Abstract
KRAS mutations have been found in duodenal adenocarcinomas and may have prognostic significance. The purpose of this study was to classify clinicopathological characteristics, microsatellite instability, and KRAS mutations and identify possible prognostic role of KRAS mutations in duodenal adenocarcinomas. Demographics, tumor characteristics and survival were recorded for 78 patients with duodenal adenocarcinomas (stages I to III). KRAS mutations were detected in 27 (34.6%) cases, of which the majority (74.1%) were G>A transitions. Multivariate logistic regression analysis showed that KRAS G>A mutation was significantly associated with late stage (p = 0.025) and poor tumor differentiation (p = 0.035), when compared with wild-type and other than G>A mutations. KRAS G>A mutation carriers were at increased risk for distant relapse (p = 0.022) and had significantly shorter overall survival (OS; log-rank p = 0.045) and a trend toward shorter relapse-free survival (RFS; log-rank p = 0.062) when compared with those who did not carry the KRAS G>A mutation. In multivariate analyses, there was a significant correlation between ≥ 3 positive lymph nodes and poor OS (p < 0.001) and RFS (p = 0.001) and KRAS G>A mutation carriers demonstrated no effect on clinical outcome. In conclusion, KRAS G>A mutation correlates significantly with late stage and poor tumor differentiation in duodenal adenocarcinoma. Among patients who undergo a curative resection of duodenal adenocarcinoma, KRAS G>A mutation carriers will more likely experience distant relapse but may not exhibit a poor prognosis. Number of positive lymph nodes should be incorporated in future staging systems.
Keywords: KRAS, mutation, duodenal adenocarcinoma, prognosis
Cancer of the small bowel is rare and accounts for less than 3% of gastrointestinal malignancies, with an estimated 7570 new cases and 1100 deaths in the United States in the year 2011.1 Adenocarcinoma is the second most commonly diagnosed histologic subtype of small bowel tumors.2 Approximately, 58.8% of the small bowel adenocarcinomas are found in the duodenum.2 Outcomes for patients with duodenal adenocarcinoma are poor, with data from a single institute reported demonstrating 5-year overall survivals of 68.6%, 43.5% and 24.6%, for stage I, stage II, and stage III, respectively.3 Curative resection does improve survival but many patients will experience local or distant recurrence.
The Ras superfamily of small GTPases comprises a group of molecular switches that regulate a large diversity of cellular functions.4 The RAS gene family consists of three genes, HRAS, NRAS, and KRAS, which encode highly homologous 21 kDa proteins.5 RAS genes become oncogenic by single point mutations, mainly in codons 12 or 13, which alter the guanine nucleotide–binding region, rendering Ras unresponsive to GTPase activating proteins and resulting in constitutive activation of Ras and aberrant downstream signaling.6 Oncogenic mutations have been found in all three members of the RAS gene family with KRAS being the most frequently mutated. KRAS mutations are found at high frequencies in pancreatic, colorectal, small intestine, lung, and liver cancers.7
KRAS mutations may also play a prognostic role in colorectal cancer (CRC).8 Previous studies have described KRAS mutations in duodenal cancers.9–12 However, these studies included a small number of patients, neither frequency assessment of KRAS mutations nor further information with regard to their prognostic significance in this disease, was available.
The aims of this study, therefore, were to assess the mutational status of the KRAS gene in the largest series of duodenal adenocarcinomas reported to date and to establish whether or not any or specific mutations of KRAS might have prognostic value or be associated with clinicopathological characteristics and microsatellite instability (MSI).
Material and Methods
Study population
Patients were identified from the Johns Hopkins Hospital Oncology Clinical Information System from October 1997 to December 2009. Formalin-fixed, paraffin-embedded tissue blocks were collected from the patients who had curative surgical resection for duodenal adenocarcinomas with stages I to III. Tissue sections from all cases in this study were reviewed by an expert gastrointestinal pathologist. Ascertainment of survival was performed by using the Johns Hopkins electronic health records, the Cancer Registry and mortality was confirmed also within the Social Security Death Index. The Johns Hopkins Hospital Institutional Review Board approved this research protocol.
Analyses of KRAS mutations
Genomic DNA from paraffin-embedded tissue was extracted by phenol-chloroform, and polymerase chain reaction (PCR) targeted for KRAS codons 12 and 13 was performed as previously described.13 PCR products were sequenced in both directions by use of a M13F primer (5′-GTAAAACGACGGCCAGT-3′) and a M13R primer (5′-CAGGAAACAGCTATGACC-3′) that were incorporated into the forward and reverse primer of each primer pair, respectively (Agencourt Bioscience Corporation, Beverly, MA). Sequence data were analyzed with SequencherTM 4.8 software (Gene Codes, Ann Arbor, MI). Verification of all mutations was accomplished by bidirectional sequencing of a second PCR product derived independently from the original template.
Analyses of microsatellites
MSI status was determined using D2S123, D5S346, D17S250, BAT25, and BAT26.14 Microsatellite sizes were compared with those of normal adjacent tissue, and tumors with 2 or more of the markers exhibiting instability were classified as high MSI (MSI-H). Tumors with only one marker exhibiting instability or no markers with instability were classified as low MSI (MSI-L) or microsatellite stable (MSS) respectively. In this study, MSI-low and MSS tumors were grouped together and henceforth are referred to as MSS, and MSI-H is referred to as MSI.
