Abstract
Objective:
To study the immunomodulatory effect of ethanolic and aqueous extract of the rhizomes of Picrorrhiza kurroa (Scrophulariaceae) in normal and immunosuppressed mice models.
Materials and Methods:
The rhizomes extract of Picrorrhiza kurroa was administered orally according to their body weight in mice. The study was carried out by various hematological and serological tests. The assessment of immunomodulatory activity on specific and non-specific immunity was studied by administration of test extract. The method of cyclophasphamide-induced immunosuppression was employed with slight modification to study the immunomodulatory potential of the extract. Plant extracts were administered by oral feeding canula to the test groups (groups III–VI), group I (control animals) and group II (model control animals) received same volume of normal saline (0.2 ml). Humoral antibody response to SRBC measurement of antibody titer by hemagglutination reaction was done. The mice belonging to the all groups were antigenically challenged with SRBC (0.5×109 cells/ml/100 g) on 10th day intraparitoneally. Cellular immune response (Foot pad reaction test) the edema was induced by injecting SRBC (0.025×109 cells) in left paw, and 0.025 ml of saline was injected in right paw.
Results:
The plant extract showed protective effects on humoral immunity. The change in percentage deduction in footpad volume was also found significant (P<0.001). Administration of extract remarkably ameliorated both cellular and humoral antibody response.
Conclusion:
It is concluded that the test extracts possessed promising immunostimulant properties. But, the alcoholic extract is more potent than aqueous extract in producing delayed type hypersensitivity response.
Keywords: Hematological and serological tests, immunomodulatory activity, Picrorrhiza kurroa, rhizomes
INTRODUCTION
On entering into the 21st century, we have subjected ourselves to such a susceptible environment that it has almost become impossible for our natural immune system to give us the fair share of protection from the antigens. Natural product resources provide excellent raw material for the discovery and development of novel immunomodulatory compounds.[1]
Immunomodulatory medicinal plants are comparatively a recent concept in pharmacognosy. In many of the diseased conditions, as mentioned above, immune response is impaired. Hence, to maintain a disease-free state, modulation of immune response, by either its stimulation or suppression, can be a helpful therapy. Immunomodulators can provide supportive therapy to the chemotherapy. Immunosuppressants have already been employed in transplantation surgery. The non-specific immune stimulation or immunopotentiation can be caused with the use of some medicinal plants. Ayurvedic concepts of preventive health care have shown certain links with non-specific immunostimulation by such medicinal plants. It has now become clear that natural products represent prominent and promising sources for interesting immunomodulating properties.[1]
The innate immune system is the first line of defense against an antigenic insult and includes physical (skin), biochemical (complement, lysozyme, and interferon), and cellular components (neutrophils, monocytes, macrophages). When the innate immune response is inadequate to cope with infection, the adaptive response culminates in the production of antibodies, which are the effectors of humoral immunity and the activation of T lymphocytes, which are the effectors of cell-mediated immunity. Humoral immunity or B-cell immunity develops circulating antibodies, which are globulin molecules that are capable of attacking the invading agent. Cell-mediated immunity is achieved by the formation of large number of activated lymphocytes that are specifically design to destroy foreign agent.[2]
Immunosuppression is a common consequence of long term cyclophosphamide (CP) therapy in cancer patient. Reduction of immunosuppressive effects may thus become beneficial to patients undergoing CP-chemotherapy. In this study, we, therefore, assessed the protective effects of Picrorrhiza kurroa against cyclophosphamide-induced immunosuppression.
Picrorrhiza kurroa is an important alpine herb of Himalayan region, growing in an altitudinal range of 3000- 5000 m in temperate belts. In Indian system of medicine, it is known as “Kutki” and constitutes an important drug out of 2000 drug items derived from vegetable sources.[3]
The rhizomes are bitter, acrid, cooling, laxative carminative digestive stomachic, anthelmintic, anti-inflammatory, depurative, cardiotonic, galactopurifier, and expectorant, anti-pyretic and anti-periodic and purgative in large doses. They are also useful in gastropathy, flatulence, colic, hypotension, cough, asthma, bronchitis, hiccough, fever, diabetes, jaundice, hemorrhoids, and impurity of breast milk and general debility.[2]
MATERIALS AND METHODS
Plant material
The rhizomes of Picrorrhiza kurroa (Scrophulariaceae) were collected locally and it was identified by a botanist, Dr. M. P. Sharma,Professor, Department of Botany, Faculty of Science, Hamdard University, New Delhi-62. The collected rhizomes were dried under shade and pulverized in a mechanical grinder and stored in closed container for further use.
