Abstract
Objective
Obtaining real-time image is essential for neurosurgeons to minimize invasion of normal brain tissue and to prompt diagnosis of intracranial event. The aim of this study was to report our three-year experience with a mobile computed tomography (mCT) for intraoperative and bedside scanning.
Methods
A total of 357 mCT (297 patients) scans from January 2009 to December 2011 in single institution were reviewed. After excluding post-operative routine follow-up, 202 mCT were included for analysis. Their medical records such as diagnosis, clinical application, impact on decision making, times, image quality and radiologic findings were assessed.
Results
Two-hundred-two mCT scans were performed in the operation room (n=192, 95%) or intensive care unit (ICU) (n=10, 5%). Regarding intraoperative images, extent of resection of tumor (n=55, 27.2%), degree of hematoma removal (n=42, 20.8%), confirmation of catheter placement (n=91, 45.0%) and monitoring unexpected complications (n=4, 2.0%) were evaluated. A total of 14 additional procedures were introduced after confirmation of residual tumor (n=7, 50%), hematoma (n=2, 14.3%), malpositioned catheter (n=3, 21.4%) and newly developed intracranial events (n=2, 14.3%). Every image was obtained within 15 minutes and image quality was sufficient for interpretation.
Conclusion
mCT is feasible for prompt intraoperative and ICU monitoring with enhanced diagnostic certainty, safety and efficiency.
Keywords: Computed tomography, Intensive care unit
INTRODUCTION
Navigation-guided procedure has been widely conducted in the neurosurgical filed to perform anatomy-oriented approach8). However, the approach cannot reflect the real-time intracranial image due to differential spatial displacement caused by cerebrospinal fluid drainage, volume change after surgical evacuation or newly developed lesions such as aggravation of brain edema or increased hematoma14). Therefore, intraoperative scanning including computed tomography (CT), magnetic resonance imaging (MRI) and ultrasound sonography have been adapted to obtain real-time findings for operators to minimize invasion of normal brain tissue.
Considering consumed time, mobility, without major reconstruction of current facility in operative room (OR) or intensive care unit (ICU), objectivity and costs, mobile CT (mCT) can be a more appropriate method13).
Since the first introduction of mCT as an intraoperative monitoring modality in our institution in late 2008, four mCT have been installed in Korea. Besides the established use of mCT in the OR, this apparatus can allow neurosurgeons to make appropriate management decisions in the ICU by obtaining relevant imaging data more safely and rapidly.
The purpose of this study was to report our 3-year experience with mCT under various clinical circumstances focusing on indications and advantages.
MATERIALS AND METHODS
The study was approved by institutional review board for the ethical committee for human research. This retrospective analysis was performed in patients who had mCT for evaluation of intracranial lesions in OR and ICU from January 2009 to December 2011. Their medical charts including diagnosis, clinical implications, further treatment, consumed times, image quality and result were reviewed.
mCT has been used with the following criteria in our hospital : 1) Decision-making for the assessment of the residual tumor or hematoma; 2) Reduction of uncertainty in the process of surgery including suspected catheter malplacement, close observation for the change of hematoma in opposite site or sudden brain swelling; 3) Prompt diagnosis and management in ICU.
Image acquisition
mCT parameters were 120 kV, 7 mA, effective radiation dose of 1.7 mSv, 10-mm-wide collimated beam and eight 1.25-mm-wide detectors with an acquisition time of 2 s5). With regard to enhancement, a total of 90 mL of contrast agent (Iopromide, Ultravist; Schering AG, Berlin, Germany) was injected through an anterior cubital vein with a 4 mL/s flow rate with a 30 s X-ray time delay. Post-processing of mCT data was simultaneously conducted on a portable workstation. The images shown as multiplanar reformation in the axial, coronal and sagittal views were reconstructed. Every mCT was performed with the CereTom (Neurologica, Danvers, MA, USA).
RESULTS
A total of 297 patients, with a male : female ratio of 1.47 : 1.0, and a mean±SD age of 57.9±17.2 years, underwent 357 mCT as an intraoperative or bedside scan. Data of 202 cases of mCT (OR, n=192, 95%; ICU, n=10, 5%) were included for review and analyses of the study after excluding post-operative routine follow-up in the OR.
