Abstract
Estrogen replacement therapy reduces the incidence of type 2 diabetes in postmenopausal women; however, the mechanism is unknown. Therefore, the aim of this study was to evaluate the metabolic effects of estrogen replacement therapy in an experimental model of menopause. At 8 weeks of age, female mice were ovariectomized (OVX) or sham (SHAM) operated, and OVX mice were treated with vehicle (OVX) or estradiol (E2) (OVX+E2). After 4 weeks of high-fat diet feeding, OVX mice had increased body weight and fat mass compared with SHAM and OVX+E2 mice. OVX mice displayed reduced whole-body energy expenditure, as well as impaired glucose tolerance and whole-body insulin resistance. Differences in whole-body insulin sensitivity in OVX compared with SHAM mice were accounted for by impaired muscle insulin sensitivity, whereas both hepatic and muscle insulin sensitivity were impaired in OVX compared with OVX+E2 mice. Muscle diacylglycerol (DAG), content in OVX mice was increased relative to SHAM and OVX+E2 mice. In contrast, E2 treatment prevented the increase in hepatic DAG content observed in both SHAM and OVX mice. Increases in tissue DAG content were associated with increased protein kinase Cϵ activation in liver of SHAM and OVX mice compared with OVX+E2 and protein kinase Cθ activation in skeletal muscle of OVX mice compared with SHAM and OVX+E2. Taken together, these data demonstrate that E2 plays a pivotal role in the regulation of whole-body energy homeostasis, increasing O2 consumption and energy expenditure in OVX mice, and in turn preventing diet-induced ectopic lipid (DAG) deposition and hepatic and muscle insulin resistance.
Estrogen loss during menopause in women is associated with increased visceral adipose tissue mass and, in turn, related metabolic disorders, such as insulin resistance, diabetes, and cardiovascular disease. The beneficial effects of estrogen on insulin action and glucose homeostasis are supported by studies showing that insulin sensitivity is greater in premenopausal women compared with age-matched men, and metabolic-related cardiovascular diseases and type 2 diabetes are less frequent in those same women (1, 2). Also, estrogen deficiency leads to increased fat mass and body weight in postmenopausal women, which was associated with increased intraabdominal fat (3). This increased fat mass observed in postmenopausal women could be explained by changes in energy expenditure, because menopausal transition leads to decreased energy expenditure (3). Interestingly, menopausal women also displayed a reduced fat oxidation and spontaneous physical activity (3). Finally, estrogen replacement therapy in postmenopausal women reduces the incidence of type 2 diabetes (4).
Experimental studies have also shown that estrogen plays an important role in the regulation of metabolic homeostasis. Increased body weight associated with increased fat mass is observed in both ovariectomized (OVX) mice (5) and rats (6). Also, both female and male aromatase knockout mice, which are unable to synthesize estrogen, display increased body weight, fat mass, and adipocyte hypertrophy compared with wild-type mice, demonstrating the impact of estrogen deficiency on fat accumulation (7).
Some studies suggest that estrogen's effects on metabolic homeostasis are mediated by estrogen receptor (ER)α. Both male and female ERα knockout mice display increased weight gain and fat mass associated with impaired glucose tolerance (8). On the other hand, ERβ knockout mice do not display changes in body weight, fat mass, or glucose tolerance (9).
However, the exact cellular mechanism by which estrogen controls metabolic homeostasis is not completely known. Thus, the aim of the present work was to evaluate the metabolic effects of estrogen replacement therapy in an experimental model of estrogen deprivation (OVX mice).
Materials and Methods
Animal care and protocol
All experimental procedures were approved by and conducted in accordance with the Institutional Animal Care and Use Committee guidelines of Yale University School of Medicine. Regular chow-fed female mice were randomly divided into 3 groups: sham (SHAM) operated, OVX, and OVX treated with estradiol (E2) (OVX+E2). At 8 weeks of age, female mice were anesthetized with a ketamine-xylazine-acepromazine mixture (64.9, 3.2, and 0.78 mg/kg, respectively, ip) and were OVX or SHAM operated. To study the effect of chronic E2 administration, OVX mice were sc implanted with pellets releasing either placebo (OVX) or E2 (OVX+E2) (0.1 mg for 60 d; ie, 70 μg/kg per day; Innovative Research of America, Sarasota, Florida) at the same time as ovariectomy. During all procedures, mice were in a constant room temperature environment, with 12-hour light, 12-hour dark cycle, with free access to water and food. High-fat diet (HFD) (60% fat, 20% carbohydrate, and 20% protein, D12492; Research Diets, New Brunswick, New Jersey), containing 90% of fat calories from lard and 10% from soybean oil, was administered after ovariectomy and pellet implantation and continued for 4 weeks before experiments. It was previously described that OVX mice and/or E2 treatment have no effect on whole-body insulin sensitivity in regular chow-fed mice. Therefore, it was decided to study just mice fed with HFD in the present work (10).
Metabolic cage studies
Fat and lean body mass were assessed by 1H-magnetic resonance spectroscopy (Bruker BioSpin, Billerica, Massachusetts). Comprehensive Animal Metabolic Monitoring System (Columbus Instruments, Columbus, Ohio) was used to evaluated O2 consumption, CO2 production, energy expenditure, activity, and food consumption. Drinking was assessed by a computer system counting consumed water droplets.
