Abstract
Background:
Pinus roxburghii Sarg. Is one of 3 species of pine found in Nepal, the oil of which is traditionally used to treat cuts, wounds, boils, and blisters.
Objective:
To obtain, analyze, and examine the anti-microbial and cytotoxic activities of the essential oils of P. roxburghii.
Materials and Methods:
Three plant parts (cone, needle, and bark) of Pinus roxburghii were collected in Biratnagar, Nepal. The essential oils were obtained by hydrodistillation, and the chemical compositions were determined by GC-MS. The needle and cone essential oils were screened for anti-microbial activity against Bacillus cereus, Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli, and Aspergillus niger; brine shrimp (Artemia salina) lethality; and in-vitro cytotoxicity against MCF-7 cells.
Results:
GC-MS analysis for the cone oil revealed 81 compounds with 78 components being identified (95.5% of the oil) while 98.3% of needle oil was identified to contain 68 components and 98.6% of the bark oil (38 components) was identified. The 3 essential oils were dominated by sesquiterpenes, particularly (E)-caryophyllene (26.8%-34.5%) and α-humulene (5.0%-7.3%) as well as monoterpene alcohols terpinen-4-ol (4.1%-30.1%) and α-terpineol(2.8%-5.0%). The monoterpene δ-3-carene was present only in needle and cone essential oils (2.3% and 6.8%, respectively). Bio-activity assays of the cone essential oil of P. roxburghii showed remarkable cytotoxic activity (100% killing of MCF-7 cells at 100 μg/mL) along with notable brine shrimp lethality (LC50 =11.8 μg/mL). The cone essential oil did not show anti-bacterial activity, but it did exhibit anti-fungal activity against Aspergillus niger (MIC=39 μg/mL).
Conclusion:
The bioactivity of P. roxburghii essential oil is consistent with its traditional medicinal use.
Keywords: Anti-fungal, brine shrimp lethality, cytotoxicity, essential oil composition, α-humulene
INTRODUCTION
Pinus roxburghii Sarg. is a species of several evergreen trees belonging to the Pinaceae and is native to the Himalayas and distributed throughout Pakistan, India, Nepal, and Bhutan.[1] With around 105 species found worldwide,[2] only 3 Pinus species (P. roxburghii, P. wallichiana, and P. wallichiana var. manangensis) are found in Nepal.[3] P. roxburghii is a large tree attaining up to 28-55 m in height with a trunk diameter reaching up to 2 m. The cones of P. roxburghii are ovoid conic and usually open up to 20 cm to release the seeds.[4] P. roxburghii oil has been traditionally used to treat cuts, wounds, boils, and blisters.[5] In addition, phytochemical screening of Pinus needles and stems have found abundant amounts of vitamin C, tannins, and alkaloids while the stem has been primarily used as a source of turpentine oil.[6,7]
Microbiological activity research into the essential oil of P. roxburghii has shown significant anti-fungal activity[8] while alcoholic extract of the needle, stem, and cones are reported to exhibit strong anti-bacterial activity.[9] Investigation into the cytotoxicity activity of twig essential oil has been also reported to show activity against human cancer cells and Ehlirch ascites carcinoma cells.[10] Furthermore, mosquito repellent and larvicidal activities[11] as well as allelopathic activities have been reported from India.[12] Essential oil compositions of needle[13] and stem[8] from Pakistan as well as twig,[10] needle, and cone[14] from Egypt have been previously reported. To our knowledge, this is the first report of the essential oil compositions of P. roxburghii from Nepal.
MATERIALS AND METHODS
Plant material
The plant materials of Pinus roxburghii were collected from city of Biratnagar (26°28′ N, 87°16′ E, 72 m above sea level), in Morang district in Koshi Zone, in Nepal on 13 May 2011. The plant was identified by Tilak Gautam (Lecturer of Botany, MMAMC Campus, Tribhuvan University, Biratnagar, Nepal), and a voucher specimen (HN669) has been deposited in the herbarium of the Tribhuvan University, Post-Graduate Campus, Botany Department, Biratnagar. The fresh needle sample (100 g), was crushed and hydrodistilled using a Clevenger type apparatus for 4 h to give clear pale yellow essential oil (0.053 g), which was stored at 4°C until analysis. Hydrodistillation of the fresh bark (100 g) gave 0.001 g clear pale yellow oil, while the fresh cone (100 g) gave 0.012 g pale yellow clear oil.
