Abstract
Background:
Diabetes mellitus, becoming the third killer of mankind after cancer and cardiovascular diseases, is one of the most challenging diseases facing health care professionals today. That is why; there has been a growing interest in the therapeutic use of natural products for diabetes, especially those derived from plants.
Aim:
To evaluate the anti-diabetic activity together with the accompanying biological effects of the fractions and the new natural compounds of Hyphaene thebaica (HT) epicarp.
Materials and Methods:
500 g of coarsely powdered of (HT) fruits epicarp were extracted by acetone. The acetone crude extract was fractionated with methanol and ethyl acetate leaving a residual water-soluble fraction WF. The anti-diabetic effects of the WF and one of its compounds of the acetone extract of the (HT) epicarp were investigated in this study using 40 adult male rats.
Results:
Phytochemical investigation of active WF revealed the presence of ten different flavonoids, among which two new natural compounds luteolin 7-O-[6”-O-α-Lrhamnopyranosyl]-β-D-galactopyranoside 3 and chrysoeriol 7-O-β-D-galactopyranosyl(1→2)-α-L-arabinofuranoside 5 were isolated. Supplementation of the WF improved glucose and insulin tolerance and significantly lowered blood glycosylated hemoglobin levels. On the other hand, compound 5 significantly reduced AST and ALT levels of liver, respectively. Likewise, the kidney functions were improved for both WF and compound 5, whereby both urea and creatinine levels in serum were highly significant
Conclusion:
The results justify the use of WF and compound 5 of the (HT) epicarp as anti-diabetic agent, taking into consideration that the contents of WF were mainly flavonoids
Keywords: Anti-diabetic activity, epicarp, hyphaene thebaica, novel flavonoids
INTRODUCTION
Diabetes have been treated with several medicinal plants for a long time, whereby the medicinal plant extracts were found to improve the diabetic control and meanwhile reduce associated side effects than the synthetic ones.[1,2] Therefore, the search for more effective and safer anti-diabetic agents has become an area of active research. Flavonoids are well-known for their multi-directional biological activities including anti-diabetic efficacy.[3–6] The Doum (HT) is a type of palm tree with edible oval fruit, popular in Egypt and originally is native to the Nile valley; its herb tea is traditionally believed to be good for treatment of hypertension. The aqueous extract of (HT)is also useful for the treatment of bilharziasis, hematuria bleeding, especially after child birth.[7–11] (HT) Epicarp, when investigated chemically, proved to contain alkaloid (s), reducing sugars, glycosides[12] and flavones glycosides.[13] Phytochemical screening of the WF afforded ten different flavonoid compounds. Elucidation of the chemical structure of the isolated compounds was determined by different spectroscopic methods in addition to the chemical and physical methods of analysis.
MATERIALS AND METHODS
Collection of Plant material
Fruits of the Hyphaene thebaica (HT) were collected from Aswan, Egypt (October, 2009). The fruits were then cracked to collect its epicarp. Authentication was performed by Dr. M. El-Gebali, former researcher of botany at the National Research Center. A voucher specimen is deposited in the National Research Centre Herbarium (CAIRC) for future references.
Apparatus and techniques
1H and 13C (500, 125 MHz) NMR: Joel spectrometer in DMSO-d6; UV: Shimadzu spectro-photometer model UV-240; column chromatography (CC): Polyamide 6S (Riedel, De Häen), cellulose (Merck) and Sephadex LH-20 (Pharmacia); paper chromatography (PC): was carried out on Whatman No.1 and preparative (PPC) on 3 MM paper using solvent systems (1) BAW (n-BuOH: AcOH: H2O, 4:1:5-upper phase); (2) H2O; (3) 15% AcOH (AcOH: H2O, 15:85); and (4)Forestal (AcOH: Conc. HCl: H2O: 30:3:10); (5) 6% AcOH (AcOH: H2O, 06:94).
Animals
40 adult Sprague-Dawely male rats, of the same age (4 months) and weight (120-150 gm), obtained from the animal house colony of the National Research Center, were used in this study. The animals were kept in stainless steel cages under the same hygienic conditions with 12 hours light/dark cycle. They were fed on a well-balanced diet and had free access to tap water.
The animals were divided into:
Group I: 10 normal healthy adult male rats served as a control group.
Group II: 30 adult male rats were rendered diabetic by intraperitoneal injection of freshly prepared alloxan monohydrate solution in a dose of 120 mg/kg body weight.[14]
Group III: 10 alloxan diabetic rats from group II were given orally a solution of WF (20 mg/Kg body weight) using an orogastric tube daily for a period of 30 days.
Group IV: 10 alloxan diabetic rats from group II were given orally a solution of compound 5 ( 20 mg/Kg body weight) daily for a period of 30 days.
Blood samples were collected using ocular vein puncture from the fasting groups I, II, III, and IV, respectively. Small portions of blood samples were placed in heparinized plastic tubes and assayed in the same day of collection to prevent the conversion of glutathione into its reduced form, for the determination of glutathione peroxidase and superoxide dismutase activities as well as lipid peroxidation. Other portions of the blood samples were left to clot and then centrifuged at 5000 r.p.m. under cooling for 10 minutes to separate the sera for the other biochemical analysis.