Statistics
Differences in categorical variables between study groups were analyzed using χ2 test for homogeneity of Fisher’s exact test. Multivariate relationships were estimated by fitting logistic regression models. Survival was estimated by using the Kaplan-Meier method and log-rank statistics computed to test for differences between survival curves for various prognostic factors. Univariate and Multivariate Cox proportional hazard regression models included KRAS mutations, sex, age, stage or positive lymph nodes, tumor differentiation, chemotherapy and/or radiotherapy, and MSI status. Results of Cox regression are reported as hazard ratio (HR) with corresponding 95% confidence intervals (CI). All hypotheses tests were two-sided, and results were considered statistically significant for p values < 0.05. Calculations were performed using SPSS 16.0 software (SPSS Inc, Chicago, IL).
Results
KRAS mutations in duodenal adenocarcinoma
Amongst 78 patients who had underwent a curative resection of stages I to III duodenal adenocarcinomas, KRAS mutations were found in 27 (34.6%) patients. KRAS mutations were seen in 14.8% of early stage (stages I and II) cancers and 85.2% of late stage (stage III) cancers (Table 1). Twenty-one of the 27 patients with KRAS mutations had codon 12 mutations. Of the 21 mutations, 66.7% (14 of 21) were G to A transitions, 4.8% (1 of 21) were G to C transitions, and 28.6% (6 of 21) were G to T transitions. Conversely, mutations in KRAS codon 13 were detected only in 6 patients, and all of these were G to A transitions (Table 2). The overall frequency of G to A transition was 25.6% (20 of 78) and comprised 74.1% (20 of 27) of patients with KRAS mutations.
Table 1.
Patient characteristics by genetic and molecular groups
| Total (%) | KRAS wild-type (%) | KRAS mutated (%) | p * | |
|---|---|---|---|---|
| No. of patients | 78 | 51 (65.4) | 27 (34.6) | |
| Age at surgery | 0.614 | |||
| <60 | 26 (33.3) | 18 (35.3) | 8 (29.6) | |
| ≥60 | 52 (66.7) | 33 (64.7) | 19 (70.4) | |
| Sex | 0.555 | |||
| Male | 44 (56.4) | 30 (58.8) | 14 (51.9) | |
| Female | 34 (43.6) | 21 (41.2) | 13 (48.1) | |
| Stage | 0.022† | |||
| Early stage (T1–4 N0 M0) | 25 (32.1) | 21 (41.2) | 4 (14.8) | |
| Late stage (anyT N1-2 M0) | 53 (67.9) | 30 (58.8) | 23 (85.2) | |
| Tumor differentiation | 0.167 | |||
| Well/Moderate | 43 (55.1) | 31 (60.8) | 12 (44.4) | |
| Poor | 35 (44.9) | 20 (39.2) | 15 (55.6) | |
| Extent of resection | 0.086† | |||
| R0 | 68 (87.2) | 47 (92.2) | 21 (77.8) | |
| R1/R2 | 10 (12.8) | 4 (7.8) | 6 (22.2) | |
| Chemotherapy/radiotherapy | 0.241 | |||
| No | 27 (34.6) | 20 (39.2) | 7 (25.9) | |
| Yes | 51 (65.4) | 31 (60.8) | 20 (74.1) | |
| MSI status | 0.390† | |||
| MSS | 61 (78.2) | 38 (74.5) | 23 (85.2) | |
| MSI | 17 (21.8) | 13 (25.5) | 4 (14.8) |
KRAS wild-type vs. KRAS mutated, χ2 test unless indicated otherwise;
Fisher’s exact test; MSS, microsatellite stable; MSI, microsatellite instability
Table 2.
Non-synonymous K-RAS mutation status of patients with duodenal adenocarcinoma in stage I, II and III
| Mutation Sequence | Amino Acid | Stage I | Stage II | Stage III |
|---|---|---|---|---|
| Codon 12 | ||||
| GGT>GAT | G12D | 0 | 1 | 12 |
| GGT>AGT | G12S | 0 | 0 | 1 |
| GGT>CGT | G12R | 0 | 0 | 0 |
| GGT>GCT | G12A | 0 | 0 | 1 |
| GGT>GTT | G12V | 0 | 2 | 4 |
| GGT>TGT | G12C | 0 | 0 | 0 |
| Codon 13 | ||||
| GGT>GAT | G13D | 1 | 0 | 5 |
| All | ||||
| G>A | 1 | 1 | 18 | |
| G>C | 0 | 0 | 1 | |
| G>T | 2 | 4 | ||
| Total | 1 | 3 | 23 | |
Clinicopathologic correlation of KRAS mutations
KRAS mutations occurred more frequently in late stage tumors (p = 0.022), and were more common in poorly differentiated tumors although this association failed to reach statistical significance (p = 0.167). No significant association was found between KRAS mutations and age, sex, extent of resection, undergoing chemotherapy/radiotherapy and MSI status (Table 1).
We next assessed the correlation of KRAS mutation subtypes with clinicopathologic variables and MSI. On univariate association analysis stratified by KRAS mutations subtypes (Table 3), KRAS G>A mutation was significantly more frequent in late stage tumors (p = 0.014) and poorly differentiated tumors (p = 0.009) when compared with KRAS wild-type and other than G>A mutations. Most of the patients with KRAS G>A mutation (18/20, 90.0%) were seen in late stage cancers, while just 2 (2/20, 10%) of the patients with KRAS G>A mutation were seen in early stage tumors. Amongst 35 patients with poorly differentiated tumors, there were 14 patients with G>A mutation, 20 patients with KRAS wild-type, and only 1 patient with other than G>A mutation. In 43 patients with well and moderately differentiated tumors, there were 31 patients with KRAS wild-type, 6 patients with G>A mutation, and 6 patients with other than G>A mutation. In a multivariate logistic model containing age, sex, stage, tumor differentiation, and MSI status (Table 3), KRAS G>A mutation remained significantly more frequent in late stage [odds ratio (OR) = 6.42, 95% CI: 1.26–32.79; p = 0.025] and poorly differentiated tumors (OR = 3.51, 95% CI: 1.09–11.26; p = 0.035).
