Vaccine-induced molecular correlates of protection against repeated low-dose rectal SIVmac251 challenges of rhesus macaques, in peripheral blood mononuclear cells.
Keywords: nonhuman primate, adenovirus, infection, vaccination, immune correlate, gene expression profiles, STEP trial
Abstract
In this study, we compared the immunogenicity and protection from repeated low-dose intrarectal SIVmac251 challenge in two groups of vaccinated RMs. Animals were immunized with live SIVmac239, which had been attenuated by a deletion of the nef sequence, or they were vaccinated twice with an E1-deleted AdHu5, expressing SIVmac239gag. The vaccinated animals and a cohort of unvaccinated control animals were then challenged 10 times in weekly intervals with low doses of SIVmac251 given rectally. Our results confirm previous studies showing that whereas SIVΔnef provides some degree of protection against viral acquisition after repeated low-dose rectal SIVmac251 challenges, vaccination with an AdHu5gag vaccine designed to induce only antiviral T cell responses is ineffective. As immunological analyses of prechallenge, vaccine-induced T and B cell responses failed to reveal correlates of protection that distinguished the more susceptible from the more resistant vaccinated animals, we carried out RNA-Seq studies of paired pre- and postvaccination samples to identify transcriptional patterns that correlated with the differences in response. We show that gene expression signatures associated with the delayed SIV infection seen in some AdHu5gag recipients were largely present in prevaccination samples of those animals. In contrast, the responding SIVΔnef-immunized animals showed a predominance of vaccine-induced changes, thus enabling us to define inherited and vaccine-induced gene expression signatures and their associated pathways that may play a role in preventing SIV acquisition.
Introduction
Despite a massive effort by the AIDS research community, a safe and effective vaccine against HIV-1 infection remains elusive. Most licensed antiviral vaccines induce protection through virus-neutralizing antibodies. However, the Env of HIV-1 is extraordinarily variable and heavily glycosylated, undergoes structural changes upon receptor binding, and is expressed at low copy numbers on the virion surface [1, 2]. For all of these reasons, it has, so far, been impossible to design Env-based immunogens that can reliably induce broadly neutralizing antibodies. Indeed, neutralizing antibody responses, when present, develop much later than Env-binding, non-neutralizing antibodies upon natural infection and consistently fail to control virus replication in vivo [3].
Many of the recent vaccine efforts have focused on inducing protection through T cells directed against more conserved epitopes of HIV-1 [4–8], with some of these candidate vaccines providing partial protection against viral acquisition or significantly reduced viral loads upon infection in NHP models. However, the large phase IIb STEP clinical trial, which was aimed at testing the concept that protection could be provided by T cells to conserved antigens of HIV-1 induced by an AdHu5, failed to reduce HIV-1 acquisition rates or viral loads, limit CD4+ T cell loss, or decrease the interval between infection and highly active antiretroviral therapy treatment in infected individuals [9]. A subsequent trial, the RV144 trial, tested an attenuated poxvirus vector [a vaccine based on a replication-defective recombinant canary poxvirus (ALVAC)] expressing Gag/Pro and Env combined with a clade C/E gp120 protein called AIDSVAX [10]. The vaccine induced mainly binding antibodies in human individuals at moderate to high risk for infection, with neutralization only observed toward isolates closely related to those used in the vaccine, as well as limited HIV-1-specific T cell responses. Despite this low immunogenicity, the vaccine provided modest but statistically significant protection from HIV-1 acquisition in the modified intend-to-treat group, although individuals that became infected developed viral loads similar to placebo recipients. Whereas the results of the RV144 trial are somewhat encouraging, the immunological and molecular correlates of vaccine-induced protection from HIV transmission remain largely unknown.
To further define correlates of protection against HIV-1, we conducted a study in RMs, comparing two vaccine regimens. The first vaccine was based on an attenuated SIVΔnef, which was shown previously to protect animals against SIVmac239 infection [11]. The second was an E1-deleted AdHu5 vector expressing only a T cell-inducing antigen, i.e., SIVgag, which based on previous animal studies [12] and the results of the STEP trial [9], was expected to fail to protect from transmission. Upon vaccination, SIV-specific B and T cell responses were monitored, and the RMs were then challenged 10 times with low intrarectal doses of SIVmac251, administered in weekly intervals. Of note, SIVmac251 is a neutralization-resistant viral swarm unlike the clonally derived SIVmac239 virus from which the immunogens were derived. As such, it is, in part, different from SIVmac239. As expected, the AdHu5gag vaccine failed to protect RMs from virus transmission, whereas the SIVΔnef vaccination delayed infection in four of six animals and prevented infection in the remaining two. To identify potential molecular correlates of protection from SIV challenge, we analyzed the transcriptome of matched pairs of PBMCs that were collected prior to vaccination and shortly before challenge. Whereas SIVΔnef and AdHu5gag induced changes in the transcriptomes, which could be linked to similar pathways, these changes were more pronounced in the SIVΔnef group. Of note, the pattern of gene expression associated with delayed SIV infection in some AdHu5gag recipients was largely present in prevaccination samples, thus implicating genetic resistance factors rather than vaccine-induced protective mechanisms. In contrast, SIVΔnef-immunized RMs showed a predominance of vaccine-induced changes that enabled us to define genes and pathways that may play a role in preventing SIV acquisition.
MATERIALS AND METHODS
Vaccine vectors
The E1- and E3-deleted Ad vectors expressing Gag were generated, purified, and quality-controlled as described [13]. SIVΔnef virus was most kindly provided by Ronald Desrosiers (New England Primate Research Center, Harvard University, Boston, MA, USA).
NHPs
Two- to 3-year-old healthy and SIV-uninfected Indian origin Macaca mulatta were purchased and housed at Bioqual (Rockville, MD, USA). Animals were typed for Mamu-A*01, A*02, A*08, A*11, B*01, B*03, B*04, B*08, and B*17 alleles (University of Wisconsin, AIDS Vaccine Research Lab, Madison, WI, USA), and results are shown in Supplemental Table 1A. All NHPs were screened prior to enrollment for neutralizing antibodies to AdHu5 virus and were found to be seronegative. All procedures involving handling and sacrifice of animals were performed according to approved protocols and upon approval by the relevant Institutional Animal Care and Use Committees.
Immunization regimen
Animals were divided into three groups (Supplemental Table 1A). One group of six animals was vaccinated twice in a 2-month interval with 1010 virus particles of an AdHu5 vector expressing gag by injecting animals with vector diluted in 1 ml saline into the quadriceps muscles. One female AdHu5-vaccinated RM died during the course of the experiment, and results obtained with this animal are not included. Another six animals were injected i.v. with 1 μg/ml p27 of the SIVΔnef stock. The six control animals were not immunized. They were enrolled just prior to challenges.
Viral challenge
Nine months after the initial vaccination, animals were challenged rectally in weekly intervals with 300 50% tissue culture infectious doses of SIVmac251 obtained from Koen Van Rampay (University of California, Davis, CA, USA). The virus stock had been titrated for repeated rectal challenge, and this dose was found suitable to cause infection of control animals upon ≤10 challenges.
Isolation and preservation of lymphocytes
PBMCs were isolated as described [14]. They were tested immediately after isolation or frozen in 90% FBS and 10% DMSF (Sigma, St. Louis, MO, USA) at −80°C until testing.
Synthetic peptides
Peptide pools of 15-mers (overlapping by 11 aa), spanning the SIVmac239 Gag protein, were reconstituted in DMSO, and pools were prepared from individual peptide stocks obtained from the National Institutes of Health AIDS Research and Reference Reagent Program (Germantown, MD, USA).
ICS
The function of SIV-specific CD8+ T cells was assessed by ICS after stimulation with the SIV Gag peptide pool. All peptides were used at a final concentration of 2 μg each peptide/ml. Frozen cells were thawed and washed immediately with HBSS, supplemented with 2 U/ml DNase I, resuspended with RPMI media, and stimulated for 6 h with anti-CD28 (clone CD28.2), anti-CD49d (clone 9F10), and Brefeldin A [13]. Cells were stained with Violet fluorescent reactive dye-Pacific Blue (Invitrogen, Carlsbad, CA, USA) and anti-CD14-Pacific Blue (clone M5E2), anti-CD16-Pacific Blue (clone 3G8), anti-CD8-APC-H7 (clone SK1), anti-CD4-Alexa700 (clone OKT4), anti-CD95-PE-Cy5 (clone DX2), and anti-CD28-Texas Red (clone CD28.2; Beckman Coulter, Fullerton, CA, USA) for 30 min at 4°C. Additionally, cells were stained with anti-CD62L-PE (clone SK11; fresh cells only) or anti-CCR7-PE (clone 150,503; used for frozen cells). After fixation and permeabilization with Cytofix/Cytoperm (BD Biosciences, San Jose, CA, USA) for 30 min at 4°C, cells were stained with anti-IFN-γ-APC (clone B27), anti-IL-2-FITC (clone MQ1-17H12), and anti-TNF-α-PE-Cy7 (clone mAb11; R&D System, Minneapolis, MN, USA) and anti-CD3-PerCp-Cy5.5 (clone SP34-2) for 30 min at 4°C. Cells were washed twice, fixed with BD Stabilizing Fixative (BD Biosciences), and then analyzed by FACS using LSRII (BD Biosciences) and DiVa software. Flow cytometric acquisition and analysis of samples were performed on at least 400,000 events. Postacquisition analyses were performed with FlowJo (TreeStar, Ashland, OR, USA). Data shown on graphs represent values of Gag peptide-stimulated wells from which background values have been subtracted. Single-color controls used CompBeads anti-mouse immunoglobulin chain (BD Biosciences) for compensation. Unless otherwise noted, antibodies were purchased from BD Biosciences.