Extraction, Fractionation, and Isolation
The (HT) epicarp (500 g) was coarsely powdered and extracted by successive maceration with acetone in a soxhlet extractor at room temperature (5 l). The extract was concentrated to dryness under reduced pressure and controlled temperature (40°C) to yield the crude extract (150 g). The acetone crude extract was successively extracted in a separating funnel with methanol and ethyl acetate (3 l each) at room temperature till exhaustion leaving a residual water-soluble fraction. The active concentrated water-soluble fraction (800 mg) was then subjected to Sephadex LH-20 column chromatography (500 g, 40 × 1000 mm) and eluted with water followed by different ratios of water/ethanol (1 l each eluent) to give rise to five fractions, which were further purified by a series of fractionations on a Sephadex LH-20 column and (PPC). Compounds (1, 26 mg and 2, 28 mg) were separated from fraction I by fractionation over Sephadex LH-20 column using MeOH/H2O (decreasing polarity) for elution then PPC to the sub-fractions using (AcHO: H2O; 6:94). Compounds (3, 88 mg and 4, 56 mg) were isolated as pure compounds from fraction II by using Sephadex LH-20 column and n-BuOH saturated with H2O as developing system. Applying the third fraction on Sephadex LH-20 column (100 g, 19 × 250 mm) and eluted by ethanol to obtain the pure natural compounds (5, 75 mg and 6, 43 mg). From the fourth fraction, compound (7, 63 mg) was separated in a pure form by applying on the Sephadex LH-20 column and eluted by 40% EtOH. Finally, the pure aglycones 8 (25 mg), 9 (23 mg), and 10 (30 mg) were obtained in a pure form from a cellulose column chromatography of fraction V using ethanol as eluent.
Luteolin 7-O-[6’-O-α-L-rhamnopyranosyl]-β-D-galactopyranoside 3
Rf -values ×100: 37 (1), 05 (2), 30 (3); UV λmax nm (MeOH): 255, 265 sh, 349; +NaOMe: 264, 299 sh, 396;+NaOAc: 259, 265 sh, 366, 403; +NaOAc/H3BO3: 260, 370;+AlCl3: 272, 296 sh, 331, 432; +AlCl3/HCl: 272, 295, 359, 389. 1H-NMR (DMSO-d6 ): aglycone moiety: δ (ppm) 7.41 (d, J=2.1 Hz, H-2’); 7.39 (dd, J=8.4 and 2.1 Hz, H-6’); 6.88 (d, J=8.4 Hz, H-5’); 6.76 (s, H-3); 6.71 (d, J=2.0 Hz, H-8); 6.42 (d, J=2.0 Hz, H-6); Sugar moieties: δ (ppm) 5.03 (d, J=7.5 Hz, H-1” of galactose); 4.5 (d, J=2.1 Hz, H-1”’ of rhamnose); 3.11-3.79 (m, rest of sugar protons); 1.02 (t, CH3); 13C-NMR (DMSO-d6): aglycone moiety: δ (ppm) 165.1 (C-2); 103.66 (C-3); 182.4 (C-4); 161.7 (C-5); 99.9 (C-6); 163.38 (C-7); 95.3 (C-8); 157.42 (C-9); 105.88 (C-10); 121.86 (C-1’); 114.0 (C-2’); 146.3 (C-3’); 150.46 (C-4’); 115.9 (C-5’); 119.76 (C-6’); Sugar moieties: β-D-galactopyranoside moiety: δ (ppm) 101.2 (C-1”); 73.59 (C-2”); 76.04 (C-3”); 70.8 (C-4”); 76.75 (C-5”); 68.84 (C-6”); α-L-rhamnopyranoside moiety: δ (ppm) 100.38 (C-1”’); 71.22 (C-2”’); 70.8 (C-3”’); 72.54 (C-4”’); 68.84 (C-5”’); 18.3 (C-6”’).
Chrysoeriol 7-O-[2”-O-β-D-galactopyranosyl]-α-L-arabinofuranoside 5
Rf -values ×100: 30(1), 59(2), 32(3)35(4); UV λmax nm (MeOH): 251, 265, 342; +NaOMe: 263,400;+NaOAc: 260, 290 sh, 396; +NaOAc/H3BO3: 265, 344;+AlCl3: 262, 296 sh, 342 sh, 378; +AlCl3/HCl: 259, 296 sh, 342 sh, 378; 1H-NMR (DMSO-d6): Aglycone moiety: δ (ppm) 7.56 (d, J=2 Hz, H-2’); 7.54 (dd, J=2 and 8 Hz, H-6’); 6.93 (d, J=8 Hz, H-5’); 6.88 (s, H-3); 6.75 (d, J=2.1 Hz, H-8); 6.44 (d, J=2.1 Hz, H-6); 3.87 (s, OCH3). Sugar moieties: δ (ppm) 5.54 (brs, H-1” of arabinose); 5.03 (d, J=7.5 Hz, H-1”’ of galactose); 4.401 (d, J=3.4, H-2”); 3.91 (m, H-4”a); 3.83 (m, H-3”); 3.81 (d, J=9.27 Hz, H-4”b), 3.7-3.35 (m, rest of sugar protons); 13C-NMR (DMSO-d6): aglycone moiety: δ (ppm) 154.6 (C-2); 103.5 (C-3); 176.9 (C-4); 162.4 (C-5); 99.2 (C-6); 168.9 (C-7); 94.6 (C-8); 156.9 (C-9); 104.1 (C-10); 131.1 (C-1’); 111.6 (C-2’); 146.8 (C-3’); 149.6 (C-4’); 116.5 (C-5’); 121.9 (C-6’); 55.6 (OCH3). Sugar moieties: α-L-arabinofuranose moiety: δ(ppm) 108.8 (C-1”), 85.6 (C-2”), 78.3 (C-3”), 86.5 (C-4”), 62.1 (C-5”); β-D-galactopyranose moiety: δ (ppm) 103.8 (C-1”’); 74.3 (C-2”’); 76.5 (C-3”’); 69.6 (C-4””); 76.5 (C-5”’); 60.7 (C-6”’).