Table 3.
Association between clinicopathologic features or microsatellite instability status and the presence of KRAS G>A mutation in 78 duodenal adenocarcinomas
| Characteristics | Frequency analysis
|
Multivariate logistic regression analysis
|
||||
|---|---|---|---|---|---|---|
| Frequency
|
p* | |||||
| No. | % | OR | 95% CI | p | ||
| Sex (female vs. male) | 10/34 vs. 10/44 | 29.4 vs. 22.7 | 0.503 | 1.71 | 0.53, 5.49 | 0.371 |
| Age (≥60 vs. <60) | 15/52 vs. 5/26 | 28.8 vs. 19.2 | 0.359 | 2.00 | 0.56, 7.13 | 0.283 |
| Stage (late vs. early) | 18/53 vs. 2/25 | 34.0 vs. 8.0 | 0.014† | 6.42 | 1.26, 32.79 | 0.025 |
| Differentiation (poor vs. well/moderate) | 14/35 vs. 6/43 | 40.0 vs. 14.0 | 0.009 | 3.51 | 1.09, 11.26 | 0.035 |
| MSI (MSS vs. MSI) | 17/61 vs. 3/17 | 27.9 vs. 17.6 | 0.536† | 1.76 | 0.40, 7.77 | 0.454 |
OR, odds ratio; CI, confidence interval; MSS, microsatellite stable; MSI, microsatellite instability;
p values were calculated by χ2 test unless indicated otherwise;
Fisher’s exact test
Pattern of relapse
Relapses were observed in 27 patients (34.6%). Relapses were local in 10 patients (37%), and distant in 17 patients (63%; including 13 cases with distant failure only, and 4 cases with both local recurrence and distant metastases). Local relapse occurred in 2 patients with KRAS G>A mutation and in 8 patients with KRAS wild-type, respectively. Distant relapse occurred in 8 patients with KRAS G>A mutation status and in 9 patients with wild-type, respectively. None of 10 patients with other than G>A mutations experienced relapse. KRAS G>A mutation carriers were not more likely to recur (p = 0.094) but prone to experience distant relapse (p = 0.022), when compared with those without KRAS G>A mutation.
Prognostic role with KRAS mutations
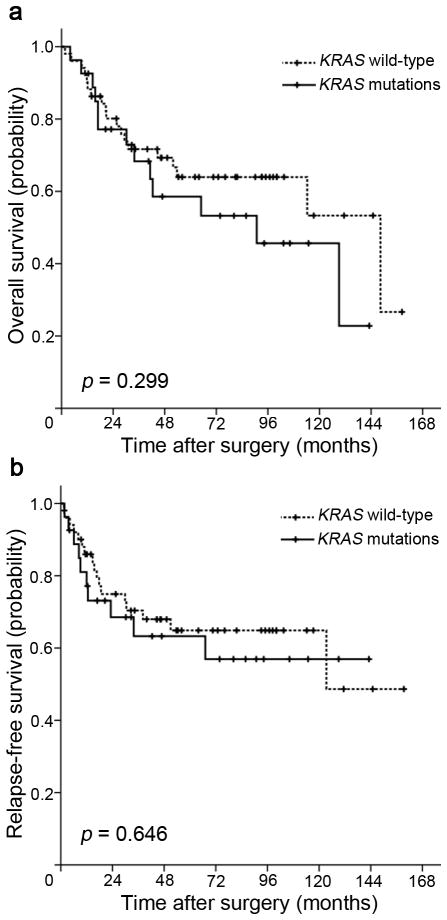
We next assessed the influence of KRAS mutation status on clinical outcomes in this cohort of duodenal adenocarcinomas. With median follow-up of 46.8 months, there were 27 events for relapse-free survival (RFS) analysis, and 32 events for overall survival (OS) analysis. In Kaplan-Meier analysis, there were no significant differences in survival time distributions between patients with KRAS mutations and those with wild-type KRAS (log-rank p = 0.299 for OS, Figure 1a; log-rank p = 0.646 for RFS, Figure 1b).
Figure 1.
Kaplan-Meier survival estimates between patients with duodenal adenocarcinomas with KRAS mutations and those with wild-type KRAS: (a) overall survival, (b) relapse-free survival. P values were based on the log-rank test.
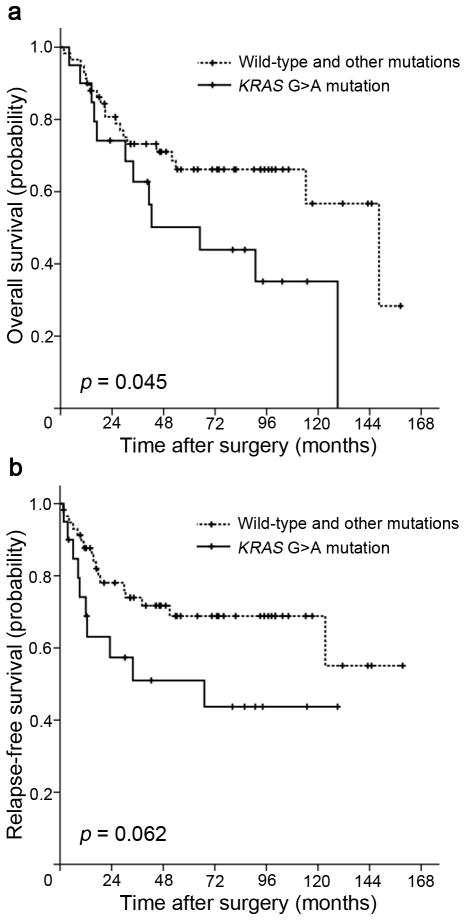
We then compared OS and RFS for patients stratified by mutation status. The median OS of all KRAS G>A mutation carriers was significantly shorter at 64.9 months compared with that of KRAS wild-type patients and other than G>A mutations carriers at 148.4 months (log-rank p = 0.045, Figure 2a). KRAS G>A mutation carriers were also associated with shorter RFS (median 67.1 years) when compared with KRAS wild-type patients and other than G>A mutations carriers (median RFS had not been reached), but this did not reach statistical significance (log-rank p = 0.062, Figure 2b).