Preparation of extract
The powdered rhizomes (1 kg) was packed and defatted with petroleum ether (60-70°C about 10-15 cycles) in a soxhlet extractor. The defatted material was subjected to ethanol and water in soxhlet extractor; the extract was filtered through whatman filter paper. The extract, which was thus obtained,was vacuum evaporated in order to make it into powder form for it to be redissolved in double-distilled water and ethanol. Alcohol is evaporated, and residue is suspended in water using Tween-80 as an emulsifying agent. Plant extracts (200 mg/kg body wt.) were administered by oral feeding canula to the test groups (groups III-VI). Group I (control animals) and group II (model control animals) received same volume of normal saline (0.2 ml).
Test animals
Healthy mice (25-30 g) of either sex were selected for the study. The animals were fed on commercial diet (Hindustan lever pellets, Bangalore) and water ad libitum. They were acclimated to laboratory hygienic condition for 10 days before starting the experiment. Each animal was used once. The experiments were performed between 10.00 and 16.00 hrs. The experimental protocol and animal house has been approved by the institutional ethical committee of Jamia Hamdard, and the study was conducted according to the Indian National Science Academy Guidelines for the use and care of experimental animals.
Anti-genic materials
For the present study, the anti-genic material used was sheep RBCs (SRBC). The blood was withdrawn from external jugular vein of sheep (Slaughter House, Pahar Ganj, New Delhi). It was mixed with Alsever's solution in 1:1 proportion and was stored at 4°C in refrigerator.

Composition of Alsevers solution
During the experimentation, adequate amount of blood was taken from the above stock solution (i.e. SRBCs, stored in Alsever's solution) and was allowed to stand at room temperature. It was washed 3 times with normal saline (0.9% w/v NaCl). The settled RBCs were then suspended in normal saline. The RBC count of this suspension was determined by hemocytometer using Neubauer Chamber. The known amount of RBCs (0.5 × 109 cells/ml/100 g) was injected intraperitoneally to the mice as an anti-genic challenge.
Immunosuppressant
In the present study,cyclophosphamide (CP) was used as immunosuppressing agent. It is available as fine white odorless crystalline powder with slightly bitter taste m.p. 45°C. It is soluble in water. It is sensitive to oxidation, moister, and light. CP is well-known alkylating agent and has high therapeutic index.[4] It is used in chronic lymphatic leukemia and monoblastic leukemia in children. CP has also a significant immunosuppressant activity. It is used in the treatment of autoimmune diseases and for renal and bone marrow transplantation. The LD 50 of CP in mice ranges from 370 mg/kg s.c. to 360 mg/kg i.p. CP is rapidly metabolized and excreted within 20-30 minutes, following intraperitoneal injection up to 75% of the radioactivity is excreted within 5-8 hours. In mice, rats, and dogs, the predominant hematological effect of CP is leukemia. Some depression of thrombocytes is also noted. The treatment of rodents with CP for a long time has produced pathological structural changes in a variety of lung, gut, gut pancrease, and liver.
Dosing schedule
Animals were divided into 6 groups. Each group comprised of a minimum of 6 animals. Group I (control) received normal saline for 14 days, group II (CP) animals also received normal saline for 14 days but were injected with a single dose of CP on the 12th day of initiation of experiment, group III (plant alcohol extract) and group IV (plant water extract) animals were given alcoholic and water extracts, respectively, of plant drug for 14 days. Group V (plant alcohol extract+CP) and group VI (plant water extract+CP) animals were administered with plant alcoholic and water extracts, respectively, for 14 days with a single injection of CP on 12th day.
Immunization schedule
The mice belonging to the above groups were antigenically challenged with SRBC (0.5 × 109 cells/ml/100 g) on 10th day intraparitoneally.
