Abstract
Introduction:
Lamiaceae herbs have are well known for their immunomodulatory effects, however, the mechanism by which they effect innate immune system is not clearly understood.
Objective:
The effect of dietary supplementation with two Lamiaceae herbs (oregano and sage) modulation of on innate immunological parameters was investigated in Lumbricus terrestris.
Materials and Methods:
Animals were fed (ad libitum) on herbs supplemented diet [(0.1% (w/v) and 0.5% (w/v)] for 6 days. Changes in immune competent cell counts, viability, and relative neutrophil-like cell counts were determined in response to herb treatment. Changes in nitric oxide, phagocytic activity, and respiratory burst index were also determined in response to herb treatment relative to control. Additionally, effect of herb co-treatment cyclophosphamide (50 mg/kg-BW) induced immunosuppression was also evaluated.
Results:
Our results suggested abrogation of CP-induced immunosuppression in response to co-treatment with herbs. Significant increase in nitric oxide-mediated immune-competent cell counts, viability, and differentiation into neutrophil-like cells were observed in response to dietary supplementation with Lamiaceae herbs. Significantly higher phagocytic activity relative to control was also noted in response to dietary intake of oregano and sage. However, the respiratory burst index did not increase exponentially in response to herb treatments, suggesting a potential enhancement in pathogen recognition and antioxidant defenses.
Conclusion:
Lamiaceae herbs may have potential immune-modulatory properties important for human health and merits further investigation.
Keywords: Cyclophosphamide, immunosuppression, innate immunity, respiratory burst, Zingiberaceae herbs
INTRODUCTION
Among the many benefits of dietary herbs and spices, the ability to modulate the innate immunological properties has received renewed attention recently.[1–3] The innate immune system is often described as the first line of defense against modified self and non-self (foreign) entities such as viruses, bacteria, parasites and their associated metabolites.[2,4–7] It is phylogenetically conserved between species and is a rapid, consistent, non-specific, and non-anticipatory system[4,8] and is comprised of anatomic and physiological barriers as well as cellular and humoral components, which collectively serve to protect the overall health and integrity of the host.[9] Innate immune response involves the detection, uptake, and destruction of altered or non-self threats to the organism via phagocytosis[10] executed primarily by macrophages and neutrophils and secreted humoral factors.[11,12]
Though broad in specificity, cells of the innate immune system are involved in initial rapid recognition of the foreign epitopes via the pathogen associated molecular patterns (PAMPs)[4–5,13–14] followed byprocessing and presentation of antigen to the adaptive immune system.[4–5] In addition, the innate immune response has also been shown to be important for removal of modified self epitopes, such as oxidized or glycated proteins and lipids generated during normal cellular processes such as apoptosis and tissue remodeling.[15–16] Accumulation of these epitopes has been implicated in a many chronic diseases including Alzheimer's disease, diabetes mellitus, and cardiovascular disease.[15–16] The causes for the accumulation of these modified epitopes has been linked to an inability of the innate immune system to process them either due to a weak quantitative (i.e. lower number of cells) or a poor qualitative, less robust, response (diminished clearance).[17–20] Therefore, having an efficient and robust innate immune system may not only improve the initial removal of the foreign antigen, but also accelerate the adaptive immune response and prevent the progression of these pathologies.[16,18] Thus, stimulation of the innate response by biological agents may have important implications in the prevention and management of multiple diseases.[2,18,21]
Herbs belonging to the Lamiaceae family, including oregano (Origanum vulgare) and sage (Salvia officinalis), are important culinary components of diets across the world and are also rich in bioactive phytochemicals with health promoting properties.[1,22–24] Lamiaceae herbs constitute natural sources of a variety of phenolic compounds such as flavonoids and phenolic acids that have been shown to exert antioxidant, anti-inflammatory, anti-microbial, anti-mutagenic, and anti-cancer protection.[25–26] They have also been implicated for the prevention and management of chronic diseases of inflammatory and oxidative origin including cardiovascular disease, diabetes mellitus, and cancer.[26] Oregano (Origanum vulgare) has been used historically to improve circulation as an emmenagogue, for infections of the oral cavity, as a carminative for digestive health, and for the treatment of inflammatory disorders such as arthritis.[27–28] Phytochemical extracts of oregano have been extensively studied for antioxidant, anti-bacterial, anti-fungal and anthelmintic activity.[29–31] Recent studies have also described immunomodulatory activity of oregano by decreasing pro-inflammatory cytokines, IL-1β, and IL-6 mRNA in colonic tissue of mice with colitis.[27] Also of the Lamiaceae family, sage (Salvia officinalis) contains a variety of bioactive phytochemicals including phenolic compounds, flavonoids, essential oils, catechins, and tannins that may confer important health benefits.[32–34] Sage has traditionally been used to enhance cognitive performance and memory but more recently for the therapeutic management of neurodegenerative diseases such as Alzheimer's disease.[32–34] Apeginin, a flavonoid in sage, has specifically been shown to reduce amyloid-β plaques in neurons of patients with Alzheimer's disease.[32–33] The abundance of phenolic compounds in sage including rosmarinc acid, genkwarin and luteolin, for example, contribute to its high antioxidant capacity.[30] Additionally, components of sage such as α-pinene have been observed to exhibit anti-inflammatory activity by inhibiting eicosinoid synthesis via suppression of cyclooxygease-2 (COX-2) expression.[33,35]
Although there is substantial evidence that Lamiaceae herbs can affect some aspects of the immune response, there is a relative lack of mechanistic and in-vivo data on the overall effect of Lamiaceae herbs on the innate immunologic parameters and is the primary objective of this study.[36]
MATERIALS AND METHODS
Growth conditions and priming of Lumbricus terrestris[37]
Cultured Lumbricus terrestris purchased from DMFB Company (Waterford, MI) were selected based on the presence of a fully developed clitellum indicative of sexual maturation. Worms were then washed in distilled water to ensure the skin was free from soil and debris. The earthworms were then transferred to petri plates containing Lumbricus Growth Medium (LGM) comprised of 1.25% agar, 0.31% Gerber single grain oatmeal (Nestle, S.A Vevey, Switzerland) and incubated at 10°C for 48 hours to clear the digestive tract of soil. After worms were primed, the animals were selected for treatment. Prior to weighing, worms were gently massaged along the posterior length of the body to clear the gut of digestive contents.