Biochemical study
Determination of Serum glucose concentration
Serum glucose concentration was determined enzymatically according to the method described by Trinder.[15]
Serum amino transferase enzyme activities
The activities of aspartate amino transferase (AST) and alanine amino transferase (ALT) were determined using kits provided by Pointe Scientific Company USA, according to the method described by Tietz.[16]
Serum protein concentration
Quantitative determination of total protein concentration in serum was carried out using kits provided by Pointe Scientific Company USA, according to the method described by Weichelbaum.[17]
Serum albumin concentration
Serum albumin concentration was determined using kits supplied by Pointe Scientific Company USA, according to the method described by Doumas.[18]
Serum cholesterol and triglycerides
Quantitative determination of total cholesterol in serum was carried out according to the method described by Richmond.[19] Triglycerides concentration in the serum was determined by enzymatic colorimetric method of Burolo and David.[20]
Lipid peroxidation
The product of lipid peroxidation was determined as thio-barbituric acid reactive substance (TBARS) according to the method of Mihara and Uchiyamo.[21]
Glutathione peroxidase activity
Erythrocyte glutathione peroxidase activity was determined using Ransel kit from Randox Laboratories according to Paglia and Valentine[22] method. The activity of glutathione peroxidase was expressed as units per gram of hemoglobin (HB). The hemoglobin concentration was determined by the cyanmet-hemoglobin method according to Mahoney.[23]
Superoxide dismutase activity
The activity of superoxide dismutase was determined using Ransel kit from Randox. This method employs xanthine and xanthine oxidase (XOD) to generate superoxide radicals, which react with 2-(4-iodophexyl)-3-(4-nitrophenol)-5-phenyl tetrazolium chloride (INT) to form a red formazan dye. The superoxide dismutase activity is measured by the degree of inhibition of this reaction.[24]
Glutathione content in liver tissue
The glutathione (GSH) content in liver tissue homogenate was estimated by the method of Beutler.[25]
Serum urea concentration
Enzymatic determination of serum urea was carried out according to the method of Fawcett and Scott[26] using Bio Merieux kits, France.
Serum creatinine concentration
Serum creatinine concentration was determined by the method described by Bartles[27] using Pointe Scientific kit, INC, USA.
Serum testosterone concentration
Enzyme immune assay kit for the quantitative measurements of testosterone in serum was provided by Biosource Company, Europe, according to the method described by Hill.[28]
Serum acid phosphatase activity
Serum acid phosphatase activity was determined by a colorimetric method described by Moss,[29] using Quimica Clinica Aplicada S. A. Spain kit.
RESULTS AND DISCUSSION
In the recent days, many researchers and investigators tested various traditional medicinal plants for their potential anti-diabetic effect in experimental animals. Working on the same line, we have undertaken a study on Hyphaene thebaica doum epicarp for its anti-diabetic property.
One of the most striking results of the present study is the improvement of the kidney functions in response to doum supplementation, with parallel reduction in the concentration of both urea and creatinine levels in serums, which were high, significantly dropped. Also, each of glutathione peroxidase and superoxide dismutase levels was increased, besides albumin and total protein levels were reduced. As we state the significant marked improvement of some biological symptoms then start to show the improvement in them individually e.g. the serum glucose, the liver function, markedly developed both by AST and ALT levels with mild decrease in both cholesterol and triglycerides levels.
Bioassay-guided phytochemical investigation of Egyptian Doum fractions with proven activity was carried out to isolate secondary metabolites. An in-depth phytochemical analysis of active WF fraction of the acetone extract of the (HT)epicarp resulted in the isolation of ten compounds; vitexin 1, isovitexin 2, luteolin 7-O-β-D-glucopyranoside 4 , chrysoeriol 7-O-[6”-O-α-L-rhamnopyranosyl]-β-D-glucopyranoside 6, kaempferol 7, 4’-dimethoxy-3-[6”-O-α-L-rhamnopyranosyl]-β-D-glucopyranoside 7, the aglycones, luteolin 8, chrysoeriol 9 and kaempferol 10 together with the two new natural compounds luteolin 7-O-[6”-O-α-L-rhamnopyranosyl]-β-D-galactopyranoside 3 and chrysoeriol 7-O-[2”-O-β-D-galactopyranosyl]-α-L-arabinofuranoside 5 [Figure 1]. Their structures were elucidated on the basis of spectroscopic analysis.
Figure 1.