Figure 2.
Kaplan-Meier survival estimates between patients with duodenal adenocarcinomas with KRAS G>A mutation and those with wild-type KRAS and other than G>A mutations: (a) overall survival, (b) relapse-free survival. P values were based on the log-rank test.
To correct for possible prognostic factors for OS and RFS, a Cox proportional hazards model that included KRAS mutation status, sex, age, stage, tumor differentiation, chemotherapy/radiotherapy, and MSI status, was used (Table 4). In univariate Cox regression analysis, KRAS G>A mutation, advanced stage, poor tumor differentiation, and undergoing chemotherapy and/or radiotherapy are associated with reduced OS, and young age, advanced stage, poor differentiation, and undergoing chemotherapy and/or radiotherapy are associated with reduced RFS. In multivariate analysis, there was no correlation between KRAS G>A mutation and poor OS (HR = 1.14, 95% CI: 0.51–2.53, p = 0.748) and RFS (HR = 1.59, 95% CI: 0.65–3.88, p = 0.309). Only undergoing chemotherapy and/or radiotherapy was significantly associated with poor OS (HR = 2.80, 95% CI: 1.02–7.69, p = 0.046). Late stage, poor differentiation and MSS were also associated with poor OS although these associations approached but failed to reach statistical significance. Poor differentiation (HR = 3.04, 95% CI: 1.28–7.19, p = 0.011) and MSS (HR = 3.62, 95% CI: 1.06–12.29, p = 0.039) were found to be independent prognostic factors for RFS. Young age, late stage and undergoing chemotherapy and/or radiotherapy were also associated with poor RFS though these were not statistically significant.
Table 4.
Univariate and multivariate Cox proportional hazard analysis of overall survival and relapse-free survival
| Characteristic | OS
|
RFS
|
||||||
|---|---|---|---|---|---|---|---|---|
| Univariate
|
Multivariate
|
Univariate
|
Multivariate
|
|||||
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | |
| KRAS mutations (G>A vs. wild-type and not G>A) | 2.06 (1.00, 4.25) | 0.049 | 1.14 (0.51, 2.53) | 0.748 | 2.07 (0.95, 4.54) | 0.068 | 1.59 (0.65, 3.88) | 0.309 |
| Sex (Female vs. Male) | 1.01 (0.50, 2.05) | 0.986 | 1.16 (0.55, 2.48) | 0.694 | 0.72 (0.33, 1.57) | 0.408 | 0.90 (0.38, 2.16) | 0.812 |
| Age (≥60 vs. <60) | 1.17 (0.55, 2.47) | 0.685 | 1.37 (0.62, 3.04) | 0.442 | 0.44 (0.21, 0.93) | 0.032 | 0.44 (0.19, 1.00) | 0.050 |
| Stage (late vs. early) | 3.09 (1.27, 7.55) | 0.013 | 2.34 (0.88, 6.22) | 0.089 | 4.31 (1.48, 12.59) | 0.008 | 2.57 (0.80, 8.31) | 0.115 |
| Differentiation (Poor vs. Well/moderate) | 2.68 (1.26, 5.71) | 0.011 | 2.05 (0.94, 4.46) | 0.071 | 3.67 (1.59, 8.48) | 0.002 | 3.04 (1.28, 7.19) | 0.011 |
| Chemotherapy/radiotherapy (yes vs. no) | 3.52 (1.33, 9.31) | 0.011 | 2.80 (1.02, 7.69) | 0.046 | 4.21 (1.41, 12.51) | 0.010 | 2.88 (0.95, 8.73) | 0.061 |
| MSI status (MSS vs. MSI) | 2.39 (0.83, 6.83 | 0.105 | 2.74 (0.95, 7.91) | 0.063 | 2.97 (0.89, 9.87) | 0.076 | 3.62 (1.06, 12.29) | 0.039 |
OS, overall survival; RFS, relapse-free survival; HR, hazard ratio; CI, confidence interval; MSS, microsatellite stable; MSI, microsatellite instability
Prognostic role with positive lymph nodes
We next assessed the prognostic role with positive lymph nodes. In Kaplan-Meier analysis, the median OS of ≥ 3 positive lymph nodes patients was significantly shorter at 20.8 months compared with that of none positive lymph nodes (stages I and II) patients and < 3 positive lymph nodes patients (median OS had not been reached; log-rank p = 0.001; Supporting Information, Figure a). Patients with ≥ 3 positive lymph nodes were also associated with shorter RFS (median 16.7 months) when compared with none positive lymph nodes patients and < 3 positive lymph nodes patients (median RFS had not been reached; log-rank p < 0.001; Supporting Information, Figure b).