With regard to intra-operative images, extent of tumor resection (n=55, 27.2%), degree of hematoma evacuation (n=42, 20.8%), confirmation of catheter placement (n=91, 45.0%) and evaluation of complications (n=4, 2.0%) were checked. An additional 14 procedures were introduced after confirmation of residual tumor (n=7, 50%), hematoma (n=2, 14.3%), malplaced catheters (n=3, 21.4%) and new developed intracranial events (n=2, 14.3%).
Detailed information on use of bedside CT scanning among 10 patients included : ventilator (n=8, 80%), sedation fluid (n=7, 70%), vasopressor administration (n=6, 60%), post-operative drains (n=7, 70%) and mean Glasgow Coma Scale score of 7.4 (range, 5-11). All images were obtained within 15 min by three staff members with sterile preparations. The quality of mCT image was sufficient in decision-making. The clinical application of mCT is summarized in Table 1.
Table 1.
The summary of clinical application of mobile CT in this study
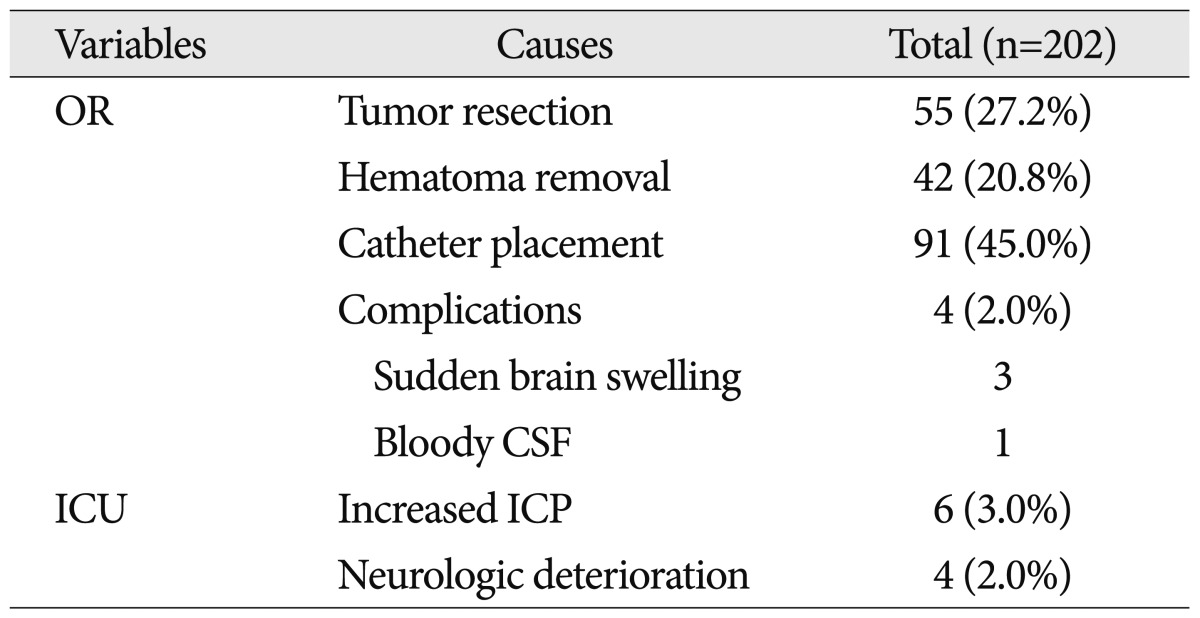
OR : operative room, ICU : intensive care unit, ICP : intracranial pressure, CSF : cerebrospinal fluid
Illustrative case 1
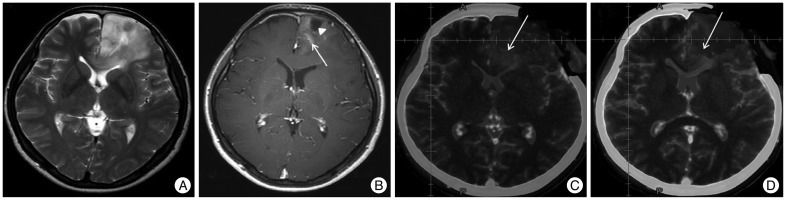
A 47-year-old female was referred to our institution for further management of diffuse astrocytoma. Axial T2WI magnetic resonance (MR) showed a diffuse hyperintense white matter mass with anterior hypointensity lesion in the left frontal lobe. Infiltrative margin with cortex and genu of the corpus callosum was observed (Fig. 1A). Contrast-enhanced axial T1WI MR revealed focal enhancement (white arrow) and low signal lesion (arrowhead), suggestive of cystic change (Fig. 1B). Intraoperative CT-MR image fusion revealed a residual mass (white arrow) located close to anterior corpus callosum (Fig. 1C). Fused CT-MR image demonstrated complete resection of remnant tumor (arrowhead) without damage to corpus callosum (Fig. 1D).