Treatment of L. terrestris
Dried turmeric and ginger (Vrdür Inc., Odessa, TX) were added directly to LGM at concentrations of 0.1% (w/v) or 0.5% (w/v) prior to pouring them into petri dishes [Table 1]. For L. terrestris cyclophosphamide treatment and spice co-treatment,[38–42] experimental worms were treated with 50 mg/kg/day cyclophosphamide (CP), which was dissolved in 0.9% NaCl and added to LGM agar just prior to pouring [Table 1]. For co-treatment, 0.1% (w/v) or 0.5% (w/v) of dried herb product plus 50 mg/kg of CP were added to LGM prior to treatment [Table 1]. A single primed worm was transferred on each treatment and control plates (LGM only).
Table 1.
Treatment of L. terrestris with different lamiaceae herbs and cyclophosphamide
For each treatment or control group, 36 (n=36) worms were allowed to feed ad libitum for 6 days at 18-20°C in the dark. Treatment plates were renewed on the fourth day of treatment.
Ethanol extrusion of coelomocytes
Coelomocytes were isolated by ethanol extrusion.[43] Fecal contamination during extrusion was avoided by gently massaging one fourth of the posterior length of the worm to expel intestinal contents of the worm. Briefly, worms were rinsed in 20 mL Ca-LBSS (1.5 mM NaCl, 4.8 mM KCl, 1.1 mM MgSO4, 0.4 mM KH2 PO4, 0.3 mM Na2 HPO4, 4.2 mM NaHCO3, 3.8 mM CaCl2, and adjusted to pH 7.3 with HCl) in a beaker to remove contaminants. Cleansed worms were placed in a glass petri dish containing 3 mL extrusion medium (5% ethanol in saline, 23.5 mg/mL EDTA, 10 mg/mL guaiacol glycerol ether, and adjusted to pH 7.3 with HCl) for a total of 3 minutes at room temperature. Whole coelomic fluid was transferred from the petri dish to a 15 mL falcon tube, and worms were discarded. Nitric oxide analysis was performed using whole coelomic fluid and is described in detail below. Finally, 10 mL Ca-LBSS was transferred to the falcon tube followed by centrifugation at 150×g for 15 minutes at 4°C. The pellet was then re-suspended in 0.425 mL Ca-LBSS and stored on ice for further analysis or total coelomocyte count, coelomocyte viability, relative neutrophil count, phagocytic activity, and respiratory burst.[44]
Total coelomocyte counts
Total coelomocyte count was determined using an Improved Neubauer 1/400 sq. mm hemocytometer.[44] Briefly, 10 μl of re-suspended coelomocytes in Ca-LBSS was transferred to the hemocytometer. Total coelomocytes were counted on two of the large outer squares, and total coelomocyte count was estimated using the formula supplied by the manufacturer (Hausser Scientific, Horsham, PA).
Coelomocyte viability
Coelomocyte viability was determined by trypan blue exclusion as previously described elsewhere.[45] Briefly, re-suspended coelomocytes were mixed at a ratio of 1:1 with 0.04% trypan blue (0.004 g/ 10 mM PBS) and vortexed. 20 μl of this mixture was transferred to a glass microscope slide with a cover slip. Live coelomocytes were determined by dye exclusion and reported as percent live coelomocytes per total cells counted. Fold changes in coelomocyte viability in response to treatments were calculated relative to control.
Relative neutrophil-like coelomocyte counts (RNLCC)
Differential coelomocyte count was determined using a modified Wright-Giemsa smear technique and visualized using bright field microscopy.[46] To a clean microscope slide, 20 μl re-suspended coelomocytes was smeared and heat fixed. The slides were stained by direct immersion in 15 mL Wright-Giemsa stain for a total of 3.5 minutes. Slides were then washed briefly with 5 mL of 10 mM phosphate buffer saline (PBS) followed by brief rinsing with 5 mL DW. Slides were then gently dried by briefly holding over a low flame. Neutrophils-like cells were identified by the presence of a prominent pink nucleus and pale pink cytoplasm upon staining and enumerated. Relative neutrophil-like coelomcyte count was reported as a percentage of total coelomocytes. Fold changes neutrophil-like coelomocyte counts in response to treatment were calculated relative to control.
Phagocytic activity
Phagocytosis was stimulated using a modified assay previously described.[46] Briefly, 100 μl re-suspended coelomocytes were incubated for 24 hours in 100 μl of a yeast/Congo red solution prepared as follows: 1 g yeast was added to 3 mL 0.87% Congo red in 10 mM PBS. 10 mL DW was then added, and the solution was autoclaved. Finally, the yeast solution was diluted to 10-2 with Ca-LBSS. After 24 hours, the reaction mixture was centrifuged at 150× g for 15 minutes at 4°C, and the pellet was re-suspended in 200 μl Ca-LBSS. 20 μl was transferred to a microscope slide for analysis. Phagocytosis was determined visually by the presence of engulfed Congo red-stained yeast particles within the coelomocytes. Phagocytic activity was calculated as the percentage of phagocytic cells of total coelomocytes. Fold changes phagocytic activity (PA) in response to treatments were calculated relative to control.
Respiratory burst index (RBI)
Respiratory burst index was indirectly determined as the absorbance of diformazan at 570 nm, formed by oxidation of Nitroblue Tetrazolium (NBT) during an NBT reduction assay.[47] Briefly, 100 μl re-suspended coelomocytes were transferred to a microcentrifuge tube, and phagocytosis was stimulated by addition of 100 μl of a yeast solution prepared as follows (1 g yeast in 3 mL 10 mM Ca-LBSS incubated for 15 minutes at room temperature. 10 mL DW was then added, and the solution was autoclaved. Finally, the yeast solution was serial diluted to 1×10-2 with Ca-LBSS). 50 μl NBT (1.5 mg/mL in Ca-LBSS) was transferred to the reaction mixture, which then incubated for 24 hours at 10°C, followed by centrifugation at 13,000 rpm for 10 minutes at 4°C. To the pellet, 120 μl of 2 M KOH and 140 μl DMSO was added to extract the pigment. Tubes were then re-centrifuged at 13,000 rpm for 10 minutes at 4°C to precipitate debris. Absorbance of supernatant was at 570 nm in a 96-well microplate (Biotek Instruments; Houston, TX). Fold changes Respiratory burst index (RBI) in response to treatments was calculated relative to control.
Nitric oxide production
Nitric oxide production was indirectly measured using a modified Griess diazotization assay for the detection of total nitrites/nitrates (NOx).[36] Briefly, 100 μl of whole coelomic fluid was transferred to a microplate followed by addition of 100 μl of vanadium chloride (0.08 g/10 mL 0.1 M HCl) and 100 μl Griess reagent. Alternatively, 50 μl sulfanilamide and 50 μl N-(1-Naphthyl) ethylendiamine dihydrochloride(NEDD) can be substituted for Griess reagent in the reaction. The microplate incubated for 30 minutes at 37°C, and absorbance was measured at 540 nm using the Biotek EL 808 microplate reader (Biotek Instruments, Houston, TX). The concentration of nitric oxide was determined by calculating the % change based on a linear standard curve: Conc (umol/ L)=(A540 -0.0344)/0.0057). Fold changes nitric oxide production in response to treatments were calculated relative to control.