Hyperinsulinemic-euglycemic clamp
Hyperinsulinemic-euglycemic clamps were performed as previously described (11). To do this, a jugular venous catheter was implanted 6-7 days before hyperinsulinemic-euglycemic clamps. To assess basal whole-body glucose turnover, [3-3H]-glucose (HPLC purified; PerkinElmer Life Sciences, Waltham, Massachusetts) was infused at a rate of 0.05 μCi/min for 120 minutes into the jugular catheter after overnight fasting condition. After the basal period, hyperinsulinemic-euglycemic clamps were conducted in conscious mice for 140 minutes with a 4-minute primed infusion of insulin (7.14 mU/[kg-min]) and [3-3H]-glucose (0.24 μCi/min), followed by a continuous (3 mU/[kg-min]) infusion of human insulin (Novolin; Novo Nordisk, Bagsværd, Denmark), and [3-3H]-glucose (0.1 μCi/min), and a variable infusion of 20% dextrose to maintain euglycemia (100-120 mg/dL); 10-μCi bolus of 2-deoxy-d-[1-14C]glucose (PerkinElmer) was injected after 85 minutes to estimate the insulin-stimulated tissue glucose uptake. Plasma samples were obtained from the tip of the tail at 0, 25, 45, 65, 80, 90, 100, 110, 120, 130, and 140 minutes. The tail incision was made at least 2 hours before the first blood sample was taken to allow for acclimatization, according to standard operating procedures (12). Also, mice received an iv albumin-containing solution (115mM NaCl, 5.9mM KCl, 1.2mM MgCl2-6H2O, 1.2mM NaH2PO4-H2O, 1.2mM Na2SO4, 2.5mM CaCl-2H2O, 25mM NaHCO3, and 4% BSA [pH 7.4]) mimicking artificial plasma, at a rate of 4.2 μL/min, during the insulin-stimulated period of the clamp to compensate for volume loss secondary to blood sampling. At the end of the clamps, mice were anesthetized with pentobarbital sodium injection (150 mg/kg), and all tissues taken were snap frozen in liquid nitrogen and stored at −80°C for subsequent use.
O2 consumption measurement in primary hepatocytes
Primary hepatocytes from OVX or E2-treated mice were isolated (by liver perfusion with collagenase digestion) at the Yale Liver Center. Briefly, cells were washed 3 times with recovery media (DMEM with high glucose plus 10% of fetal bovine serum), and equal amounts of cells (12 000) were seeded in each well of a Seahorse XF24 cell culture plate (Seahorse Bioscience, North Billerica, Massachusetts). These experiments were repeated 6 times under the same conditions. The cells were kept in recovery media for 4-6 hours, then they were washed with DMEM (low glucose plus 10% of fetal bovine serum) and incubated overnight. The next morning, cells were washed with prewarmed (∼37°C) XF24 Assay media, and 600 μL of assay media were added to each well for basal O2 consumption measurement and palmitate oxidation test, respectively. Before measurements, cells with assay media were placed in an unbuffered, humidified incubator at 37°C for 1 hour to allow temperature and pH equilibration. Three measurements of basal O2 consumption rates (picomoles per minute) were recorded using 3-minute mix, 2-minute wait, and 3-minute measure cycles, before the addition of palmitate (200μM), and the O2 consumption rates were recorded using the same cycles as used for basal measurements. The average of 3 measurements was used for analysis. These experiments were repeated 6 times using 6 different mice per group under the same conditions.
Liver and skeletal muscle lipid measurements
Tissue triglycerides (TGs) were extracted using the method of Bligh and Dyer (13) and measured using a DCL TG reagent (Diagnostic Chemicals, Charlottetown, Prince Edward Island, Canada). For diacylglycerol (DAG) extraction, livers were homogenized in a buffer solution (20mM Tris-HCl, 1mM EDTA, 0.25mM EGTA, 250mM sucrose, and 2mM phenylmethylsulfonyl fluoride) containing a protease inhibitor mixture (Roche, Indianapolis, Indiana), and samples were centrifuged at 100 000g for 1 hour. The supernatants containing the cytosolic fraction were collected. DAG levels were then measured by liquid chromatography/mass spectrometry/mass spectrometry as previously described (13). Total cytosolic DAG content is expressed as the sum of individual species. Ceramide was measured as previously described (14). All lipid measurements were done from tissues of animals after a 6-hour fast.