In multivariate analysis including positive lymph nodes, KRAS mutation status, sex, age, tumor differentiation, chemotherapy/radiotherapy, and MSI status, there was a significant correlation between ≥ 3 positive lymph nodes and poor OS (HR = 4.46, 95% CI: 2.03 – 9.77, p < 0.001) and RFS (HR = 4.79, 95% CI: 1.85–12.45, p = 0.001). KRAS G>A mutation was not associated with OS (HR = 1.12, 95% CI: 0.53–2.39, p = 0.769) and RFS (HR = 1.63, 95% CI: 0.68–3.88, p = 0.273; Supporting Information, Table).
Discussion
Approximately 30–40% of duodenal adenocarcinoma patients undergoing curative-intent surgery for primary cancer will relapse.15–17 More than half of the recurrences occur distantly, and the most common sites of distant failure are liver and lung.18, 19 Several studies have tried to better define clinicopathologic variables associated with the risk of recurrence with the goal of being able to supply effective therapeutic strategies in patients with high risk small bowel adenocarcinoma.15, 19–24 Risk classification based on clinicopathologic criteria and the use of radiotherapy and/or chemotherapy has been proven unsuccessful.25–27 Therefore, molecular characterization offers an attractive alternative to direct conventional adjuvant therapies and promote the development of novel targeted treatments for this malignancy.
KRAS mutation represents one of the most common molecular defects in colorectal cancer and small bowel cancer, and tumors with these defects are readily identifiable through direct sequencing. But due to rarity of duodenal adenocarcinomas, the distribution or contribution of KRAS mutation in this disease remains unknown. In CRC, there has been a considerable controversy in the literature regarding prognostic value of KRAS mutations. It was demonstrated that patients with G>A mutations and G>C mutations tended to have a worse prognosis than those with G>T mutations.28 In addition, it was found that the presence of KRAS mutations increased risk of recurrence and death. In particular, any mutation of G>T but not G>A or G>C increased the risk of recurrence and death.8 A subsequent analysis found only the codon 12 mutation leading to a glycine to valine substitution (G12V) of KRAS to be prognostic in stage III only.29 More recently, several large randomized trials have failed to consistently demonstrate a meaningful effect of KRAS mutations on prognosis in CRC.30–32 KRAS mutations are, however, now widely recognized as a predictive marker of resistance to EGFR-targeted therapy in CRC.33–35
The discovery of possible prognostic implications for KRAS mutations in colorectal cancer prompted this KRAS mutations study in duodenal adenocarcinoma. To our knowledge, the current analysis included the largest number of patients with duodenal adenocarcinoma in any single study to date.
There have been few studies on KRAS mutations in duodenal adenocarcinomas. Younes et al. first reported that 4 out of 12 patients with duodenal adenocarcinoma exhibited KRAS mutations, and all detected mutations were glycine to aspartate on codon 12 (G12D).9 Rashid and colleagues found 3 out of 8 tumors with one G12D, one glycine to aspartate on codon 13 (G13D) and one glycine to serine on codon 12 (G12S).11 Achille et al. detected 4 tumors with G12D and one tumor with glycine to alanine on codon 12 (G12A) in 12 tumors.12 Muneyuki et al. found 2 cases with G12V in 10 cases.10 Taken together, the frequency of KRAS mutations is nearly 30% and the predominant mutations are G>A transitions. However, all these studies included a small number of patients and had no classification of clinicopathological characteristics, MSI, and clinical outcomes in regard to KRAS mutations.
In the present study, KRAS mutations were found in 27 (34.6%) of the 78 patients with duodenal adenocarcinoma and most of them (74.1%) were G to A transitions, similar to what has been shown for CRC.29, 30 Analysis of the KRAS mutation subtypes and patterns of histopathology revealed that KRAS G>A mutation was significantly more common in tumors with late stage and poor differentiation as compared with wild-type and other than G>A mutations. Remarkably, KRAS G>A mutation not only correlates with the loss of tumor differentiation but also shows a significant association with the presence of distant metastases when compared with those with wild-type and other subtypes of KRAS mutations. Therefore, presence of KRAS G>A mutation in tumor may have prognostic value.
In Kaplan-Meier analysis, using OS and RFS, KRAS G>A mutation carriers correlated with a worse prognosis as compared with those wild-type patients and other than G>A mutation carriers, whereas multiple mutations did not have an influence on survival. All KRAS mutation subtypes identified in this study have been shown to contribute to malignant transformation in various malignancies.36–39 We speculate that the causes of KRAS G>A mutation, rather than the mutation itself, are more likely to lead to poor differentiation and worse prognosis in duodenal adenocarcinomas. If this is true, then perhaps tumors with KRAS G>A mutation arising from duodenum develop from a different pathway. The etiology of KRAS mutations is unclear and this may partially explain the failure to develop effective anti-KRAS therapies. Success in this endeavor would hinge on an in-depth analysis of distinct mechanisms for differences in KRAS mutations within a specific cancer type. It was reported that inactivation of MGMT by promoter hypermethylation was theoretically associated with the presence of KRAS G>A transitions in CRC.40 We do not know whether this is true in duodenal cancers. Future studies of relevant lesions will be necessary to address these questions.