Baculovirus-produced SIV gp160 protein
A recombinant baculovirus plasmid (pFastBac flag), carrying gp160 of SIVmac239, was constructed and transposed into DH10 Bac cells, and bacmid DNA was isolated. The bacmid DNA was then transfected into Sf9 cells following the Bac-to-Bac method (Invitrogen). Baculovirus was produced to a P2 amplification following Bac-to-Bac methods. One liter of Sf9 cells was infected with baculovirus at a multiplicity of infection of 1.0 for 48 h at 27°C. After 48 h, cells were lysed with Sf9 cell lysis buffer (100 mM Na2H2 PO40, 10 mM Tris, 500 mM NaCl, 1% Triton, 10% glycerol+protease inhibitors). Lysate was bound to ANTI-FLAG M2 resin, washed, and eluted with 800 μg/ml FLAG peptide. Eluted protein was dialyzed with 1× PBS, 500 mM NaCl, pH 8.0, overnight at 4°C.
ELISA for antibodies to gp160
Briefly, 96-well plates (Nunc-Immuno MicroWell) were coated overnight at 4°C with buffer (0.1 M NaHCO3, pH 9.6) containing SIVmac239 gp160 protein (150 ng/well). Wells were washed three times with PBS/0.05% Tween-20, followed by overnight blocking at 4°C with 200 μl PBS/3% BSA/0.05% Tween-20. Wells were then washed four times with PBS/0.05% Tween 20 and incubated with serial-diluted plasma samples (in duplicates) for 2 h at room temperature. Wells were subsequently washed, and bound IgG was detected with a rabbit anti-monkey IgG alkaline phosphatase conjugate (Sigma). Bound enzyme was detected with phosphatase substrate tablets (Sigma) diluted in substrate buffer (1% MgCl2, 10% NaH3, pH 9.8) and read on a microplate reader at 405 nm.
Avidity ELISA
Avidity of antibodies to gp160 was assessed by NaSCN-displacement ELISA. Briefly, plasma samples were diluted to a level calculated to have a remaining titer of 1:4000 and incubated on ELISA plates coated with SIVmac239 gp160 for 2 h at room temperature. Then, ascending concentrations of the chaotropic agent NaSCN were added to the wells (0–8 M). Plates were incubated for 15 min at room temperature before washing. They were then treated with the secondary antibody and substrate as described above.
Plasma viral load
Plasma SIV viral load was determined by quantitative real-time RT-PCR, as described previously [13]. To distinguish between SIVmac251 and SIVΔnef, RNA was isolated from blood samples of SIVΔnef-immunized animals that were positive for SIV sequences by regular PCR. These samples were tested by a nested PCR for the presence of the nef coding sequence using the QIAamp Viral RNA Mini Kit (Qiagen, Valencia, CA USA). RNA was mixed with buffer AVL containing carrier RNA precipitated with ethanol (96–100%), and purified through mini columns. The sequence was amplified with the following primers: forward primer, TGACCTACCTACAATATGGGTG; reverse primer, TCCCCTTGTGGAAAGTCCCTGCT, using Platinum Taq RT. Samples were amplified through 42 cycles at temperatures of 50°C, 72°C, and 94°C. The nested PCR used the forward primer, CGTGGRGAGACTTATGGGAGACT, and the reverse primer, AAGGCCTCTTGCGGTTAGCCTTC, for 35 cycles at 54°C, 72°C, and 94°C. Amplicons were analyzed by gel electrophoresis. Samples that showed a band at 667 bp were scored as being positive for SIVmac251.
Sample preparation for RNA-Seq
RNA was extracted from PBMCs using Tri-Reagent, as described previously [14]. RNA was quality-assessed by Bioanalyzer analysis, and RNA-Seq was prepared using the Illumina TruSeq library preparation kits, as recommended by the manufacturer.
Statistical analysis
t-Tests were used for pairwise comparisons. Standard descriptive statistics were used to summarize the efficacy of vaccine regimens. Mixed effects models, with nonlinear responses over time, were used to model variables measured repeatedly over the course of the study, such as viral loads. These models are similar to repeated ANOVA models but allow for incomplete data as a result of a missing time-point for a particular animal. Tukey adjustment for multiple comparisons was used when conducting pairwise comparisons among the three groups overall and at each time-point. Associations between variables measured at a single time-points were assessed using Spearman correlation coefficients. Survival analysis of differences between numbers of challenges required for infection for two vaccines and control was performed using a log-rank test with Bonferroni correction for multiple testing. Hazard ratios were used as a measurement of reduction of risk to be infected after each challenge. Analyses were conducted using Excel, GraphPad Prism 5, and SAS 9.2.
RNA-Seq data preprocessing and analysis
RNA sequencing data, obtained from base-calling of raw data produced by the Genome Analyzer II, was preprocessed using the Galaxy analysis package [15]. TopHat v1.2.0 algorithm [16] was used to align the data against the RheMac2 genome. RefSeq information about 28,908 known transcripts was obtained through the Browser and Cufflinks v0.9.3 algorithm (University of California, Santa Cruz, CA, USA) [17] and was used to quantify transcript abundances in FPKM units. Transcripts (19,062) that were found to be expressed significantly (lower FPKM confidence level >0) in at least four samples were used for further analysis. Zero FPKM value was floored to 0.0023, a value equal to the minimal significant FPKM found across all transcripts and samples. The data were submitted to the ArrayExpress database with Accession Number E-MTAB-1130.
To visualize gene expression similarities between samples, we performed PCA using expression of all 19,062 significantly detected transcripts and projected each sample on the identified first and second principal components. Significance of overlap between any two gene lists was estimated using a hypergeometric test. The difference between protected and unprotected animals was tested by t-test and concordance with weeks of protection by Pearson correlation. Estimation of FDR for multiple testings was carried out according to the Storey and Tibshirani procedure [18]. Values for heatmaps were generated by normalizing each transcript's FPKM expression values over the FPKM sum across all samples for the transcript. Fisher exact test was used to compare samples from SIVΔnef and AdHu5 vaccinated RMs for proportions of genes already correlated in prevaccinated samples among genes correlated in postvaccinated samples. All tests were two-tailed and done using Matlab v.7.2 (R2006a), with significance threshold set at P < 0.05.
Pathway and functional analysis was carried out with IPA software (http://www.ingenuity.com/) using Ingenuity Core Analysis (IPA 8.0; Ingenuity Systems, Redwood City, CA, USA). Results were corrected for multiple testing by the Benjamini-Hochberg procedure, using FDR <5% as a significance threshold.
RESULTS
Study design
The study was undertaken to define correlates of protection against repeated low-dose rectal SIVmac251 challenges in RMs vaccinated with two regimens. To this end, two groups of six RMs were immunized with the attenuated SIVΔnef virus (Group 1), which establishes a persistent, low-level infection and induces protective immunity against high-dose challenges with SIVmac, or with two doses of an AdHu5 vector expressing Gag (Group 2), which was given at a 2-month interval, with the first dose given at the time of the SIVΔnef inoculation to Group 1. The study also included a group of six unvaccinated, control animals (Group 3). All RMs were challenged with 10 weekly intrarectal low doses of SIVmac251, starting 9 months after the initial vaccination. One of the animals that received the AdHu5gag vaccine died for non-vaccine-related reasons during the immunization phase and was thus excluded from the analysis. Supplemental Table 1 shows the Mamu-genotype of the RMs. Figure 1 shows the immunization/challenge schedule and the schedule for sample collection. Animals with the protective alleles B*17 and B*08 were distributed equally among the groups; one A*01+ animal was enrolled into the SIVΔnef group.
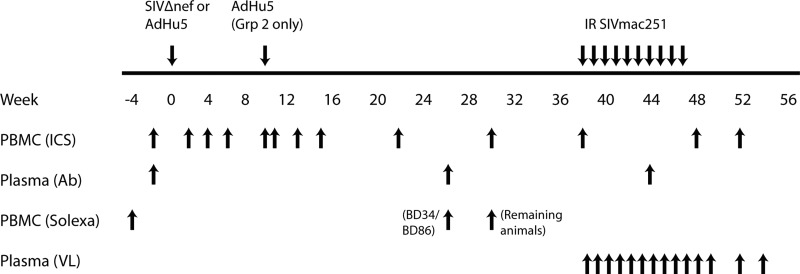
Figure 1. Schedule of vaccinations, bleeds, and challenges.