Gas chromatographic - mass spectral analysis
The essential oils of P. roxburghii were analyzed by GC-MS using an Agilent 6890 GC with Agilent 5973 mass selective detector [MSD, operated in the EI mode (electron energy=70 eV), scan range=45-400 amu, and scan rate=3.99 scans/sec], and an Agilent ChemStation data system. The GC column was an HP-5ms fused silica capillary with a (5% phenyl)-polymethylsiloxane stationary phase, film thickness of 0.25 μm, a length of 30 m, and an internal diameter of 0.25 mm. The carrier gas was helium with a column head pressure of 48.7 kPa and a flow rate of 1.0 mL/min. Injector temperature was 200°C, and detector temperature was 280°C. The GC oven temperature program was used as follows: 40°C initial temperature, hold for 10 min; increased at 3°C min to 200°C; increased 2°/min to 220°C. A 1% w/v solution of each sample in CH 2 Cl 2 was prepared, and 1 μL was injected using a splitless injection technique.
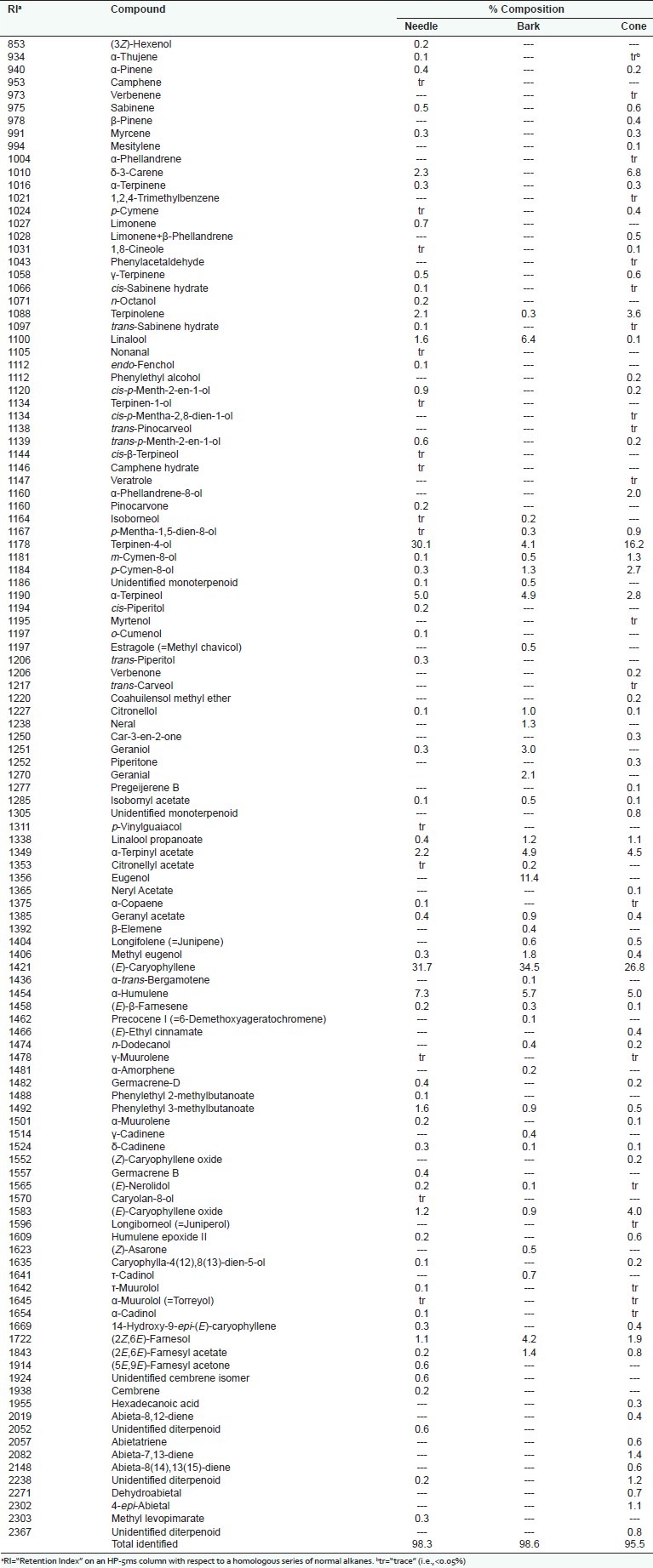
Identification of the oil components was based on their retention indices determined by reference to a homologous series of n-alkanes and by comparison of their mass spectral fragmentation patterns with those reported in the literature[15] and stored on the MS library [NIST database (G1036A, revision D.01.00)/ChemStation data system (G1701CA, version C.00.01.080)]. The percentages of each component are reported as raw percentages based on total ion current without standardization. The essential oil compositions of P. roxburghii from Nepal are summarized in Table 1.