Statistical analysis
Statistical significance was determined using a Student's one-tailed t-test. Treatment with CP was compared to control worms feeding on LGM only. Worms treated with CP plus herb or spice were compared to control worms feeding on LGM only and worms feeding on CP only. Statistical significance was indicated by P values of<0.05
RESULTS
Effect of herbs on coelomocyte viability
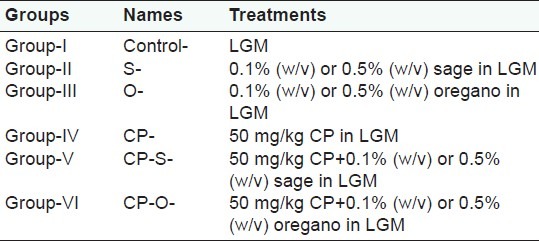
In control worms feeding on LGM only, coelomocyte viability (CV) was 52.7% over the duration of the treatment. CV significantly increased following treatment with oregano at 0.1% (w/v) and 0.5% (w/v) by 37% (P=0.021), and 43% (P=0.032), respectively, compared to control [Figure 1a]. Treatment with sage resulted in significantly higher percentages of viable coelomocytes in all treatment groups at both concentrations, compared to the control [Figure 1]. For worms feeding on 0.1% (w/v) sage, CV increased by 35% (P=0.03), in worms that consumed 0.5% (w/v) sage, this increase was 28% (P=0.025) relative to control [Figure 1a]. Following treatment with cyclophosphamide (CP), CV decreased significantly by 42% (P=0.018) relative to control [Figure 1a]. Supplementation of CP containing LGM with oregano (CP-O) at 0.1% (w/v) and 0.5% (w/v) resulted in a 43.1% (P=0.032) and 48.5% (P=0.039) increase in cell viability relative to coelomcyte viability of CP-treated worms [Figure 1b]. Supplementation of CP containing LGM with sage (CP-S) at 0.1% (w/v) and 0.5% (w/v) resulted in a 40.6% (P=0.027) and 44.8% (P=0.028) increase in cell viability,relative to coelomcyte viability of CP-treated worms [Figure 1b].
Figure 1.
Change in coelomocyte viability (CV) (% Control) in L. terrestris in response to oregano (O), sage (S), and (a) cyclophosphamide (CP) treatment, (b) cyclophosphamide-herb (CP-S and CP-O) co-treatment. The data is represented as mean+SEM. *-indicates significant difference from the control (P<0.05). Length of experiment-6 days, n=36. Concentration: Herbs: 0.1% (w/v) and 0.5% (w/v), cyclophosphamide 50mg/kg-BW
Effect of herbs on total coelomocyte count
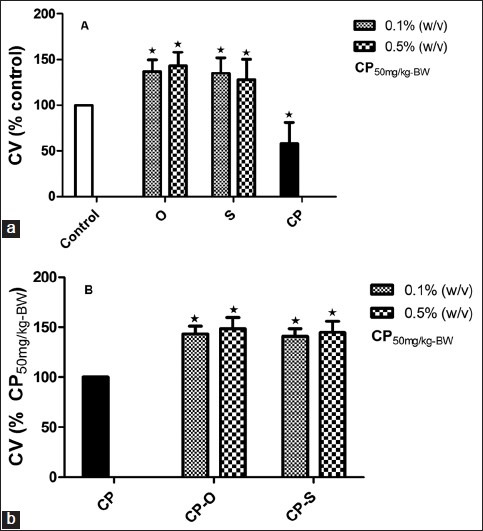
In control worms feeding on Lumbricus Growth Medium, the TCC was 2.9 × 106. For worms that fed on 0.1% (w/v) oregano, TCC increased 153% (P=0.020) and with 0.1% (w/v) sage, TCC increased by 135% (P=0.025) compared to control [Figure 2a]. At 0.5% (w/v), oregano and sage did not have any significant effect on TCC compared to control [Figure 2a]. Treatment with CP at 50 mg/kg bw did not affect the total coelomcyte count relative to control [Figure 2a]. In worms that fed on CP-O,the total cell count was significantly higher only at 0.1% (w/v) and was 46.9% (P=0.027) higher relative to CP [Figure 2b]. In worms that fed on CP-S at 0.1% (w/v), the total cell count increased by 17.1% (P=0.042) and at 0.5% (w/v), the cell viability increased by 46.9% (P=0.024) relative to total coelomcytes in CP-treated worms [Figure 2b].
Figure 2.
Change in total coelomocyte count (TCC) (% Control) in L. terrestris in response to oregano (O), sage (S), and (a) cyclophosphamide (CP) treatment,(b) cyclophosphamide-herb (CP-S and CP-O) co-treatment. The data is represented as mean+SEM. *-indicates significant difference from the control (P<0.05). Length of experiment-6 days, n=36. Concentration: Herbs: 0.1% (w/v) and 0.5% (w/v), cyclophosphamide 50mg/kg-BW
Effect of herbs on relative neutrophil-like coelomocyte count
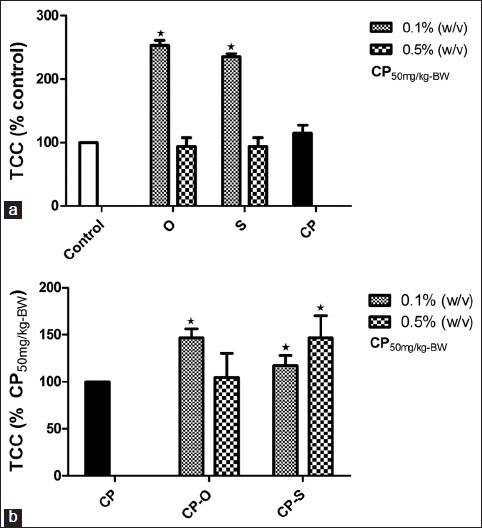
Relative to control, neutrophil-like coelomocyte count was significantly different only at 0.1% (w/v) of sage or oregano. In both cases, the NLCC was 20% (P=0.034) higher relative to control worms that fed on LGM only plates [Figure 3a]. The relative neutrophil-like coelomocyte in response to CP treatment decreased by 11% (P=0.048) relative to control [Figure 3a]. In worms that fed on CP-O and CP-S at 0.1% (w/v), the RNLCC was 35.9% (P=0.041) and 40.4% (P=0.034) higher than the RNLCC in CP-treated worms [Figure 3b]. At a concentration of 0.5% (w/v), CP-O treatment resulted in 13.4% (P=0.046) increase in RNLCC compared to CP-treated worms [Figure 3b]. CP-S at this dosage did not have a significant effect on RNLCC.