Fig. 1.
Mobile CT for diffuse astrocytoma. A and B : Axial T2WI magnetic resonance (MR) and Contrast-enhanced axial T1WI MR show a diffuse hyperintense lesion in the left frontal lobe with focal enhancement (white arrow) and low signal lesion (arrowhead). C : Intra-operative CT-MR image fusion discloses a residual mass (white arrow) close to anterior corpus callosum. D : Fused CT-MR image illustrates complete resection of remnant tumor (arrow) without injury to corpus callosum.
Illustrative case 2
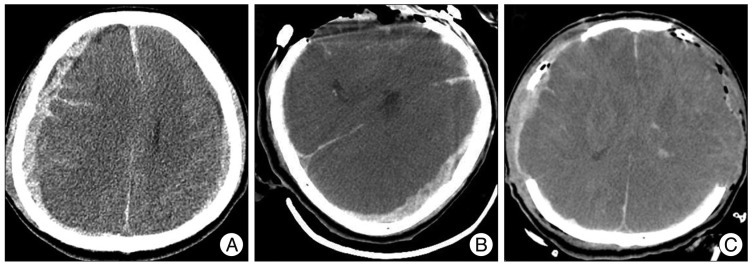
A 49-year-old male was transferred for deterioration in conscious level. Initial CT showed acute subdural hemorrhage on both side with midline shift to the left (Fig. 2A). After mCT scan, additional surgical evacuation of hematoma was performed on the left side due to brain shift towards the right (Fig. 2B, C).
Fig. 2.
Mobile CT for the detection of increased subdural hematoma in the contralateral side. A : Initial CT showed acute subdural hemorrhage on both side with midline shift to the left. B : Mobile CT scan revealed the brain shift towards the right by increased hematoma. C : Subdural hematoma on the left side was evacuated without delay.
Illustrative case 3
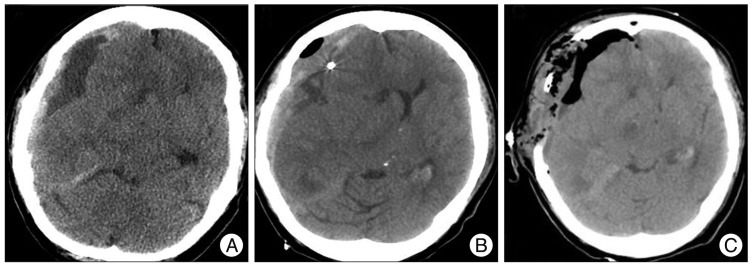
A 74-year-old female presented with headache. Intracranial CT revealed mixed density of subdural hemorrhages (Fig. 3A). Burr-hole craniotomy and closed system drainage was conducted under local anesthesia. Just before leaving the OR, the patient vomited and the patient's condition deteriorated. Emergent mCT showed acute subdural hematoma (Fig. 3B) and an open craniotomy followed without delay (Fig. 3C).
Fig. 3.
Mobile CT (mCT) for the detection of acute hematoma on the same side of operation. A : Initial CT revealed mixed density of subdural hemorrhages, and followed by closed drainage insertion under local anesthesia. B : Emergent mCT showed acute subdural hematoma on the operation side. C : Open craniotomy and hematoma evacuation was performed without delay.
DISCUSSION
Real-time monitoring of change in brain is particular crucial in decision-making when uncertainty is present in various clinical settings including residual tumor, malplacement of drainage catheter or combination with other intra-cranial lesions during the procedures, as well as increased intracranial pressure or deteriorated in conscious level in ICU. Our experience with mCT demonstrates that more complete and safe surgery can be achieved in OR and prompt diagnosis and proper management in ICU by obtaining relevant imaging data more safely and rapidly.