A large proportion of patients with a KRAS G>A mutation presented with late stage and poorly differentiated disease, and therefore to ensure that the prognostic effect associated with KRAS G>A mutation was not just a reflection of later stage and poor tumor differentiation, we carried out a multivariate analysis. We developed models correcting for age, sex, stage, tumor differentiation, chemotherapy/radiotherapy, and MSI status and analyzed the data using Cox regression. On multivariate analysis, undergoing chemotherapy/radiotherapy seemed to be associated with both poor OS and RFS. It is reasonable because patients who had undergone chemotherapy/radiotherapy were usually at advanced stages and had more lymph node involvement. As anticipated, late stage, poor differentiation and MSS were independently associated with, or at least, showed a trend toward worse OS and RFS. There was no correlation between KRAS G>A mutation and both OS and RFS. The KRAS G12V mutation, which has been associated with poor survival in CRC,29 was present in six of our patients, five of them were still alive at the end of this study (data not shown), indicating that there are differences between duodenal adenocarcinoma and CRC.
It was reported that stratifying patients with stage III small bowel adenocarcinomas into those with < 3 positive lymph nodes and ≥ 3 positive lymph nodes significantly improved prognostication.22 We looked into whether this is true in this cohort of patients with duodenal adenocarcima. Our results validated that patients with ≥ 3 positive lymph nodes have worst prognosis in patients with stages I to III tumor. Future staging systems should incorporate the number of positive lymph nodes.22
In summary, this is the first study to classify clinicopathological characteristics, MSI, and KRAS mutations and examine possible prognostic role of KRAS mutations in duodenal adenocarcinomas. We show that KRAS mutations are present in 34.6% of duodenal cancers. The predominant KRAS mutations are G>A transitions (74.1%). Furthermore, KRAS G>A mutation is associated with late stage and poor tumor differentiation. Among patients who undergo a curative resection of duodenal adenocarcinoma, KRAS G>A mutation carriers will more likely experience distant relapse but may not exhibit a poor prognosis. Patients with ≥ 3 positive lymph nodes have worst prognosis, and number of positive lymph nodes should be incorporated in future staging systems.
Supplementary Material
Study highlights.
What is current knowledge
KRAS mutations have been described in duodenal adenocarcinomas but have not been further characterized due to the rarity of this disease.
The relevance of KRAS mutations to clinicopathological characteristics and prognosis remains unclear in duodenal adenocarcinomas.
What is new here
KRAS is mutated in 34.6% of duodenal adenocarcinomas based on our analysis in the largest cohort described, to date. The predominant KRAS mutations are G>A transitions.
KRAS G>A mutation correlates significantly with late stage and poor tumor differentiation in duodenal adenocarcinomas.
KRAS G>A mutation favors distant relapse but may not be associated with poor prognosis in patients who undergo a curative resection for duodenal adenocarcinoma.
Acknowledgments
Grant sponsor: National Institutes of Health, Grant number: CA140599, CA127141; Grant sponsor: National Natural Science Foundation of China, Grant number: 81000898.
We would like to thank Kathy Bender for administrative support. We would also like to thank Sharon Metzger-Gaud, Theresa Sanlorenzo-Caswell, and the Johns Hopkins Cancer Registry for assistance with the primary cancer databases.
Abbreviations
- CRC
colorectal cancer
- MSI
microsatellite instable
- MSS
microsatellite stable
- PCR
polymerase chain reaction
- HR
hazard ratio
- OR
odds ratio
- CI
confidence interval
- RFS
relapse-free survival
- OS
overall survival
Footnotes
Potential competing interests: None.
References
- 1.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–36. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 2.Bilimoria KY, Bentrem DJ, Wayne JD, Ko CY, Bennett CL, Talamonti MS. Small bowel cancer in the United States: changes in epidemiology, treatment, and survival over the last 20 years. Ann Surg. 2009;249:63–71. doi: 10.1097/SLA.0b013e31818e4641. [DOI] [PubMed] [Google Scholar]
- 3.Halfdanarson TR, McWilliams RR, Donohue JH, Quevedo JF. A single-institution experience with 491 cases of small bowel adenocarcinoma. Am J Surg. 2010;199:797–803. doi: 10.1016/j.amjsurg.2009.05.037. [DOI] [PubMed] [Google Scholar]
- 4.Macara IG, Lounsbury KM, Richards SA, McKiernan C, Bar-Sagi D. The Ras superfamily of GTPases. FASEB J. 1996;10:625–30. doi: 10.1096/fasebj.10.5.8621061. [DOI] [PubMed] [Google Scholar]
- 5.Malumbres M, Barbacid M. RAS oncogenes: the first 30 years. Nat Rev Cancer. 2003;3:459–65. doi: 10.1038/nrc1097. [DOI] [PubMed] [Google Scholar]
