Abstract
Vasospasm following aneurysmal subarachnoid hemorrhage (SAH) occurs in the extraparenchymal vessels in the subarachnoid space at the base of the brain. Ischemia/ Infarction affecting primarily the perforator vessels in isolation, following aneurysmal SAH is uncommon. A 28-year-old man with a ruptured middle cerebral artery aneurysm underwent clipping of the aneurysm. He developed delayed bilateral deep seated infarcts involving both internal capsular regions, the thalamus and basal ganglia without any major vessel infarct. The patient was managed with triple H (hypertensive hypervolemic hemodilutional) therapy and calcium channel antagonists but did not show any improvement and remained in poor neurological status. Perforator vasospasm occurring secondary to aneurysmal SAH, though documented in experimental animal studies, has rarely been reported in humans in a clinical setting. The present case provides evidence, albeit indirect, of isolated perforator vasospasm, which possibly should be the target of future therapeutic strategies.
Keywords: Aneurysmal subarachnoid haemorrhage, Infarcts, perforator vasospasm
Introduction
Vasospasm following subarachnoid hemorrhage (SAH) due to aneurysmal rupture primarily affects the larger basal intracerebral arteries.[1] The intraparenchymal course of penetrating arteries supposedly protects them from thick blood clot and therefore isolated lenticulostriate or thalamoperforating infarcts are rare. Direct clinical evidence of spasm of perforators of brain in human has rarely been reported, though has been shown in animal models.[2,3]
Case Report
A 28 -year- old man was admitted with SAH of 24 h duration in Hunt and Hess grade III, WFNS grade II. The admission blood pressure was around 150/90 mm Hg. The CT scan head showed subarachnoid hemorrahge mainly in the right sylvian fissure (both lateral and basal), suprasellar, anterior interhemispheric fissure and ambient cistern. The amount of blood in subarachnoid space according to Hijdra scale was 11.[4] There was intraventricular hemorrhage and the modified Graeb score was 19.[5] CT-Angiogram demonstrated a right middle cerebral artery (MCA) bifurcation aneurysm [Figure 1]. The patient was operated upon and the aneurysm was clipped within 36 h after ictus. In the post-operative period, he was conscious but disoriented and had spontaneous motor activity of all 4 limbs. Serial CT scans done in the post-operative period [Figure 1] showed gradual resolution of the intracerebral and intraventricular blood. There was no infarct or hydrocephalus. The GCS was 14 in post-operative period and remained in the same status till postictal day (i.e. GCS: 13). He was on triple H therapy [hypervolaemia (Central venous pressure >10cm), hemodilution (Hematocrit < 35) and hypertension (systolic BP> 180)] and oral nimodipine (60mg four hourly).
Figure 1.
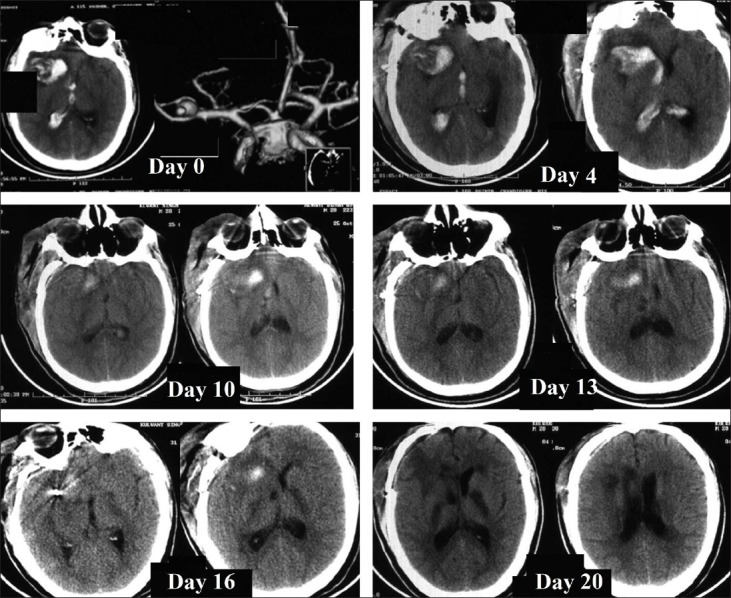
CT scan on Day 0 shows subarachnoid hemorrhage with intraventricular and intraparenchymal hemorrhage. CT angio on day 0 showed right middle cerebral artery aneurysm. On day 4 and 10, CT scan showed resolving hematoma without hydrocephalus. On day 13, an infarct was seen in the right basal ganglia with resolving hematoma. On day 16 and 20, bilateral infarcts were seen in both the basal ganglia and internal capsule.