Table 1.
Chemical compositions of Pinus roxburghii essential oils from Nepal
Anti-microbial screening
The essential oil was screened for anti-microbial activity against Gram-positive bacteria, Bacillus cereus (ATCC No. 14579), and Staphylococcus aureus (ATCC No. 29213); Gram-negative bacteria, Pseudomonas aeruginosa(ATCC No. 27853) and Escherichia coli (ATCC No. 10798). Minimum inhibitory concentrations (MICs) were determined using the microbroth dilution technique as described previously.[16] Dilutions of the crude extracts were prepared in cation-adjusted Mueller Hinton broth (CAMHB) beginning with 50 μL of 1% w/w solutions of crude extracts in DMSO plus 50 μL CAMHB. The extract solutions were serially diluted (1:1) in CAMHB in 96-well plates. Organisms at a concentration of approximately 1.5×108 colony-forming units (CFU)/mL were added to each well. Plates were incubated at 37°C for 24 hours; the final minimum inhibitory concentration (MIC) was determined as the lowest concentration without turbidity. Gentamicin was used as a positive antibiotic control; DMSO was used as a negative control. Anti-fungal activity against Aspergillus niger (ATCC No. 16888) was determined as above using YM broth inoculated with A. niger hyphal culture diluted to a McFarland turbidity of 1.0. Amphotericin B was the positive control.
Brine shrimp lethality assay
The brine shrimp (Artemia salina) lethality test was carried out using a modification of the procedure by McLaughlin as previously described.[16] Artemia salina eggs were hatched in a sea salt solution (Instant Ocean®, 38 g/L) with an incandescent light bulb as the heat source. After 48 hours, the newly hatched nauplii were counted using a micropipette and transferred to 20-mL vials. Nine vials each containing 10 A. salina nauplii in 10 mL of sea salt solution (same as the hatching solution) were prepared. Three vials were labeled as controls with first one containing no DMSO, another with 10 μL, and the last one with 100 μL DMSO. Three replicate vials contained 10 μL of 1% essential oil solution in DMSO, and the other 3 were prepared by adding 100 μL of 1% essential oil solution in DMSO. Surviving A. salina were counted after 24 hours.
Cytotoxicity screening
Human MCF-7 breast adenocarcinoma cells (ATCC No. HTB-22)[17] were grown in a 3% CO2 environment at 37°C in RPMI-1640 medium, supplemented with 10% fetal bovine serum, 100,000 units penicillin and 10.0 mg streptomycin per liter of medium, 15 mM of Hepes, and buffered with 26.7 mM NaHCO3, pH 7.35. Cells were plated into 96-well cell culture plates at 2.5 × 104 cells per well. The volume in each well was 100 μL. After 48 h, supernatant fluid was removed by suction and replaced with 100 μL growth medium containing 1.0 μL of DMSO solution of the essential oil (1% w/w in DMSO). This gave a final concentration of 100 μg/mL in each well. Solutions were added to wells in four replicates. Medium controls and DMSO controls (10 μL DMSO/mL) were used. Tingenone[18] was used as a positive control. After the addition of compounds, plates were incubated for 48 h at 37°C in 5% CO2; medium was then removed by suction and 100 μL of fresh medium was added to each well. In order to establish percent kill rates, the MTT assay for cell viability was carried out.[19] After colorimetric readings were recorded (using a Molecular Devices Spectra MAX Plus microplate reader, 570 nm), average absorbances, standard deviations, and percent kill ratios (%killcmpd/%killDMSO) were calculated.