Fifty-five (28.6%) mCT procedures were conducted for estimating tumor resection. Among them, additional tumor removals for seven cases were performed. This result agrees with that of Gumprecht and Lumenta2). The feasibility of mCT for assessing residual suprasellar tumors such as macroadenoma or meningioma and cerebellopontine angle tumor including vestibular schwanoma has been described in our previous report5). On the other hand, mCT has relative limitation for depicting poorly enhanced lesion such as low grade glioma5). However, with reconstruction of three-dimensional images from mCT data, image fusion based on preoperative MRI findings can provide more valuable information on tumor margin assessment (Fig. 1C, D). Hosoda et al.3) reported that surgical resection with intraoperative CT prolongs survival by acquiring more complete surgical resection, which was confirmed during or immediately after the procedure.
External ventricular drainage (EVD) is a widely performed neurosurgical procedure under anatomic landmarks, but freehand EVD does not show high accuracy1). Saladino et al.9) described 26 cases (12.3%) malplaced ventriculostomy catheters of EVD and shunt; five (2.4%) of required additional adjustment. In our cohort, three cases of incomplete intra-ventricular catheter apposition were retargeted. With the guidance of mCT, more precise operations such as unusual insertion to the cyst or Ommaya reservoir placement can be attained5). Not limited to the neurosurgical filed, mCT can be used in other surgeries including reconstruction surgery of skull base, orbital wall12) and sinus surgery4). Another advantage of mCT is cost. Masaryk et al.7) suggested that the total economic benefit of mCT for 5 years would be more than US$2 million dollars due to dedication of conventional CT for outpatients without unnecessary delay. In addition, cost savings can be achieved by eliminating needless complications in transfer patients. No data regarding the incidence of adverse events in transfer process for CT imaging has been reported in Korea. Smith et al.11) described that untoward incidents can be observed in up to 71% of ICU cases. Therefore, post-operative patients with unstable vital signs are more likely to have complications due to their multiple connections with intravenous lines and drains. The average procedure times of conventional CT scanning from ordering to arrival at ICU was 28 min (range, 18-35 min) in our institution. Mean transport time for CT image from operation room to ICU was 16 min (range, 14-27 min). This result may agree with that of other university hospitals because of their similar location of ICU, OR and CT rooms. In addition, considering the current status in Korea, conveyance of patients for neuroimaging is usually conducted with an intern doctor or a physician assistant. If case critical life events happen during the transfer, appropriate management cannot be done. In comparison with conventional intracranial CT, interruption for mCT scanning can be limited to within 15 min. Less manpower to shift the patients was presently needed for mCT (three staff in mCT vs. four-to-six staff in routine CT examination). Consequently, mCT can reduce the workload of the ICU staff members7).
Concerns remain about radiation exposure of mCT. Our protocol comprised an effective radiation dose of 1.7 mSV (approximately 45 mGy), which was lower or comparable with conventional head CT scan of 60 mGy10) or 47 mGy of multi-detector CT scan in our hospital. Because of the short source image distance, scattered radiation in mCT can be within acceptable limits for radiation safety as other portable X-ray devices in OR and ICU.
Incidence of infection after mCT remains unclear. Five patients who underwent mCT experienced surgical infection in our series. Three of them had cerebrospinal fluid (CSF) leakage, and two did re-operation due to delayed hemorrhage. Korinek et al.6) reported that CSF leakage and early re-operation were independent risk factors of infection after craniotomy. Thus, we could not confirm mCT as a cause of infection since the patient had high risk factor such as CSF leakage and re-operation. Therefore, prospective data about infection rate associated with mCT are needed further.
There are some shortcomings in the clinical application of mCT. Contrast-enhanced image of mCT is done by manual timing. Thus, suboptimal enhancement of cerebral vascular structure can be obtained in cases of cardiac dysfunction or vascular stenosis, which affect cerebral blood flow velocity7). And algorithm enhancing image quality of mCT angiography needs to be investigated further.
CONCLUSION
Our 3-year experience with mCT shows that more safe and complete surgery can be achieved without the need for transfer in the OR as well as faster diagnosis and proper management in the ICU.
Acknowledgements
We thank Hee Jeong Kim, Sung Eun Kim, and Min-Jae Cho for help with data collection, valuable contribution to image preparation and review of the image acquisition data.