Humoral antibody response to SRBC[5,6]
Measurement of antibody titer by hemagglutination reaction was performed by using method of Bin-Hafeez with some modification. The mice were lightly anesthetized with anesthetic ether. A fine capillary was gently inserted into the lower angle of eye at 45 o, and the blood was obtained from retro-orbital plexus. The blood was collected into vial and centrifuged for separating serum.[5] The serum of mice was used for determination of hemagglutination titer. Micro titration plate having 98 cups was used for carrying out titration. Each cup was filled with 25±l of normal saline. 25±l of serum obtained from mice blood was added to 1st cup and was mixed with 25±l of normal saline present in microtitration plate. In this way, serial two fold dilutions of serum were prepared. The dilutions were made as follows:
25±l of serum+25±l of saline →50±l of 2-fold dilution (Grade I)
25±l of 2-fold dilution+25±l of saline→50±l of 4-fold dilution (Grade II)
25±l of 4-fold dilution+25±l of saline→50±l of 8-fold dilution (Grade III)
→continued to get higher dilution
The lowest dilution, i.e. 1:2, is graded as 1. In this way, each cup was graded according to serial dilution of serum.
To each cup, 25±l of 1% v/v SRBC was added. The plate was incubated at 37°C for one hr. and then was observed for agglutination. Positive hamagglutination reaction was visualized as a mat formation at the bottom, whereas button formation indicated negative hemagglutination reaction. The antibody titer was expressed in terms of maximum dilution, which gave positive hemagglutination reaction. The minimum dilution (1:2) was ranked as one, and the antibody titer was expressed in terms of rank of different groups, and these ranks were compared for statistical significance.
Cellular immune response (foot pad reaction test)[7,8]
Cell-mediated immune response was assessed by footpad reaction test. On 14th day, after measuring volume of footpad of both legs, SRBC (0.025×109 cells) were injected in left paw and 0.025 ml of saline was injected in right paw. On 15th day, after 24 hours, the paw volume was measured again to check the increase or decrease in volume. The increase in paw volume was considered as an index of cell-mediated immunity (delayed type hypersensitivity).
Total leukocytes count (TLC)[7,8]
The blood withdrawn from the above antigenically challenged mice on 15th day were used to estimate total leukocytes count. The TLC count of the blood was determined by routine hematological method using Neubauer Chamber with hemocytometer.
Body weight and relative organ weight[5]
Animals of all groups were sacrificed on 5thday of immunization. Body weight gain (percentage) and relative organ weight (organ weight/100 g of body weight) of kidney, liver, and spleen were determined for each animal.
Statistical analysis
Data were statistically analyzed using student's t-test to determine significant differences in data of various groups; P values less than 0.5 were considered significant. The values are expressed as means±SE.
RESULTS
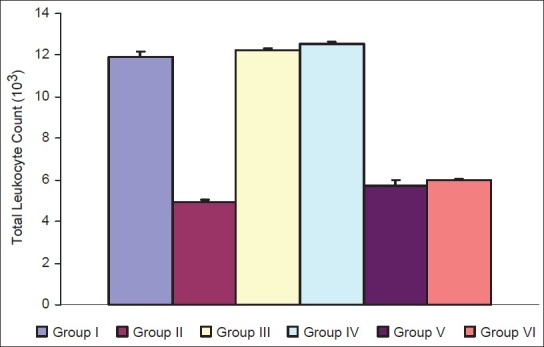
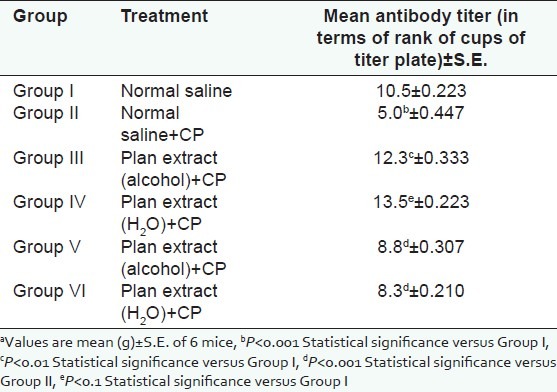
The effect on humoral immunity has been shown in Table 1, [Figures 1 and 2]. As is evident from graphs, antibody titer of the test group of immunosuppressed animals (group IV) was increased as compared to immunosuppressed control group (group II). The increase was found highly significant. Thus, the plant extract showed protective effects on humoral immunity.