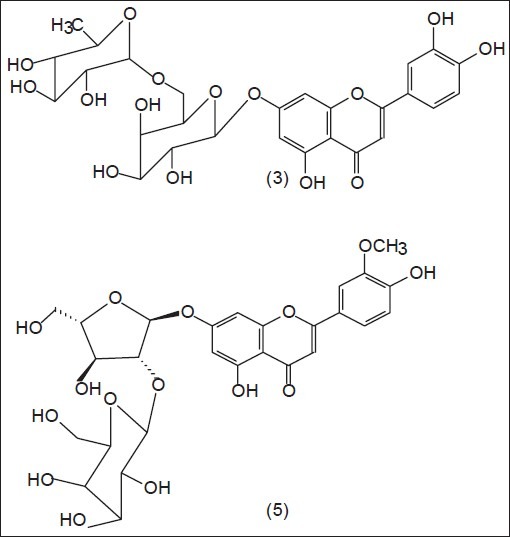
Structure of the isolated new natural flavonoidsww
Compound 3 was obtained as an amorphous yellow powder. The UV spectrum exhibited absorption maxima (255, 265 sh, 349 nm) together with that of diagnostic reagents, suggested a 7-substituted flavone structure. Complete acid hydrolysis of 3 yielded luteolin, galactose, and rhamnose, identified by Co-PC with authentic samples in different solvents. The 1 H NMR spectrum of 3 exhibited an ABX system at δH 7.41 (d, J=2.1 Hz, H-2’), 7.39 (dd, J=8.4, 2.1 Hz, H-6’) and 6.88 (d, J=8.4 Hz, H-5’) due to a 3’, 4’-disubstitution pattern of ring B. Moreover, two meta-coupled doublets at δH 6.71 (J=2.0 Hz, H-8) and 6.42 (J=2.0 Hz, H-6) were consistent with 5, 7-dioxygenated ring A. These resonances together with the singlet signal at δH 6.76 (H-3) revealed the presence of the aglycone luteolin.[30] Additionally, the resonances of two anomeric protons at δH 5.03 (d, J=7.5 Hz, H-1”) and δH 4.5 (d, J=1.8 Hz, H-1”’), respectively, were indicative of the presence of one β-and one α-linked sugar unit together with the methyl rhamnose proton, which resonate at δ 1.02 ppm (d, J=6.15 Hz, CH3) and revealed the disaccharide moiety as rhamnosyl (1→6) galactoside. Assignments for all carbon resonances were achieved by 13C-NMR, which confirmed the presence of a β-galactose and one α-rhamnose as sugar units. The appearance of a downfield signal at δc 68.84 ppm for the C-6” of the galactose moiety confirmed (1→6) glycosidic linkage between them. The C-7 of the aglycone resonated at δC 163.38 ppm indicated that the disaccharide unit was attached to C-7(OH), whereby the galactose moiety at δC 101.02 ppm attached directly to the aglycone and the rhamnose is terminal at δC 100.38 ppm. Thus, compound 3 was identified as the new natural compound: Luteolin 7-O-[6”-O-α-L-rhamnopyranosyl]-β-D-galactopyranoside.
The new natural glycoside 5 was identified through Rf -values, color reactions, and UV spectral data as a flavone type substituted at 7 and 3’ positions since the addition of NaOAc and NaOAc/H3BO3 produced no shift in band II and I, respectively, indicating the absence of a free 7-OH group in ring A or a free 3’,4’-dihydroxyl group in ring B. Addition of NaOMe led to a bathochtomic shift in band I (58 nm) without decrease in intensity, suggesting a substitution in position 3’. Upon complete acid hydrolysis of 5 yielded chrysoeriol as the aglycone and galactose, arabinose as the sugar moieties indicating it to be in position 7. β-galactosidase enzymatic hydrolysis gave an intermediate, which was identified as chrysoeriol 7-arabino-furanoside [identified by Co-PC, UV spectral data and 1H-NMR] i.e. arabinose was directly attached to the aglycone, and galactose was terminal. The 1H-NMR spectrum confirmed the above features and revealed the arabinosyl moiety to be α-linked to the aglycone at 7-position (br s, δ 5.54 ppm) and galactosyl anomeric proton at δ 5.03 ppm (J=7.5 Hz) to be β-linked to the arabinose hydroxyl group at H-2’, whereas its doublet signal appeared at δ 4.40 ppm (J=3.4 Hz). The 13C NMR shifts of the aglycone moiety of 5 corresponded well with the signals of chrysoeriol, with the only significant difference being those corresponding to C-6, C-7, and C-8. These shifts are analogous to those reported when the 7-hydroxy group is glycosylated in a flavones glycoside.[31] Two anomeric protons, assigned to the C-1 protons of an arabinofuranosyl and a galactosyl units, were easily identified in the spectra of 5 as they resonated at δ 108.8 and 103.8 ppm, respectively, with C-2” at δ 85.6 ppm, confirming the disaccharide unit to be galactosyl (1→2) arabinofuranoside attached to C-7 of the aglycone. Therefore, the structure of 5 was determined as the new natural flavone glycoside chrysoeriol 7-O-β-D-galactopyranosyl (1→2) α-L-arabinofuranoside.
[Table 1] represents serum glucose level of control rats, alloxan-diabetic rats, and diabetic rats treated with fraction WF and compound 5 . The results show highly significant increase in serum glucose level in alloxan-diabetic rats as compared to control ones, while diabetic rats treated with fraction WF show highly significant decrease in serum glucose level as compared to alloxan-diabetic ones.
Table 1.