- 6.Bos JL. ras oncogenes in human cancer: a review. Cancer Res. 1989;49:4682–9. [PubMed] [Google Scholar]
- 7.Karnoub AE, Weinberg RA. Ras oncogenes: split personalities. Nat Rev Mol Cell Biol. 2008;9:517–31. doi: 10.1038/nrm2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andreyev HJ, Norman AR, Cunningham D, Oates JR, Clarke PA. Kirsten ras mutations in patients with colorectal cancer: the multicenter “RASCAL” study. J Natl Cancer Inst. 1998;90:675–84. doi: 10.1093/jnci/90.9.675. [DOI] [PubMed] [Google Scholar]
- 9.Younes N, Fulton N, Tanaka R, Wayne J, Straus FH, 2nd, Kaplan EL. The presence of K-12 ras mutations in duodenal adenocarcinomas and the absence of ras mutations in other small bowel adenocarcinomas and carcinoid tumors. Cancer. 1997;79:1804–8. [PubMed] [Google Scholar]
- 10.Muneyuki T, Watanabe M, Yamanaka M, Isaji S, Kawarada Y, Yatani R. Combination analysis of genetic alterations and cell proliferation in small intestinal carcinomas. Dig Dis Sci. 2000;45:2022–8. doi: 10.1023/a:1005560428937. [DOI] [PubMed] [Google Scholar]
- 11.Rashid A, Hamilton SR. Genetic alterations in sporadic and Crohn’s-associated adenocarcinomas of the small intestine. Gastroenterology. 1997;113:127–35. doi: 10.1016/s0016-5085(97)70087-8. [DOI] [PubMed] [Google Scholar]
- 12.Achille A, Baron A, Zamboni G, Orlandini S, Bogina G, Bassi C, Iacono C, Scarpa A. Molecular pathogenesis of sporadic duodenal cancer. Br J Cancer. 1998;77:760–5. doi: 10.1038/bjc.1998.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yachida S, Mudali S, Martin SA, Montgomery EA, Iacobuzio-Donahue CA. β-catenin nuclear labeling is a common feature of sessile serrated adenomas and correlates with early neoplastic progression after BRAF activation. Am J Surg Pathol. 2009;33:1823–32. doi: 10.1097/PAS.0b013e3181b6da19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dietmaier W, Wallinger S, Bocker T, Kullmann F, Fishel R, Ruschoff J. Diagnostic microsatellite instability: definition and correlation with mismatch repair protein expression. Cancer Res. 1997;57:4749–56. [PubMed] [Google Scholar]
- 15.Bakaeen FG, Murr MM, Sarr MG, Thompson GB, Farnell MB, Nagorney DM, Farley DR, van Heerden JA, Wiersema LM, Schleck CD, Donohue JH. What prognostic factors are important in duodenal adenocarcinoma? Arch Surg. 2000;135:635–41. doi: 10.1001/archsurg.135.6.635. discussion 41–2. [DOI] [PubMed] [Google Scholar]
- 16.Lee HG, You DD, Paik KY, Heo JS, Choi SH, Choi DW. Prognostic factors for primary duodenal adenocarcinoma. World J Surg. 2008;32:2246–52. doi: 10.1007/s00268-008-9678-6. [DOI] [PubMed] [Google Scholar]
- 17.Joesting DR, Beart RW, Jr, van Heerden JA, Weiland LH. Improving survival in adenocarcinoma of the duodenum. Am J Surg. 1981;141:228–31. doi: 10.1016/0002-9610(81)90163-x. [DOI] [PubMed] [Google Scholar]
- 18.Nicholl MB, Ahuja V, Conway WC, Vu VD, Sim MS, Singh G. Small bowel adenocarcinoma: understaged and undertreated? Ann Surg Oncol. 2010;17:2728–32. doi: 10.1245/s10434-010-1109-x. [DOI] [PubMed] [Google Scholar]
- 19.Agrawal S, McCarron EC, Gibbs JF, Nava HR, Wilding GE, Rajput A. Surgical management and outcome in primary adenocarcinoma of the small bowel. Ann Surg Oncol. 2007;14:2263–9. doi: 10.1245/s10434-007-9428-2. [DOI] [PubMed] [Google Scholar]
- 20.Wu TJ, Yeh CN, Chao TC, Jan YY, Chen MF. Prognostic factors of primary small bowel adenocarcinoma: univariate and multivariate analysis. World J Surg. 2006;30:391–8. doi: 10.1007/s00268-005-7898-6. discussion 9. [DOI] [PubMed] [Google Scholar]
- 21.Cunningham JD, Aleali R, Aleali M, Brower ST, Aufses AH. Malignant small bowel neoplasms: histopathologic determinants of recurrence and survival. Ann Surg. 1997;225:300–6. doi: 10.1097/00000658-199703000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Overman MJ, Hu CY, Wolff RA, Chang GJ. Prognostic value of lymph node evaluation in small bowel adenocarcinoma: analysis of the surveillance, epidemiology, and end results database. Cancer. 2010;116:5374–82. doi: 10.1002/cncr.25324. [DOI] [PubMed] [Google Scholar]
- 23.Dabaja BS, Suki D, Pro B, Bonnen M, Ajani J. Adenocarcinoma of the small bowel: presentation, prognostic factors, and outcome of 217 patients. Cancer. 2004;101:518–26. doi: 10.1002/cncr.20404. [DOI] [PubMed] [Google Scholar]
- 24.Abrahams NA, Halverson A, Fazio VW, Rybicki LA, Goldblum JR. Adenocarcinoma of the small bowel: a study of 37 cases with emphasis on histologic prognostic factors. Dis Colon Rectum. 2002;45:1496–502. doi: 10.1097/01.DCR.0000034134.49346.5E. [DOI] [PubMed] [Google Scholar]
- 25.Thomsen Lonborg J, Vilmar A, Mard D, Astrup Jensen S, Sorensen JB. Prognosis of small bowel adenocarcinoma treated with Mayo or Xelox regimen: a matched case-control study from a database of 581 patients with colorectal cancer. Oncol Rep. 2007;18:1023–9. [PubMed] [Google Scholar]