Table 1.
Effect of P. kurroa on total leukocyte count
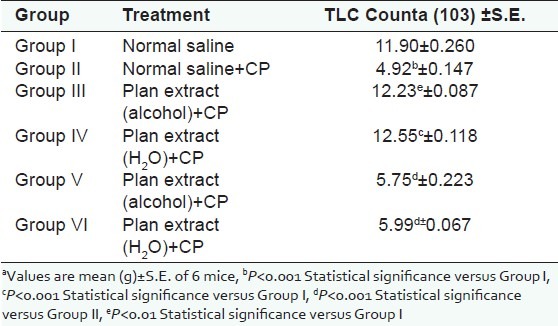
Figure 1.
Effect of P. kurroa on total leukocyte count
Figure 2.
Effect of P. kurroa on humoral immunity
For cell-mediated response, footpad volume was taken by digital plethysmometer, which showed the further deduction in footpad volume [Table 2] in immunosuppressed test animal receiving the regular dose of plant extract when compared with immunosuppressed animals receiving normal saline only. The change in percentage deduction in footpad volume was also found significant (P<0.001).
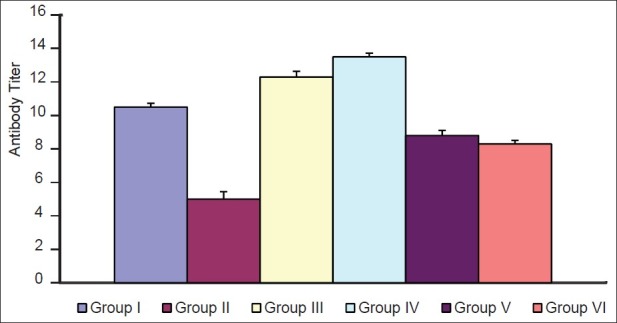
Table 2.
Effect of P. kurroa on humoral immunity
Although CP injection caused a prominent reduction (P<0.001) in relative organ weight (kidney, liver, and spleen) of the mice [Table 3 and Figure 3], recovery was also observed significant (P<0.001) in each organ.
Table 3.
Effect of P. kurroa on delayed type hypersensitivity response
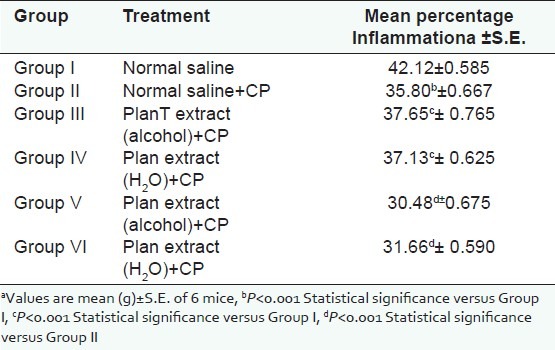
Figure 3.
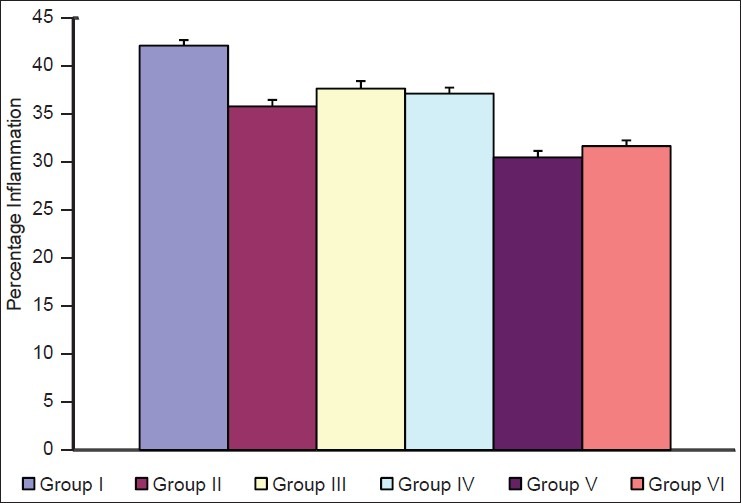
Effect of P.kurroa on delayed type hypersensitivity
Considering the structural data, we could state that Picrorhiza kurroa possess the immunomodulatory principles. The results obtained shows that Picrorhiza kurroa given to the mice increases the number of antibodies in their serum while produces an inhibitory effect on cell-mediated immune response. As the results were significant, previous reports on its immunomodulation were confirmed, and the drug can be suggested for its therapeutic usefulness in various infections and inflammatory diseases.