Represents serum glucose level of control rats, alloxan-diabetic rats, and diabetic rats treated with WF and 5 at the end of 30 days

The production of glucose by gluconeogenesis is energy expensive process since the production of one mole of glucose from pyruvat will require six moles of ATP.[32] It is, therefore, likely that the necessary energy was peroxided by the increased rate of lipid oxidation. Reduction of serum glucose from 281.3+1.2 to 137.2+0.1 mg/dl after treatment of alloxan-diabetic rats with fraction WF indicates that this fraction could bring about blood glucose homeostasis through regeneration of endocrine pancreas and increasing insulin secretion and stimulating the enzyme glycogen synthetase, which traps glucose moieties into pre-existing glycogen chains.[33]
Liver function assessments for control rats, alloxan-diabetic rats, and diabetic rats treated with WF and 5 are depicted in [Table 2], whereby it depicts the serious de-arrangement in liver functions. Each of the serum level of AST, ALT, triglycerides, and cholesterol significantly raised to 62.1+3.1 μ/L, 57.2+1.3 μ/L, 140.3+3.1 mg/dl, and 131+0.2 mg/dl, respectively, in alloxan-diabetic rats as compared to control ones, while serum albumin and total protein levels markedly dropped to 3.1+0.4 mg/dl and 6.31+0.2 mg/dl, respectively. The high level of ALT is indicated of the serious hepatocellular damage since it is more liver specific than AST. Triglycerides are synthesized in the liver from fatty acids and glycerol and as such they are transported as very low density lipoproteins (V-LDL) to a dipose tissue store.[34] However, the significantly high level of serum triglycerides to 140.3+3.1 mg/dl in alloxan-diabetic rats is said to be associated with diminished triglycerides content of muscle, a matter, which reflects rapid disordering of the glucose fatty acid cycle.[35]
Table 2.
Liver profile for control group, alloxan-diabetic rats, and diabetic rats treated with fraction WF and compound 5 at the end of 30 days
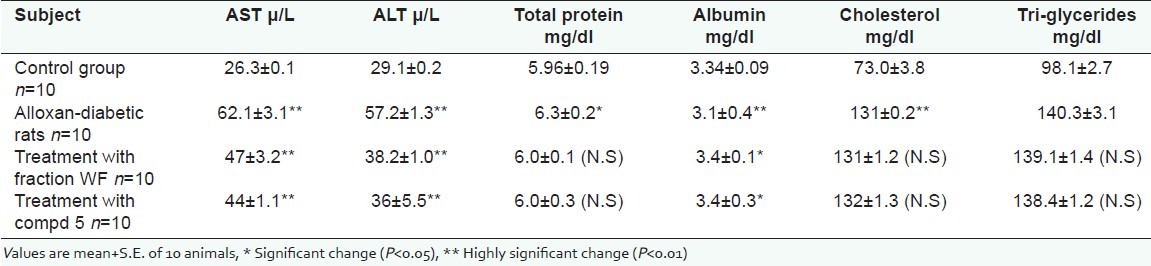
A great improvement in liver function of diabetic male rats was achieved after treatment with WF and 5. The serum level of AST was highly significant; reduced from 62.1+3.1 μ/L to 47+3.2 μ/L and 44+1.1 μ/L for WF and 5, respectively. The serum level of ALT was high; significantly reduced from 57.2+1.3 μ/L to 38.2+1.0 μ/L and 36.0+5.5 μ/L for WF and 5, respectively. While mild decrease in serum level of each of cholesterol and triglycerides was observed in [Table 2], the protein content was unchanged or decreased in alloxan-diabetic rats that's in liver of diabetic animals. However, skeletal and cardiac muscles are most likely the major recorded sites of net protein less during diabetes.[36,37] [Table 2] also shows how a lowering in the level of total protein in diabetic rats mechanisms for intracellular protein break down involve cytosolic ATP-dependent. It has been reported that the plasma concentration of a number of the regulatory substances: Glucagons, glucocorticoids, and branched chain amino acids, which also affected protein metabolism, are altered during the insulin-deficient state. Noteworthy, the presence in-vivo of other hormones, particularly alkysoid and corticosteroid hormone, can affect protein turnover either alone or in contact with insulin.[36] In fact, treatment of diabetic rats with WF and 5 has raised the level of total protein to 6.0+0.1 and 6.0+0.3, respectively. A drop in serum albumin level in diabetic rats as compared to the control group was recorded from 3.34+0.09 mg/dl to 3.1+0.4 mg/dl, while a mild increase was observed after treatment of the diabetic rats with WF and 5 to 3.4+0.1 mg/dl and 3.4+0.3 mg/dl, respectively. This fraction and compound have shown protective effect against the oxidation stress and has been found to be mainly due to an increased production of free radicals attached with a sharp reduction of antioxidant defenses.[38]