Figure 3.
Change in total relative neutrophil like coelomocyte count (RNLCC) (% Control) in L. terrestris in response to oregano (O), sage (S), and (a) cyclophosphamide (CP) treatment, (b) cyclophosphamide-herb (CP-S and CP-O) co-treatment. The data is represented as mean+SEM. *-indicates significant difference from the control (P<0.05). Length of experiment-6 days, n=36. Concentration: Herbs: 0.1% (w/v) and 0.5% (w/v), cyclophosphamide 50mg/kg-BW
Effect of herbs on phagocytic activity
When worms were allowed to feed on LGM prepared with oregano at 0.1% (w/v) and 0.5% (w/v), a significant increase in PA by 17% (P=0.017) and 19% (P=0.018) respectively, relative to control, was observed [Figure 4a]. In response to supplementation of LGM with sage, the PA increased by 23% and 21% at a dosage of1% (w/v) and 0.5% (w/v), respectively, relative to control [Figure 4a]. Following treatment with CP, phagocytic activity decreased 19% (P=0.041) relative to control [Figure 4a]. However, in worms that consumed CP-O and CP-S at 0.1% (w/v), the phagocytic activity increased by 17.6% (P=0.046) and 13.6% (P=0.52), relative to phagocytic activity of coelomocytes in CP-treated worms [Figure 4b]. At a dosage of 0.5% (w/v), the PA increased by 1.2% (P=0.043) for the CP-S-treated worms and in the CP-O-treated worms, it was 17.6% (P=0.046) higher than in CP-treated worms [Figure 4b].
Figure 4.
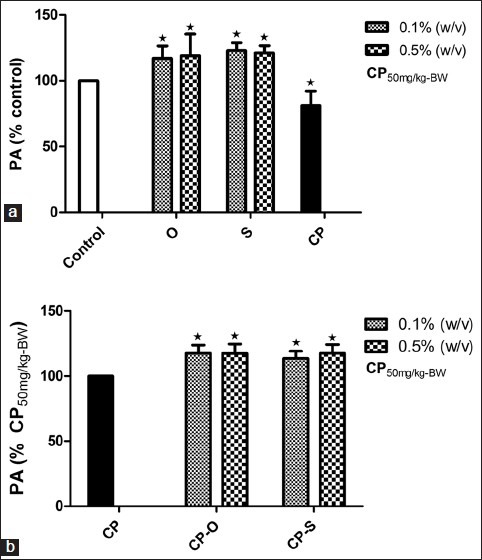
Change in phagocytic activity (PA) (% Control) of yeast by coelomocytes in L. terrestris in response to oregano (O), sage (S), and (a) cyclophosphamide (CP) treatment (b) cyclophosphamide-herb (CP-S and CP-O) co-treatment. The data is represented as mean+SEM. *-indicates significant difference from the control (P<0.05). Length of experiment-6 days, n=36. Concentration: Herbs: 0.1% (w/v) and 0.5% (w/v), cyclophosphamide 50mg/kg-BW
Effect of herbs on respiratory burst index
Relative to control, respiratory burst index (RBI) significantly increased by relative to control; in worms that fed on LGM supplemented with 0.1% (w/v) oregano, the RBI increased by 26% (P=0.039) and at 0.5% (w/v) oregano, the RB increased by 55% (P=0.027) [Figure 5a]. In coelomcytes from worms that fed on sage, RBI increased in all experimental groups relative to control [Figure 5a]. The RBI was 42% (P=0.024) at 0.1% (w/v) and 57% (P=0.007) at 0.5% (w/v) sage concentration, respectively [Figure 5a]. The respiratory burst index in coelomcytes increased significantly by 50% (P=0.019) in CP-treated worms relative to control [Figure 5a]. When worms were allowed to feed on CP-O at 0.1% (w/v), the respiratory burst index of coelomcytes was not significantly different relative to CP treatment [Figure 5b]. At 0.5% (w/v), the RBI in CP-O-treated worms was significantly higher (19.4%, P=0.037) compared to RBI in CP-treated worms [Figure 5b]. When worms were allowed to feed on CP-S at 0.1% (w/v) or 0.5% (w/v), the respiratory burst index of coelomcytes was not significantly different relative to CP treatment [Figure 5b].
Figure 5.
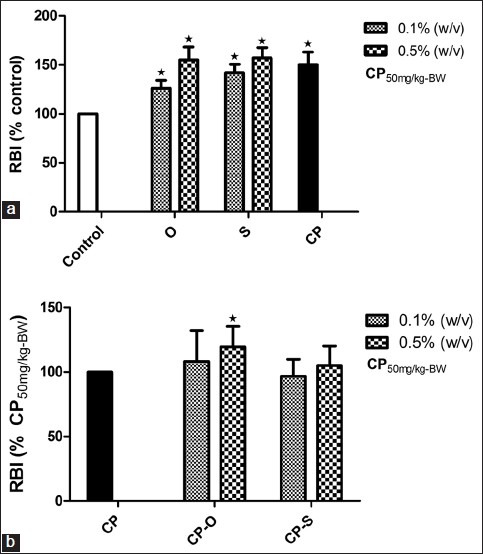
Change in respiratory burst index (RBI) (% Control) in coelomocytes of L. terrestris in response to oregano (O), sage (S), and (a) cyclophosphamide (CP) treatment, (b) cyclophosphamide-herb (CP-S and CP-O) co-treatment. The data is represented as mean+SEM. *-indicates significant difference from the control (P<0.05). Length of experiment-6 days, n=36. Concentration: Herbs: 0.1% (w/v) and 0.5% (w/v), cyclophosphamide 50mg/kg-BW
Effect of herbs on nitric oxide production
Following treatment with oregano at 0.1% (w/v) and 0.5% (w/v), total nitrites/nitrates (NOx) increased by 38% (P=0.025) and 13% (P=0.025) relative to control [Figure 6a]. In worms consuming LGM prepared with 0.1% (w/v) sage treatment, a 30% increase in total nitrites/ nitrates (NOx) (P=0.034) was observed relative to control [Figure 6a]. In worms that fed on 0.5% (w/v) sage, the increase in NOx was 31% (P=0.021) relative to control [Figure 6a]. The total nitrates in coelomic fluid did not change in response to CP treatment [Figure 6b]. Total nitrates in worms that fed on CP-O at 0.1% (w/v),were 89% (P=0.013) and at 0.5% (w/v),81% (P=0.011) higher relative to CP treatment. Feeding worms with CP-S at 0.1% (w/v) and 0.5% (w/v) resulted in a 28% (P=0.036) and 55% (P=0.021) increase in total nitrates relative to control and CP treatment.