Immunoblotting analysis
Tissues were homogenized in radioimmunoprecipitation assay lysis buffer supplemented with protease inhibitor cocktail (Roche) for protein isolation. Proteins from homogenized liver, skeletal muscle, or brown adipose tissue (30 μg of protein extracts) were electrophoretically separated by 4%–12% SDS-PAGE (Invitrogen, Carlsbad, California) and then transferred to polyvinylidene difluoride membranes (Millipore, Bedford, Massachusetts) using a semidry transfer cell (Bio-Rad, Hercules, California) for 120 minutes. After blockade of nonspecific sites with 5% nonfat dry milk Tris-buffered saline and Tween 20 (10mM Tris, 100mM NaCl, and 0.1% Tween 20) solution, membranes were incubated overnight at 4°C with the following primary antibodies: phopho-AKT2ser473 (Signalway Antibody, Pearland, Texas), AKT2 (serine-threonine kinase 2) (Cell Signaling Technology, Inc, Danvers, Massachusetts), protein kinase C (PKC)ϵ (BD Transduction Laboratories, Lexington, Kentucky), PKCθ (BD Transduction Laboratories), or glyceraldehyde-3-phosphate dehydrogenase (Santa Cruz Biotechnology, Inc, Santa Cruz, California). After washing with Tris-buffered saline and Tween 20, membranes were incubated with peroxidase-conjugated antirabbit, or antimouse, or antigoat antibody. Membranes were thoroughly washed, and immune complexes were detected using an enhanced luminol chemiluminescence system (ECL; Thermo Scientific, Rockford, Illinois) and subjected to photographic films. Signals on the immunoblot were quantified by optical densitometry (Scion Image Software, Frederick, Maryland).
Total RNA preparation and real-time quantitative PCR analysis
Total RNA was extracted from frozen livers using RNeasy 96 kit (QIAGEN, Valencia, California), then 1 μg of RNA was reverse transcribed into cDNA with the use of the Quantitect RT kit (QIAGEN) as per the manufacturer's protocol. The abundance of transcripts was assessed by real-time PCR on a 7500 Real-Time PCR system (Applied Biosystems, Foster City, California) with a SYBR Green detection system. Samples were run in duplicate for both the gene of interest and glyceraldehyde-3-phosphate dehydrogenase, and data were normalized for the efficiency of amplification, as determined by a standard curve included on each run. Primers used are shown in Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org.
Statistical analysis
Data are expressed as the mean ± SEM. Results were assessed using 1-way ANOVA (Prism 5; GraphPad, San Diego, California) followed by Tukey's multiple comparison test, as appropriate. A P value less than .05 was considered significant.
Results
Plasma substrate and hormone concentrations
Before surgery and the HFD intervention, there were no differences in body weight and fat mass between the groups, and all groups had similar plasma concentrations of glucose, insulin, nonesterified fatty acids, and TG levels (Table 1). After ovariectomy and 4-week HFD feeding, OVX mice gained more weight than the SHAM mice (Table 1), and they also had increased fat mass (Table 1), suggesting that estrogen deprivation leads to obesity in rodents. The E2 treatment in OVX mice prevented both these increases in body weight and fat mass (Table 1). Finally, as expected, the OVX mice had significantly reduced plasma E2 levels compared with SHAM and OVX+E2 mice (Table 1). OVX+E2 mice displayed modestly elevated plasma E2 levels compared with SHAM (Table 1); however, the absolute values for both groups were within the physiological range (5, 15). Progesterone levels were reduced in both OVX and OVX+E2 mice, as expected (Table 1).
Table 1.
Physiological Parameters and Plasma Analysis
| SHAM | OVX | OVX+E2 | |
|---|---|---|---|
| E2 (pg/mL) | 24.1 ± 2.6 | 8.3 ± 0.3a | 39.1 ± 5.2ab |
| Progesterone (ng/mL) | 28.2 ± 3.7 | 7.1 ± 0.9a | 6.0 ± 1.1a |
| Initial body weight (g) | 17.0 ± 0.1 | 16.8 ± 0.2 | 16.8 ± 0.2 |
| Final body weight (g) | 22.8 ± 0.2 | 30.2 ± 1.1a | 23.3 ± 0.3b |
| Body fat (%) | 11.1 ± 0.6 | 28.0 ± 1.8a | 5.8 ± 0.2ab |
| Basal glucose (mg/dL) | 107 ± 6.6 | 132 ± 14 | 110 ± 7.7 |
| Basal insulin (μU/mL) | 12.8 ± 3.1 | 12.8 ± 3.0 | 12.7 ± 2.5 |
| NEFA (mmol/L) | 0.86 ± 0.08 | 0.61 ± 0.04a | 0.91 ± 0.13b |
| Plasma TG (mg/dL) | 58.3 ± 8.3 | 61.5 ± 4.9 | 60.3 ± 6.0 |
NEFA, nonesterified fatty acids. Data represent the mean ± SEM.
P < .05 compared with SHAM.
P < .05 compared with OVX (n = 8).