In order to assess the indices of oxidative stress, which is associated with the development of complications in diabetes, the thiobarbituric acid reactive substances (TBARS) were measured as an index of malondialdehyde production. Hence, lipid peroxidation, compared with control diabetic liver and pancreas, showed significant increase in TBARS level at all time intervals.[39]
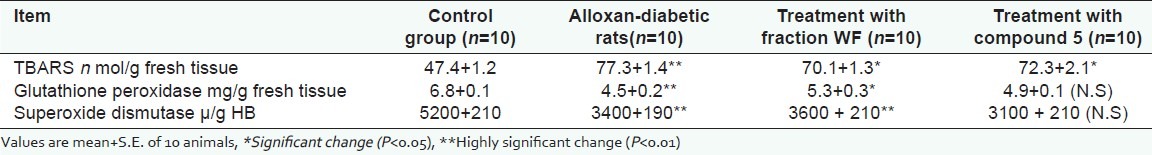
The data of lipid peroxidation as thiobarbituric acid reactive substance (TBARS), glutathione peroxidase (GPX), and superoxide dismutase (SOD) diabetic rats and diabetic rats treated with WF and 5 are shown in [Tables 3 and 4]. Highly significant increase in lipid peroxidation was observed in alloxan-diabetic male rats as compared with the corresponding control ones. The data in [Tables 3 and 4] shows the levels of TBARS in the whole blood (88.3+4.1 n mol/ g HB) and in the liver (77.3+1.4 n mol/g fresh tissue). Highly significant decrease in lipid peroxidation was detected after treatment with WF and 5 (73.3+1.2 and 76.1+1.4 n mol/ g HB) and (70.1+1.3 and 72.3+2.1 n mol/g fresh tissue), respectively. In contrast, highly significant decrease in both glutathione peroxidase and superoxidase dismutase activities were detected in alloxan-diabetic rats as compared to control rats in the whole blood (42.2+1.7 and 2.0+0.13 μ/g HB, respectively) and in the liver (4.5+0.2 and 3400+190 μ/g HB, respectively). Meanwhile, the activity of glutathione peroxidase and superoxidase dismutase showed moderate increase after treatment with WF (47.2+2.1 and 2.82+0.09 μ/g HB, respectively, in the whole blood and 5.3+0.3 and 3600+210 μ/g HB, respectively, in liver) and 5 ( 45.1+1.0 and 2.7+0.17 μ/g HB, respectively, in the whole blood and 4.9+0.1 and 3100+210 μ/g HB, respectively, in liver).
Table 3.
Lipid peroxidation activities in whole blood of control group, alloxan-diabetic rats and diabetic rats after treatment with fraction WF and compound 5 (20 mg/Kg) at the end of 30 days
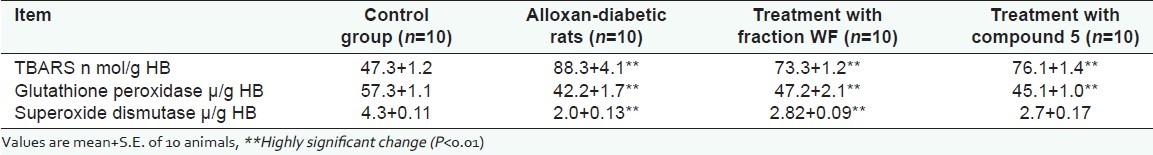
Table 4.
Level of TBARS, Glutathione (GSH) and superoxide dismutase (SOD) in liver of control group, alloxan-diabetic rats, and diabetic rats treat with fraction WF and compound 5 (20 mg/Kg) at the end of 30 days
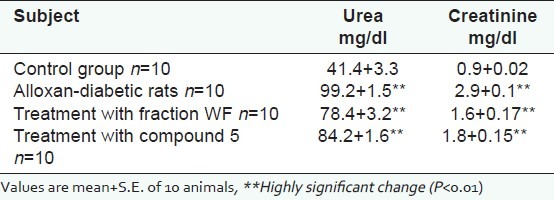
[Table 5] illustrates the kidney function profile of control rats, alloxan-diabetic rats, and diabetic rats treated with WF and 5. Highly significant increases in serum urea (99.2+1.5 mg/ dl) and creatinine concentration (2.9+0.1 mg/dl) observed in alloxan-diabetic rats as compared to control ones. Urea measurements have come to be accepted as giving a means of renal function since 50% or more of urea filtered to the glamorous is passively reabsorbed through the tubules.[40] Severe hyperglycemia has driven anosmatic diverse resulting in loss of extracellular fluid and electrolytes with consequent reduction in the glomerular filtration rate and retention of urea as well as increased plasma creatinine an indication of a full in glomerular filtration rate.[34] After treatment with WF and 5, highly significant decreases in serum urea and creatinine concentration were detected from 99.2+1.5 mg/dL to 78.4+3.2 mg/dL and from 2.9+0.1 mg/dL to 1.6+0.17 mg/dL, respectively, for WF and from 99.2+1.5 mg/dL to 84.2+1.6 mg/dL and from 2.9+0.1 mg/dL to 1.8+0.15 mg/dL, respectively, for compound 5.
Table 5.
Kidney function profile for control group, alloxan-diabetic rats, and diabetic rats treated with fraction WF and compound 5 at the end of 30 days
The levels of serum testosterone, total and prostatic acid phosphates of control rats, alloxan-diabetic rats, and diabetic rats treated with WF and 5 are illustrated in [Table 6]. Highly significant decrease in serum testosterone level with concentrate increase in total and prostatic acid phosphates activities were observed in alloxan-diabetic rats as compared to control rats. After treatment with WF and 5, highly significant increases in serum testosterone level accompanied with highly significant decrease in total and prostatic acid phosphatase activities were achieved.
Table 6.
