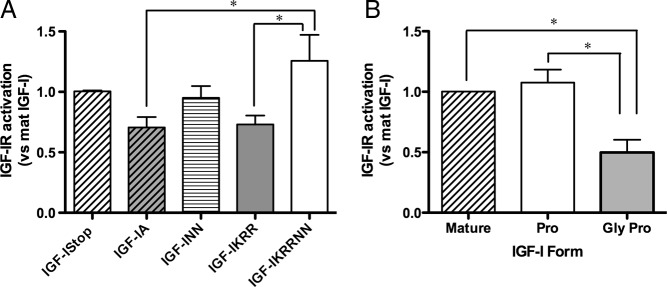
Comparison of IGF-IR Activation by IGF-I Forms. A, KIRA assay IGF-IR phosphorylation levels by IGF-I in enriched media quantified by immunoblotting. Results are compared with mature IGF-I activation within each KIRA experiment, where each sample was measured in triplicate. Data shown are mean ± SEM for four experiments. B, Calculated contribution of mature IGF-I, pro-IGF-I, and gly-pro-IGF-I to IGF-IR activation. Pro-IGF-I displays equivalent activation of the receptor compared with mature IGF-I, whereas gly-pro-IGF-I has a significantly reduced ability to activate the IGF-IR. *P < .05 for comparisons between conditions.
Because many of the enriched media samples contained multiple forms of IGF-I, we took advantage of knowing the proportion of each species (Figure 3B) and the apparent receptor activation to calculate the contribution of each IGF-I form to the final IGF-IR response by setting up a series of simultaneous equations and solving for apparent activity of mature, pro-, and gly-pro-IGF-I in each replicate (Supplemental Appendix 1). As shown in Figure 4B, we found that pro-IGF-I could, in theory, activate the IGF-IR as well as mature IGF-I, yet gly-pro-IGF-I was 2-fold less potent than mature IGF-I. Thus, our calculated comparisons supported that both pro-IGF-I and mature IGF-I were potent ligands for the IGF-IR.
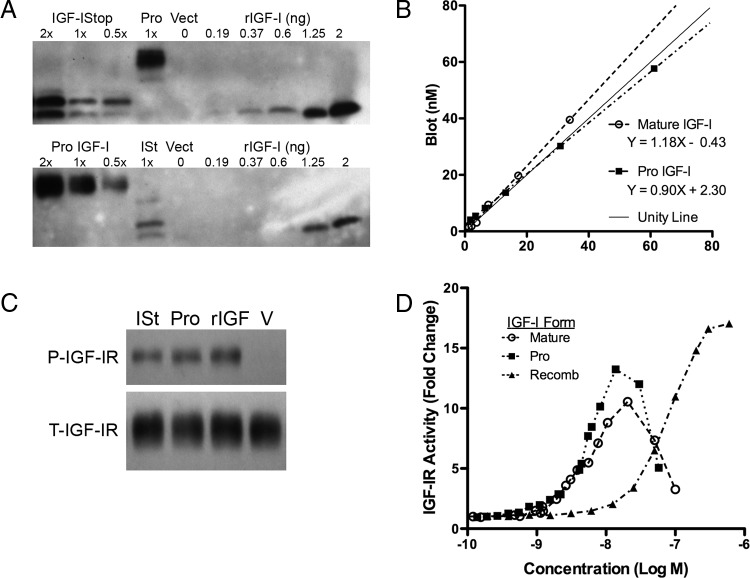
Even though transfections of many of our constructs resulted in a mixed population of IGF-I forms (Figure 3B), transfection of IGF-IStop and IGF-IKRRNN routinely produced pure mature and pro-IGF-I, respectively. Because the initial measurements were not at maximal activation levels, we extended the analysis of mature IGF-I and pro-IGF-I activity to cover a wide range of concentrations. We generated a dose-response curve of IGF-IR activation for mature and pro-IGF-I. First, we concentrated or diluted the conditioned media produced after transfection into 3T3 cells and estimated the IGF-I content by immunoblotting and ELISA (Figure 5, A and B). Although both comparisons were close to unity, they were significantly different, with mature IGF-I values higher when quantified by immunoblot than by ELISA (both mature bands used for quantification), and with pro-IGF-I values lower by immunoblot than by ELISA. This comparison suggested that it was the state of glycosylation that impaired accurate ELISA measurements of IGF-I when all forms were present.