References
- 1.Abdoh MG, Bekaert O, Hodel J, Diarra SM, Le Guerinel C, Nseir R, et al. Accuracy of external ventricular drainage catheter placement. Acta Neurochir (Wien) 2012;154:153–159. doi: 10.1007/s00701-011-1136-9. [DOI] [PubMed] [Google Scholar]
- 2.Gumprecht H, Lumenta CB. Intraoperative imaging using a mobile computed tomography scanner. Minim Invasive Neurosurg. 2003;46:317–322. doi: 10.1055/s-2003-812496. [DOI] [PubMed] [Google Scholar]
- 3.Hosoda T, Takeuchi H, Hashimoto N, Kitai R, Arishima H, Kodera T, et al. Usefulness of intraoperative computed tomography in surgery for low-grade gliomas : a comparative study between two series without and with intraoperative computed tomography. Neurol Med Chir (Tokyo) 2011;51:490–495. doi: 10.2176/nmc.51.490. [DOI] [PubMed] [Google Scholar]
- 4.Jackman AH, Palmer JN, Chiu AG, Kennedy DW. Use of intraoperative CT scanning in endoscopic sinus surgery : a preliminary report. Am J Rhinol. 2008;22:170–174. doi: 10.2500/ajr.2008.22.3153. [DOI] [PubMed] [Google Scholar]
- 5.Kim JW, Lee SH, Son YJ, Yang HJ, Chung YS, Jung HW. Mobile computed tomography : early experience in Korea. J Korean Neurosurg Soc. 2010;48:31–36. doi: 10.3340/jkns.2010.48.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Korinek AM, Golmard JL, Elcheick A, Bismuth R, van Effenterre R, Coriat P, et al. Risk factors for neurosurgical site infections after craniotomy : a critical reappraisal of antibiotic prophylaxis on 4,578 patients. Br J Neurosurg. 2005;19:155–162. doi: 10.1080/02688690500145639. [DOI] [PubMed] [Google Scholar]
- 7.Masaryk T, Kolonick R, Painter T, Weinreb DB. The economic and clinical benefits of portable head/neck CT imaging in the intensive care unit. Radiol Manage. 2008;30:50–54. [PubMed] [Google Scholar]
- 8.Nakao N, Nakai K, Itakura T. Updating of neuronavigation based on images intraoperatively acquired with a mobile computerized tomographic scanner : technical note. Minim Invasive Neurosurg. 2003;46:117–120. doi: 10.1055/s-2003-39344. [DOI] [PubMed] [Google Scholar]
- 9.Saladino A, White JB, Wijdicks EF, Lanzino G. Malplacement of ventricular catheters by neurosurgeons : a single institution experience. Neurocrit Care. 2009;10:248–252. doi: 10.1007/s12028-008-9154-z. [DOI] [PubMed] [Google Scholar]
- 10.Shrimpton PC, Hillier MC, Lewis MA, Dunn M. National survey of doses from CT in the UK : 2003. Br J Radiol. 2006;79:968–980. doi: 10.1259/bjr/93277434. [DOI] [PubMed] [Google Scholar]
- 11.Smith I, Fleming S, Cernaianu A. Mishaps during transport from the intensive care unit. Crit Care Med. 1990;18:278–281. doi: 10.1097/00003246-199003000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Stieve M, Schwab B, Haupt C, Bisdas S, Heermann R, Lenarz T. Intraoperative computed tomography in otorhinolaryngology. Acta Otolaryngol. 2006;126:82–87. doi: 10.1080/00016480510040119. [DOI] [PubMed] [Google Scholar]
- 13.Uhl E, Zausinger S, Morhard D, Heigl T, Scheder B, Rachinger W, et al. Intraoperative computed tomography with integrated navigation system in a multidisciplinary operating suite. Neurosurgery. 2009;64:231–239. doi: 10.1227/01.NEU.0000340785.51492.B5. discussion 239-240. [DOI] [PubMed] [Google Scholar]
- 14.Willems PW, van der Sprenkel JW, Tulleken CA, Viergever MA, Taphoorn MJ. Neuronavigation and surgery of intracerebral tumours. J Neurol. 2006;253:1123–1136. doi: 10.1007/s00415-006-0158-3. [DOI] [PubMed] [Google Scholar]