Energy expenditure and food intake
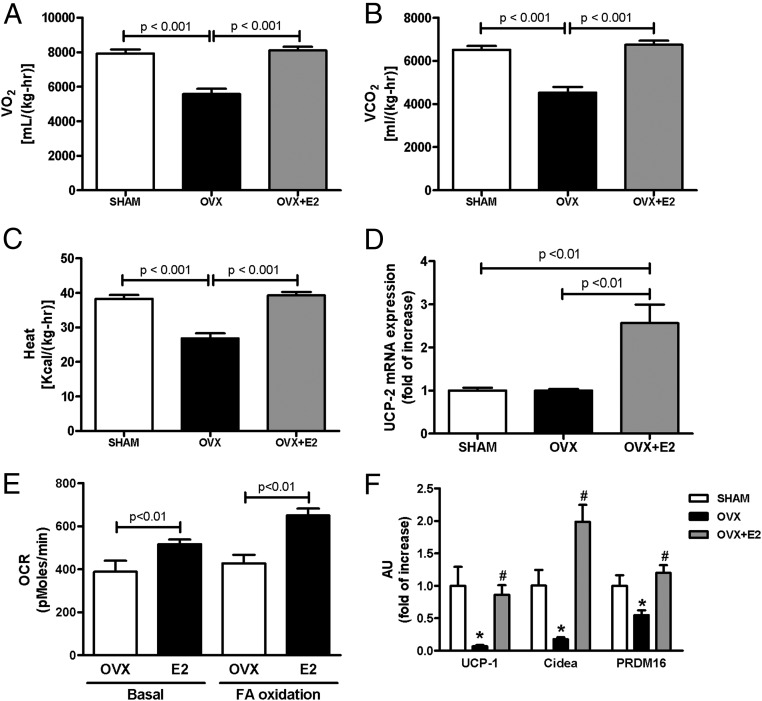
OVX mice displayed reduced O2 consumption and CO2 production and whole-body energy expenditure, as compared with SHAM and OVX+E2 mice (Figure 1, A–C). Notably, OVX+E2 and SHAM mice had similar rates of O2 consumption, CO2 production, and energy expenditure, suggesting that E2 treatment prevented the effects of ovariectomy (Figure 1). Interestingly, during the light hours, the activity of the OVX and SHAM mice was similar, whereas during the dark hours, the OVX mice were less active than SHAM mice (Supplemental Figure 1, C and D). These results suggest that the reduced energy expenditure observed in OVX mice was not due solely to reduced activity. Also of interest, although 24-hour O2 consumption and energy expenditure were higher in the OVX+E2 mice compared with OVX mice, they were less active than OVX mice during the light hours and had similar activity during the dark hours (Supplemental Figure 1, C and D), suggesting that the E2 treatment per se enhanced oxidative metabolism in these mice.
Figure 1.
E2 regulates whole-body energy expenditure. (A) Whole-body O2 consumption. (B) Whole-body CO2 production. (C) Whole-body energy expenditure. (D) mRNA UCP-2 expression in liver. (E) O2 consumption in isolated hepatocytes. (F) mRNA UCP-1, Cidea, and PRDM16 expression in WAT. Significance between groups was determined by 1-way ANOVA (n = 8). Data represent the mean ± SEM. *P < .01 compared with SHAM, #P < .01 compared with OVX. AU, arbitrary units; OCR, oxygen consumption rate; VO2;VCO2, volume of O2, volume of CO2.
Although the OVX mice were obese compared with SHAM mice, their food intake was slightly lower than the SHAM and OVX+E2 mice (Supplemental Figure 1E), suggesting increased energy efficiency in these mice and that the observed differences in activity between groups may be due to reduced feeding behavior. There was no difference in drinking behavior among the groups (Supplemental Figure 1F).
We measured the expression of key genes to further explore the mechanism whereby estrogen may regulate energy expenditure. OVX+E2 mice had increased mRNA expression of uncoupling protein (UCP)-2 in liver as well as increased O2 consumption in isolated hepatocytes in both basal condition and fatty acid oxidation (Figure 1, D and E). In white adipose tissue (WAT), the mRNA expression of UCP-1, cell death-inducing DFFA-like effector a (Cidea), and PR domain containing 16 (PRDM16) was reduced in OVX mice (Figure 1F), suggesting reduced oxidative metabolism in adipose tissue from OVX mice.
E2 treatment improves glucose homeostasis and insulin sensitivity in OVX mice
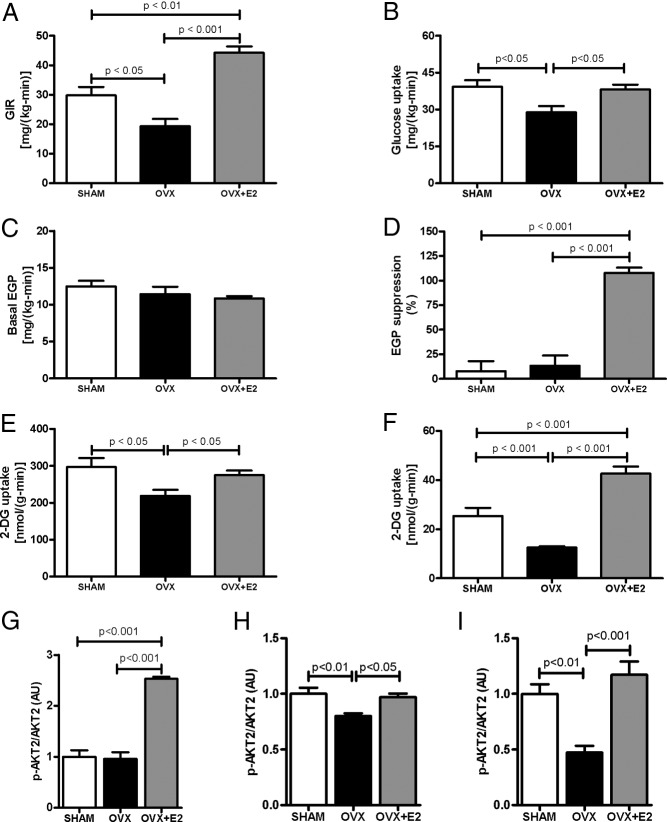