The graph shows time-points for vaccination and challenges of animals above the line and for collection of samples for the different types of analyses below the line. IR, intrarectal; ICS, testing PBMCs for T cells by ICS; Ab, testing sera for antibodies by ELISAs; Solexa, testing PBMCs for gene expression profiles; VL, testing plasma for viral loads.
Vaccine-induced, SIV-specific T cell responses
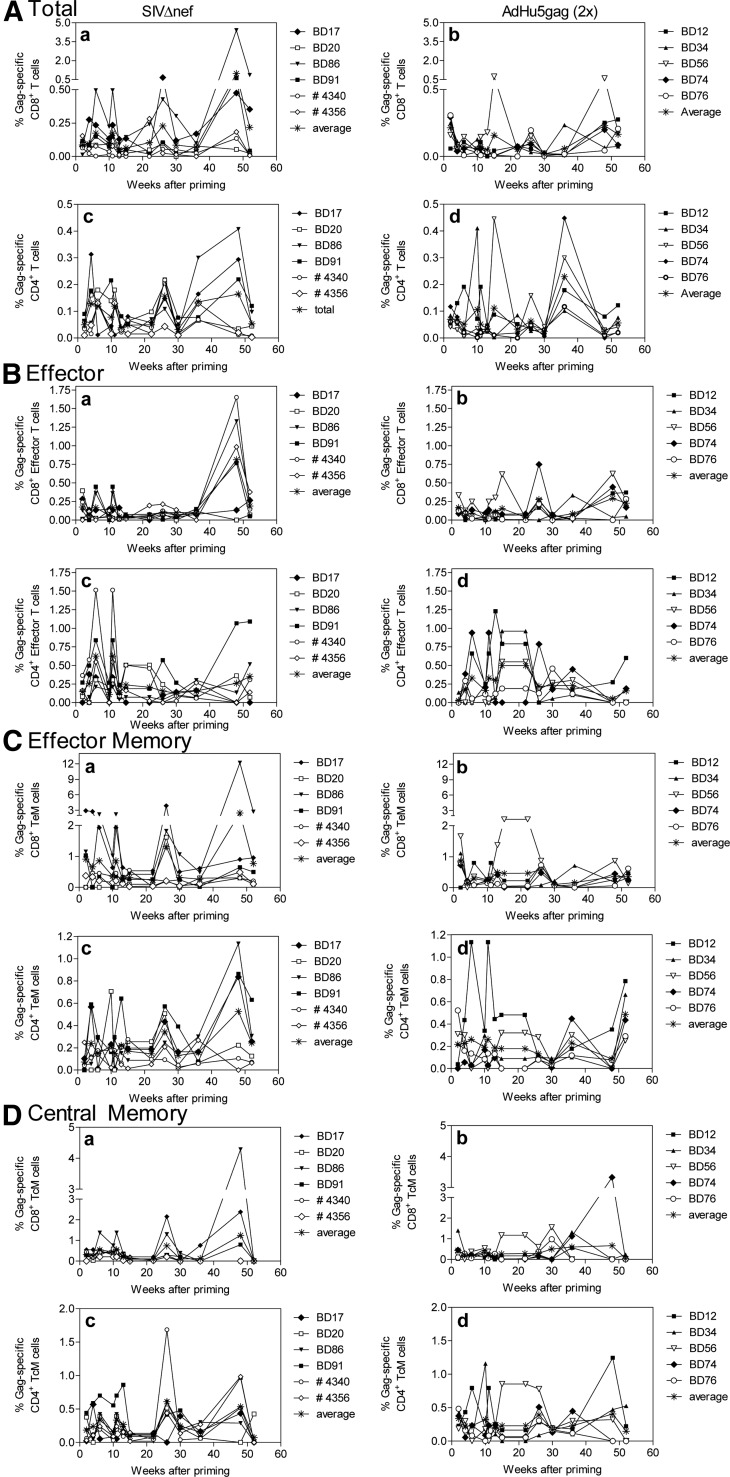
To determine how the two used immunization regimens elicited SIV-specific cellular immune responses, we tested Gag-specific CD8+ and CD4+ (Fig. 2) T cell responses before and after challenges by ICS. Samples were stained for CD95, CD28, and CD62L or CCR7 to determine the differentiation status of T cells following immunization and to characterize the pattern of cytokine secretion by different T cell subpopulations (all T cells in Fig. 2A; Teff in Fig. 2B: CD95+CD28−CD62Llow or CCR7low; TEM in Fig. 2C: CD95+CD28+CD62Llow or CCR7low; and TCM in Fig. 2D: CD95+CD28+CD62Lhigh or CCR7high). The analysis revealed no significant differences in frequencies of CD8+ T cells or individual CD8+ T cell subsets among the groups at any of the time-points tested (Fig. 2A–D, a and b). Frequencies of total CD4+ T cells and CD4+ Teff (Fig. 2A and B, d) were higher in the AdHu5 group early after vaccinations (weeks 2 and 4, respectively, with P values <0.05 by t-tests). The same group showed higher frequencies of CD4+ TCM cells (Fig. 2D, d) after the boost at weeks 26 and 30.
Figure 2. Gag-specific CD8+ and CD4+ T cell responses.
PBMCs from vaccinated RMs were tested by ICS for Gag-specific T cells producing IFN-γ, TNF-α, or IL-2. Graphs show T cell frequencies for each animal after subtraction of background for different time-points after priming (i.e., vaccination with SIVΔnef or the first dose of AdHu5). (A) Results for total T cells, (B) Teff, (C) TEM, and (D) TCM. The graphs (a and b) show results for CD8+ T cells and (c and d) CD4+ T cells. (a and c) Results for SIVΔnef-immunized animals; (b and d) results for AdHu5-vaccinated animals.
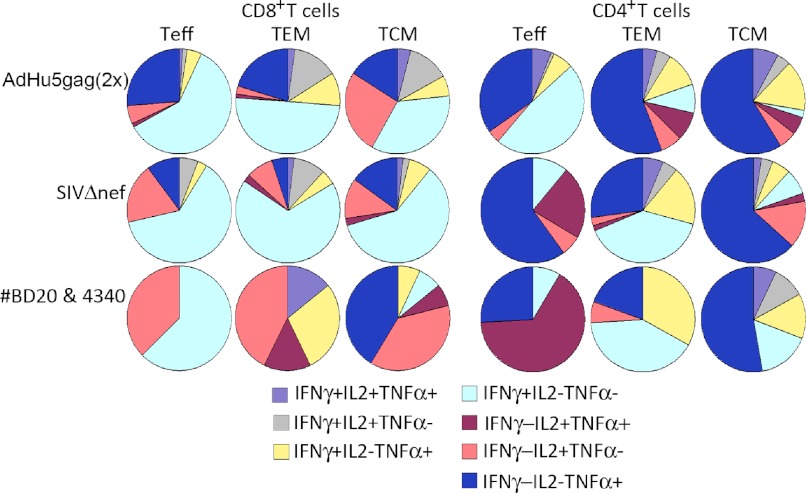
T cell functionality was assessed by measuring production of IFN-γ, TNF-α, and IL-2 in response to the Gag peptide pool. Results for week 13 after priming, i.e., week 3 after the second dose of AdHu5gag, are shown in Fig. 3. Average distribution of Gag-specific responses of the different CD8+ and CD4+ T cell subsets is shown for all of the AdHu5gag-vaccinated RMs, whereas for SIVΔnef-inoculated RMs, results from the four animals that became infected and the two that remained uninfected are shown separately. By week 13 after immunization, the functional profiles of Gag-specific CD8+ T cell responses in AdHu5gag-vaccinated RMs and unprotected SIVΔnef-inoculated animals were similar, with the only difference consisting of higher levels of IFN-γ only producing CD8+ T cells in TEM and TCM of SIVΔnef-inoculated RMs. Interestingly, more pronounced differences were observed between protected and unprotected RMs of the SIVΔnef group. Protected RMs showed increased frequencies of SIV-specific CD8+ T cells producing IL-2 alone or in combination with other cytokines as compared with unprotected animals of Groups 1 and 2. Within the CD4+ T cell population, the main difference was that protected RMs showed a higher frequency of IL-2- and TNF-α-producing CD4+ Teff cells as compared with unprotected SIVΔnef-vaccinated animals.
Figure 3. Cytokine profiles of Gag-specific CD8+ and CD4+ T cells subsets.
The graphs show averages for all of the AdHu5gag-vaccinated animals in the top rows, for the four SIVΔnef-vaccinated RMs that became infected in the middle rows, and for the two SIVΔnef-vaccinated RMs that resisted infection in the lower rows. Results were obtained with samples harvested 13 weeks after the first vaccine dose. The different colors of the pie chart reflect the relative percentages of T cells producing cytokines or a combination of cytokines: light purple, cells producing IFN-γ, IL-2, and TNF-α; gray, cells producing IFN-γ and IL-2; yellow, cells producing IFN-γ and TNF-α; light blue, cells producing IFN-γ; magenta, cells producing IL-2 and TNF-α; pink, cells producing IL-2; dark blue, cells producing TNF-α.