In case of chemotherapy of cancer patients, it can be a beneficial tool to reduce the humoral immunosuppressive effects. Humoral antibodies that are capable of killing free tumor cells in blood and in serosal cavities have been suggested to play a very important role in cancer. The result obtained shows that Picrorhiza kurroa given to the immunosuppressed mice increases the number of antibodies in their serum.
At times, prominent inflammatory reaction is present in and around the tumor that could possibly be due to cell-mediated immunological response by the host in an attempt to destroy the tumor. The result shows that Picrorhiza kurroa given to the immunosuppressed mice produces an inhibitory effect on cell-mediated immune response decreasing inflammation.
During chemotherapy, cylophosphamide also causes hepatic damage as well as urotoxicity, which is a problem associated with internal organs due to its cytotoxic nature. Significant improvements were found in relative organ weights of kidney, liver, and spleen;therefore, Picrorhiza kurroa could be suggested for the drug-induced immunopathy in the organs [Tables 1–4].
Table 4.
Effect of P. kurroa on Relative organ weight
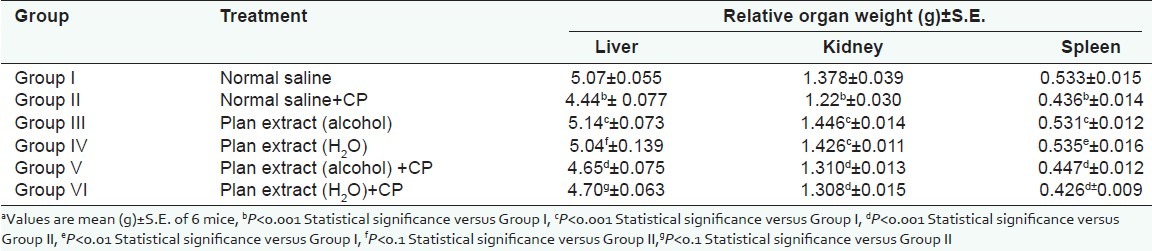
DISCUSSION
The immune system is a complex system, involving an interwoven network of biochemical mechanisms. The modulation of immune response by various agents in order to alleviate the disease has been of interest since many years and the concept of Indian Rasayana in Ayurveda has similarity with it.[9]
Immunomodulators are being used as an adjuvant in conditions of immunodeficiency in cancer and to a limited extent in acquired immunodeficiency syndrome. Many of the presently available immunomodulators such as levamisole, glucans, teleronesz, L-fucose as well as Corynebacterium parvum bacterium, are not free from side effects, which include fever, neutropenia, leucopenia and, at times, allergic reactions. Hence, screening for new immunomodulators is an urgent need.[10] Immunomodulatory agents of plant origin enhance the immune responsiveness of the organism against a pathogen by activating the immune system. However, these agents should be subjected to systemic studies to substantiate the therapeutic claims made with regard to their clinical utility.[11] Picrorhiza kurroah as ability to modulate humoral immune responses by acting at various levels in immune mechanism such as antibody production, release of mediators of hypersensitivity reactions, and tissue responses to these mediators in the target organs. Ethanolic extract and aqueous extracts of Picrorhiza kurroa rhizomes have ability to stimulate humoral response. The present study was carried out to compare the immunomodulatory activity between alcoholic and aqueous extracts of Picrorhiza kurroa. Antibody molecules, a product of B-lymphocytes and plasma cells, are central to humoral immune responses. IgG and IgM are the major immunoglobulins, which are involved in the complement activation, opsonization, neutralization of toxins etc. Alcoholic and aqueous extracts of Picrorhiza kurroashowed significant increase in hemagglutination titer. In case of aqueous extract, the increase in hemagglutination titer is lower compared to alcoholic extract, showing that alcoholic extract (200 mg/kg) may be superior compared to aqueous extract.