The patient deteriorated on day 13 of ictus and developed left hemiplegia with a little deterioration in the conscious level (GCS 13). CT scan done on the same day (12 h later) demonstrated ill defined hypodensities in the internal capsular region and in right anterior thalamic area. [Figure 1] The patient had no episode of hypotension or hypoxia. The electrolytes and other metabolic parameters were within normal limits. There were no seizures. The systolic BP was raised up to 200mmHg. The transcranial doppler revealed normal flow velocities. Unfortunately, CT angiogram could not be done due to non availability of software to avoid clip artifacts. Digital subtraction angiogram was not attempted in view of rapid deterioration. Despite raising the blood pressure further, the patient continued to deteriorate progressively over the next 7-10 days and had decerebrate motor response. Serial CT scans over this period [Figure 1] showed well defined infarcts developing in both internal capsules and right thalamus without any infarct in major vessel territory, suggesting no major vessel spasm. He showed no clinical improvement and remained static. The modified Rankin scale score was 5 at 6 months follow-up.
Discussion
Cerebral vasospasm in aneurysmal SAH is usually considered as narrowing of angiographically visible large extraparenchymal vessels. The morphological and microcirculatory changes in the perforating intraparenchymal vessels after SAH and their role in development of cerebral ischemia in clinical setting is still not fully understood.[1]
In our patient, the development of bilateral multiple deep infarcts in the brain involving the internal capsules and basal ganglia coinciding with clinical deterioration, suggests isolated perforator involvement. These infarcts were seen when the mass effect secondary to cerebral hemorrhage was regressing and the basal cisterns and surface sulci were well visualized, therefore, it is unlikely that they were pressure related. Delayed deterioration and development of bilateral infarcts without involvement of any major vessel territory, ruled out direct complication of surgical procedure. Moreover, the complication first appeared 13 days after surgery suggesting that the time course of involvement of perforating vessels may be different from that of major extraparenchymal vessels.
It is possible that vasospasm affecting penetrating of arteries may account for the paradoxical phenomenon that occurs in some clinical cases, like clinical deterioration without angiographic vasospasm or failure of clinical improvement following angioplasty or improvement with drugs like Nimodipine despite the absence of apparent effect on angiographic vasospasm.[2,3]
MRI has shown that delayed ischemic lesions after SAH are usually bilateral, multifocal tending to involve the territory of deep perforating arteries though many of them are asymptomatic.[6] These deep subcortical lesions do not match with the Transcranial doppler and angiographic findings, suggesting a mechanism other than that causing vasospasm of larger vessels. Experimental data in animal models has demonstrated endothelial dysfunction and histopathological evidence of luminal narrowing in intraparenchymal small arteries.[6] Moreover, preliminary data from human studies indicate that autoregulatory responses are impaired after SAH and microcirculatory changes manifested by prolonged cerebral circulation time may lead to decreased regional cerebral blood flow. Furthermore, microembolism could contribute to the occurrence of small infarcts in patients with SAH.[6]
The case reported here provides some clinical evidence in humans, though indirect, supporting the possible occurrence of isolated vasospasm in penetrating arteries. Unfortunately, other causes of isolated perforator ischemia could not be ruled out due to lack of angiography and no response to raised blood pressure. Perforator spasm and ischemia following subarachnoid hemorrhage needs further research and should be the target of future therapeutic strategies.
Footnotes
Source of Support: Nil.
Conflict of Interest: Nil.
References
- 1.Ohkuma H, Itoh K, Shibata S, Suzuki S. Morphological changes of intraparenchymal arterioles after experimental subarachnoid haemorrhage in Dogs. Neurosurgery. 1997;91:230–6. doi: 10.1097/00006123-199707000-00036. [DOI] [PubMed] [Google Scholar]
- 2.Zubkov AY, Tibbs RE, Aoki K, Zhang JH. Morphological changes of cerebral penetrating arteries in a canine double haemorrhage model. Surg Neurol. 2000;54:212–20. doi: 10.1016/s0090-3019(00)00305-0. [DOI] [PubMed] [Google Scholar]
- 3.Zubkov AY, Tibbs RE, Aoki K, Zhang JH. Prevention of vasospasm in penetrating arteries with MAPK inhibition in Dog.Double Haemorrhage Model. Surg Neurol. 2000;54:221–8. doi: 10.1016/s0090-3019(00)00290-1. [DOI] [PubMed] [Google Scholar]
- 4.Hijdra A, Brouwers PJ, Vermeulen M, van Gijn J. Grading the amount of blood on computed tomograms after subarachnoid hemorrhage. Stroke. 1990;21:1156–61. doi: 10.1161/01.str.21.8.1156. [DOI] [PubMed] [Google Scholar]
- 5.Hwang BY, Bruce SS, Appelboom G, Piazza MA, Carpenter AM, Gigante PR, et al. Evaluation of intraventricular hemorrhage assessment methods for predicting outcome following intracerebral hemorrhage. J Neurosurg. 2012;116:185–92. doi: 10.3171/2011.9.JNS10850. [DOI] [PubMed] [Google Scholar]
- 6.Rabinstein AA, Weigand S, Atkinson JL, Wijdicks EF. Patterns of cerebral infarction in aneurysmal subarachnoid hemorrhage. Stroke. 2005;36:992–7. doi: 10.1161/01.STR.0000163090.59350.5a. [DOI] [PubMed] [Google Scholar]