Figure 6.
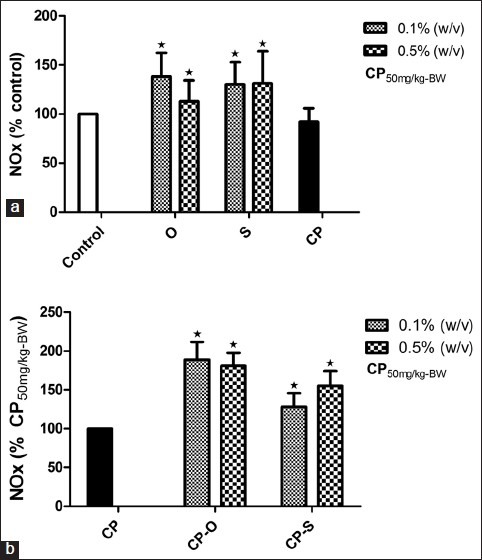
Change in total nitrates (NOx) (% Control) in coelomic fluid of L. terrestris in response to oregano (O), sage (S), and (a) cyclophosphamide (CP) treatment,(b) cyclophosphamide-herb (CP-S and CP-O) co-treatment. The data is represented as mean+SEM. *-indicates significant difference from the control (P<0.05). Length of experiment-6 days, n=36. Concentration: Herbs: 0.1% (w/v) and 0.5% (w/v), cyclophosphamide 50mg/kg-BW
DISCUSSION
Nitric oxide plays an important role in maintenance and regulation of normal cell cycle in a dose-dependent manner via inhibition of apoptosis at low doses and facilitating apoptosis at higher doses.[49,50–52] Emerging research also indicates that nitric oxide formation is necessary for the mobilization of undifferentiated progenitor cells in circulation.[49,53–54] Additionally, immunopoietic reconstitution and mobilization have been shown to be significantly mediated by nitric oxide.[49,53–54] Treatment with oregano and sage resulted in a significant increase in coelomocyte viability and total number of coelomocytes compared to control worms. An intake of sage and oregano appeared to increase the relative distribution of neutrophil-like coelomocytes and was accompanied by increased levels of nitric oxide in the coelomic fluid. This concurrent increase in total coelomocyte count, cell viability, and nitric oxide in the present study is consistent with recent findings and may be a result of nitric oxide-mediated inhibition of premature apoptotic signaling in coelomocytes. An overall increase in the total number of coelomocytes in the coelomic fluid of worms co-treated with cyclophosphamide (CP) and either oregano (CP- O) or sage (CP-S) compared to worms treated with CP alone was also observed in this study. Since treatment with CP is associated with increased susceptibility to infection due to widespread cytoxicity and leucopenia, an increase in total immunogenic cells in response to herb co-treatment may confer benefits against CP-induced immunosuppression.[55–56] Additionally, viability of coelomocytes, relative distribution of neutrophil-like coelomocytes also increased in the coelomic fluid of worms co-treated with CP and herbs compared to worms treated with CP only. Here also, a concomitant increase of total nitrites/nitrates was observed in the coelomic fluid in response to the treatments with CP-S or CP-O relative to CP only treated worms. Similar to our observation, previous studies with other dietary and medicinal have shown to increase populations of immunocompetent cells in animals treated with CP.[39–40,57] It is likely that co-treatment with oregano and sage may ameliorate CP-induced cytopenia via induction of immunopoiesis progenitor cell differentiation.[58]
Coupled to increased cell viability, cell count, and distribution of neutrophil-like cells, we also observed increases in phagocytic activity and respiratory index in response to feeding sage and oregano. Overall, phagocytic activity of coelomocytes was also enhanced in CP-treated worms supplemented with sage and oregano, compared to those treated with CP only. As respiratory burst in phagocytic cells is a consequence of a phosphorylation cascade-mediated cytosolic assembly and activation of NADPH oxidases,[58–61] an increase in phagocytosis as noted in this study may suggest a more robust foreign epitope recognition, perhaps due to increased expression of genes such as β-1, 4-mannosyl-glycoprotein 4-β-N-acetylglucosaminyltransferase-3 and toll-like receptors crucial for recognition and clearance of foreign material.[37,62–64]
Similar studies in cell culture models and in vivo models with other dietary plants have shown to increase phagocytosis and release of cytokines crucial to the mediation of immune response.[62,65–67] Interestingly, in this study, enhanced phagocytic activity upon feeding sage and oregano was associated with non-exponential increase in respiratory burst index. This lower production of reactive oxygen species may be due lower number of phagosomes per cell, resulting in fewer number of active NADPH oxidase enzymes assembled. Alternatively, a more controlled respiratory burst may also result from a robust antioxidant defense responses mediated by glutathione S-transferase, NADPH: Quinine oxidoreducatase-1 and other phase II enzymes in response to oregano and sage. Plant secondary metabolites have been shown to increase NRF2 (nuclear factor E2-related factor)-antioxidant response element (ARE)-mediated expression of antioxidant genes facilitating efficient removal of oxidants and recycling of antioxidants and may be responsible in controlling the rate of oxidant formation in this study.[68–71] An overall increase in respiratory burst index, relative to control, was also observed upon treatment with CP. It is well-known antioxidant enzyme defenses in immune cells that are inhibited by the metabolites formed from the microsomal metabolism of CP.[72] In our study, immune-competent cells from CP-S and CP-O worms had higher phagocytic activity than CP-treated worms. However, respiratory burst index of immune-competent cells from CP-S and CP-O worms was not significantly different than CP-treated worms, suggesting a potential protective effect of sage and oregano on the detrimental effect on antioxidant enzyme response induced by CP metabolites.
CONCLUSION
Our results indicate that dietary intake of Lamiaceae herbs (oregano and sage) improved innate immune system functions and also reduced the immunosuppresive effects of cyclophopshamide in vivo. These herbs may have potential applications in antibiotic and cancer chemotherapy as well as in diabetes and Alzheimer's disease induced by accumulation of modified self-epitopes and merits further investigation.