RESULTS AND DISCUSSION
Needle, bark, and cone essential oils of P. roxburghii were obtained in 0.053%, 0.001%, and 0.012%, respectively. Chemical compositions of P. roxburghii cone, needle, and bark essential oils revealed a total of 117 components, of which 111 were identified. A total of 95.5% of the cone essential oil of P. roxburghii was identified with major components being (E)-caryophyllene (26.8%), terpinen-4-ol (16.2%), with smaller amounts of δ-3-carene (6.8%) and α-humulene (5.0%). Examination of the needle essential oil yielded 98.3% identified components, mostly comprised of (E)-caryophyllene (31.7%), terpinen-4-ol (30.1%), α-humulene (7.3%), and α-terpineol (5.0%). A total of 38 components were identified in the bark essential oil accounting for 98.6% of the composition. The major components were (E)-caryophyllene (34.5%) and eugenol (11.4%), along with linalool (6.4%), α-humulene (5.7%), α-terpineol (4.9%), and terpinen-4-ol (4.1%).
The P. roxburghii essential oil compositions in this present study are very different in comparison with previous reports from Egypt[10,14] and Pakistan.[8,13] The needle and cone essential oils from Egypt indicated a remarkably different chemotype led by δ-3-carene, comprising 26.3% and 45.87% of the needle and cone, respectively, as oppose to the Nepalese sample, which contained only 2.3% and 6.8% of needle and cone oil, respectively. In addition, the presence of α-pinene (29.3% in the needle[13] and 41.9% in the stem[8] ) in essential oils from Pakistan is in stark contrast to the trace amount found in the P. roxburghii samples from Nepal. P. roxburghii needle and stem oils from Pakistan were also rich in δ-3-carene (14.2% and 16.3%, respectively) and (E)-caryophyllene (21.9% and 12.3%, respectively).
Both, the needle and cone essential oils of P. roxburghii, were screened for anti-microbial activity. Neither of the oils exhibited anti-bacterial activity (MIC ≥625 μg/mL against B. cereus, S. aureus, P. aeruginosa, and E. coli). The major components (E)-caryophyllene and α-humulene had previously shown moderate anti-bacterial activity against B. cereus and S. aureus, while terpinen-4-ol was inactive.[20] Both the needle and cone oils, on the other hand, were notably anti-fungal against A. niger (MIC=156 and 39 μg/mL, respectively), and α-humulene was previously shown to be anti-fungal against A. niger.[20] Both the needle and bark oils also showed in-vitro cytotoxic activity against MCF-7 cells at 100 μg/mL concentrations (70.9 ± 1.4% and 100% kill, respectively). The cytotoxicities of these essential oils are likely due to the high concentrations of terpinen-4-ol, (E)-caryophyllene, and α-humulene, which have been shown to be cytotoxic on MCF-7 cells.[21] P. roxburghii cone oil was toxic to Artemia salina with LC50=11.8 μg/mL. The biological activities of P. roxburghii oil in this study are consistent with previous literature reports[8,13] and are consistent with the traditional medicinal uses of this plant.
ACKNOWLEDGMENTS
PS and PP are grateful to Tribhuvan University for helping with the plant collection and access to laboratory equipment. WNS is grateful to an anonymous private donor for the gift of the GC-MS instrumentation. We all are thankful to Tilak Gautam for identification of plant sample. We thank Dr. Bernhard Vogler for technical assistance with GC-MS measurements and Dr. Debra Moriarity for technical assistance with cell culture and cytotoxicity assays.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Conifer Specialist Group 1998. Pinus roxburghii. In: IUCN 2011. IUCN red list of threatened species. Version. 2011. [cited 2012 Feb 13]. Available from: http:\\www.iucn redlist.org .