Whole-body and tissue-specific insulin sensitivity was assessed using the hyperinsulinemic-euglycemic clamp. During the clamp, the glucose infusion rate required to maintain euglycemia was significantly reduced by approximately 30% and approximately 55% in OVX compared with SHAM and E2-treated mice (Figure 2A; Supplemental Table 2), respectively, demonstrating impaired whole-body insulin sensitivity in OVX mice. When comparing SHAM and OVX mice, the insulin resistance in OVX mice was accounted for by reduced insulin-stimulated whole-body glucose uptake (Figure 2B), whereas there was no difference in the suppression of endogenous (primarily hepatic) glucose production (Figure 2D). E2 replacement prevented the development of peripheral insulin resistance. However, E2 also protected mice from diet-induced hepatic insulin resistance, a notable difference from the SHAM animals that retained intact ovaries. Indeed, the improvements in hepatic insulin response along with insulin-stimulated whole-body glucose uptake accounted for the increase in the insulin-stimulated glucose infusion rate in the OVX+E2 mice (Figure 2A) and may result from the modest difference in plasma E2 levels between these 2 groups or difference in plasma progesterone levels (Table 1). Interesting, the enzyme estrogen sulfotransferase (EST) mRNA expression was reduced in OVX and OVX+E2 mice compared with SHAM mice (Supplemental Figure 2). It might increase the E2 inactivation in liver from SHAM mice, leading to reduced protection from diet-induced liver insulin resistance.
Figure 2.
E2 regulates whole-body insulin sensitivity. (A) Average of glucose infusion rate during the last 40 minutes of euglycemic-hyperinsulinemic clamp. (B) Insulin-stimulated whole-body glucose uptake during the euglycemic-hyperinsulinemic clamp. (C) Basal endogenous glucose production. (D) Insulin-stimulated endogenous glucose production suppression during the euglycemic-hyperinsulinemic clamp. (E) Insulin-stimulated 2-DG uptake in skeletal muscle (Gastro). (F) Insulin-stimulated 2-DG uptake in WAT. (G) Insulin-stimulated AKT2 phosphorylation in liver. (H) Insulin-stimulated AKT2 phosphorylation in skeletal muscle (Gastro). (I) Insulin-stimulated AKT2 phosphorylation in WAT. Significance between groups was determined by 1-way ANOVA (n = 8). Data represent the mean ± SEM. GIR, glucose infusion rate; EGP, endogenous glucose production; gastro, gastrocnemius; AU, arbitrary units.
Tissue-specific 2-deoxyglucose (2-DG) uptake was measured to quantify alterations in muscle and adipose glucose uptake. Skeletal muscle 2-DG glucose uptake, which accounts for most glucose disposal during a clamp, was decreased in OVX mice and accounts for the reduced whole-body glucose uptake observed in OVX compared with SHAM and OVX+E2 mice (Figure 2E). Also, 2-DG uptake was reduced in WAT of OVX compared with SHAM and OVX+E2 (Figure 2F), indicating insulin resistance in adipose tissue. There was no difference in brown adipose tissue 2-DG uptake (data not shown).
Also, insulin-stimulated AKT2 serine phosphorylation was increased in liver from OVX+E2 mice (Figure 2G), corroborating the clamp data, and OVX mice displayed reduced insulin-stimulated AKT2 phosphorylation in skeletal muscle and WAT (Figure 2, H and I). The time-course for plasma glucose and glucose infusion rate during the hyperinsulinemic-euglycemic clamp is shown in Supplemental Figure 3.