Testosterone, total and prostatic acid phosphatase levels in serum of control group, alloxandiabetic rats, and diabetic rats treat with fraction WF and compound 5 at the end of 30 days
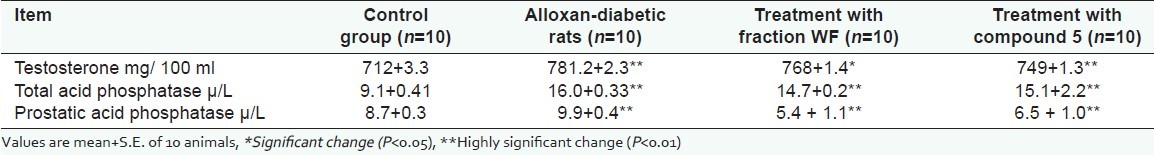
It has been reported by Bala Subramanian[41] that diabetes mellitus is associated with significant reduction in serum testosterone level and accessory sex gland weight. The sperm content of tepidity male regions also decreased. These results were in consistence with our data [Table 6] where we found highly significant decrease in serum testosterone level in alloxan-diabetic rats in concentrate with highly significant rise in total acid phosphatase and prostatic acid phosphatase activities. However, treatment of alloxan-diabetic rats with WF and 5 resulted in highly significant increase in serum testosterone level and marked modulation in the level of both total acid phosphatase and prostatic acid phosphatase activities.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Bolkent S, Yanardag R, Tabakoglu-Oguz A, Ozsoy-Sacan O. Effect of chard (Beta vulgaris L. Var. Cicla) extract on pancreatic b cells in streptozotocin-diabetic rats: A morphological and biochemical study. J Ethnopharmacol. 2000;73:251–9. doi: 10.1016/s0378-8741(00)00328-7. [DOI] [PubMed] [Google Scholar]
- 2.Aybar MJ, Sánchez Riera AN, Grau A, Sánchez SS. Hypoglycemic effect of the water extract of Smallantus sonchifolius (yacon) leaves in normal and diabetic rats. J Ethnopharmacol. 2001;74:125–32. doi: 10.1016/s0378-8741(00)00351-2. [DOI] [PubMed] [Google Scholar]
- 3.Brahmachari G. Mother nature: An inexhaustible source of drugs and lead molecules. In: Brahmachari G, editor. Chemistry, Biochemistry and Pharmacology. 1st ed. New Delhi: Narosa Publishing House Pvt. Ltd; 2009. pp. 1–20. [Google Scholar]
- 4.Brahmachari G, Gorai D. Progress in the research of naturally occurring flavones and flavonols: An overview. Curr Org Chem. 2006;10:873–98. [Google Scholar]
- 5.Brahmachari G, Gorai D. Progress in the research of natural flavonoids: An overview. In: Brahmachari G, editor. Chemistry of Natural Products: Recent Trends and Developments. 1st ed. Trivandrum: Research Signpost; 2006. pp. 78–168. [Google Scholar]
- 6.Brahmachari G. Naturally occurring flavanones: An overview. Nat Prod Commun. 2008;3:1337–54. [Google Scholar]
- 7.Irobi ON, Adedayo O. Antifungal activity of aqueous extract of dormant fruits of Hyphaene thebaica (Palmae) Pharm Biol. 1999;37:114–7. [Google Scholar]
- 8.Adaya AL, Bitrus H, Fanjoji H, Eaton M, Gambo D. Hidden harvest project in research series. Compiled by 11ED and HNNCP. (47-53).1977:14–27. [Google Scholar]
- 9.Burkill HM. The useful plants of West Tropical Africa. 2nd ed. Kew: Royal Botanical Garden; 1994. pp. 371–3. [Google Scholar]
- 10.Hetta MH, Yassin NZ. Comparative studies on hypocholesterolemic effect of different fractions of Hyphaene thebaica (Doum) in experimental animals. Pharmazie. 2006;61:230–2. [PubMed] [Google Scholar]
- 11.Kamis AB, Modu S, Zanna H, Oniyangi TA. Preliminary biochemical and haematological effects of aqueous suspension of pulp of Hyphaene thebaica (L.)Mart in rats. Biokemistri. 2003;13:1–7. [Google Scholar]
- 12.Shariff ZU. Nature Pharmacy Series. Vol. 1. UK: Spectrum Books Ltd., Ibadan, Nigeria in Association with Safari Books (Export) Ltd; 2001. Modern herbal therapy for common ailments; pp. 9–84. [Google Scholar]
- 13.Hashim A. Growing in Egypt family Palmae. Giza, Egypt: Thesis for master, National Research Center; 1994. Phytochemical investigation of the fruit of Hyphaene thebaica (L) Mart. [Google Scholar]
- 14.Lazarow A, Palay SL. The production and course of alloxan diabetes in the rat. J Lab Clin Med. 1946;31:1004–15. [PubMed] [Google Scholar]
- 15.Trinder P. Determination of blood glucose using an oxidase/peroxidase system with a non-carcinogenic chromogen. J Clin Pathol. 1969;22:158–61. doi: 10.1136/jcp.22.2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tietz NW. In: Fundamentals of Clinical Chemistry. 2nd ed. Tietz NW, editor. Philadelphia: WB Saunders Co; 1976. pp. 657–80. [Google Scholar]
- 17.Weichselbaum TE. An accurate and rapid method for the determination of proteins in small amounts of blood serum and plasma. Am J Clin Pathol. 1946;7:40–9. [PubMed] [Google Scholar]