- 2.Farjon A. Brill. 2nd ed. Leiden: 2005. Pines. [Google Scholar]
- 3.Press JR, Shrestha KK, Sutton DA. Annotated Checklist of the Flowering Plants of Nepal. The Natural History Museum. 2000 [Google Scholar]
- 4.Wu Z, Raven PH. Flora of China. Vol. 4. Beijing Science Press; 1999. [Google Scholar]
- 5.Gewali MB. Institute of Natural Medicine. Japan: University of Toyama; 2008. Aspects of Traditional Medicine in Nepal; pp. 19–20. [Google Scholar]
- 6.Vallejo MCN, Evandro A, Sergio ALM. Volatile wood oils of the Brazilian Pinus caribaea var. hondurensis and Spanish Pinus pinaster var. mediterranea. J Braz Chem Soc. 1994;5:107–112. [Google Scholar]
- 7.Asta J, Jurgita S, Aida S, Eugenija K. Characteristics of essential oil composition in the needles of young scots pine (Pinus sylvestris L.) stands growing along and ariel ammonia gradient. Chemija. 2006;17:67–73. [Google Scholar]
- 8.Hassan A, Amjid I. Gas chromatography-mass spectrometric studies of essential oil of Pinus roxburghaii stems and their antibacterial and antifungal activities. J Med Plant Res. 2009;3:670–3. [Google Scholar]
- 9.Parihar P, Parihar L, Bohara A. Antibacterial activity of extracts of Pinus roxburghii Sarg. Bangladesh J Bot. 2006;35:85–6. [Google Scholar]
- 10.Islam WT. Composition and bioactivities of the essential oils of twigs of four Pinus species cultivated in Egypt. Egypt J Biomed Sci. 2004;15:452–64. [Google Scholar]
- 11.Ansari MA, Mittal PK, Razdan RK, Sreehari U. Larvicidal and mosquito repellent activities of pine (Pinus longifolia, family: Pinaceae) oil. J Vector Borne Dis. 2005;42:95–9. [PubMed] [Google Scholar]
- 12.Melkania NP, Singh JS, Bisht KK. Allelopathic potential of Artemisia vulgaris and Pinus roxburghii Sarg: A bioassay study. Proc Indian Natl Sci Acad B. 1982;48:685–8. [Google Scholar]
- 13.Zafar I, Fatima A, Khan SJ, Rehman Z, Mehmud S. GC-MS studies of needles essential oil of Pinus roxburghii and their antimicrobial activity from Pakistan. Elec J Environ Agric Food Chem. 2010;9:468–73. [Google Scholar]
- 14.Islam WT. Volatile oils from needles and cones of Egyptian chir pine (Pinus roxburghii Sarg) Bull Fac Pharm Cairo Univ. 2006;44:77–83. [Google Scholar]
- 15.Adams RP. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry. 4th ed. Carol Stream, Illinois: Allured Publishing Corporation; 2007. [Google Scholar]
- 16.Satyal P, Paudel P, Lamichhane B, Setzer WN. Volatile constituents and biological activities of the leaf essential of Jasminum mesnyi growing in Nepal. J Chem Pharm Res. 2012;4:437–9. [Google Scholar]
- 17.Soule HD, Vazguez J, Long A, Albert S, Brennan M. A human cell line from a pleural effusion derived from a breast carcinoma. J Natl Cancer Inst. 1973;51:1409–16. doi: 10.1093/jnci/51.5.1409. [DOI] [PubMed] [Google Scholar]
- 18.Setzer WN, Setzer MC, Hopper AL, Moriarity DM, Lehrman GK, Niekamp KL, et al. The cytotoxic activity of a Salacia liana species from Monteverde, Costa Rica, is due to a high concentration of tingenone. Planta Med. 1998;64:583. doi: 10.1055/s-2006-957524. [DOI] [PubMed] [Google Scholar]
- 19.Ferrari M, Fornasiero MC, Isetta AM. MTT colorimetric assay for testing macrophage cytotoxic activity in vitro. J Immunol Methods. 1990;131:165–72. doi: 10.1016/0022-1759(90)90187-z. [DOI] [PubMed] [Google Scholar]
- 20.Schmidt JM, Noletto JA, Vogler B, Setzer WN. Abaco bush medicine: Chemical composition of the essential oils of four aromatic medicinal plants from Abaco Island, Bahamas. J Herbs Spices Med Plants. 2006;12:43–65. [Google Scholar]
- 21.Wright BS, Bansal A, Moriarity DM, Takaku S, Setzer WN. Cytotoxic leaf essential oils from Neotropical Lauraceae: Synergistic effects of essential oil components. Nat Prod Commun. 2007;2:1241–4. [Google Scholar]