SIVΔnef induced antibodies to Env
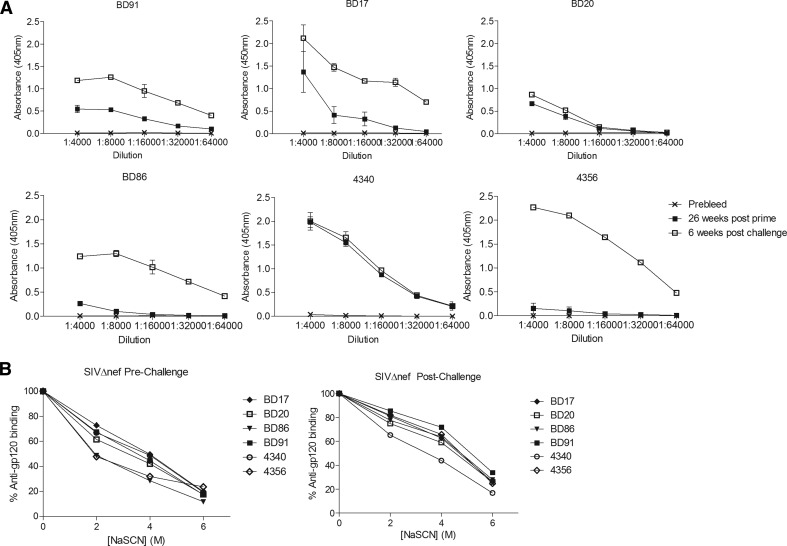
To determine the level of humoral immune responses induced by SIVΔnef inoculation, Group 1 RMs were tested for the presence of circulating antibodies to gp160 by an ELISA. Figure 4A shows results for individual RMs tested just prior to challenges and then at 8 weeks after they had been challenged 10 times. Prior to challenges, all RMs developed detectable Env-binding antibodies, demonstrating that all animals had become infected. Two of the animals, i.e., BD86 and 4356, had comparatively low titers. After challenges, four RMs showed increased antibody titers whereas the two animals that resisted infection, i.e., BD20 and 4340, did not develop a booster response.
Figure 4. gp160-Specific antibody responses in SIVΔnef-vaccinated RMs.
(A) Antibody titers: plasma samples were harvested before (×), 26 weeks after vaccination (■), and 6 weeks after completion of challenges (▫) and tested for antibody responses to SIVmac239 Gp160 by an ELISA. Graphs show average results ± sd for each sample for individual animals. (B) Antibody avidity: plasma samples harvested 32 weeks after vaccination and 8 weeks after completion of challenges were diluted in PBS/3% BSA/0.05% Tween-20 and incubated in wells coated with Gp160. Wells were then incubated with various concentrations of NaSCN. Graphs show percent adsorbance (determined by adding an enzyme-labeled secondary antibody and then substrate) of anti-Gp160 antibodies in the presence of different concentrations of NaSCN as compared with binding without NaSCN (the latter normalized to 100%). Symbols identifying samples from specific animals are shown next to the graphs.
The avidity of NHP sera of SIVΔnef-immunized RMs was tested in pre- and postchallenge samples by measuring the amount of antibodies that remained bound to an ELISA plate coated with gp160 after addition of increasing concentrations of NaSCN (Fig. 4B). Sera of all RMs, except for #4340, collected postchallenge, showed increased avidity compared with sera collected prior to challenge. Antibodies in prechallenge sera from RMs BD91, BD20, and 4340 had higher avidity to gp160 compared with those from the other three animals.
Repeated low-dose rectal challenges with SIVmac251
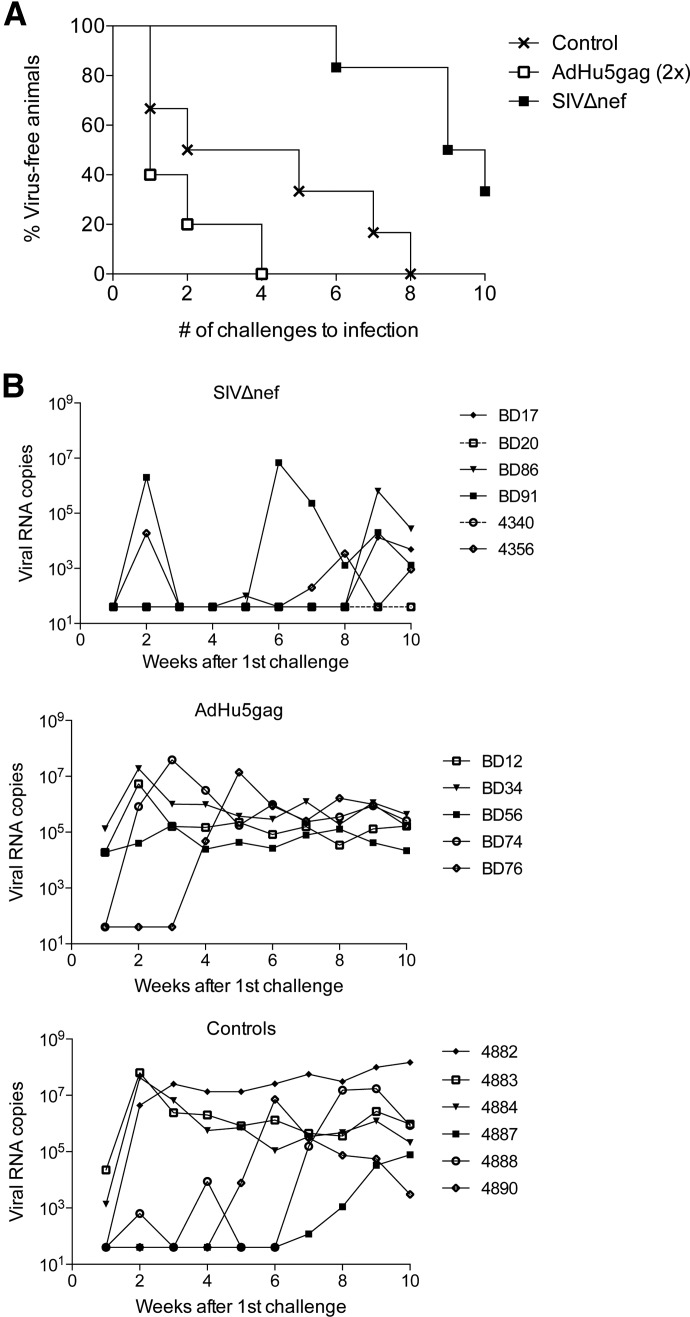
All RMs included in this study were challenged intrarectally 10 times at a week interval with a low dose of SIVmac251. During this period and for an additional 8 weeks afterward, viral loads were measured by RT-PCR. As expected, all of the control RMs became infected after the seventh challenge, requiring, on average, 3.8 doses of virus (median=3.5) until infection. AdHu5gag-vaccinated RMs showed a trend toward lower resistance to SIVmac251, with all animals becoming infected after the fourth challenge (Fig. 5A), requiring, on average, 1.8 doses of virus (median=1). Whether the trend toward accelerated infection related to increased CD4+ T cell responses at the port of viral entrance in the AdHu5 group, which could potentially provide targets for SIV infection, remains to be investigated. In the SIVΔnef group, two RMs tested positive for SIV sequences after the second challenge but became negative again the following week (Fig. 5B). However, Nef sequence-specific PCR analysis of week 2 plasma samples indicated that the observed viral blips most likely reflected reactivation of SIVΔnef rather than de novo infection with SIVmac251. By the sixth challenge, one animal, BD91, became infected with SIVmac251. This animal had an average antibody response to Env and was only remarkable in that his numbers of Gag-specific CD8+ T cells were very low before challenges. An additional three SIVΔnef-inoculated RMs became infected by the ninth or 10th challenges, i.e., BD17, BD86, and 4356. Of those, 4356 showed a viral blip at weeks 7 and 8, which most likely originated from the vaccine virus, as a PCR was negative for Nef sequences. The two remaining animals, i.e., BD20 and 4340, remained uninfected, as again, the Nef sequence-specific PCR indicated that the observed low levels of viral RNA originated from SIVΔnef rather than from SIVmac251. The average number of challenges for SIVΔnef-vaccinated animals that remained susceptible was 8.5 (median=9). These two “protected” RMs did not mount a SIV-specific B or T cell recall response. By 8 weeks after completion of challenges, the viral loads of infected RMs were similar among groups (Fig. 5B). We next compared the number of challenges required for infection among the three groups using a log-rank test. AdHu5gag was not significantly different from controls (nominal P=0.248); the SIVΔnef group was 1.5× less likely to get infected after each challenge compared with controls (Bonferroni corrected P=0.033) and 4.0× less likely compared with the AdHu5gag (Bonferroni corrected P=0.012) group. Taken together, these data indicate that SIVΔnef inoculation, but not AdHu5gag vaccination, conferred protection from low-dose intrarectal SIVmac251 challenge.
Figure 5. Time to infection and viral loads upon SIVmac251 challenges.