- 18.Doumas BT, Watson WA, Biggs HG. Albumin standards and the measurement of serum albumin with bromcresol green. Clin Chem Acta. 1971;31:87–96. doi: 10.1016/0009-8981(71)90365-2. [DOI] [PubMed] [Google Scholar]
- 19.Richmond W. Preparation and properties of a cholesterol oxidase from Nocradia sp.and its application to the enzymatic assay to total cholesterol in serum. Clin Chem. 1973;19:1350–6. [PubMed] [Google Scholar]
- 20.Bucolo G, David H. Quantitative determination of serum triglycerides by the use of enzymes. Clin Chem. 1973;19:476–82. [PubMed] [Google Scholar]
- 21.Mihara M, Uchiyama M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem. 1978;86:271–8. doi: 10.1016/0003-2697(78)90342-1. [DOI] [PubMed] [Google Scholar]
- 22.Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;70:158–69. [PubMed] [Google Scholar]
- 23.Mahoney JJ, Vreman HJ, Stevenson DK, Van Kessel AL. Measurement of carboxyhemoglobin and total hemoglobin by five specialized spectrophotometers (CO-oximeters) in comparison with reference methods. Clin Chem. 1993;39:1693–700. [PubMed] [Google Scholar]
- 24.Suttle NF. Copper deficiency in ruminants; recent developments. Vet Rec. 1986;119:519–22. doi: 10.1136/vr.119.21.519. [DOI] [PubMed] [Google Scholar]
- 25.Beutler E. A Manual of Biochemical Methods. 2nd ed. New York: Grune and Stration; 1975. Red cell metabolism; pp. 69–70. [Google Scholar]
- 26.Fawcett JK, Scott JE. A rapid and precise method for the determination of urea. J Clin Pathol. 1960;13:156–9. doi: 10.1136/jcp.13.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bartels H, Böhmer M, Heierli C. [Serum creatinine determination without protein recipitation] Clin Chem Acta. 1972;37:193–7. doi: 10.1016/0009-8981(72)90432-9. [DOI] [PubMed] [Google Scholar]
- 28.Hill P, Garbaczewski L, Kasumi F. Plasma testosterone and breast cancer. Eur J Cancer Clin Oncol. 1985;21:1265–6. doi: 10.1016/0277-5379(85)90025-2. [DOI] [PubMed] [Google Scholar]
- 29.Moss DW. In: Methods of enzymatic analysis. 3rd ed. Bergmeyer HU, editor. Verlag-Chemie; 1984. pp. 92–106. [Google Scholar]
- 30.Markham KR. Techniques of flavonoid identification. London: Academic Press; 1982. [Google Scholar]
- 31.Agrawal PK. Carbon 13-NMR of flavonoids. Amsterdam, Oxford, Tokyo, New York: Elsevier Science; 1989. [Google Scholar]
- 32.Franssila-Kallunki A, Groop L. Factors associated with basal metabolic rate in patients with type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia. 1992;35:962–6. doi: 10.1007/BF00401426. [DOI] [PubMed] [Google Scholar]
- 33.Mitra SK, Gopumadhavan S, Muralidhar TS. Effect of D-400, an Ayurvedic herbal formulation on experimentally induced-diabetes mellitus. Phytother Res. 1996;10:433–5. [Google Scholar]
- 34.Smith AF, Beckett GJ, Walker SW, Rae PW. Disorders of Carbohydrate Metabolism. Oxford: Blackewell Science; 1998. Lecture Notes on Clinical Biochemistry; p. 149. chap 1. [Google Scholar]
- 35.Karageuzyan KG, Vartanyan GS, Agadjanov MI, Panossian AG, Hoult JR. Restoration of the disordered glucose-fatty acid cycle in alloxan-diabetic rats by trihydroxyoctadecadienoic acids from Bryonia alba, a native Armenian medicinal plant. Planta Med. 1998;64:417–22. doi: 10.1055/s-2006-957472. [DOI] [PubMed] [Google Scholar]
- 36.Warram JH, Rich SS, Krolewski A. In: Joslin's Diabetes Mellitus. 13th ed. Khan CR, Weir GC, editors. Philadelphia: Lea and Febiger; 1994. pp. 116–38. chap. 7. [Google Scholar]
- 37.Hay AM, Waterlow JC. The effect of alloxan protein synthesis in the rat measured by constant infusion of L-(C-14) lysine. J Physiol. 1967;191:111–2. [PubMed] [Google Scholar]
- 38.Baynes JW. Role of oxidative stress in development of complications in diabetes. Diabetes. 1992;40:405–12. doi: 10.2337/diab.40.4.405. [DOI] [PubMed] [Google Scholar]
- 39.Kakkar R, Mantha SV, Radhi J, Prasad K, Kalra J. Increased oxidative stress in rat liver and pancreas during progression of streptozotocin-induced diabetes. Clin Sci (Lond) 1998;94:623–32. doi: 10.1042/cs0940623. [DOI] [PubMed] [Google Scholar]
- 40.Smith AF, Beckett GJ, Walker SW, Rae PW. Renal Disease. 6th ed. Oxford: Blackewell Science; 1998. Lecture notes on clinical biochemistry; pp. 53–4. chap 4. [Google Scholar]
- 41.Balasubramanian K, Sivashanmugam P, Thameemdheen S, Govindarajulu P. Effect of diabetes mellitus on epididymal enzymes of adult rats. Indian J Exp Biol. 1991;29:907–9. [PubMed] [Google Scholar]