Footnotes
Source of Support: Vrdür Inc. Odessa, TX.,
Conflict of Interest: None declared
REFERENCES
- 1.Craig WJ. Health-promoting properties of common herbs. Am J Clin Nutr. 1999;70:491S–499S. doi: 10.1093/ajcn/70.3.491s. [DOI] [PubMed] [Google Scholar]
- 2.Haddad PS, Azar GA, Groom S, Boivin M. Natural health products, modulation of immune function and prevention of chronic diseases. Evid Based Complement Alternat Med. 2005;2:513–20. doi: 10.1093/ecam/neh125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tejasari D. Evaluation of Ginger (Zingiber officinale Roscoe) Bioactive Compounds in Increasing the Ratio of T-cell Surface Molecules of CD3+CD4+:CD3+CD8+ In-Vitro. Malays J Nutr. 2007;13:161–70. [PubMed] [Google Scholar]
- 4.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 5.Singh BP, Chauhan RS, Singhal LK. Toll-like receptors and their role in innate immunity. Curr Sci. 2003;85:1156–63. [Google Scholar]
- 6.Portnoy DA. Manipulation of innate immunity by bacterial pathogens. Curr Opin Immunol. 2005;17:25–8. doi: 10.1016/j.coi.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Brown GE, Stewart MQ, Bissonnette SA, Elia AE, Wilker E, Yaffe MB. Distinct ligand-dependent roles for p38 MAPK in priming and activation of the neutrophil NADPH oxidase. J Biol Chem. 2004;279:27059–68. doi: 10.1074/jbc.M314258200. [DOI] [PubMed] [Google Scholar]
- 8.Hoffmann JA, Kafatos FC, Janeway CA, Ezekowitz RA. Phylogenetic perspectives in innate immunity. Science. 1999;284:1313–8. doi: 10.1126/science.284.5418.1313. [DOI] [PubMed] [Google Scholar]
- 9.Plowden J, Renshaw-Hoelscher M, Engleman C, Katz J, Sambhara S. Innate immunity in aging: Impact on macrophage function. Aging Cell. 2004;3:161–7. doi: 10.1111/j.1474-9728.2004.00102.x. [DOI] [PubMed] [Google Scholar]
- 10.Henneke P, Golenbock DT. Phagocytosis, innate immunity, and host-pathogen specificity. J Exp Med. 2004;199:1–4. doi: 10.1084/jem.20031256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Segal AW. How neutrophils kill microbes. Annu Rev Immunol. 2005;23:197–223. doi: 10.1146/annurev.immunol.23.021704.115653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sheppard FR, Kelher MR, Moore EE, McLaughlin NJ, Banerjee A, Silliman CC. Structural organization of the neutrophil NADPH oxidase: Phosphorylation and translocation during priming and activation. J Leukoc Biol. 2005;78:1025–42. doi: 10.1189/jlb.0804442. [DOI] [PubMed] [Google Scholar]
- 13.Janeway CA., Jr Approaching the asymptote. Evolution and revolution in immunology? Cold Spring Harb Symp Quant Biol. 1989;54(Pt 1):1–13. doi: 10.1101/sqb.1989.054.01.003. [DOI] [PubMed] [Google Scholar]
- 14.Fearon DT. Innate immunity and the biological relevance of the acquired immune response. QJM. 1999;92:235–7. doi: 10.1093/qjmed/92.5.235. [DOI] [PubMed] [Google Scholar]
- 15.Binder CJ, Chang MK, Shaw PX, Miller YI, Hartvigsen K, Dewan A, et al. Innate and acquired immunity in atherogenesis. Nat Med. 2002;8:1218–26. doi: 10.1038/nm1102-1218. [DOI] [PubMed] [Google Scholar]
- 16.Miller YI, Worrall DS, Funk CD, Feramisco JR, Witztum JL. Actin polymerization in macrophages in response to oxidized LDL and apoptotic cells: Role of 12/15-lipoxygenase and phosphoinositide 3-kinase. Mol Biol Cell. 2003;14:4196–206. doi: 10.1091/mbc.E03-02-0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laske C, Stransky E, Leyhe T, Eschweiler GW, Schott K, Langer H, et al. Decreased brain-derived neurotrophic factor (BDNF)- and beta-thromboglobulin (beta-TG)- blood levels in Alzheimer's disease. Thromb Haemost. 2006;96:102–3. doi: 10.1160/TH06-03-0173. [DOI] [PubMed] [Google Scholar]
- 18.Fiala M, Liu PT, Espinosa-Jeffrey A, Rosenthal MJ, Bernard G, Ringman JM, et al. Innate immunity and transcription of MGAT-III and Toll-like receptors in Alzheimer's disease patients are improved by bisdemethoxycurcumin. Proc Natl Acad Sci U S A. 2007;104:12849–54. doi: 10.1073/pnas.0701267104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cashman JR, Ghirmai S, Abel KJ, Fiala M. Immune defects in Alzheimer's disease: New medications development. BMC Neurosci. 2008;9(Suppl 2):S13. doi: 10.1186/1471-2202-9-S2-S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Avagyan H, Goldenson B, Tse E, Masoumi A, Porter V, Wiedau-Pazos M, et al. Immune blood biomarkers of Alzheimer disease patients. J Neuroimmunol. 2009;210:67–72. doi: 10.1016/j.jneuroim.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 21.Zhou HL, Deng YM, Xie QM. The modulatory effects of the volatile oil of ginger on the cellular immune response in vitro and in vivo in mice. J Ethnopharmacol. 2006;105:301–5. doi: 10.1016/j.jep.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 22.Kris-Etherton PM, Hecker KD, Bonanome A, Coval SM, Binkoski AE, Hilpert KF, et al. Bioactive compounds in foods: Their role in the prevention of cardiovascular disease and cancer. Am J Med. 2002;113(Suppl 9B):71S–88S. doi: 10.1016/s0002-9343(01)00995-0. [DOI] [PubMed] [Google Scholar]
- 23.Moreno S, Scheyer T, Romano CS, Vojnov AA. Antioxidant and antimicrobial activities of rosemary extracts linked to their polyphenol composition. Free Radic Res. 2006;40:223–31. doi: 10.1080/10715760500473834. [DOI] [PubMed] [Google Scholar]
- 24.Aherne SA, Kerry JP, O’Brien NM. Effects of plant extracts on antioxidant status and oxidant-induced stress in Caco-2 cells. Br J Nutr. 2007;97:321–8. doi: 10.1017/S0007114507250469. [DOI] [PubMed] [Google Scholar]