Vaccinated and control RMs were challenged 10 times with SIVmac251 given rectally. Viral titers were measured, and for SIVΔnef-vaccinated RMs, positive samples were retested by a nef-specific nested PCR. (A) Percent of animals that remained virus-free after each challenge. (B) Viral titers measured after each challenge. Transient viral spikes in SIVΔnef-immunized RMs and low titers at the end in BD20 and 4340 could not be amplified by the nef-specific PCR. The two animals of the SIVΔnef group that resisted infection are shown with dotted lines.
Gene expression analyses: data overview
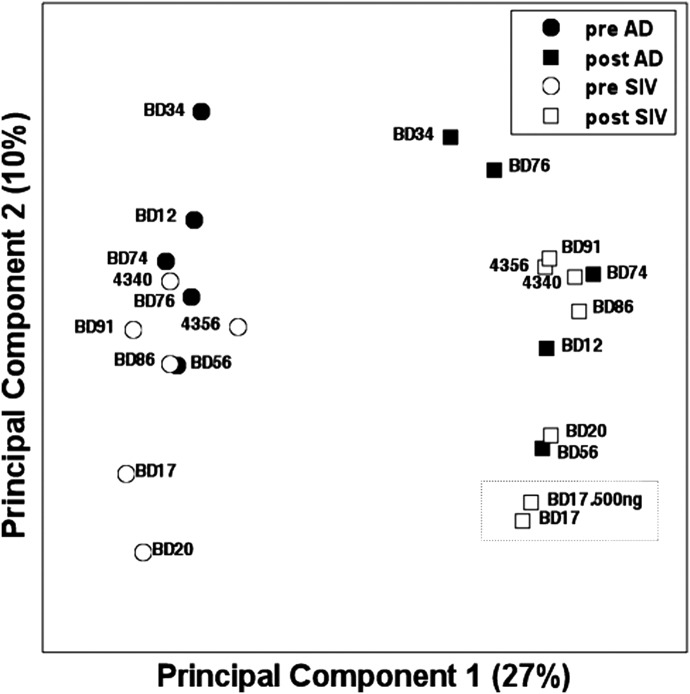
As none of the immunological assays that we performed to measure SIV-specific immune responses allowed us to identify clear correlates of vaccine-induced resistance to infection, we analyzed gene expression in pre- and postvaccination PBMC samples using RNA-Seq to identify differences in transcriptional patterns induced by the SIVΔnef and AdHu5gag vector that might correlate with resistance (or lack thereof) to challenge. RNA isolated from PBMC collected before and 26 weeks (BD34 and BD86) or 30 weeks (remaining animals) after vaccination were analyzed by RNA-Seq. Aliquots of the same samples were tested for frequencies of T cell subsets, including Gag-specific T cells and non-T cell populations, and showed no significant differences between the two vaccine groups. An initial PCA performed using data from all detected transcripts (Fig. 6) was carried out to assess gene expression differences among the study animals. Although differences were not evident in the functional studies described above, PCA revealed that clear, major differences in global gene expression existed between pre- and postvaccination samples, and this effect was evident for the AdHu5gag and SIVΔnef vaccine groups. Additional data analysis studies were then carried out to assess these differences.
Figure 6. PCA performed on pre- and postvaccinated PBMC samples using expression of all 19,062 informative transcripts.
Samples are plotted against first and second principal components and Pc1-captured common differences between effects of vaccines, i.e., differences between pre- and postvaccination samples. Dotted box shows two replicates from the same sample done with different initial RNA concentrations.
Effects of vaccination on gene expression signatures
We identified 734 transcripts that significantly changed at least twofold between pre- and postvaccination samples in RMs that received the AdHu5gag vaccine (P<0.005; FDR=2%) and 1841 transcripts that changed at least twofold between pre- and postinoculation samples in the SIVΔnef group (P<0.005; FDR=0.7%). There was a significant (P<10−12 by hypergeometric test) overlap of 463 transcripts between the two comparisons that were affected by both vaccines, but that number is 63% of genes that changed upon AdHu5gag vaccination and only 25% of changed genes in SIVΔnef-inoculated RMs. The large number of changes in gene expression specific to SIVΔnef reflects the more pronounced immunological effect of SIVΔnef. Although SIVΔnef is known to persist, we were unable to detect any SIV transcripts in PBMC RNA of RMs that had received this virus.
We next identified pathways that the differentially expressed genes represented using IPA. Pathways that were modulated upon vaccination in both groups (with FDR<5%) are shown in Supplemental Table 2. Both groups showed common differences before versus after vaccination in pathways indicative for activation of T and B cells and cells of the innate immune system, as well as several metabolic pathways. Changes in those pathways were more pronounced in the SIVΔnef group. For example, whereas overall changes in chemokine signaling affecting leukocyte trafficking patterns were similar pre- versus postvaccination in both groups, signaling through CCR5, in particular, was markedly reduced in the SIVΔnef group (14/79 genes; P=0.002) and only moderately reduced in the AdHu5gag group (six of 79 genes; P=0.03). Genes associated with CXCR4 signaling were also increased in both, but activation of this pathway was more pronounced in the SIVΔnef group (24/159 genes; P=0.003) than in the AdHu5gag group (11/159 genes; P=0.02). Of note, we found no statistically significant pathways that were uniquely affected by the AdHu5gag vaccine. By contrast, eight pathways were affected significantly in SIVΔnef-vaccinated RMs (FDR<5%) but not in AdHu5gag-vaccinated RMs (nominal P>0.05). Several of these pathways were linked to apoptosis, such as Huntington's disease signaling (38 out of 228 genes), Nur77 signaling, which specifically controls T cell apoptosis (13/57 genes), myc-mediated apoptosis signaling (13/60 genes), or OX40 signaling (12/61 genes). Consistent with an overall increase in apoptotic pathways was an effect on pathways associated with xenobiotic metabolism signaling and the cellular stress response to exogenous or endogenous toxin (36/265 genes). Other pathways that were modulated upon SIVΔnef vaccination reflected effects on cell mobility or migration, such as CDC42 signaling, which affects actin localization and directed cell movement (23/143 genes; P=0.0007), and glycerophospholipid metabolism, which also affects cell movement by producing a crucial component of the cell membrane and controlling actin localization (20/119 genes; P=0.003).
Postvaccination gene expression and correlation with protection from challenge
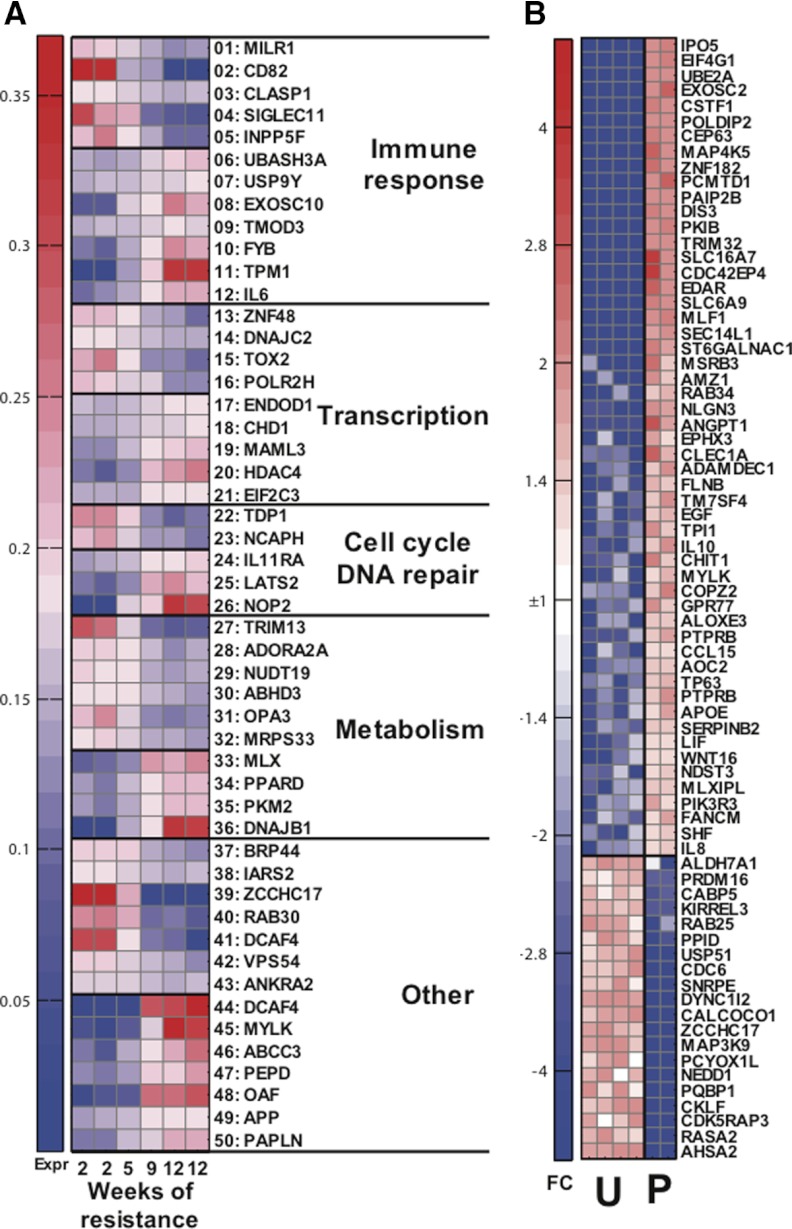
We next attempted to correlate resistance to infection (i.e., number of challenges needed to achieve infection) with gene expression profiles measured after vaccination but prior to challenge. We identified genes whose expression was highly correlated with weeks of protection in postvaccination samples of the AdHu5gag and SIVΔnef groups individually. There were 185 genes that were highly correlated (Pearson |r|>0.95) with delayed infection in AdHu5gag postvaccine samples. However, 46 of those 185 genes had also shown correlation with protection (Pearson |r|>0.8), in AdHu5gag prevaccine samples. In contrast, for genes highly correlated with protection in SIVΔnef postvaccine samples (77 genes with |r|>0.95), there were only four genes that held at least |r| > 0.8 for prevaccination samples. This is a highly significant difference between vaccines (P=0.0002 by Fisher exact test) and indicates that SIVΔnef samples exhibit primarily acquired changes in gene expression as a result of vaccination, which are connected to protection. A significantly higher proportion of genes in AdHu5gag-vaccinated RMs were correlated with protection, even without taking into account the effects of vaccination, and most likely reflecting genetic resistance factors in these animals rather than vaccine-induced protective mechanisms. Upon SIVΔnef inoculation, genes that correlated with protection expressed in prevaccination samples (four genes) were different from those that were seen in the AdHu5gag vaccination group (46 genes). Out of those 50 vaccine-independent, protective genes, 34 had at least 10 mapped sequence reads, had known functions, and are listed in Supplemental Table 3. Interestingly, the SIVΔnef group showed a reduction in expression of the Flapendonuclease 1 gene that encodes for a diacylglycerol kinase involved in DNA replication and repair and may promote HIV-1 integration [19]. In the AdHu5gag group, vaccine-independent resistance to infection correlated with gene products that play a role in immune responses, transcription, translation, metabolism, and others. Among these, we observed: (i) increased expression of the Duffy antigen/chemokine receptor, which encodes the Duffy antigen, known to decrease resistance to infection with plasmodium and may decrease susceptibility to HIV-1 infections [20]; (ii) increased expression of STAU1, Staufen homolog 1, which encodes a dsRNA-binding protein that may help encapsidation of the HIV-1 genome [21]; and (iii) decreased expression of the NACA, an α-chain of the nascent-polypeptide-associated complex, which encodes a polypeptide that has been reported to reduce oxidative stress to HIV-1 infection [22]. Differences in vaccine-induced transcripts in the SIVΔnef vaccine group (Fig. 7A and Supplemental Table 4) included those that could be linked to adaptive and innate immunity, transcription, translation, cell cycle and DNA repair, metabolism, and others. Of note, we observed a strong correlation between protection and expression of IL-6 and FYB. This latter Fyn-binding protein gene encodes an adaptor molecule, which through the Fyn pathway, modulates IL-2 expression and may regulate transport of HIV-1 viral protein X from the nucleus [23]. CD82, which can act as a T cell costimulator and may facilitate fusion and cell entry of HIV-1 particles [24], was reduced in the more resistant SIVΔnef-inoculated RMs. Noteworthy were also positive correlations with histone deacetylase 4, which may be involved in DNA repair following HIV-1 integration [25], and chromodomain-helicase-DNA-binding protein 1, a chromatin-remodeling factor that promotes HIV-1 integration [26].
Figure 7. Expression of transcripts induced by SIVΔnef vaccines and indicative of protection.
(A) Groups of transcripts correlated with weeks of resistance, also listed in Supplemental Table 4. (B) Transcripts differentially expressed between two protected (P) and among four unprotected (U) animals, also listed in Supplemental Table 5. FC, fold change.
Postvaccination profile gene expression of protected SIVΔnef-inoculated RMs
We next directly assessed differences between postvaccination gene expression profiles of PBMCs derived from protected (n=2) versus nonprotected (n=4) SIVΔnef-inoculated RMs. A total of 864 transcripts showed significance at P < 0.05, and Fig. 7B displays the expression heatmap for 74 more expressed transcripts (10 reads or more in at least one sample) that differed at least threefold between protected and unprotected animals, with details for individual genes changed at least fivefold, as shown in Supplemental Table 5. Nineteen genes showed reduced expression in protected animals, and three could potentially be linked to immune responses: (i) CKLF, a chemokine-like factor that may be involved in inflammatory responses; (ii) MAP3K9, which activates the Jun pathway downstream of TCR signaling; and (iii) RAB25, a RAS family member that promotes cell migration. We also noted a decrease in SNRPE, a gene that encodes a small nuclear ribonucleoprotein polypeptide E, which as part of the U7 small nuclear ribonucleoprotein complex, interacts with HIV-1 tat [22]. Several of the genes, whose expressions were increased in protected RMs, could relate to innate or adaptive immunity. Three are associated with DC functions—i.e., TM7SF4, transmembrane 7 superfamily member 4, which is down-regulated upon CD40 ligation in DCs; ADAMDEC1, a disintegrin and metalloprotease -like decysin 1, which becomes up-regulated following DC maturation; and CLEC1A, C-type lectin domain family 1, member A, which may play a role in DC functions. Another gene, CHIT1, chitinase 1 (chitotriosidase), is up-regulated upon macrophage maturation. Several of the increased transcripts reflected chemotactic factors or chemokine receptors, such as IL-8, LIF, the chemokine receptor CCL15, and GPCR 77. The latter encodes a receptor for chemotactic and inflammatory peptides of the complement cascade. Two other gene products—EDAR, the ectodysplasin A receptor, which promotes activation of JNK and NF-κB, and MAP4K5, which is induced by environmental stress and activates the Jun pathway—are related to inflammatory responses. Only two genes may reflect adaptive immune responses—i.e., IL-10, a Th2 cytokine that is produced by T cells and cells of the innate immune system, down-regulates Th1-linked responses and promotes B cell responses; and PIK3R3, one of the regulatory subunits of phosphoinositide-3-kinase that is instrumental in linking TCR signaling to the AKT/mammalian target of rapamycin pathway, which in turn, adjusts cell metabolism to meet the increased energetic demands of activated lymphocytes. Accordingly, several genes involved in sugar and lipid metabolism were up-regulated in protected animals, specifically, SLC16A7, a solute carrier protein, which removes monocarboxylic acid produced during glycolysis; TPI1, triosephosphate isomerase 1, which plays a role in glycolysis and glucogenesis; MLXIPL, Max-like protein-interacting protein-like, which binds to carbohydrate response element sequences in promoters of triglyceride synthesis genes; APOE, apolipoprotein E, which mediates the binding, internalization, and catabolism of lipoprotein particles; and ALOXE3, arachidonate lipoxygenase 3, which introduces molecular oxygen into polyunsaturated fatty acids. Several of the increased genes have been linked previously to the life cycle of HIV-1. LIF and SEC14L1, SEC14-like protein 1, have been reported to inhibit replication of HIV-1 [27, 28]; SERPINB2, serpin peptidase inhibitor, clade B (OVA), member 2, enhances HIV-1 replication [29]; and TRIM32, a zinc finger protein, interacts with HIV-1 Tat [30].
DISCUSSION
In this study, we compared the immunogenicity and protection from repeated low-dose intrarectal SIVmac251 challenge in two groups of RM vaccinated with SIVΔnef or AdHu5gag. The rationale for selecting a live attenuated SIV vaccine was based on previous studies showing at least partial efficacy in preventing viral acquisition, thus enabling us to investigate potential correlates of protection as compared with an AdHu5 vector expressing only Gag, which was not expected to confer protection from SIV acquisition. We then set to identify potential correlates of protection at the cellular immunology level by comparing SIV-specific T and B cell responses and at the molecular/transcriptional level in PBMCs obtained at baseline and after vaccination (i.e., shortly before challenges). We compared RMs belonging to the two vaccine groups with animals that were resistant versus susceptible to infection within the same group.
As expected, SIVΔnef-immunized RMs were relatively resistant to SIVmac251 challenges, although in the end, four out of six became infected, thus demonstrating that even a live attenuated vaccine that is effective against high-dose i.v. SIVmac239 challenge provides incomplete resistance to viral acquisition upon repeated low-dose rectal challenges with SIVmac251. Whereas there were statistically significant differences in the number of challenges needed to infect SIVΔnef-inoculated RMs as compared with AdHu5gag-vaccinated or control animals, the fact that the majority of SIVΔnef-immunized RMs became infected demonstrates the stringency of the chosen challenge model.