Figure 5.
Quantification and Activity of IGF-I Forms. A, Immunoblot of concentrated conditioned media from 3T3 cells transfected with IGF-IStop, IGF-IKRRNN (Pro-IGF-I), or vector only (Vect). Media were concentrated using microcentrifugal filters and then serially diluted. Serial dilution of murine recombinant IGF-I was used as a standard. B, Comparison of ELISA and immunoblotting measurements for the same IGF-I samples. C, Immunoblot for IGF-IR phosphorylation after 20 minutes exposure to IGF-IStop media (ISt), pro-IGF-I media (Pro), or 2 nM murine recombinant IGF-I. D, KIRA assay for media from IGF-I Stop transfected 3T3 cells (Mature IGF-I), media from IGF-IKRRNN transfected 3T3 cells (Pro IGF-I), and murine recombinant IGF-I (Recomb IGF-I), based on the amount of IGF-I in each sample (by blot). Both bands were combined for mature IGF-I levels. Data are generated from three independent transfections for each construct, and three independent KIRA assays, as well as immunoblot quantification for all samples as in panels A and B. Each point represents the mean of duplicate measurements for any given sample. The fold change increase in IGF-IR phosphorylation is calculated from raw OD450 values for each sample compared with negative control (no IGF-I added).
We confirmed that these forms caused phosphorylation of the IGF-IR by immunoblotting, and saw that both generated robust phosphorylation of IGF-IR (Figure 5C). Aliquots of the same media were used in a KIRA assay, and we found that exposure of cells to pro-IGF-I resulted in similar IGF-IR activity as mature IGF-I (Figure 5D). The maximum activation we measured for mature IGF-I was 10 ± 1×, for pro-IGF-I was 14 ± 3×, and for recombinant murine IGF-I was 17 ± 2× (mean ± SEM for four experiments), which were not significantly different from each other. To exclude that additional factors in the conditioned media may have altered activity, the same assays were performed on conditioned media obtained from 3T3 cells transfected with vector only. We found that vector-only samples did not increase IGF-IR activity even when it was concentrated 4-fold (Supplemental Figure 2) in contrast to IGF-I containing samples, nor did the addition of concentrated media to recombinant IGF-I impair activity (data not shown). This supports that the forms of IGF-I were the only agents in the conditioned media that activated the IGF-IR. Based on the results of this study, we assert that both mature and pro-IGF-I are ligands for the IGF-IR. In addition, because gly-pro-IGF-I is a less potent ligand for the receptor, we propose that it may serve as a reservoir for IGF-I that can be stored in the extracellular matrix (ECM) until needed, when the glycosylated C terminus is removed to release mature IGF-I.
Discussion
The goals of this study were to identify the forms of IGF-I produced in muscle, and to clarify which of these forms were able to activate the IGF-IR. We found that untreated muscle retains predominantly the glycosylated pro- and pro-IGF-I forms, and when Igf1a is overexpressed by viral delivery, both pro- and glycosylated pro-IGF-I are present at high levels, but there is little mature IGF-I in either case. Cell-based assays for IGF-IR activation demonstrated that pro-IGF-I was as efficient at receptor activation as mature IGF-I, yet gly-pro-IGF-I was significantly less effective. To our knowledge, this is the first study to distinguish the potency of the different species of IGF-I produced, and helps not only to explain our previous observations, but also establishes new potential ways to optimize IGF-I activity.
The additional production of gly-pro-IGF-I by IGF-IA expression may act as a ligand reservoir, although this was not directly addressed in this study. Because it is less efficient at activating the IGF-IR, the glycosylation of the E-peptide may inhibit receptor binding. Although the crystal structure of mature IGF-I, as well as mutational analysis of the protein, clearly delineates the surface in mature IGF-I that is important for ligand binding (21, 23), the structure of the E-peptide extension has not been resolved. Our KIRA measurements suggest that the EA-peptide glycosylation covers the ligand-binding site for IGF-I to the receptor. However, whereas the nonglycosylated E-peptide in the pro-IGF-I species may also have a similar conformation, it does not seem to impede receptor phosphorylation. The recent development of PEGylated IGF-I also supports that a C-terminal extension does not necessarily block ligand binding, which is consistent with our observations (18). However, the affinity of the PEG-IGF-I was less than that of mature IGF-I, suggesting that it may be more similar to gly-pro-IGF-I.