- 26.Gibson MK, Holcroft CA, Kvols LK, Haller D. Phase II study of 5-fluorouracil, doxorubicin, and mitomycin C for metastatic small bowel adenocarcinoma. Oncologist. 2005;10:132–7. doi: 10.1634/theoncologist.10-2-132. [DOI] [PubMed] [Google Scholar]
- 27.Zaanan A, Gauthier M, Malka D, Locher C, Gornet JM, Thirot-Bidault A, Tougeron D, Taieb J, Bonnetain F, Aparicio T Association des Gastro Enterologues O. Second-line chemotherapy with fluorouracil, leucovorin, and irinotecan (FOLFIRI regimen) in patients with advanced small bowel adenocarcinoma after failure of first-line platinum-based chemotherapy: a multicenter AGEO study. Cancer. 2011;117:1422–8. doi: 10.1002/cncr.25614. [DOI] [PubMed] [Google Scholar]
- 28.Span M, Moerkerk PT, De Goeij AF, Arends JW. A detailed analysis of K-ras point mutations in relation to tumor progression and survival in colorectal cancer patients. Int J Cancer. 1996;69:241–5. doi: 10.1002/(SICI)1097-0215(19960621)69:3<241::AID-IJC15>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 29.Andreyev HJ, Norman AR, Cunningham D, Oates J, Dix BR, Iacopetta BJ, Young J, Walsh T, Ward R, Hawkins N, Beranek M, Jandik P, et al. Kirsten ras mutations in patients with colorectal cancer: the ‘RASCAL II’ study. Br J Cancer. 2001;85:692–6. doi: 10.1054/bjoc.2001.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roth AD, Tejpar S, Delorenzi M, Yan P, Fiocca R, Klingbiel D, Dietrich D, Biesmans B, Bodoky G, Barone C, Aranda E, Nordlinger B, et al. Prognostic role of KRAS and BRAF in stage II and III resected colon cancer: results of the translational study on the PETACC-3, EORTC 40993, SAKK 60-00 trial. J Clin Oncol. 2010;28:466–74. doi: 10.1200/JCO.2009.23.3452. [DOI] [PubMed] [Google Scholar]
- 31.Richman SD, Seymour MT, Chambers P, Elliott F, Daly CL, Meade AM, Taylor G, Barrett JH, Quirke P. KRAS and BRAF mutations in advanced colorectal cancer are associated with poor prognosis but do not preclude benefit from oxaliplatin or irinotecan: results from the MRC FOCUS trial. J Clin Oncol. 2009;27:5931–7. doi: 10.1200/JCO.2009.22.4295. [DOI] [PubMed] [Google Scholar]
- 32.Ogino S, Meyerhardt JA, Irahara N, Niedzwiecki D, Hollis D, Saltz LB, Mayer RJ, Schaefer P, Whittom R, Hantel A, Benson AB, 3rd, Goldberg RM, et al. KRAS mutation in stage III colon cancer and clinical outcome following intergroup trial CALGB 89803. Clin Cancer Res. 2009;15:7322–9. doi: 10.1158/1078-0432.CCR-09-1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qiu LX, Mao C, Zhang J, Zhu XD, Liao RY, Xue K, Li J, Chen Q. Predictive and prognostic value of KRAS mutations in metastatic colorectal cancer patients treated with cetuximab: a meta-analysis of 22 studies. Eur J Cancer. 2010;46:2781–7. doi: 10.1016/j.ejca.2010.05.022. [DOI] [PubMed] [Google Scholar]
- 34.Lievre A, Bachet JB, Boige V, Cayre A, Le Corre D, Buc E, Ychou M, Bouche O, Landi B, Louvet C, Andre T, Bibeau F, et al. KRAS mutations as an independent prognostic factor in patients with advanced colorectal cancer treated with cetuximab. J Clin Oncol. 2008;26:374–9. doi: 10.1200/JCO.2007.12.5906. [DOI] [PubMed] [Google Scholar]
- 35.Amado RG, Wolf M, Peeters M, Van Cutsem E, Siena S, Freeman DJ, Juan T, Sikorski R, Suggs S, Radinsky R, Patterson SD, Chang DD. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:1626–34. doi: 10.1200/JCO.2007.14.7116. [DOI] [PubMed] [Google Scholar]
- 36.Aguirre AJ, Bardeesy N, Sinha M, Lopez L, Tuveson DA, Horner J, Redston MS, DePinho RA. Activated Kras and Ink4a/Arf deficiency cooperate to produce metastatic pancreatic ductal adenocarcinoma. Genes Dev. 2003;17:3112–26. doi: 10.1101/gad.1158703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tuveson DA, Shaw AT, Willis NA, Silver DP, Jackson EL, Chang S, Mercer KL, Grochow R, Hock H, Crowley D, Hingorani SR, Zaks T, et al. Endogenous oncogenic K-ras(G12D) stimulates proliferation and widespread neoplastic and developmental defects. Cancer Cell. 2004;5:375–87. doi: 10.1016/s1535-6108(04)00085-6. [DOI] [PubMed] [Google Scholar]
- 38.Guerra C, Mijimolle N, Dhawahir A, Dubus P, Barradas M, Serrano M, Campuzano V, Barbacid M. Tumor induction by an endogenous K-ras oncogene is highly dependent on cellular context. Cancer Cell. 2003;4:111–20. doi: 10.1016/s1535-6108(03)00191-0. [DOI] [PubMed] [Google Scholar]
- 39.Choi YL, Moriuchi R, Osawa M, Iwama A, Makishima H, Wada T, Kisanuki H, Kaneda R, Ota J, Koinuma K, Ishikawa M, Takada S, et al. Retroviral expression screening of oncogenes in natural killer cell leukemia. Leuk Res. 2005;29:943–9. doi: 10.1016/j.leukres.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 40.Esteller M, Toyota M, Sanchez-Cespedes M, Capella G, Peinado MA, Watkins DN, Issa JP, Sidransky D, Baylin SB, Herman JG. Inactivation of the DNA repair gene O6-methylguanine-DNA methyltransferase by promoter hypermethylation is associated with G to A mutations in K-ras in colorectal tumorigenesis. Cancer Res. 2000;60:2368–71. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.