- 25.Miura K, Kikuzaki H, Nakatani N. Antioxidant activity of chemical components from sage (Salvia officinalis L.) and thyme (Thymus vulgaris L.) measured by the oil stability index method. J Agric Food Chem. 2002;50:1845–51. doi: 10.1021/jf011314o. [DOI] [PubMed] [Google Scholar]
- 26.Kwon YI, Vattem DA, Shetty K. Evaluation of clonal herbs of Lamiaceae species for management of diabetes and hypertension. Asia Pac J Clin Nutr. 2006;15:107–18. [PubMed] [Google Scholar]
- 27.Bukovská A, Cikos S, Juhás S, Il’ková G, Rehák P, Koppel J. Effects of a combination of thyme and oregano essential oils on TNBS-induced colitis in mice. Mediators Inflamm. 2007;2007:23296. doi: 10.1155/2007/23296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strange R. A history of herbal plants. New York, NY: Arco Publishing Co; 1977. [Google Scholar]
- 29.Rinzler CA. The new complete book of herbs, spices and condiments. New York: Checkmark Books; 2001. [Google Scholar]
- 30.Dragland S, Senoo H, Wake K, Holte K, Blomhoff R. Several culinary and medicinal herbs are important sources of dietary antioxidants. J Nutr. 2003;133:1286–90. doi: 10.1093/jn/133.5.1286. [DOI] [PubMed] [Google Scholar]
- 31.Eskin M, Tamir S. Dictionary of nutraceuticals and functional foods. Boca Raton, FL: CRC Press; 2006. [Google Scholar]
- 32.Wang CN, Chi CW, Lin YL, Chen CF, Shiao YJ. The neuroprotective effects of phytoestrogens on amyloid beta protein-induced toxicity are mediated by abrogating the activation of caspase cascade in rat cortical neurons. J Biol Chem. 2001;276:5287–95. doi: 10.1074/jbc.M006406200. [DOI] [PubMed] [Google Scholar]
- 33.Perry NS, Bollen C, Perry EK, Ballard C. Salvia for dementia therapy: Review of pharmacological activity and pilot tolerability clinical trial. Pharmacol Biochem Behav. 2003;75:651–9. doi: 10.1016/s0091-3057(03)00108-4. [DOI] [PubMed] [Google Scholar]
- 34.Kennedy DO, Scholey AB. The psychopharmacology of European herbs with cognition-enhancing properties. Curr Pharm Des. 2006;12:4613–23. doi: 10.2174/138161206779010387. [DOI] [PubMed] [Google Scholar]
- 35.Scheckel KA, Degner SC, Romagnolo DF. Rosmarinic acid antagonizes activator protein-1-dependent activation of cyclooxygenase-2 expression in human cancer and nonmalignant cell lines. J Nutr. 2008;138:2098–105. doi: 10.3945/jn.108.090431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suhaj M. Spice antioxidants isolation and their antiradical activity: A review. J Food Compos Anal. 2006;19:531–7. [Google Scholar]
- 37.Townsend D, White L, Cisneros I, Richardson CR, Vattem DA. Evaluation of potential redox modulatory and chemotherapeutic effects of a proprietary bioactive silicate Alka-Vita™/Alka-V6™/Alkahydroxy™ (AVAH) IJARNP. 2010;3:5–18. [Google Scholar]
- 38.Bharani SE, Asad M, Dhamanigi SS, Chandrakala GK. Immunomodulatory activity of methanolic extract of Morus alba Linn.(mulberry) leaves. Pak J Pharm Sci. 2010;23:63–8. [PubMed] [Google Scholar]
- 39.Bin-Hafeez B, Ahmad I, Haque R, Raisuddin S. Protective effect of Cassia occidentalis L.on cyclophosphamide-induced suppression of humoral immunity in mice. J Ethnopharmacol. 2001;75:13–8. doi: 10.1016/s0378-8741(00)00382-2. [DOI] [PubMed] [Google Scholar]
- 40.Chu DT, Wong WL, Mavligit GM. Immunotherapy with Chinese medicinal herbs. II. Reversal of cyclophosphamide-induced immune suppression by administration of fractionated Astragalus membranaceus in vivo. J Clin Lab Immunol. 1988;25:125–9. [PubMed] [Google Scholar]
- 41.Kumar KB, Kuttan R. Chemoprotective activity of an extract of Phyllanthus amarus against cyclophosphamide induced toxicity in mice. Phytomedicine. 2005;12:494–500. doi: 10.1016/j.phymed.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 42.Thatte UM, Dahanukar SA. Comparative study of immunomodulating activity of Indian medicinal plants, lithium carbonate and glucan. Methods Find Exp Clin Pharmacol. 1988;10:639–44. [PubMed] [Google Scholar]
- 43.Eyambe GS, Goven AJ, Fitzpatrick LC, Venables BJ, Cooper EL. A non-invasive technique for sequential collection of earthworm (Lumbricus terrestris) leukocytes during subchronic immunotoxicity studies. Lab Anim. 1991;25:61–7. doi: 10.1258/002367791780808095. [DOI] [PubMed] [Google Scholar]
- 44.Burch SW, Fitzpatrick LC, Goven AJ, Venables BJ, Giggleman MA. In vitro earthworm Lumbricus terrestris coelomocyte assay for use in terrestrial toxicity identification evaluation. Bull Environ Contam Toxicol. 1999;62:547–54. doi: 10.1007/s001289900910. [DOI] [PubMed] [Google Scholar]
- 45.Kirk CJ, Peel RN, James KR, Kershaw Y. Basic medical laboratory technology. New York: John Wiley and Sons; 1975. [Google Scholar]
- 46.Xing K, Yang HS, Chen MY. Morphological and ultrastructural characterization of the coelomocytes in Apostichopus japonicas. Aquatic Biol. 2008;2:85–92. [Google Scholar]
- 47.Rainard P. A colorimetric microassay for opsonins by reduction of NBT in phagocytosing bovine polymorphs. J Immunol Methods. 1986;90:197–201. doi: 10.1016/0022-1759(86)90076-1. [DOI] [PubMed] [Google Scholar]
- 48.Miranda KM, Espey MG, Wink DA. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide. 2001;5:62–71. doi: 10.1006/niox.2000.0319. [DOI] [PubMed] [Google Scholar]
- 49.Shami PJ, Weinberg JB. Differential effects of nitric oxide on erythroid and myeloid colony growth from CD34+ human bone marrow cells. Blood. 1996;87:977–82. [PubMed] [Google Scholar]
- 50.Albina JE, Cui S, Mateo RB, Reichner JS. Nitric oxide-mediated apoptosis in murine peritoneal macrophages. J Immunol. 1993;150:5080–5. [PubMed] [Google Scholar]