Although the in vivo measurements show stable accumulation of the pro- and gly-pro-IGF forms in the muscle lysates, we cannot confirm that the location of the IGF-I pool. Classically, IGF-I posttranslational processing includes intracellular cleavage of mature IGF-I from the E-peptide, leading to mature peptide secretion, and an unknown destination for the E-peptide (10). However, the highly orchestrated process of glycosylation poses another order of complexity onto IGF-I processing. The forms we have observed appear to be stable intermediates or final products of posttranslational processing, given that furin and glycosidase digests confirm the identity of each band. Further, our cell-based measurements clearly show that all IGF-I species can be secreted, and substantiate the previous observations of pro-IGF-I in cell media (10, 12, 13, 19). In fact, most of the IGF-I is pro-IGF-I or gly-pro-IGF-I in the media from IGF-IA-transfected cells, suggesting that only a small portion of IGF-I cleavage occurs intracellularly, or that cleavage occurs outside of the cell. In addition, comparison of the media from IGF-IA and IGF-INN transfections show that blockade of glycosylation did not affect the final amount of mature IGF-I secreted (Figure 3B). This suggests that the cell may reserve a portion of the nascent IGF-I propeptides for intracellular cleavage, and the rest is directed for glycosylation and/or secretion. In muscle, these pro-forms could be stored in the ECM for subsequent activity by cleavage, which has been proposed recently (24). However, the extent of glycosylation or cleavage may vary across different cell types. These possibilities will require further experiments to delineate where each form of IGF-I resides in the muscle.
The murine IGF-IA has two potential N-glycosylation sites, N92 and N100, both of which follow the consensus sequence NXS/T, where X can be any residue except proline. In humans and nonhuman primates, only the sequence surrounding N92 is conserved (25), suggesting that if the same glycosylation patterns occurred in all species, N100 may not be used. Therefore, we anticipated that mutation of N92 would block glycosylation, whereas mutation of N100 would not. However, site-directed mutagenesis of either asparagine only removed the presence of the faint bands above 17 kDa, and instead the migration patterns of N92A and N100A mutants were indistinguishable. Only when both sites were mutated did all gly-pro-IGF-I bands disappear. Based on these results, it appeared that both residues could be glycosylated in mouse 3T3 cells, and therefore in order to generate pro-IGF-I for the KIRA assay, we used the double mutant. Although not directly addressed in this study, differential glycosylation of IGF-I may occur in different tissue types and provide another point for regulating stability and/or local retention of the growth factor. Further studies are needed to address the tissue specificity of the posttranslational modifications of IGF-I, and to determine whether these modifications change IGF-I actions in vivo.
How the pro- and gly-pro-IGF forms reside in the muscle matrix is an open question. Like the receptor-binding assays, these forms may be able to associate with IGFBPs, which are known stabilizers of IGF-I, but this was not addressed in the current study. Although there was no apparent change in the level of IGFBP3 in conditioned media, this does not address differential binding affinity of the various forms of IGF-I to IGFBPs. One advantage of utilizing conditioned media is that the native glycosylation pattern and folding of IGF-I is preserved. However, the disadvantage of this approach is that additional factors are also present to alter receptor activation, and measurement of IGF-I content must be done indirectly. As such, we cannot distinguish between a model in which pro-IGF-I has lower affinity for IGFBPs leading indirectly to strong receptor activation vs a model in which pro- and mature IGF-I have similar potencies. Future studies to address these distinctions will require pure preparations.
The glycosylated residues may also associate directly with the ECM, serving as an alternate additional reservoir of this growth factor. Cleavage of the entire E-peptide by extracellular proteases could then release active mature IGF-I for receptor binding when needed. There is evidence for candidate furin-like proteases residing in the ECM that could cleave mature IGF-I from the E-peptide. The proprotein convertase subtilisin/kexin type 6, commonly known as PACE4, is a likely candidate to cleave pro-IGF-I, given the importance of this proprotein convertase for muscle differentiation (26), and the fact that it can perform the same reaction intracellularly (10). Alternatively, the glycosylation could be clipped from the core protein, releasing pro-IGF-I for receptor activation. However, there is little evidence for extracellular glycosidases that could achieve this alternative path of regulation and remove glycosylation from the IGF-I C terminus.