In this study, T cell responses specific to Gag, i.e., the most abundantly expressed protein of SIV, were measured by ICS. The analysis showed CD4+ T cell responses were transiently higher at some of the tested time-points before challenges in AdHu5gag-immunized RMs. There were no statistically significant differences in challenge frequencies of CD8+ Teff or TEM cells between resistant or susceptible animals, thus suggesting that vaccine-induced CD8+ T cell responses do not correlate with protection in this experimental model. This finding is in contrast to results obtained with other vaccines, and in particular, with vaccination with a recombinant rhesus CMV expressing SIV antigens, whose protection was linked to vaccine-induced CD8+ TEM cell responses [5]. Similarly, our data do not provide strong evidence in support of the role of specific features of the vaccine-induced CD4+ T cell response as a correlate of protection from SIVmac251 challenge, although they do suggest that increased frequencies of Gag-specific CD4+ TCM cells do not contribute to vaccine-induced resistance against SIVmac251 challenges.
In this study, we also assessed the presence of Env-specific antibodies, although this analysis was limited to the SIVΔnef-inoculated RMs, as no Env immunogens were included in the AdHu5 group. All of the SIVΔnef-immunized RMs developed antibodies to Env, which in nonprotected animals, increased upon challenges. One of the protected RMs (i.e., 4340) had the highest titers, whereas the other (i.e., BD20) had titers comparable with susceptible RMs, such as BD91, which was the first RM of the SIVΔnef vaccine group to develop a sustained SIVmac251 infection. Prechallenge testing of the two protected animals as well as two of the susceptible animals (i.e., BD91 and BD17) had higher avidity antibodies to gp160 compared with two of the other animals. The lack of a correlation between Env-specific antibody titers or avidity to gp160 and protection contrasts with data obtained in which increased resistance to rectal challenges with SIVmac251 was obtained upon vaccinations with human serotype 26 Ad vectors used in combination with modified vaccinia Ankara vectors; in this study, protection correlated with antibodies, especially those to the V2 loop of Env [4]. Whereas the current set of results does not suggest that the levels of Env-specific binding (i.e., non-neutralizing) antibodies play a major role in protecting from SIVmac251 challenge, the relatively low number of animals does not allow us to reach a firm conclusion in that regard.
Although the analysis of potential correlates of resistance to SIVmac251 challenge failed to identify markers that emerged by conventional immunological assays (i.e., ICS and serology), a comparative, longitudinal analysis of the effects of the used vaccines on the transcriptional profiles of PBMCs revealed more interesting results. AdHu5gag and SIVΔnef had a significant effect on gene expression, as in both groups, pre- and postvaccination samples clustered separately by PCA gene expression profiling. However, the two vaccine groups failed to form distinct clusters from each other pre- or postvaccination. Pathway analyses suggested that both vaccines affected similar pathways that play roles in adaptive and innate immunity, although postvaccination changes of most pathways were more pronounced in SIVΔnef-immunized RMs. Pathways that were altered only significantly in SIVΔnef-immunized RMs were apoptotic pathways, as well as pathways related to cell migration and mobility. Interestingly, relative resistance to SIV acquisition within the AdHu5gag group was largely linked to differences in gene expression profiles that were already present prior to vaccination, thus suggesting a key role played by genetic factors. In contrast, relative resistance to SIV acquisition in the SIVΔnef-immunized group was mainly related to vaccination, even though only few of the vaccine-induced resistance markers of the SIVΔnef group corresponded to genes with known functions in innate or adaptive immunity. The most significant differentiator was IL-6, which is an inflammatory cytokine that is increased upon HIV-1 infection and whose increase in SIVΔnef infected that animals may reflect virus persistence. Only one of the up-regulated genes was indicative of B cell functions, i.e., EXOSC10, exosome component 10, which is involved in Ig class-switching and hypermutation of Ig variable genes, whereas several factors involved in T cell responses (i.e., CD82; CLASP1, cytoplasmic linker-associated protein 1; and INPP5F, inositol polyphosphate-5-phosphatase F) were down-regulated. It is possible that more gene expression changes related to adaptive immunity could be detected by conducting an analysis that includes more frequent blood sampling and/or sampling of tissues such as bone marrow, LNs, and rectal biopsies. Remarkably, several of the genes that correlated with relative resistance (i.e., number of challenges needed to obtain infection) or that distinguished the two completely protected animals from the others in the SIVΔnef group have known effects on HIV-1 replication and infectivity.
Previous studies have analyzed the transcriptome of PBMCs or T cell subsets from vaccinated RMs prior to their challenge with SIV. A study analyzing the transcriptome of RMs immunized with DNA vaccines, given alone or with a chemokine adjuvant [31] and comparing postvaccination samples between immunized and control animals, revealed differences in the expression of a number of genes that were distinct from those linked to resistance in our current study. Another study used a replication-competent AdHu5 vaccine given mucosally, followed by a protein boost [32]. This vaccine regimen, which did not protect against acquisition of SHIV89.6P but rather reduced viral loads postinfection, showed that reduced viral loads correlated with increases in the expression of genes related to B cell development and lymphocyte survival.
As SIVΔnef establishes a benign but persisting infection, we next compared our data set with those that evaluated gene expression profiles, which showed sustained control of virus replication as well as those that progressed rapidly following HIV-1 infection, in HIV-infected humans. In one study [33], human elite controllers compared with rapid progressors showed a reduction of genes of the IFN response. A similar observation [33] was made comparing T cells from SIV-infected RMs with those of AIDS-resistant SMs, which are the natural host of SIV and experience chronic nonpathogenic infection. RMs and SMs developed a rapid IFN response after infection, which within weeks, returned to baseline in SMs but remained up-regulated in RMs. Pathway analyses in the SIVΔnef-inoculated RMs revealed significant differences in IFN signaling (P=0.005) and activation of IRF by cytosolic PRRs (P=0.0009) but failed to support the possibility that decreases in IFN responses prior to challenges contributed to protection. Specifically, only four of the IFN response transcripts that were linked to resistance in humans were differentially expressed in protected versus nonprotected SIVΔnef-immunized RMs, i.e., IFIT5, IFN-induced protein with tetratricopeptide repeats 5 (difference in expression between protected and nonprotected RMs: 1.5), IRF8 (−2.0), IRF9 (−1.4), and SOCS3, suppressor of cytokine signaling (1.8). Along the same lines, only two of the genes that differentiated SIV-infected RMs from SMs were shown to be significant in our analyses, i.e., CCR2, which was increased 2.2-fold in the two protected RMs of our study, and TRIM25, which was moderately (1.2-fold) decreased in IFN-induced protein with tetratricopeptide repeat 5 in the more resistant AdHu5gag-vaccinated animals.
In summary, the current study, which was only conducted with small numbers of RMs, confirmed that vaccination with SIVΔnef provides some degree of protection against viral acquisition following repeated low-dose rectal SIVmac251 challenges, whereas vaccination with an AdHu5gag vaccine designed to induce only antiviral T cell responses is ineffective. Although conventional immunological assays failed to reveal a clear correlate of protection, analyses of gene expression profiles prior to challenges revealed changes in a number of transcripts encoding host proteins that have previously been linked to the host immune response or the HIV-1/SIV life cycle. Further analysis of these genes and gene products, preferentially with larger numbers of animals, will provide further information on their potential role as correlates of vaccine-induced protection from SIV acquisition.
ACKNOWLEDGMENTS
This work was supported by P01 AI082282 from the U.S. National Institute of Allergy and Infectious Diseases/Division of Acquired Immunodeficiency Syndrome. The project used the Wistar Institute Genomics and Bioinformatics facilities, supported by a Wistar Cancer Center support grant (P30 CA010815).
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
- Ad
- adenoviral
- AdHu5
- adenovirus of human serotype 5
- AdHu5gag
- AdHu5 expressing gag of SIVmac239
- APC
- allophycocyanin
- Bac
- baculovirus
- CD62L
- CD62 ligand
- Env
- envelope protein
- FDR
- false discovery rate
- FPKM
- fragments/kb transcript/million sequencing reads
- ICS
- intracellular cytokine staining
- IPA
- Ingenuity Pathway Analysis
- IRF
- IFN-regulatory factor
- LIF
- leukemia inhibitory factor
- MAP3K9/MAP4K5
- MAPKK kinase
- NaSCN
- sodium thiocyanate
- NHP
- nonhuman primate
- PCA
- principle component analysis
- RM
- rhesus macaque
- SIVΔnef
- nef deletion mutant of SIVmac239
- SM
- sooty mangabey
- TCM
- central memory T cells
- Teff
- effector T cells
- TEM
- effector memory T cells
- TRIM
- tripartite motif
AUTHORSHIP
R.K., S.T., and A.V.K. conducted experiments and wrote the paper. M.S., L.H.H., and M.O.L. conducted experiments. S.J.R. performed biostatistics. S.E.B., D.G.C., and L.C.S. performed analyses. M.L. supervised the NHP study. L.C.S., G.S., and H.C.J.E. designed experiments and wrote paper.