Liver and skeletal muscle lipids content and novel PKC (nPKC) activation
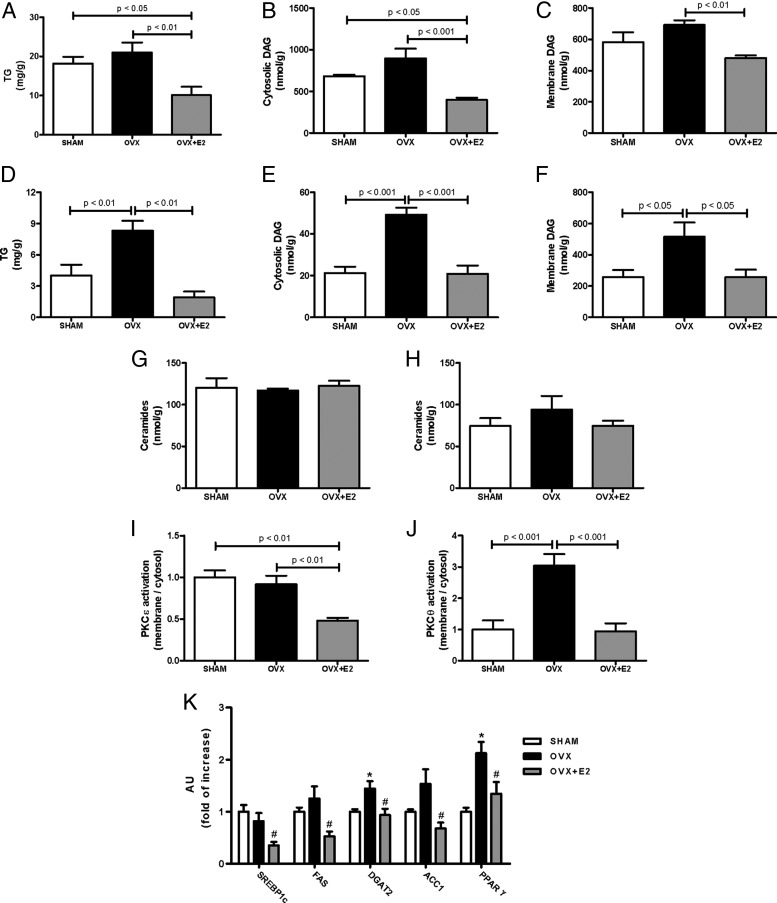
Because tissue lipid levels, such as TG and DAG, are strongly associated with insulin resistance in human subjects and rodent models of type 2 diabetes (16), hepatic and skeletal muscle lipid contents were determined. There was no difference in liver TG levels between SHAM and OVX mice; however, OVX+E2 mice has significantly reduced liver TG compared with OVX and SHAM mice (Figure 3A). OVX+E2 mice displayed reduced hepatic DAG content in both cytosolic and membrane fractions compared with OVX mice (Figure 3, B and C), consistent with changes in hepatic insulin sensitivity observed during the clamp (Figure 2A). Skeletal muscle TG levels were 2-fold greater in OVX compared with SHAM mice, whereas E2 treatment (OVX+E2) prevented this increase, such that SHAM and OVX+E2 muscle TG content was approximately equal (Figure 3D). Similar to skeletal muscle TG, DAG levels in muscle increased in OVX mice in both cytosolic and membrane fractions compared with SHAM, and E2 treatment (OVX+E2) reversed this effect (Figure 3, E and F). There were no differences in ceramide content in liver or muscle between the groups (Figure 3, G and H).
Figure 3.
OVX mice display increased ectopic lipid accumulation and nPKC activation. (A) Triacylglycerol (TG) content in liver. (B) Cytosolic DAG content in liver. (C) Membrane DAG content in liver. (D) TG content in skeletal muscle (Gastro). (E) Cytosolic content in skeletal muscle (Gastro). (F) Membrane DAG content in skeletal muscle (Gastro, gastrocnemius). (G) Ceramides content in liver. (H) Ceramides content in skeletal muscle (Gastro). (I) PKCϵ activation in liver. (J) PKCθ activation in skeletal muscle (Gastro). (K) mRNA expression of some lipids synthesis enzymes in liver. Significance between groups was determined by 1-way ANOVA (n = 8). Data represent the mean ± SEM. *P < .01 compared with SHAM, #P < .01 compared with OVX.
DAGs are proposed to activate nPKC isoforms, which in turn interfere with insulin signaling, contributing to insulin resistance (17). Thus, consistent with the observed changes in hepatic insulin sensitivity and DAG levels, OVX+E2 mice displayed reduced PKCϵ activation (translocation to membrane) in liver compared with OVX mice (Figure 3I). Also, consistent with changes in skeletal muscle insulin sensitivity and DAG levels, OVX mice showed increased PKCθ activation in skeletal muscle compared with SHAM, and E2 treatment (OVX+E2) reversed this effect (Figure 3J).
Due to the observed increase in lipid content in liver from SHAM and OVX mice, we also evaluated mRNA expression of several protein related to lipid synthesis (Figure 3K). E2 treatment decreased expression of several proteins that play a role on lipid synthesis, among them sterol regulatory element-binding protein 1c, a key transcriptional regulator of lipid synthesis.
Discussion
Epidemiological, clinical, and molecular studies have shown that estrogen plays an important role on metabolic homeostasis (18), and the loss of estrogen may have profound effects on glucose homeostasis and body composition in both menopause women (3) and rodents (5, 7). In the present study, we showed that endogenous estrogen is important to protect against HFD-induced skeletal muscle insulin resistance, whereas E2 treatment in estrogen-deprived mice increased insulin sensitivity in both liver and skeletal muscle. Also, we showed that estrogen plays an important role modulating the whole-body O2 consumption, energy expenditure, and accumulation of ectopic lipids.
With menopause, women can have an increase in body fat mass and visceral fat (3). The increased fat mass could be explained by the reduced energy expenditure in postmenopausal women (3). Those observations in women fit with the present study, which shows that OVX mice had reduced whole-body O2 consumption and energy expenditure, leading to increased body weight associated with increased fat mass.
The importance of estrogen on body weight and fat mass maintenance is also observed in transgenic mice. Both ERα knockout mice and aromatase knockout mice displayed increased body weight and fat mass in both male and female (7, 8). Also, those results were associated with reduced energy expenditure in transgenic mice (19). As observed in this work, Rogers et al (5) also observed reduced energy expenditure in OVX mice associated with increased fat mass. However, here, we demonstrate that chronic E2 replacement therapy corrected the reduced energy expenditure and whole-body O2 consumption. The increased UCP-2 in liver and O2 consumption in isolated hepatocytes from E2-treated mice could account for part of the increased energy expenditure as well as reduced content of liver lipids. Also, the reduced expression of UCP-1, Cidea, and PRDM16 in WAT from OVX may account for the decreased energy expenditure in those mice, because it is known that those proteins can improve whole-body energy metabolism when highly expressed in WAT (20).