In summary, we have identified multiple species of IGF-I that are produced by muscle both endogenously and after viral expression of IGF-IA. The major forms that accumulate in the tissue are not mature IGF-I, but instead pro-IGF-I and gly-pro-IGF-I. In addition, we have shown that the species of IGF-I produced by the Igf1a isoform have differential ability to activate the IGF-IR, where pro-IGF-I and mature IGF-I are more efficient at receptor activation than glycosylated pro-IGF-I. In future studies of IGF actions in muscle, it will be important to account for the forms produced and how they behave in physiologic and pathologic situations.
Supplementary Material
Acknowledgments
We thank R. Baserga for providing the P6 cells. We also thank R. Ma for generating the mutant IGF-I constructs. We are indebted to Drs. K. Speicher and H. Tang for extensive advice on quantification of IGF-I.
This work was supported by National Institutes of Health Grant AR057363 (to E.R.B.). B.K.B. was supported by a fellowship (AR053461) from the Pennsylvania Muscle Institute, University of Pennsylvania, Philadelphia, Pennsylvania.
Current address for A.P.: Department of Experimental Physiology, Medical School, National & Kapodistrian University of Athens, Goudi-Athens, Greece.
J.D. is a visiting scholar whose permanent appointment is the Department of Molecular Virology, Institute of Experimental Biology, Adam Mickiewicz University, Poznań, Poland.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AAV
- adeno-associated virus
- ECM
- extracellular matrix
- GFP
- green fluorescent protein
- IGFBP
- IGF-binding protein
- IGF-IR
- IGF-I receptor
- KIRA
- kinase receptor activation
- PEG
- polyethylene glycol
- PNGase F
- N-glycosidase F
- TA
- tibialis anterior.
References
- 1. Barton ER, Morris L, Musaro A, Rosenthal N, Sweeney HL. Muscle-specific expression of insulin-like growth factor I counters muscle decline in mdx mice. J Cell Biol. 2002;157:137–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Coleman ME, DeMayo F, Yin KC, et al. Myogenic vector expression of insulin-like growth factor I stimulates muscle cell differentiation and myofiber hypertrophy in transgenic mice. J Biol Chem. 1995;270:12109–12116 [DOI] [PubMed] [Google Scholar]
- 3. Musaro A, McCullagh K, Paul A, et al. Localized Igf-1 transgene expression sustains hypertrophy and regeneration in senescent skeletal muscle. Nat Genet. 2001;27:195–200 [DOI] [PubMed] [Google Scholar]
- 4. Barton ER. Viral expression of insulin-like growth factor-I isoforms promotes different responses in skeletal muscle. J Appl Physiol. 2006;100:1778–1784 [DOI] [PubMed] [Google Scholar]
- 5. Barton-Davis ER, Shoturma DI, Musaro A, Rosenthal N, Sweeney HL. Viral mediated expression of insulin-like growth factor I blocks the aging-related loss of skeletal muscle function. Proc Natl Acad Sci USA. 1998;95:15603–15607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stevens-Lapsley JE, Ye F, Liu M, et al. Impact of viral-mediated IGF-I gene transfer on skeletal muscle following cast immobilization. Am J Physiol Endocrinol Metab. 2010;299:E730–E740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Adams GR, McCue SA. Localized infusion of IGF-I results in skeletal muscle hypertrophy in rats. J Appl Physiol. 1998;84:1716–1722 [DOI] [PubMed] [Google Scholar]
- 8. Lynch GS, Cuffe SA, Plant DR, Gregorevic P. IGF-I treatment improves the functional properties of fast- and slow-twitch skeletal muscles from dystrophic mice. Neuromuscul Disord. 2001;11:260–268 [DOI] [PubMed] [Google Scholar]
- 9. Barton ER. The ABCs of IGF-I isoforms: impact on muscle hypertrophy and implications for repair. Appl Physiol Nutr Metab. 2006;31:791–797 [DOI] [PubMed] [Google Scholar]