REFERENCES
- 1. Hu S. L., Stamatatos L. (2007) Prospects of HIV Env modification as an approach to HIV vaccine design. Curr. HIV Res. 5, 507–513 [DOI] [PubMed] [Google Scholar]
- 2. Roux K. H., Taylor K. A. (2007) AIDS virus envelope spike structure. Curr. Opin. Struct. Biol. 17, 244–252 [DOI] [PubMed] [Google Scholar]
- 3. Gray E. S., Moore P. L., Choge I. A., Decker J. M., Bibollet-Ruche F., Li H., Leseka N., Treurnicht F., Mlisana K., Shaw G. M.., et al. (2007) Neutralizing antibody responses in acute human immunodeficiency virus type 1 subtype C infection. J. Virol. 81, 6187–6196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barouch D. H., Liu J., Li H., Maxfield L. F., Abbink P., Lynch D. M., Iampietro M. J., SanMiguel A., Seaman M. S., Ferrari G.., et al. (2012) Vaccine protection against acquisition of neutralization-resistant SIV challenges in rhesus monkeys. Nature 482, 89–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hansen S. G., Vieville C., Whizin N., Coyne-Johnson L., Siess D. C., Drummond D. D., Legasse A. W., Axthelm M. K., Oswald K., Trubey C. M.., et al. (2009) Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nat. Med. 15, 293–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shiver J. W., Fu T. M., Chen L., Casimiro D. R., Davies M. E., Evans R. K., Zhang Z. Q., Simon A. J., Trigona W. L., Dubey S. A.., et al. (2002) Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature 415, 331–335 [DOI] [PubMed] [Google Scholar]
- 7. Wilson N. A., Keele B. F., Reed J. S., Piaskowski S. M., MacNair C. E., Bett A. J., Liang X., Wang F., Thoryk E., Heidecker G. J.., et al. (2009) Vaccine-induced cellular responses control simian immunodeficiency virus replication after heterologous challenge. J. Virol. 83, 6508–6521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yin J., Dai A., Kutzler M. A., Shen A., Lecureux J., Lewis M. G., Waldmann T., Weiner D. B., Boyer J. D. (2008) Sustained suppression of SHIV89.6P replication in macaques by vaccine-induced CD8+ memory T cells. AIDS 22, 1739–1748 [DOI] [PubMed] [Google Scholar]
- 9. Buchbinder S. P., Mehrotra D. V., Duerr A., Fitzgerald D. W., Mogg R., Li D., Gilbert P. B., Lama J. R., Marmor M., Del Rio C.., et al. (2008) Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet 372, 1881–1893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rerks-Ngarm S., Pitisuttithum P., Nitayaphan S., Kaewkungwal J., Chiu J., Paris R., Premsri N., Namwat C., de Souza M., Adams E.., et al. (2009) Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N. Engl. J. Med. 361, 2209–2220 [DOI] [PubMed] [Google Scholar]
- 11. Johnson R. P., Desrosiers R. C. (1998) Protective immunity induced by live attenuated simian immunodeficiency virus. Curr. Opin. Immunol. 10, 436–443 [DOI] [PubMed] [Google Scholar]
- 12. Casimiro D. R., Wang F., Schleif W. A., Liang X., Zhang Z. Q., Tobery T. W., Davies M. E., McDermott A. B., O'Connor D. H., Fridman A.., et al. (2005) Attenuation of simian immunodeficiency virus SIVmac239 infection by prophylactic immunization with DNA and recombinant adenoviral vaccine vectors expressing Gag. J. Virol. 79, 15547–15555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lasaro M. O., Haut L. H., Zhou X., Xiang Z., Zhou D., Li Y., Giles-Davis W., Li H., Engram J. C., Dimenna L. J.., et al. (2011) Vaccine-induced T cells provide partial protection against high-dose rectal SIVmac239 challenge of rhesus macaques. Mol. Ther. 19, 417–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Showe M. K., Vachani A., Kossenkov A. V., Yousef M., Nichols C., Nikonova E. V., Chang C., Kucharczuk J., Tran B., Wakeam E.., et al. (2009) Gene expression profiles in peripheral blood mononuclear cells can distinguish patients with non-small cell lung cancer from patients with nonmalignant lung disease. Cancer Res. 69, 9202–9210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Goecks J., Nekrutenko A., Taylor J. (2010) Galaxy: a comprehensive approach for supporting accessible, reproducible, and transparent computational research in the life sciences. Genome Biol. 11, R86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Trapnell C., Pachter L., Salzberg S. L. (2009) TopHat: discovering splice junctions with RNA-Seq. Bioinformatics 25, 1105–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Trapnell C., Williams B. A., Pertea G., Mortazavi A., Kwan G., van Baren M. J., Salzberg S. L., Wold B. J., Pachter L. (2010) Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 28, 511–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Storey J. D., Tibshirani R. (2003) Statistical methods for identifying differentially expressed genes in DNA microarrays. Methods Mol. Biol. 224, 149–157 [DOI] [PubMed] [Google Scholar]
- 19. Rumbaugh J. A., Fuentes G. M., Bambara R. A. (1998) Processing of an HIV replication intermediate by the human DNA replication enzyme FEN1. J. Biol. Chem. 273, 28740–28745 [DOI] [PubMed] [Google Scholar]
- 20. Ramsuran V., Kulkarni H., He W., Mlisana K., Wright E. J., Werner L., Castiblanco J., Dhanda R., Le T., Dolan M. J.., et al. (2011) Duffy-null-associated low neutrophil counts influence HIV-1 susceptibility in high-risk South African black women. Clin. Infect. Dis. 52, 1248–1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chatel-Chaix L., Clement J. F., Martel C., Beriault V., Gatignol A., DesGroseillers L., Mouland A. J. (2004) Identification of Staufen in the human immunodeficiency virus type 1 Gag ribonucleoprotein complex and a role in generating infectious viral particles. Mol. Cell. Biol. 24, 2637–2648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Price T. O., Uras F., Banks W. A., Ercal N. (2006) A novel antioxidant N-acetylcysteine amide prevents gp120- and Tat-induced oxidative stress in brain endothelial cells. Exp. Neurol. 201, 193–202 [DOI] [PubMed] [Google Scholar]
- 23. Singhal P. K., Rajendra Kumar P., Subba Rao M. R., Mahalingam S. (2006) Nuclear export of simian immunodeficiency virus Vpx protein. J. Virol. 80, 12271–12282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Krementsov D. N., Weng J., Lambele M., Roy N. H., Thali M. (2009) Tetraspanins regulate cell-to-cell transmission of HIV-1. Retrovirology 6, 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Smith J. A., Yeung J., Kao G. D., Daniel R. (2010) A role for the histone deacetylase HDAC4 in the life-cycle of HIV-1-based vectors. Virol. J. 7, 237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gallastegui E., Millan-Zambrano G., Terme J. M., Chavez S., Jordan A. (2011) Chromatin reassembly factors are involved in transcriptional interference promoting HIV latency. J. Virol. 85, 3187–3202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tjernlund A., Walther-Jallow L., Behbahani H., Screpanti V., Nowak P., Grandien A., Andersson J., Patterson B. K. (2007) Leukemia inhibitor factor (LIF) inhibits HIV-1 replication via restriction of stat 3 activation. AIDS Res. Hum. Retroviruses 23, 398–406 [DOI] [PubMed] [Google Scholar]
- 28. Urano E., Ichikawa R., Morikawa Y., Yoshida T., Koyanagi Y., Komano J. (2010) T cell-based functional cDNA library screening identified SEC14-like 1a carboxy-terminal domain as a negative regulator of human immunodeficiency virus replication. Vaccine 28 (Suppl. 2), B68–B74 [DOI] [PubMed] [Google Scholar]
- 29. Darnell G. A., Schroder W. A., Gardner J., Harrich D., Yu H., Medcalf R. L., Warrilow D., Antalis T. M., Sonza S., Suhrbier A. (2006) SerpinB2 is an inducible host factor involved in enhancing HIV-1 transcription and replication. J. Biol. Chem. 281, 31348–31358 [DOI] [PubMed] [Google Scholar]
- 30. Fridell R. A., Harding L. S., Bogerd H. P., Cullen B. R. (1995) Identification of a novel human zinc finger protein that specifically interacts with the activation domain of lentiviral Tat proteins. Virology 209, 347–357 [DOI] [PubMed] [Google Scholar]
- 31. Belisle S. E., Yin J., Shedlock D. J., Dai A., Yan J., Hirao L., Kutzler M. A., Lewis M. G., Andersen H., Lank S. M.., et al. (2011) Long-term programming of antigen-specific immunity from gene expression signatures in the PBMC of rhesus macaques immunized with an SIV DNA vaccine. PLoS One 6, e19681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Palermo R. E., Patterson L. J., Aicher L. D., Korth M. J., Robert-Guroff M., Katze M. G. (2011) Genomic analysis reveals pre- and postchallenge differences in a rhesus macaque AIDS vaccine trial: insights into mechanisms of vaccine efficacy. J. Virol. 85, 1099–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rotger M., Dalmau J., Rauch A., McLaren P., Bosinger S. E., Martinez R., Sandler N. G., Roque A., Liebner J., Battegay M.., et al. (2011) Comparative transcriptomics of extreme phenotypes of human HIV-1 infection and SIV infection in sooty mangabey and rhesus macaque. J. Clin. Invest. 121, 2391–2400 [DOI] [PMC free article] [PubMed] [Google Scholar]