The obesity observed in OVX mice was also associated with whole-body insulin resistance and impaired glucose tolerance (data not shown). Riant et al (10) also observed impaired glucose tolerance and insulin resistance in OVX mice fed with HFD, and E2 replacement reversed these effects. However, we found that endogenous estrogen protected female mice from HFD-induced insulin resistance only in skeletal muscle, displaying hepatic insulin resistance as well as OVX mice. E2 treatment, on the other hand, was able to protect OVX mice from insulin resistance in both liver and skeletal muscle. This difference in results between the SHAM and OVX+E2 groups may be explained by the differences in plasma E2 concentrations. Although still physiological (5, 15), E2 replacement did result in slightly higher plasma concentrations than were measured in the SHAM animals. This could have lead to increased E2 action in liver and protection from hepatic insulin resistance.
Another possibility is the difference in plasma progesterone levels. SHAM mice displayed higher progesterone concentrations compared with OVX and OVX+E2 mice. Nelson et al (21) showed that progesterone administration in rats reduced the insulin-induced suppression of hepatic glucose production but did not have any effects on insulin-mediated peripheral glucose utilization. Therefore, the higher level of plasma progesterone in SHAM mice might negate the beneficial effects of endogenous E2 effects on liver insulin action. In contrast, E2 was unopposed by progesterone in the OVX+E2 mice. The possible mechanism by which progesterone prevented the E2 effects is through increased expression of the enzyme EST, a primary enzyme responsible for the inactivation of estrogen (22, 23). Consistent with this possibility, we found that OVX mice have reduced hepatic EST expression compared with SHAM mice. Interestingly, recent studies have also found that female mice lacking EST expression in liver displayed increased whole-body energy expenditure and improved hepatic insulin sensitivity (24), supporting the hypothesis that plasma progesterone levels might explain the observed differences between SHAM and E2-treated mice.
The development of insulin resistance fat-fed OVX mice was associated with ectopic lipid accumulation. OVX mice had increased TG and DAG content in skeletal muscle compared with SHAM and E2-treated mice. In liver, both SHAM and OVX mice showed higher TG and DAG content compared with E2-treated mice. Previous studies have demonstrated that cellular DAG accumulation can activate nPKCs (PKCϵ in liver and PKCθ in muscle) (17) and impair proximal insulin signaling (17, 25, 26), leading to reduced insulin-stimulated AKT2 activation, as observed in our study, and insulin resistance. In the present study, the changes in cellular DAG content paralleled nPKC activation, suggesting that this mechanism may explain the changes in insulin action in liver and skeletal muscle seen in these mice.
In summary, we found that estrogen plays a pivotal role on whole-body energy homeostasis, increasing whole-body O2 consumption and energy expenditure, leading to increased whole-body insulin sensitivity in OVX mice. Also, this study showed that estrogen is able to reduce the ectopic lipid accumulation, leading to reduced DAG content in both liver and muscle, which, in turn, leads to reduced nPKC activation and increased insulin sensitivity in these tissues. Therefore, these studies provide a cellular mechanism by which estrogen replacement therapy prevents insulin resistance and type 2 diabetes in postmenopausal women.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants R01 DK-40936, R01 AG-23686, R24 DK-059635, P30 DK-45735, P30 DK-034989, and U24 DK-059635 and by a Distinguish Clinical Investigator award (K.F.P.) and a Mentor-Based Postdoctoral fellowship award (G.I.S.) from the American Diabetes Association. J.P.G.C. was a fellow from Coordenaçào de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Brazil.
Disclosure Summary: The authors have nothing to disclose.
For editorial see page 989
- AKT2
- serine-threonine kinase 2
- Cidea
- cell death-inducing DFFA-like effector a
- DAG
- diacylglycerol
- 2-DG
- 2-deoxyglucose
- E2
- estradiol
- ER
- estrogen receptor
- EST
- estrogen sulfotransferase
- HFD
- high-fat diet
- nPKC
- novel PKC
- OVX
- ovariectomized
- PKC
- protein kinase C
- PRDM16
- PR domain containing 16
- TG
- triglyceride
- UCP
- uncoupling protein
- WAT
- white adipose tissue.