- 10. Duguay SJ, Milewski WM, Young BD, Nakayama K, Steiner DF. Processing of wild-type and mutant proinsulin-like growth factor-IA by subtilisin-related proprotein convertases. J Biol Chem. 1997;272:6663–6670 [DOI] [PubMed] [Google Scholar]
- 11. Conover CA, Baker BK, Bale LK, Clarkson JT, Liu F, Hintz RL. Human hepatoma cells synthesize and secrete insulin-like growth factor Ia prohormone under growth hormone control. Regul Pept. 1993;48:1–8 [DOI] [PubMed] [Google Scholar]
- 12. Conover CA, Baker BK, Hintz RL. Cultured human fibroblasts secrete insulin-like growth factor IA prohormone. J Clin Endocrinol Metab. 1989;69:25–30 [DOI] [PubMed] [Google Scholar]
- 13. Wilson HE, Westwood M, White A, Clayton PE. Monoclonal antibodies to the carboxy-terminal Ea sequence of pro-insulin-like growth factor-IA (proIGF-IA) recognize proIGF-IA secreted by IM9 B-lymphocytes. Growth Horm IGF Res. 2001;11:10–17 [DOI] [PubMed] [Google Scholar]
- 14. Bach MA, Roberts CT, Jr, Smith EP, LeRoith D. Alternative splicing produces messenger RNAs encoding insulin-like growth factor-I prohormones that are differentially glycosylated in vitro. Mol Endocrinol. 1990;4:899–904 [DOI] [PubMed] [Google Scholar]
- 15. Barton ER, DeMeo J, Lei H. The insulin-like growth factor (IGF)-I E-peptides are required for isoform-specific gene expression and muscle hypertrophy after local IGF-I production. J Appl Physiol. 2010;108:1069–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Forbes BE, Hartfield PJ, McNeil KA, et al. Characteristics of binding of insulin-like growth factor (IGF)-I and IGF-II analogues to the type 1 IGF receptor determined by BIAcore analysis. Eur J Biochem. 2002;269:961–968 [DOI] [PubMed] [Google Scholar]
- 17. De Meyts P, Whittaker J. Structural biology of insulin and IGF1 receptors: implications for drug design. Nat Rev Drug Discov. 2002;1:769–783 [DOI] [PubMed] [Google Scholar]
- 18. Metzger F, Sajid W, Saenger S, et al. Separation of fast from slow anabolism by site-specific PEGylation of insulin-like growth factor I (IGF-I). J Biol Chem. 2011;286:19501–19510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pfeffer LA, Brisson BK, Lei H, Barton ER. The insulin-like growth factor-I E-peptides modulate cell entry of the mature IGF-I protein. Mol Biol Cell. 2009;20:3810–3817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brisson BK, Barton ER. Insulin-like growth factor-I E-peptide activity is dependent on the IGF-I receptor. PLoS ONE. 2012;7:e45588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Denley A, Wang CC, McNeil KA, et al. Structural and functional characteristics of the Val44Met insulin-like growth factor I missense mutation: correlation with effects on growth and development. Mol Endocrinol. 2005;19:711–721 [DOI] [PubMed] [Google Scholar]
- 22. Blat C, Villaudy J, Delbe J, Troalen F, Golde A, Harel L. Purification from transformed mouse fibroblast of a cell growth inhibitor which is an IGF-binding protein. Growth Factors. 1992;6:65–75 [DOI] [PubMed] [Google Scholar]
- 23. Vajdos FF, Ultsch M, Schaffer ML, et al. Crystal structure of human insulin-like growth factor-1: detergent binding inhibits binding protein interactions. Biochemistry. 2001;40:11022–11029 [DOI] [PubMed] [Google Scholar]
- 24. Hede MS, Salimova E, Piszczek A, et al. E-Peptides control bioavailability of IGF-1. PLoS ONE. 2012;7:e51152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wallis M. New insulin-like growth factor (IGF)-precursor sequences from mammalian genomes: the molecular evolution of IGFs and associated peptides in primates. Growth Horm IGF Res. 2009;19:12–23 [DOI] [PubMed] [Google Scholar]
- 26. Yuasa K, Masuda T, Yoshikawa C, Nagahama M, Matsuda Y, Tsuji A. Subtilisin-like proprotein convertase PACE4 is required for skeletal muscle differentiation. J Biochem. 2009;146:407–415 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.