- 51.Genaro AM, Hortelano S, Alvarez A, Martínez C, Boscá L. Splenic B lymphocyte programmed cell death is prevented by nitric oxide release through mechanisms involving sustained Bcl-2 levels. J Clin Invest. 1995;95:1884–90. doi: 10.1172/JCI117869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Taylor EL, Megson IL, Haslett C, Rossi AG. Nitric oxide: A key regulator of myeloid inflammatory cell apoptosis. Cell Death Differ. 2003;10:418–30. doi: 10.1038/sj.cdd.4401152. [DOI] [PubMed] [Google Scholar]
- 53.Aicher A, Heeschen C, Mildner-Rihm C, Urbich C, Ihling C, Technau-Ihling K, et al. Essential role of endothelial nitric oxide synthase for mobilization of stem and progenitor cells. Nat Med. 2003;9:1370–6. doi: 10.1038/nm948. [DOI] [PubMed] [Google Scholar]
- 54.Thum T, Tsikas D, Stein S, Schultheiss M, Eigenthaler M, Anker SD, et al. Suppression of endothelial progenitor cells in human coronary artery disease by the endogenous nitric oxide synthase inhibitor asymmetric dimethylarginine. J Am Coll Cardiol. 2005;46:1693–701. doi: 10.1016/j.jacc.2005.04.066. [DOI] [PubMed] [Google Scholar]
- 55.Anderson D, Bishop JB, Garner RC, Ostrosky-Wegman P, Selby PB. Cyclophosphamide: Review of its mutagenicity for an assessment of potential germ cell risks. Mutat Res. 1995;330:115–81. doi: 10.1016/0027-5107(95)00039-l. [DOI] [PubMed] [Google Scholar]
- 56.Haque R, Bin-Hafeez B, Ahmad I, Parvez S, Pandey S, Raisuddin S. Protective effects of Emblica officinalis Gaertn.in cyclophosphamide-treated mice. Hum Exp Toxicol. 2001;20:643–50. doi: 10.1191/096032701718890568. [DOI] [PubMed] [Google Scholar]
- 57.Kobrinsky NL, Sjolander DE, Cheang MS, Levitt R, Steen PD. Granulocyte-macrophage colony-stimulating factor treatment before doxorubicin and cyclophosphamide chemotherapy priming in women with early-stage breast cancer. J Clin Oncol. 1999;17:3426–30. doi: 10.1200/JCO.1999.17.11.3426. [DOI] [PubMed] [Google Scholar]
- 58.Rittenhouse JR, Lui PD, Lau BH. Chinese medicinal herbs reverse macrophage suppression induced by urological tumors. J Urol. 1991;146:486–90. doi: 10.1016/s0022-5347(17)37830-8. [DOI] [PubMed] [Google Scholar]
- 59.Babior BM, Kipnes RS, Curnutte JT. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973;52:741–4. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lacy P, Abdel-Latif D, Steward M, Musat-Marcu S, Man SF, Moqbel R. Divergence of mechanisms regulating respiratory burst in blood and sputum eosinophils and neutrophils from atopic subjects. J Immunol. 2003;170:2670–9. doi: 10.4049/jimmunol.170.5.2670. [DOI] [PubMed] [Google Scholar]
- 61.Robinson JM. Phagocytic leukocytes and reactive oxygen species. Histochem Cell Biol. 2009;131:465–9. doi: 10.1007/s00418-009-0565-5. [DOI] [PubMed] [Google Scholar]
- 62.Fiala M, Liu PT, Espinosa-Jeffrey A, Rosenthal MJ, Bernard G, Ringman JM, et al. Innate immunity and transcription of MGAT-III and Toll-like receptors in Alzheimer's disease patients are improved by bisdemethoxycurcumin. Proc Natl Acad Sci U S A. 2007;104:12849–54. doi: 10.1073/pnas.0701267104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim AJ, Kim YO, Shim JS, Hwang JK. Immunostimulating activity of crude polysaccharide extract isolated from Curcuma xanthorrhiza Roxb. Biosci Biotechnol Biochem. 2007;71:1428–38. doi: 10.1271/bbb.60241. [DOI] [PubMed] [Google Scholar]
- 64.Avagyan H, Goldenson B, Tse E, Masoumi A, Porter V, Wiedau-Pazos M, et al. Immune blood biomarkers of Alzheimer disease patients. J Neuroimmunol. 2009;210:67–72. doi: 10.1016/j.jneuroim.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 65.Shao BM, Xu W, Dai H, Tu P, Li Z, Gao XM. A study on the immune receptors for polysaccharides from the roots of Astragalus membranaceus, a Chinese medicinal herb. Biochem Biophys Res Commun. 2004;320:1103–11. doi: 10.1016/j.bbrc.2004.06.065. [DOI] [PubMed] [Google Scholar]
- 66.Tan BK, Vanitha J. Immunomodulatory and antimicrobial effects of some traditional chinese medicinal herbs: A review. Curr Med Chem. 2004;11:1423–30. doi: 10.2174/0929867043365161. [DOI] [PubMed] [Google Scholar]
- 67.Schepetkin IA, Xie G, Kirpotina LN, Klein RA, Jutila MA, Quinn MT. Macrophage immunomodulatory activity of polysaccharides isolated from Opuntia polyacantha. Int Immunopharmacol. 2008;8:1455–66. doi: 10.1016/j.intimp.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dhakshinamoorthy S, Long DJ 2nd, Jaiswal AK. Antioxidant regulation of genes encoding enzymes that detoxify xenobiotics and carcinogens. Curr Top Cell Regul. 2000;36:201–16. doi: 10.1016/s0070-2137(01)80009-1. [DOI] [PubMed] [Google Scholar]
- 69.Nelson SK, Bose SK, Grunwald GK, Myhill P, McCord JM. The induction of human superoxide dismutase and catalase in vivo: A fundamentally new approach to antioxidant therapy. Free Radic Biol Med. 2006;40:341–7. doi: 10.1016/j.freeradbiomed.2005.08.043. [DOI] [PubMed] [Google Scholar]
- 70.Nguyen T, Nioi P, Pickett CB. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem. 2009;284:13291–5. doi: 10.1074/jbc.R900010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Steinkamp-Fenske K, Bollinger L, Völler N, Xu H, Yao Y, Bauer R, et al. Ursolic acid from the Chinese herb danshen (Salvia miltiorrhiza L.) upregulates eNOS and downregulates Nox4 expression in human endothelial cells. Atherosclerosis. 2007;195:e104–11. doi: 10.1016/j.atherosclerosis.2007.03.028. [DOI] [PubMed] [Google Scholar]
- 72.Klein C, Sato T, Meguid MM, Miyata G. From food to nutritional support to specific nutraceuticals: A journey across time in the treatment of disease. J Gastroenterol. 2000;35(Suppl 12):1–6. [PubMed] [Google Scholar]