References
- 1. Nuutila P, Knuuti MJ, Mäki M, et al. Gender and insulin sensitivity in the heart and in skeletal muscles. Studies using positron emission tomography. Diabetes. 1995;44(1):31–36 [DOI] [PubMed] [Google Scholar]
- 2. Donahue RP, Bean JA, Donahue RA, Goldberg RB, Prineas RJ. Insulin response in a triethnic population: effects of sex, ethnic origin, and body fat. Miami Community Health Study. Diabetes Care. 1997;20(11):1670–1676 [DOI] [PubMed] [Google Scholar]
- 3. Lovejoy JC, Champagne CM, de Jonge L, Xie H, Smith SR. Increased visceral fat and decreased energy expenditure during the menopausal transition. Int J Obes (Lond). 2008;32(6):949–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Margolis KL, Bonds DE, Rodabough RJ, et al. ; Women's Health Initiative Investigators Effect of oestrogen plus progestin on the incidence of diabetes in postmenopausal women: results from the Women's Health Initiative Hormone Trial. Diabetologia. 2004;47(7):1175–1187 [DOI] [PubMed] [Google Scholar]
- 5. Rogers NH, Perfield JW, 2nd, Strissel KJ, Obin MS, Greenberg AS. Reduced energy expenditure and increased inflammation are early events in the development of ovariectomy-induced obesity. Endocrinology. 2009;150(5):2161–2168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Camporez JP, Akamine EH, Davel AP, Franci CR, Rossoni LV, Carvalho CR. Dehydroepiandrosterone protects against oxidative stress-induced endothelial dysfunction in ovariectomized rats. J Physiol. 2011;589(10):2585–2596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jones ME, Thorburn AW, Britt KL, et al. Aromatase-deficient (ArKO) mice have a phenotype of increased adiposity. Proc Natl Acad Sci USA. 2000;97(23):12735–12740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. Increased adipose tissue in male and female estrogen receptor-α knockout mice. Proc Natl Acad Sci USA. 2000;97(23):12729–12734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Foryst-Ludwig A, Clemenz M, Hohmann S, et al. Metabolic actions of estrogen receptor β (ERβ) are mediated by a negative cross-talk with PPARγ. PLoS Genet. 2008;4(6):e1000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Riant E, Waget A, Cogo H, Arnal JF, Burcelin R, Gourdy P. Estrogens protect against high-fat diet-induced insulin resistance and glucose intolerance in mice. Endocrinology. 2009;150(5):2109–2117 [DOI] [PubMed] [Google Scholar]
- 11. Jurczak MJ, Lee AH, Jornayvaz FR, et al. Dissociation of inositol-requiring enzyme (IRE1α)-mediated c-Jun N-terminal kinase activation from hepatic insulin resistance in conditional X-box-binding protein-1 (XBP1) knock-out mice. J Biol Chem. 2012;287(4):2558–2567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ayala JE, Samuel VT, Morton GJ, et al. ; National Institutes of Health Mouse Metabolic Phenotyping Center Consortium Standard operating procedures for describing and performing metabolic tests of glucose homeostasis in mice. Dis Model Mech. 2010;3(9-10):525–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37(8):911–917 [DOI] [PubMed] [Google Scholar]
- 14. Yu C, Chen Y, Cline GW, et al. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J Biol Chem. 2002;277(52):50230–50236 [DOI] [PubMed] [Google Scholar]
- 15. Bryzgalova G, Lundholm L, Portwood N, et al. Mechanisms of antidiabetogenic and body weight-lowering effects of estrogen in high-fat diet-fed mice. Am J Physiol Endocrinol Metab. 2008;295(4):E904–E912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Samuel VT, Petersen KF, Shulman GI. Lipid-induced insulin resistance: unravelling the mechanism. Lancet. 2010;375(9733):2267–2277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Erion DM, Shulman GI. Diacylglycerol-mediated insulin resistance. Nat Med. 2010;16(4):400–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Barros RP, Gustafsson JA. Estrogen receptors and the metabolic network. Cell Metab. 2011;14(3):289–299 [DOI] [PubMed] [Google Scholar]
- 19. Ribas V, Nguyen MT, Henstridge DC, et al. Impaired oxidative metabolism and inflammation are associated with insulin resistance in ERα-deficient mice. Am J Physiol Endocrinol Metab. 2010;298(2):E304–E319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Boström P, Wu J, Jedrychowski MP, et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481(7382):463–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nelson T, Shulman G, Grainger D, Diamond MP. Progesterone administration induced impairment of insulin suppression of hepatic glucose production. Fertil Steril. 1994;62(3):491–496 [DOI] [PubMed] [Google Scholar]
- 22. Clark CL, Adams JB, Wren BG. Induction of estrogen sulfotransferase in the human endometrium by progesterone in organ culture. J Clin Endocrinol Metab. 1982;55(1):70–75 [DOI] [PubMed] [Google Scholar]
- 23. Falany JL, Falany CN. Regulation of estrogen sulfotransferase in human endometrial adenocarcinoma cells by progesterone. Endocrinology. 1996;137(4):1395–1401 [DOI] [PubMed] [Google Scholar]
- 24. Gao J, He J, Shi X, et al. Sex-specific effect of estrogen sulfotransferase on mouse models of type 2 diabetes. Diabetes. 2012;61(6):1543–1551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jornayvaz FR, Birkenfeld AL, Jurczak MJ, et al. Hepatic insulin resistance in mice with hepatic overexpression of diacylglycerol acyltransferase 2. Proc Natl Acad Sci USA. 2011;108(14):5748–5752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lee HY, Choi CS, Birkenfeld AL, et al. Targeted expression of catalase to mitochondria prevents age-associated reductions in mitochondrial function and insulin resistance. Cell Metab. 2010;12(6):668–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.