In our study, foot volume was enhanced after Picrorhiza kurroa treatment, suggesting cell-mediated immune enhancement. It was found that alcoholic and aqueous extracts of Picrorhiza kurroa potentates the DTH reaction induced by SRBC. Increase in DTH reaction in mice in response to thymus-dependent antigen by the stimulatory effect of alcoholic and aqueous extracts might indicate the involvement of T lymphocytes and accessory cell types, which are required for the DTH reaction. The present investigation suggests that alcoholic and aqueous extracts of Picrorhiza kurroa stimulate the cell-mediated immunity significantly and also stimulated the humoral immunity. Further studies are to be conducted to evaluate the effect on humoral and non-specific immunity on chronic treatment.
CONCLUSION
From the experimental study, it can be concluded that the aqueous extract of Picrorhiza kurroa showed an increase in delayed type hypersensitivity response to SRBC's, but the alcoholic extract is more potent than aqueous extract in producing delayed type hypersensitivity response. Both extracts have marginal stimulatory effect on humoral immunity.[12]
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Kokate CK, Prohit AP, Gokhale JB. Pharmacognosy. 3rd edition. Pune: Nirali Publishers; 1998. p. 14. [Google Scholar]
- 2.Rang HP, Dale MM, Ritter JM, Moore PK. Pharmacology. 5th edition. London: Churchill Livingstone; 2003. p. 230. [Google Scholar]
- 3.Chopra RN, Nayar SL, Chopra IC. Glossary of Indian Medicinal Plants (CSIR) New Delhi: 2003. pp. 11–255. 202. [Google Scholar]
- 4.Alan MK, Terry BS, Jeffrey AB. Immunomodulators: Immunosuppressive agents, tolerogens and immunostimulants, Goodman and Gilman's the pharmacological basis of therapeutics. 10th ed. United States: Mc Graw Hill Companies; 2001. p. 432. [Google Scholar]
- 5.Bin-Hafeez B, Ahmad I, Haque R, Raisuddin S. Protective effect of Cassia occidentalis L. on cyclophosphamide-induced suppression of humoral immunity in mice. J Ethnopharmacol. 2001;75:13–8. doi: 10.1016/s0378-8741(00)00382-2. [DOI] [PubMed] [Google Scholar]
- 6.Makare N, Bodhankar S, Rangari V. Immunomodulatory activity of alcoholic extract of Mangifera indica L. in mice. J Ethnopharmacol. 2001;78:133–7. doi: 10.1016/s0378-8741(01)00326-9. [DOI] [PubMed] [Google Scholar]
- 7.Joharapurkar AA, Zambad SP, Wanjari MM, Umathe SN. In vivo evaluation of antioxidant activity of alcoholic extract of Rubia cordifolia linn and its influence on ethanol-induced immunosuppression. Indian Journal of Pharmacology. 2003;35:232–6. [Google Scholar]
- 8.Puri A, Saxena R, Saxena RP, Saxena KC, Srivastava V, Tandon JS. Immunostimulant agents from Andrographis paniculata. J Nat Prod. 1993;56:995–9. doi: 10.1021/np50097a002. [DOI] [PubMed] [Google Scholar]
- 9.Tortora G. Principles of anatomy and physiology. 10th ed. New Jersy: John Willey and sons; 2003. p. 286. [Google Scholar]
- 10.Mathew S, Kuttan G. Immunomodulatory and antitumour activities of tinospora cardifolia. Fitoterpia. 1999;70:35–43. [Google Scholar]
- 11.Jeena KJ, Joy KL, Kuttan R. Effect of Emblica officinalis, Phyllanthus amarus and Picrorrhiza kurroa on N-nitrosodiethylamine induced hepatocarcinogenesis. Cancer Lett. 1999;136:11–6. doi: 10.1016/s0304-3835(98)00294-8. [DOI] [PubMed] [Google Scholar]
- 12.Joy KL, Kuttan R. Anti-diabetic activity of Picrorrhiza kurroa extract. J Ethnopharmacol. 1999;67:143–8. doi: 10.1016/s0378-8741(98)00243-8. [DOI] [PubMed] [Google Scholar]


